Abstract
Background
Deregulation of microRNA may contribute to hematopoietic malignancies. MicroRNA-196b (miR-196b) is highly expressed in MLL-rearranged leukemia and has been shown to be activated by MLL and MLL-fusion genes.
Design and Methods
In order to determine whether high expression of miR-196b is restricted to MLL-rearranged leukemia, we used quantitative stem-loop reverse transcriptase polymerase chain reaction to measure the expression of this microRNA in 72 selected cases of pediatric acute lymphoblastic leukemia i.e. MLL-rearranged and non-MLL-rearranged precursor B-cell and T-cell acute lymphoblastic leukemias. We also determined the expression of HOXA-genes flanking miR-196 by microarray and real-time quantitative polymerase chain reaction. Furthermore, we used CpG island-arrays to explore the DNA methylation status of miR-196b and HOXA.
Results
We demonstrated that high expression of miR-196b is not unique to MLL-rearranged acute lymphoblastic leukemia but also occurs in patients with T-cell acute lymphoblastic leukemia patients carrying CALM-AF10, SET-NUP214 and inversion of chromosome 7. Like MLL-rearrangements, these abnormalities have been functionally linked with up-regulation of HOXA. In correspondence, miR-196b expression in these patients correlated strongly with the levels of HOXA family genes (Spearman’s correlation coefficient ≥ 0.7; P≤0.005). Since miR-196b is encoded on the HOXA cluster, these data suggest co-activation of miR-196b and HOXA genes in acute lymphoblastic leukemia. Up-regulation of miR-196b coincides with reduced DNA methylation at CpG islands in the promoter regions of miR-196b and the entire HOXA cluster in MLL-rearranged cases compared to in cases of non-MLL precursor B-cell acute lymphoblastic leukemia and normal bone marrow (P<0.05), suggesting an epigenetic origin for miR-196b over-expression. Although patients with MLL-rearranged acute lymphoblastic leukemia are highly resistant to prednisolone and L-asparaginase, this resistance was not attributed to miR-196b expression.
Conclusions
High expression of miR-196b is not exclusively MLL-driven but can also be found in other types of leukemia with aberrant activation of HOXA genes. Since miR-196b has been shown by others to exert oncogenic activity in bone marrow progenitor cells, the findings of the present study imply a potential role for miR-196b in the underlying biology of all HOXA-activated leukemias.
Keywords: miR-196b, HOXA, acute lymphoblastic leukemia
Introduction
MicroRNA (miRNA) were discovered to be small non-protein coding RNA molecules that post-transcriptionally regulate the expression of many protein-coding genes by complementary binding to their targeted mRNA.1 Subsequently, the bound mRNA is cleaved or, in the majority of cases, its translation into protein is repressed.2 Despite their name, miRNA play major roles in biological processes such as cell proliferation, differentiation and apoptosis and aberrant activities of miRNA have been found in a variety of malignancies. For example, let-7 has been identified as a tumor suppressor since let-7 down-regulates the expression of the oncoprotein Ras. Consequently, reduced expression of let-7 miRNA was associated with an increased expression of Ras in patients with lung cancer, which may explain their unfavorable prognosis.3,4
Recent studies also showed that aberrantly expressed miRNA contribute to hematopoietic malignancies. For example, over-expression of the oncogenic miR-17–92 polycistron accelerated the formation of lymphomas in an Eμ-Myc transgenic mice model.5 This miR-17–92 cluster is often amplified in human B-cell lymphomas, suggesting a role in lymphomagenesis.6 Enforced expression of miR-155 in Eμ-miR-155 transgenic mice resulted in preleukemic pre-B-cell proliferation followed by mature B-cell leukemia, implying a leukemogenic contribution of miR-155.7 Epigenetic silencing of miR-124a caused up-regulation of its target CDK6 in acute lymphoblastic leukemia (ALL), which may drive proliferation of leukemia cells, and is associated with higher relapse and mortality rates among patients with ALL.8
In general, the 5-year disease-free survival is 85% for children with ALL on contemporary treatment protocols. However, patients with some subtypes such as those with a rearrangement of the Mixed Lineage Leukemia (MLL) gene and BCR-ABL1-positive ALL have a much worse 5-year disease-free survival rate of approximately 50%.9–11 We previously observed aberrant miRNA expression patterns in different genetic subtypes of pediatric ALL.12 One of the miRNA that was most aberrantly expressed in MLL-rearranged cases was miR-196b. The expression level of miR-196b was up-regulated by 500- to 800-fold in the majority of cases of MLL-rearranged precursor B-ALL and in about one-third of selected T-ALL cases compared to precursor B-ALL patients without MLL translocations. In addition, the expression of miR-196b in these cases of leukemia was also higher than in normal bone marrow cells.12 Interestingly, in a recent report it was postulated that miR-196b may be involved in leukemogenesis and that its expression is induced by normal MLL and MLL fusion products such as MLL-AF4.13 The MLL-AF4 fusion is most frequently found in infants with MLL-rearranged leukemia,14 who were included in our previous study.12 MLL normally regulates the expression of the homeobox domain (HOX) gene family which plays an important role in regulating normal hematopoiesis.15 The HOXA cluster genes, and especially HOXA4, HOXA5, HOXA9 and HOXA10, are over-expressed in MLL-rearranged ALL.16–18 However, aberrant expression of HOXA genes is not restricted to MLL-rearranged precursor B-ALL cases and has also been reported to occur in T-ALL patients carrying MLL- or HOXA-rearrangements e.g. inversion of chromosome 7, or fusion products including CALM-AF10 and SET-NUP214.19,20 As miR-196b is mapped between the HOXA9 and HOXA10 genes on chromosome 7p15.2, the level of expression of miR-196b may be linked to HOXA gene transcription, irrespective of the HOXA activating mechanism (MLL fusions or other factors). To test this hypothesis, we measured the expression levels of miR-196b and HOX gene-family members in cases of MLL-rearranged and non-MLL-rearranged precursor B-ALL and T-ALL carrying different genetic abnormalities leading to HOXA gene activation. In addition, since the level of expression of various miRNA may be regulated by gene methylation,21 we investigated the methylation status upstream of the miR-196b locus in MLL-rearranged cases. Since MLL-rearranged ALL cases are often highly resistant to prednisolone and L-asparaginase,22 two drugs that form major components of current ALL treatment, we also investigated whether miR-196b expression levels were linked to responsiveness to these two drugs.
Design and Methods
Patients’ samples
Leukemic cell samples from children with newly diagnosed ALL were obtained after informed consent from the children’s parents or guardians and approval by the institutional review board. The immunophenotype of the samples was determined by flow cytometry (T-ALL or precursor B-ALL), and the genetic subtype by fluorescence in situ hybridization (FISH) and/or reverse transcriptase polymerase chain reaction (PCR).11,20 In total 12 MLL-rearranged precursor B-ALL i.e. five patients carrying t(4;11), six patients carrying t(11;19) and one positive for t(9;11), 38 non-MLL precursor B-ALL and 22 T-ALL cases were included. The HOXA-linked T-ALL subgroup consisted of selected cases characterized by the fusion genes MLL-AF6 (n=2), CALM-AF10 (n=5) and SET-NUP214 (n=3) as well as one case with an inversion of chromosome 7 [inv(7)(p15q35)]. The HOXA-negative T-ALL group consisted of TAL/LMO-rearranged (n=4), TLX3-rearranged (n=2) and T-ALL cases negative for the above-mentioned abnormalities (n=5). Mononuclear cells were isolated from bone marrow or peripheral blood samples using sucrose density centrifugation.23,24 The percentage of leukemic cells was determined on May-Grünwald-Giemsa (Merck, Darmstadt, Germany) stained cytospins. If the percentage was below 90%, samples were enriched by eliminating non-malignant cells with immunomagnetic beads.23,24
Quantitative stem-loop real-time polymerase chain reaction analysis of miRNA and HOXA expression levels
Total RNA was extracted with TRIzol reagent (Invitrogen, Leek, the Netherlands) according to the manufacturer’s guidelines with minor modifications as described before.25 The 2100 bioanalyzer (Agilent, Amstelveen, the Netherlands) was used to determine the quality of total RNA. All RNA samples had an RNA integrity number of 7.5 or greater. MiR-196b expression was measured by real-time quantitative PCR (RT-qPCR) using a specific stem-loop primer and probe combination designed by Applied Biosystems, USA.26 Endogenous small nucleolar RNA 1 (RNU24) was used as the reference for small RNA-input. The levels of expression of HOXA3, HOXA9 and HOXA10 transcripts were quantified relative to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using cDNA synthesized from total RNA, as described previously.20 Primer and probe sequences are presented in Online Supplementary Table S1. All RT-qPCR were performed on an Applied Biosystems 7900HT system. Details on sample preparation, primers, probes and the real-time procedure are given in the Online Supplementary Design and Methods.
Gene expression microarray analysis
Affymetrix U133A and U133 plus 2.0 GeneChips (Santa Clara, CA, USA) were used to determine the expression of all HOXA, HOXB and HOXC-family genes in pediatric ALL cases, according to the manufacturer’s guidelines. Data extraction and normalization procedures of the 22,283 probe sets that both arrays have in common have been extensively described elsewhere.23 The data collected are part of a larger data set that has been deposited in NCBI’s Gene Expression Omnibus (GEO)27 and is accessible via GEO numbers GSE13351 and GSE13425.23
Assessment of methylation status
The methylation status of miR-196b and the HOXA cluster was assessed by the differential methylation hybridization (DMH) procedure using 244K CpG island microarrays (Agilent Technologies, Santa Clara, USA). The microarray labeling and hybridization procedures were performed according to Yan et al.28 as described elsewhere.29 The high-resolution microarrays contain 243,497 60-mer oligonucleotide probes, including numerous CpG island probes related to miRNA. For the present study, the probes containing multiple CpG islands located at chromosome 7p15 in the 5’ promoter region of the miR-196b and HOXA cluster genes were used. A pool of genomic DNA derived from ten healthy individual (five males and five females, Promega Benelux BV, Leiden, the Netherlands) constituted a common reference. Data were extracted using Agilent Feature Extraction 9.5.3 software. Subsequently, the data were normalized and differential methylation assessed in the R and Bioconductor Statistical environment, as described elsewhere.29 Methylation data are presented as ratios of the patient’s signal divided by the common reference signal. For nine MLL-rearranged cases, both miRNA methylation and matching miRNA expression levels were measured. Unprocessed genome-wide DNA methylation data were uploaded in the NCBI Gene Expression Omnibus under the GEO Series accession number GSE18400 as part of a previous study.29
Drug resistance assay
Responsiveness to prednisolone or L-asparaginase was determined by a 4-day in vitro methyl thiazolyl tetrazolium (MTT) drug resistance assay as described elsewhere.30,31 The concentration ranges tested were 0.008–250 μg/mL for prednisolone and 0.003–10 IU/mL for L-asparaginase. The concentration of prednisolone or L-aspariginase that was lethal to 50% of the ALL cells (LC50) was taken as a measure of the cellular drug resistance. LC50 values are known to be predictive of clinical outcome30 and are used to adapt treatment regimens.32,33
Statistics
Differences in the distribution of variables between groups of patients were analyzed by the Mann-Whitney U test. Correlations between miRNA and mRNA levels were determined using the Spearman’s correlation coefficient (Rs). P values were two-tailed and considered statistically significant when less than 0.05.
Results
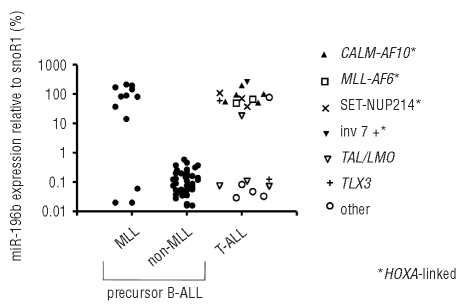
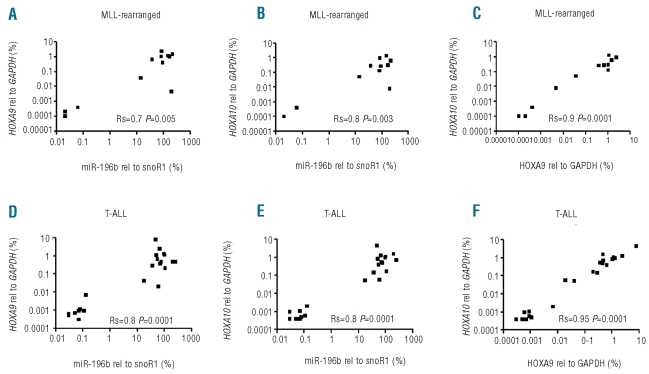
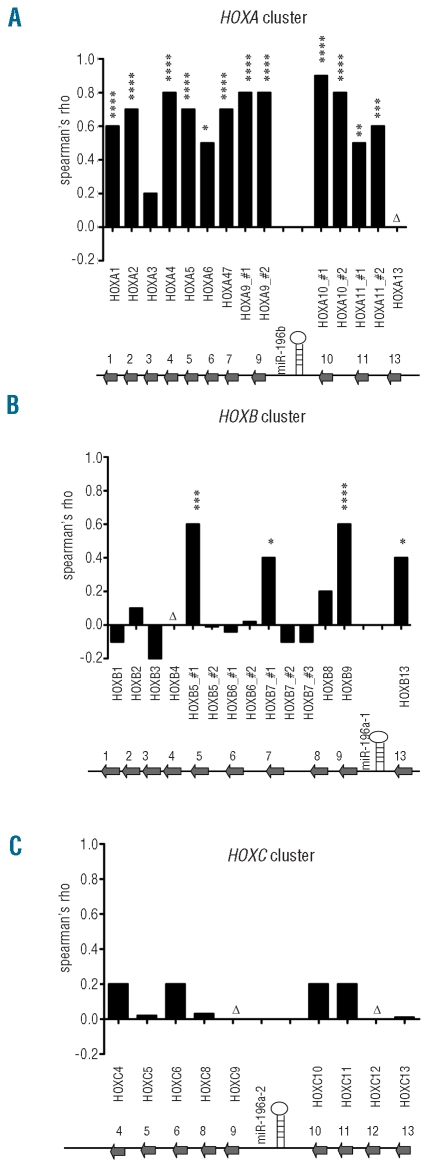
The expression of miR-196b was measured in 72 pediatric ALL cases at diagnosis. Figure 1 shows that miR-196b was highly expressed in nine out of 12 MLL-rearranged ALL cases and in 14 out of 22 cases of T-ALL. In particular, all CALM-AF10 (n=5), MLL-AF6 (n=2), SET-NUP214 (n=3) and inv(7) (n=1) positive T-ALL cases showed high levels of expression of miR-196b comparable to the levels found in MLL-rearranged cases (Figure 1). Since these specific chromosomal abnormalities are linked to the activation of HOXA genes19,20 and since miR-196b is mapped between HOXA9 and HOXA10, we quantified the expression of the HOXA9 and HOXA10 transcripts by RT-qPCR. A strong correlation between the level of expression of miR-196b and HOXA9 and HOXA10 expression (Rs ≥ 0.7; P≤0.005) was found in MLL-rearranged precursor B-ALL as well as in cases of T-ALL (Figure 2). Figure 2 and Online Supplementary Figure S1 illustrate that patients with low levels of expression of miR-196b (such as non-MLL precursor B-ALL and the majority of T-ALL cases) also had low expression of HOXA genes. In those cases in which the levels of miR-196b were higher (such as the majority of MLL-rearranged precursor B-ALL and one third of T-ALL cases), HOXA levels were also elevated. The levels of HOXA9 and HOXA10 were also significantly correlated with each other, as shown in Figure 2C and 2F (Rs ≥ 0.90; P<0.0001). These data suggest co-expression of miR-196b with HOXA cluster genes. To determine whether this was only restricted to the two adjacent HOXA genes, we also investigated the expression pattern of other HOX-family genes using a second technique. i.e. the Affymetrix human genome microarray platform. The results confirmed the strong correlation between the level of expression of miR-196b and that of HOXA9 (Rs = 0.8; P≤ 0.0001) and HOXA10 (0.8≤ Rs ≤ 0.9; P≤ 0.0001) and also revealed that miR-196b levels correlated with the expression levels of nearly all other HOXA genes represented on the array platform (0.5<Rs<0.8, P<0.05, Figure 3A for all cases and Online Supplementary Figures S2 and S3 for MLL-rearranged ALL and T-ALL cases, separately).
Figure 1.
Expression of miR-196b in pediatric ALL. The miR-196b level was measured in leukemic cells of 12 cases of MLL-rearranged precursor B-ALL, 38 of non-MLL precursor B-ALL and 22 T-ALL. * Refers to T-ALL cases that have genetic aberrations that are associated with activation of HOXA cluster genes. Dots represent the individual miR-196b levels as a percentage of the expression level of the endogenous reference, snoRNA-1. MLL-rearranged versus non-MLL precursor B-ALL P=0.003; T-ALL versus non-MLL precursor BALL P=0.001.
Figure 2.
Correlation between expression levels of miR-196b, HOXA9 and HOXA10 in ALL patients. The expression levels are compared between miR-196b and HOXA9 (A, D), between miR-196b and HOXA10 (B, E), as well as between HOXA9 and HOXA10 (C, F) in 12 MLL-rearranged precursor patients (upper panel, A–C) and 22 T-ALL patients (lower panel D–F). The expression level of miR-196b is normalized for the expression level of snoRNA-1 as measured by quantitative stem-loop RT-qPCR whereas the expression of HOXA9 and HOXA10 transcripts is normalized for GAPDH mRNA expression levels as measured by quantitative RT-qPCR. Rs and P values are indicated in each panel.
Figure 3.
MiR-196b and HOXA cluster genes are co-transcribed in pediatric ALL. The expression levels of miR-196b were compared to the expression of different members of the HOXA (A), HOXB (B) and HOXC (C) cluster in 12 MLL-rearranged B-ALL patients and 18 T-ALL patients. Spearman’s correlation coefficient was calculated and plotted as bars. # 1, 2 and 3 refer to the different probe sets for the specified genes on the Affymetrix U133A platform (Online Supplementary Table S2). *P≤0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001. Genomic location of miR-196b on 7p15.2 within the HOXA cluster (A), miR-196a-1 on 17q21.32 within the HOXB cluster (B) and miR-196a-2 on 12q13.13 within the HOXC cluster (C) is indicated at the bottom of each graph. Δ indicates genes for which no probe sets were available on the U133A microarray.
Whereas miR-196b is encoded within the HOXA cluster, another family member, miR-196a, which differs by only one nucleotide from miR-196b, is encoded by miR-196a-1 located in the HOXB cluster (17q21.32) and miR-196a-2 located in the HOXC cluster (12q13.13). The high homology between miR-196a and miR-196b may hamper the discriminative power of the stem-loop RT-qPCR procedure (and any other quantifying method) for determining solely miR-196b expression levels. However, no significant correlations were found between expression levels of miR-196b and those of members of the HOXB and HOXC cluster, except for HOXB5, HOXB7, HOXB9, and HOXB13 (Figure 3B and 3C). Only one out of two and one out of three probe sets designed for HOXB5 and HOXB7, respectively, showed a significant correlation with miR-196b (0.4 ≤ Rs ≤ 0.6; P<0.05, Figure 3B). These correlations were less significant and less strong than the association observed between expression levels of miR-196b and family members of the HOXA-cluster. This suggests that miR-196b and the HOXA cluster are more likely to be co-transcribed than miR-196b and HOXB or HOXC family genes.
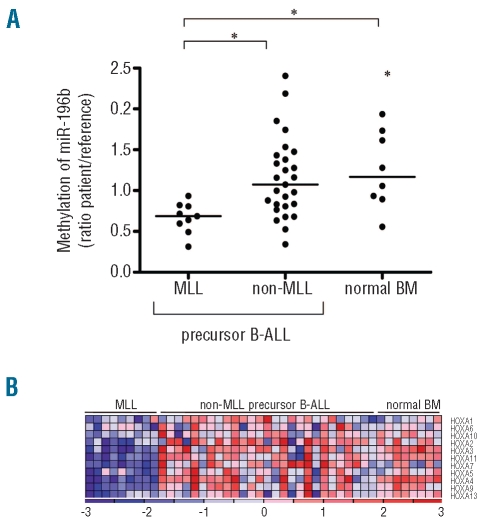
Since the expression of miRNA genes and protein-coding genes can be affected by DNA methylation of promoter regions, we analyzed the methylation state of the 5’ region upstream of miR-196b and the promoter region of all HOXA cluster genes. The methylation of the 5’region of miR-196b (Figure 4A) and of the entire HOXA cluster (Figure 4B) was reduced in MLL-rearranged cases compared to in cases of B-ALL without a MLL-translocation as well as in normal bone marrow (P≤0.01 and P<0.05, respectively), which may explain the increased expression levels of both miR-196b and the HOXA cluster in these cases.
Figure 4.
The promoters of miR-196b and HOXA genes have a lower level of methylation in MLL-rearranged precursor B-ALL patients than in non-MLL precursor B-ALL patients and normal bone marrow. (A) Methylation status of the probe covering multiple CpG islands (y-axis) within the 5’ region of miR-196b (27,175,990–27,176,034) was analyzed in nine MLL-rearranged precursor B-ALL patients, 27 non-MLL precursor B-ALL and eight normal bone marrow (BM) samples. Dots represent individual patients *P≤0.01. (B) Heat map displaying methylation status of the HOXA cluster in the same patients as for Figure 4A. Columns represent patients’ samples and rows represent the different HOXA cluster genes. Relative DNA methylation levels are shown in red (high) and blue (low). Gene names are listed at the right. The heat map was generated in GenePattern version 3.1.2.43 MLL-rearranged versus non-MLL precursor B-ALL, P<0.0001; MLL-rearranged precursor B-ALL versus normal BM, P<0.0002.
We also investigated whether the level of expression of miR-196b was linked to the sensitivity of the MLL-rearranged ALL and T-ALL patients to prednisolone and L-asparaginase, since resistance to these drugs is indicative of an unfavorable outcome.30,31 Figure 5A and 5C show that the in vitro cytotoxicity (LC50 values) for both drugs did not differ between patients with high and low expression levels of miR-196b. In correspondence, patients who were sensitive, intermediately sensitive or resistant to prednisolone or L-asparaginase did not have significantly different levels of miR-196b (Figure 5B and 5D, P>0.05).
Figure 5.
Expression levels of miR-196b are not associated with resistance to prednisolone and L-asparaginase in pediatric ALL cells. In vitro cytotoxicity (represented by LC50 value) of prednisolone (A) and L-asparaginase (C) was measured in 11 MLL-rearranged precursor BALL and 11 T-ALL patients. Patients were separated according to their miR-196b expression level into two groups, i.e. low miR-196b (<1% of snoR-1) and high miR-196b ( >1% of snoR-1). In B and D miR-196b expression is plotted against degree of resistance towards prednisolone (B) and L-asparaginase (D), i.e. sensitive, intermediately sensitive or resistant samples based upon previously established cut-off values.30,44 P>0.05 for all comparisons.
Discussion
In this study we demonstrated a strong association between the expression levels of miR-196b and genes belonging to the HOXA cluster in pediatric ALL. This co-transcription was not restricted to MLL-rearranged cases, but was also found for T-ALL cases characterized by activation of HOXA genes due to non-MLL mechanisms. Hypomethylation of CpG islands in the 5’ upstream/promoter regions of miR-196b and HOXA cluster genes, as demonstrated in MLL-rearranged cases, may explain the high expression levels of this cluster and embedded miR-196b. In vitro resistance to prednisolone and L-asparaginase could not be explained by differential levels of miR-196b expression.
Popovic et al. reported that the expression of miR-196b is regulated by MLL and MLL fusion products.13 In correspondence, we observed high-level expression in MLL-rearranged cases. However, we demonstrated that high expression of miR-196b is not restricted to MLL-rearranged cases but can also be found in patients with other cytogenetic abnormalities that are known to activate HOXA cluster genes, i.e. CALM-AF10, SET-NUP214 and inv(7)(p15q35). The mechanism by which the HOXA cluster is transcriptionally activated may differ between these patients. It has been demonstrated that CALM-AF10, SET-NUP214 and MLL fusions recruit the DOT1L histone methyltransferase that facilitates gene transcription of the HOXA cluster by dimethylation of histone H3 lysine 79 residues (H3K79). The H3K79 dimethylation possibly allows further epigenetic modification that opens up the entire HOXA locus.20,34–36 We here demonstrate CpG island hypomethylation of the HOXA cluster in MLL-rearranged patients suggesting the existence of additional mechanisms driving HOXA expression. Moreover, inv(7) cases have elevated HOXA10 and HOXA11 expression due to the rearrangement of the T-cell receptor beta locus into this region of the HOXA cluster.36 Taken together these findings indicate that high levels of expression of miR-196b and HOXA cluster genes are not exclusively MLL-driven but can also be due to other routes of HOXA locus activation. It should also be noted that not all MLL-rearranged cases have high expression levels of miR-196b and HOXA cluster genes (Figures 1 and 2). This corresponds with the fact that two distinct subgroups, which are separated based on the expression signature of HOXA cluster genes, have been found in MLL-rearranged ALL.17,37
We demonstrated that the expression of miR-196b was strongly correlated with that of most members of the HOXA cluster (Figure 3) whereas there was a less pronounced correlation with the HOXB and HOXC cluster genes in pediatric ALL cells. HOXA3 microarray-based expression levels did not correlate with miR-196b expression levels. This lack of correlation was due to a less optimal array-probe design for probe set 208604_s_at, exemplified by the fact that microarray and quantitative Taqman-based RT-qPCR data did not correlate for HOXA3 whereas these data were highly correlated for other HOXA cluster genes such as HOXA9 and HOXA10 (Online Supplementary Figure S4). Since we cannot rule out the possibility of non-optimal design for other array-probes, correlations between miR-196b and additional HOX genes may have been missed. However, since the miR-196b gene is positioned between HOXA9 and HOXA10 and is transcribed from the same DNA strand as the HOXA cluster, the high co-expression between the miRNA and HOXA genes suggests co-transcriptional activation. Correspondingly, the expression levels of both miR-196b and HOXA9 are restored upon re-expression of Mll in Mll-deficient mouse embryonic fibroblasts.13 A similar co-activation may explain the strong association for miR-10a (positioned between HOXB4 and HOXB5) as well as miR-196a (encoded between HOXB9 and HOXB13) and the HOXB cluster as observed in acute myeloid leukemia.38,39 Recent studies suggest that miRNA, in general, are often expressed at lower levels in cancer cells than in their normal counterparts.40 In the case of ALL, this phenomenon may be caused by a high frequency of CpG island hypermethylation.21 However, the fact that we observed that miR-196b and HOXA-genes are highly co-transcribed may indicate that this region has reduced DNA methylation. We demonstrated that the level of methylation of the CpG islands in the 5’ region of miR-196b and in the promoter region of the entire HOXA cluster is lower in MLL-rearranged cases than in precursor B-ALL patients without MLL-rearrangements and in healthy individuals. Since MLL-rearranged ALL is characterized by hypermethylation of CpG islands across the genome,29 the hypomethylation of the miR-196b/HOXA region is remarkable. Whether this locus displays a similar methylation status in HOXA-linked T-ALL cases needs to be explored.
Both MLL-rearranged and presumably HOXA-linked CALM-AF10-positive T-ALL patients have a poor clinical outcome.11,41 It has previously been shown that both MLL-rearranged and T-ALL pediatric ALL cases are more resistant to prednisolone and L-asparaginase, as determined by in vitro drug cytotoxicity assays.42 These two drugs are extensively used in the treatment of pediatric ALL and resistance to them is indicative of an unfavorable prognosis.30 However, we did not find evidence that miR-196b contributes to resistance to these drugs since patients with high miR-196b expression were not more resistant to both drugs than patients with low miR-196b expression levels. In contrast to a role in drug responsiveness, miR-196b may have leukemogenic potential since ectopic expression of miR-196b resulted in increased proliferation and reduced differentiation of c-Kit+ bone marrow cells of mice.13
In conclusion, we found that aberrant over-expression of miR-196b is not restricted to MLL-rearranged ALL cases (T-ALL or precursor B-ALL) but also occurs in T-ALL patients with other genetic abnormalities that activate the HOXA gene cluster. This observation is of great importance since miR-196b is known to have oncogenic activity13 and may, therefore, play a role in the biology underlying HOXA-activated precursor B-ALL and T-ALL. The high expression of miR-196b has no effect on the level of cellular responsiveness to prednisolone and L-asparaginase in pediatric ALL. The role of miR-196b in leukemogenesis and survival of HOXA-expressing ALL deserves further studies since targeting miR-196b by ‘antagomirs’ reduced the proliferation capacity of MLL-rearranged bone marrow cells of mice.13
Footnotes
Funding: the work described in this paper was financially supported by the Netherlands Organization for Scientific Research (MLDB, DS), the Sophia Foundation for Medical Research (RWS, RP, DJPMS), the Pediatric Oncology Foundation Rotterdam – Quality of Life Foundation (MLDB, ELT, RP) and the Dutch Cancer Society (grant EMCR 2005-3662, MLdB, RP, DS, JBG, RWS).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Jackson RJ, Standart N. How do microRNAs regulate gene expression? Sci STKE. 2007;2007(367):re1. doi: 10.1126/stke.3672007re1. [DOI] [PubMed] [Google Scholar]
- 2.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 3.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, et al. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64(11):3753–6. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 5.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435(7043):828–33. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mendell JT. miRiad roles for the miR-17–92 cluster in development and disease. Cell. 2008;133(2):217–22. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, et al. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci USA. 2006;103(18):7024–9. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agirre X, Vilas-Zornoza A, Jimenez-Velasco A, Martin-Subero JI, Cordeu L, Garate L, et al. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res. 2009;69(10):4443–53. doi: 10.1158/0008-5472.CAN-08-4025. [DOI] [PubMed] [Google Scholar]
- 9.Meijerink JP, den Boer ML, Pieters R. New genetic abnormalities and treatment response in acute lymphoblastic leukemia. Semin Hematol. 2009;46(1):16–23. doi: 10.1053/j.seminhematol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Arico M, Valsecchi MG, Camitta B, Schrappe M, Chessells J, Baruchel A, et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2000;342(14):998–1006. doi: 10.1056/NEJM200004063421402. [DOI] [PubMed] [Google Scholar]
- 11.Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370(9583):240–50. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 12.Schotte D, Chau JC, Sylvester G, Liu G, Chen C, van der Velden VH, et al. Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia. 2009;23(2):313–22. doi: 10.1038/leu.2008.286. [DOI] [PubMed] [Google Scholar]
- 13.Popovic R, Riesbeck LE, Velu CS, Chaubey A, Zhang J, Achille NJ, et al. Regulation of mir-196b by MLL and its overexpression by MLL fusions contributes to immortalization. Blood. 2009;113(14):3314–22. doi: 10.1182/blood-2008-04-154310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jansen MW, Corral L, van der Velden VH, Panzer-Grumayer R, Schrappe M, Schrauder A, et al. Immunobiological diversity in infant acute lymphoblastic leukemia is related to the occurrence and type of MLL gene rearrangement. Leukemia. 2007;21(4):633–41. doi: 10.1038/sj.leu.2404578. [DOI] [PubMed] [Google Scholar]
- 15.Argiropoulos B, Humphries RK. Hox genes in hematopoiesis and leukemogenesis. Oncogene. 2007;26(47):6766–76. doi: 10.1038/sj.onc.1210760. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30(1):41–7. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 17.Trentin L, Giordan M, Dingermann T, Basso G, Te Kronnie G, Marschalek R. Two independent gene signatures in pediatric t(4;11) acute lymphoblastic leukemia patients. Eur J Haematol. 2009;83(5):406–19. doi: 10.1111/j.1600-0609.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- 18.Zangrando A, Dell’orto MC, Te Kronnie G, Basso G. MLL rearrangements in pediatric acute lymphoblastic and myeloblastic leukemias: MLL specific and lineage specific signatures. BMC Med Genomics. 2009;2:36. doi: 10.1186/1755-8794-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soulier J, Clappier E, Cayuela JM, Regnault A, Garcia-Peydro M, Dombret H, et al. HOXA genes are included in genetic and biologic networks defining human acute T-cell leukemia (T-ALL) Blood. 2005;106(1):274–86. doi: 10.1182/blood-2004-10-3900. [DOI] [PubMed] [Google Scholar]
- 20.Van Vlierberghe P, van Grotel M, Tchinda J, Lee C, Beverloo HB, van der Spek PJ, et al. The recurrent SET-NUP214 fusion as a new HOXA activation mechanism in pediatric T-cell acute lymphoblastic leukemia. Blood. 2008;111(9):4668–80. doi: 10.1182/blood-2007-09-111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roman-Gomez J, Agirre X, Jimenez-Velasco A, Arqueros V, Vilas-Zornoza A, Rodriguez-Otero P, et al. Epigenetic regulation of microRNAs in acute lymphoblastic leukemia. J Clin Oncol. 2009;27(8):1316–22. doi: 10.1200/JCO.2008.19.3441. [DOI] [PubMed] [Google Scholar]
- 22.Pieters R, Carroll WL. Biology and treatment of acute lymphoblastic leukemia. Pediatr Clin North Am. 2008;55(1):1–20. doi: 10.1016/j.pcl.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–34. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stam RW, den Boer ML, Schneider P, Nollau P, Horstmann M, Beverloo HB, et al. Targeting FLT3 in primary MLL-gene-rearranged infant acute lymphoblastic leukemia. Blood. 2005;106(7):2484–90. doi: 10.1182/blood-2004-09-3667. [DOI] [PubMed] [Google Scholar]
- 25.Stam RW, den Boer ML, Meijerink JP, Ebus ME, Peters GJ, Noordhuis P, et al. Differential mRNA expression of Ara-C-metabolizing enzymes explains Ara-C sensitivity in MLL gene-rearranged infant acute lymphoblastic leukemia. Blood. 2003;101(4):1270–6. doi: 10.1182/blood-2002-05-1600. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33(20):e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30(1):207–10. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan PS, Potter D, Deatherage DE, Huang TH, Lin S. Differential methylation hybridization: profiling DNA methylation with a high-density CpG island microarray. Methods Mol Biol. 2009;507:89–106. doi: 10.1007/978-1-59745-522-0_8. [DOI] [PubMed] [Google Scholar]
- 29.Stumpel DJ, Schneider P, van Roon EH, Boer JM, de Lorenzo P, Valsecchi MG, et al. Specific promoter methylation identifies different subgroups of MLL-rearranged infant acute lymphoblastic leukemia, influences clinical outcome, and provides therapeutic options. Blood. 2009;114(27):5490–8. doi: 10.1182/blood-2009-06-227660. [DOI] [PubMed] [Google Scholar]
- 30.Den Boer ML, Harms DO, Pieters R, Kazemier KM, Gobel U, Korholz D, et al. Patient stratification based on prednisolone-vincristine-asparaginase resistance profiles in children with acute lymphoblastic leukemia. J Clin Oncol. 2003;21(17):3262–8. doi: 10.1200/JCO.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 31.Pieters R, Kaspers GJ, van Wering ER, Huismans DR, Loonen AH, Hahlen K, et al. Cellular drug resistance profiles that might explain the prognostic value of immunophenotype and age in childhood acute lymphoblastic leukemia. Leukemia. 1993;7(3):392–7. [PubMed] [Google Scholar]
- 32.Escherich G, Göbel U, Graubner U, Pekrun A, Jorch N, Kaspers GJL, et al. In vitro drug testing in acute lymphoblastic leukemia of childhood allows for therapy reduction without loss of efficacy – results of study COALL 06-97. Submitted 2010. [Google Scholar]
- 33.Janka-Schaub GE, Harms DO, den Boer ML, Veerman AJ, Pieters R. In vitro drug resistance as independent prognostic factor in the study COALL-O5-92 Treatment of childhood acute lymphoblastic leukemia; two-tiered classification of treatments based on accepted risk criteria and drug sensitivity profiles in study COALL-06-97. Klin Padiatr. 1999;211(4):233–8. doi: 10.1055/s-2008-1043794. [DOI] [PubMed] [Google Scholar]
- 34.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7(11):823–33. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 35.Okada Y, Jiang Q, Lemieux M, Jeannotte L, Su L, Zhang Y. Leukaemic transformation by CALM-AF10 involves upregulation of Hoxa5 by hDOT1L. Nat Cell Biol. 2006;8(9):1017–24. doi: 10.1038/ncb1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speleman F, Cauwelier B, Dastugue N, Cools J, Verhasselt B, Poppe B, et al. A new recurrent inversion, inv(7)(p15q34), leads to transcriptional activation of HOXA10 and HOXA11 in a subset of T-cell acute lymphoblastic leukemias. Leukemia. 2005;19(3):358–66. doi: 10.1038/sj.leu.2403657. [DOI] [PubMed] [Google Scholar]
- 37.Stam RW, Schneider P, Hagelstein JA, van der Linden MH, Stumpel DJ, de Menezes RX, et al. Gene expression profiling-based dissection of MLL translocated and MLL germline acute lymphoblastic leukemia in infants. Blood. 115(14):2835–44. doi: 10.1182/blood-2009-07-233049. [DOI] [PubMed] [Google Scholar]
- 38.Debernardi S, Skoulakis S, Molloy G, Chaplin T, Dixon-McIver A, Young BD. MicroRNA miR-181a correlates with morphological sub-class of acute myeloid leukaemia and the expression of its target genes in global genome-wide analysis. Leukemia. 2007;21(5):912–6. doi: 10.1038/sj.leu.2404605. [DOI] [PubMed] [Google Scholar]
- 39.Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, et al. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA. 2008;105(10):3945–50. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 41.van Grotel M, Meijerink JP, Beverloo HB, Langerak AW, Buys-Gladdines JG, Schneider P, et al. The outcome of molecular-cytogenetic subgroups in pediatric T-cell acute lymphoblastic leukemia: a retrospective study of patients treated according to DCOG or COALL protocols. Haematologica. 2006;91(9):1212–21. [PubMed] [Google Scholar]
- 42.Pieters R, den Boer ML, Durian M, Janka G, Schmiegelow K, Kaspers GJ, et al. Relation between age, immunophenotype and in vitro drug resistance in 395 children with acute lymphoblastic leukemia–implications for treatment of infants. Leukemia. 1998;12(9):1344–8. doi: 10.1038/sj.leu.2401129. [DOI] [PubMed] [Google Scholar]
- 43.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38(5):500–1. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 44.Holleman A, Cheok MH, den Boer ML, Yang W, Veerman AJ, Kazemier KM, et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351(6):533–42. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]