Abstract
Background
CD30+ T-cell lymphoproliferations comprise a spectrum of clinically heterogeneous entities, including systemic anaplastic large cell lymphomas (ALK− and ALK+) and primary cutaneous CD30+ T-cell lymphoproliferative disorders. While all these entities are characterized by proliferation of highly atypical, anaplastic CD30+ T cells, the expression of T-cell specific antigens in the tumor cells is not consistently detectable.
Design and Methods
We evaluated biopsies from 19 patients with primary cutaneous CD30+ lymphoproliferative disorders, 38 with ALK− and 33 with ALK+ systemic anaplastic large cell lymphoma. The biopsies were examined for the expression of T-cell receptorαβ/CD3 complex (CD3γ, δ, ɛ, ζ), transcription factors regulating T-cell receptor expression (ATF1, ATF2, TCF-1, TCF-1α/LEF-1, Ets1), and molecules of T-cell receptor-associated signaling cascades (Lck, ZAP-70, LAT, bcl-10, Carma1, NFATc1, c-Jun, c-Fos, Syk) using immunohistochemistry.
Results
In comparison to the pattern in 20 peripheral T-cell lymphomas, not otherwise specified, we detected a highly disturbed expression of the T-cell receptor/CD3 complex, TCF-1, TCF-1α/LEF-1, Lck, ZAP-70, LAT, NFATc1, c-Jun, c-Fos and Syk in most of the systemic anaplastic large cell lymphomas. In addition, primary cutaneous CD30+ lymphoproliferative disorders showed such a similar expression pattern to that of systemic anaplastic large cell lymphomas, that none of the markers we investigated can reliably distinguish between these CD30+ T-cell lymphoproliferations.
Conclusions
Severely altered expression of the T-cell receptor/CD3 complex, T-cell receptor-associated transcription factors and signal transduction molecules is a common characteristic of systemic and cutaneous CD30+ lymphoproliferations, although the clinical behavior of these entities is very different. Since peripheral T-cell lymphomas, not otherwise specified retain the full expression program required for functioning T-cell receptor signaling, the differential expression of a subset of these markers might be of diagnostic utility in distinguishing peripheral T-cell lymphomas, not otherwise specified from the entire group of CD30+ lymphoproliferations.
Keywords: systemic and cutaneous CD30+ lymphoproliferations, anaplastic large cell lymphoma, lymphomatoid papulosis, ALCL, LyP, TCR
Introduction
CD30+ T-cell lymphoproliferations comprise a spectrum of clinically heterogeneous entities, including systemic anaplastic large cell lymphomas (ALCL) as well as primary cutaneous CD30+ T-cell lymphoproliferative disorders. Morphologically, these entities are characterized by the proliferation of highly atypical, anaplastic CD30+ T cells. Despite the frequent detection of clonally rearranged T-cell receptor (TCR) genes, the expression of T-cell-specific antigens in the tumor cells is not consistently detectable.1
Systemic ALCL comprise approximately 15% of all peripheral T-cell lymphomas (PTCL) in Europe2 and are divided into anaplastic lymphoma kinase (ALK) positive and negative subgroups (ALK+ and ALK− systemic ALCL), whereas primary cutaneous CD30+ T-cell lymphoproliferative disorders represent a spectrum of skin lesions with diverging and in part overlapping clinical and morphological features. CD30+ T-cell lymphoproliferative disorders include primary cutaneous ALCL, lymphomatoid papulosis and so-called borderline lesions,3–5 but even with the knowledge of the clinical presentation and a detailed morphological and immunohistochemical picture it is sometimes difficult, if not impossible, to classify a particular cutaneous lesion correctly.
While the transforming properties of ALK over-expression have been clearly demonstrated6,7 the transformation mechanisms in ALK− systemic ALCL and primary cutaneous CD30+ T-cell lymphoproliferative disorders are poorly understood. CD30 itself, a cytokine receptor belonging to the tumor necrosis factor receptor superfamily, has been a research focus over the past years, but whether CD30 signaling leads to cell cycle arrest, apoptosis or even proliferation in CD30+ lymphoproliferations is still a matter of debate.8–11
We previously showed that a unifying molecular defect in ALCL, regardless of ALK-expression status, was a TCR and CD3ɛ expression deficiency, which - in most cases -was associated with lost or markedly reduced expression of ZAP-70.1 The present study had three aims. First, we wanted to expand our investigation of TCR-associated molecules in systemic ALCL to include the expression of the entire TCR/CD3 complex (i.e. all different CD3 molecules), transcription factors responsible for TCR expression (ATF-1, ATF-2, TCF-1, TCF-1α/LEF-1, and Ets-1) as well as key molecules involved in the signaling of the TCR/CD3 complex (LAT, ZAP70, Syk, Lck, bcl-10, Carma1, and the transcription factors NFATc1, c-Jun, c-Fos). Second, we included primary cutaneous CD30+ T-cell lymphoproliferative disorders in our study, since virtually nothing is known about the expression of these molecules in these lymphoproliferations. Finally, we wanted to identify differentially expressed markers that might be of help in the routine setting in the differential diagnosis between systemic ALCL and primary cutaneous CD30+ T-cell lymphoproliferative disorders, but also between CD30+ T-cell proliferations and peripheral T-cell lymphomas, not otherwise specified (PTCL-NOS).
Design and Methods
Characteristics of the study set
We analyzed paraffin-embedded lymph node or skin biopsies from 38 patients with ALK− systemic ALCL, 33 with ALK+ systemic ALCL and 19 with primary cutaneous CD30+ T-cell lymphoproliferative disorders selected from the files of the Institute of Pathology, University of Wuerzburg. Twenty lymph node biopsies with infiltration by PTCL-NOS served as controls. Approval for the entire study was obtained from the Ethics Committee, Medical Faculty, University of Wuerzburg, Germany.
All biopsies were stained with hematoxylin and eosin, Giemsa, periodic acid Schiff and an appropriate panel of immunohistochemical stains to establish a diagnosis according to the World Health Organization classification.3 Based on histological and clinical features the 19 primary cutaneous CD30+ T-cell lymphoproliferative disorders could be classified as lymphomatoid papulosis type A (n=7), lymphomatoid papulosis type C (n=2), cutaneous ALCL (n=6), and lymphomatoid papulosis-like cutaneous ALCL (n=4). All cases were reviewed independently by up to four expert hematopathologists (HKM-H, TR, AR, EG) and cases for which there was not a consensus were excluded from the study.
Immunohistochemical analyses
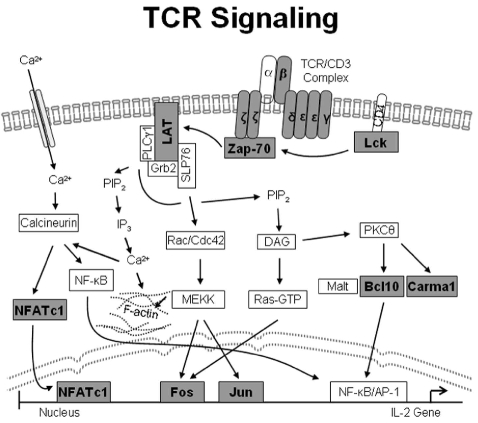
Using conventional immunohistochemical single stains, we investigated the expression of CD30 and ALK, as well as the expression of the TCRα/β (βF1) and CD3 subunits (γ, δ, ɛ, ζ) that compose the TCR-complex. Due to the lack of frozen tumor tissue, the expression of TCRγ/δ could not be evaluated. In addition, we analyzed the expression of the TCR-associated signal transduction molecules ZAP-70 (ζ-associated protein of 70 kDa), LAT (linker for the activation of T cells), Syk (spleen tyrosine kinase), Lck, bcl-10, Carma1/CARD11 (caspase recruitment domain (CARD), and membrane-associated guanylate kinase (MAGUK)-containing scaffold protein) and the transcription factors NFATc1 (nuclear factor of activated T cells), c-Jun, and c-Fos. A schematic overview of the TCR/CD3 associated signaling cascades is presented in Figure 1 in which the molecules that were investigated in the present study are highlighted. Finally, we also studied the expression levels of the transcription factors ATF-1, ATF-2 (activating transcription factors 1 and 2), TCF-1 (T-cell factor-1), TCF-1α/LEF-1 (lymphoid enhancer factor-1) and Ets-1, which are involved in the regulation of the expression of the various TCR chains. All immunohistochemical analyses were performed according to standard protocols using the Advance™ HRP Detection kit (K4068) from Dako. The sources of primary antibodies and their dilutions are listed in Online Supplementary Table S1.
Figure 1.
Schematic overview of the TCR/CD3-associated signaling cascades. Molecules investigated immunohistochemically are highlighted in dark gray boxes.
All staining results were recorded semi-quantitatively as negative (0), weakly positive (independently of the amount of positive tumor cells) or moderately positive in less than 40% of tumor cells (1), moderately or strongly positive in at least 40% of tumor cells (2), and strongly positive in more than 80% of tumor cells (3). Staining intensities in reactive cells (T cells for most of the markers, but also B cells and histiocytes, e.g. for Syk) were used as internal controls.
Statistics
Based on approximately equal variances of expression values between different entities, statistical analysis was done using the Wilcoxon-Mann-Whitney two-sample rank-sum test. P values less than 0.05 were considered statistically significant.
Results
Immunohistochemical analyses of the T-cell receptor/CD3 complex
Since a functional TCR/CD3 complex is composed of a heterodimeric TCR (either α/β or γ/δ) and three dimers of the four CD3 subunits (γ, δ, ɛ, ζ), we performed immunohistochemical stains for all of these components, except for the γ/δ-TCR, since the respective antibody cannot be used on paraffin-embedded tissue. Based on the established idea that the TCR/CD3 complex can only be assembled and transported to the cell membrane when all components consist of correctly folded proteins, we expected a membranous staining pattern for all or none of the proteins. Interestingly, we detected weak to moderate cytoplasmic staining or a pattern, sometimes with a strong staining, restricted to the Golgi complex/endoplasmic reticulum for various combinations of the five molecules, which is likely due to a prolonged degradation process. Surprisingly, we detected clear, but unexplained membranous staining for only one of the five molecules in 4/71 of the systemic and 3/19 of the cutaneous CD30+ T-cell lymphoproliferative disorders. Moreover, in six of these seven cases, CD3ɛ was the sole molecule showing a pattern of membranous staining.
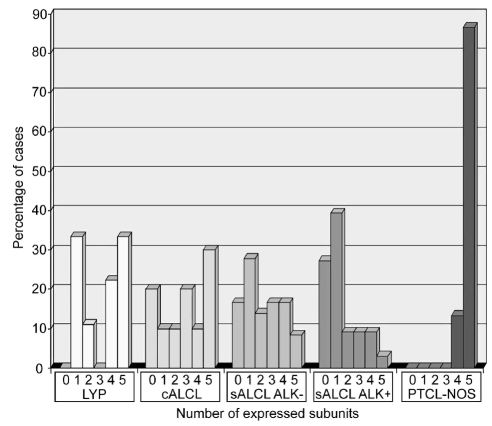
Complete negativity for all five molecules was detected in 7/38 ALK− systemic ALCL, 9/33 ALK+ systemic ALCL, 2/10 cutaneous ALCL, and 0/9 lymphomatoid papulosis. Cytoplasmic positivity for only one component was found in 9/38 ALK− systemic ALCL, 14/33 ALK+ systemic ALCL, 0/10 cutaneous ALCL, and 2/9 lymphomatoid papulosis, and was most often CD3ζ (12/26 cases) since this is the only component that forms a homodimer and is, therefore, likely protected from rapid degradation. Cytoplasmic positivity for two, three or four components in random combinations was seen in 16/38 ALK− systemic ALCL, 9/33 ALK+ systemic ALCL, 3/10 cutaneous ALCL, and 4/9 lymphomatoid papulosis. Only 2/38 ALK− systemic ALCL, 0/33 ALK+ systemic ALCL, 3/10 cutaneous ALCL, and 2/9 lymphomatoid papulosis showed cytoplasmic positivity for all five molecules. These results are graphically summarized in Figure 2 and representative immunohistochemical staining results are depicted in Figure 3. A detailed statistical analysis of these expression results revealed no significant differences among the various CD30+ lymphoproliferations.
Figure 2.
Bar chart of the TCR/CD3 complex expression data. For each entity, the percentage of cases that express a certain number of subunits (TCRβ, CD3δ, ɛ, γ, ζ) is plotted. Only cases for which expression data for all five TCR/CD3 subunits could be obtained are included [9 cases of lymphomatoid papulosis (LYP), 10 of cutaneous ALCL (cALCL), 36 ALK- systemic ALCL (sALCL), 33 ALK+ systemic ALCL (sALCL), 15 PTCL-NOS].
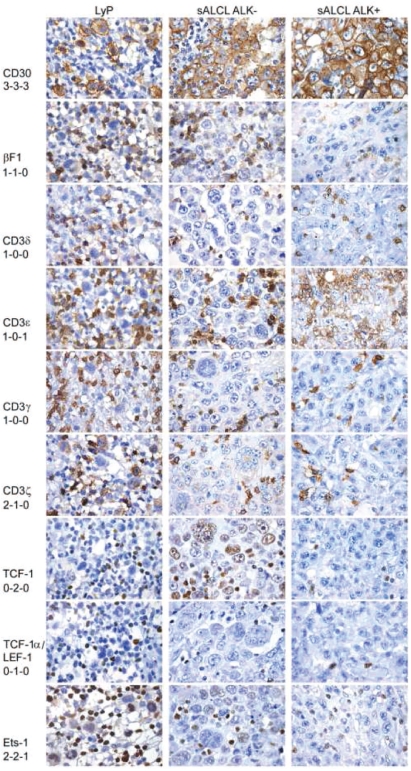
Figure 3.
Immunostaining of TCR/CD3 complex subunits and transcription factors regulating TCR expression. The left column shows characteristic staining results for lymphomatoid papulosis, the middle column shows results for an ALK− systemic ALCL and the right column shows results for an ALK+ systemic ALCL. Scoring results are provided below the respective molecule (from left to right).
Among the 20 PTCL-NOS cases, we found two cases without TCRβ expression but strong positivity for all CD3 molecules; thus, in these cases, the tumor cells most likely belong to a TCRγ/δ population. In general, none of the PTCL-NOS had loss of any CD3 molecule; however, in 5/20 PTCL-NOS the TCRβ expression could not be examined due to technical reasons.
Immunohistochemical analyses of transcription factors regulating T-cell receptor expression
We next investigated five transcription factors known to be involved in the regulation of the expression of the four different TCR chains. As detailed in Online Supplementary Table S2, we detected significantly reduced expression of TCF-1 and TCF-1α/LEF-1 in the systemic and cutaneous CD30+ lymphoproliferations as compared to in the PTCL-NOS cases, while there were no significant differences in the expression of ATF-1, ATF-2, and Ets-1. Moreover, there was significantly lower expression for TCF-1 and TCF-1α/LEF-1 in ALK+ systemic ALCL [complete TCF-1 negativity in 27/33 (82%) cases, complete TCF-1α/LEF-1 negativity in 32/33 (97%) cases] than in ALK− systemic ALCL [complete TCF-1 negativity in 12/38 (32%) cases, complete TCF-1α/LEF-1 negativity in 15/38 (40%) cases]. Interestingly, the level of expression of Ets-1 was significantly higher in primary cutaneous CD30+ T-cell lymphoproliferative disorders than in systemic ALCL, irrespective of the ALK status. Characteristic stains for the transcription factors can be viewed in Figure 3.
Immunohistochemical analyses of key molecules involved in the T-cell receptor/CD3 complex signaling cascades
Analyzing key molecules involved in the signal transduction of the TCR/CD3 complex, we found the LAT, ZAP-70, and NFATc1 were expressed at significantly lower levels in systemic and cutaneous CD30+ T-cell proliferations than in PTCL-NOS, whereas bcl10 and Carma1 expression were not significantly different. Interestingly, the level of expression of bcl-10 was slightly, but significantly, lower in ALK+ systemic ALCL than in the ALK− form. Moreover, when systemic ALCL (irrespective of their ALK status) were compared with primary cutaneous CD30+ T-cell lymphoproliferative disorders, the former showed a slight but significantly decreased expression of ZAP-70, NFATc1, bcl-10, and Carma1.
Syk, a protein kinase highly related to ZAP-70 but physiologically not expressed in mature T cells, was expressed to various degrees in 11/38 ALK− systemic ALCL, 21/33 ALK+ systemic ALCL, 3/10 cutaneous ALCL, and 3/9 lymphomatoid papulosis, while all 20 PTCL-NOS remained negative, thus revealing a significant difference not only between the CD30+ T-cell lymphoproliferations and PTCL-NOS cases, but also between ALK− and ALK+ systemic ALCL. There was no mutually exclusive staining pattern for ZAP-70 and Syk within the CD30+ T-cell lymphoproliferations.
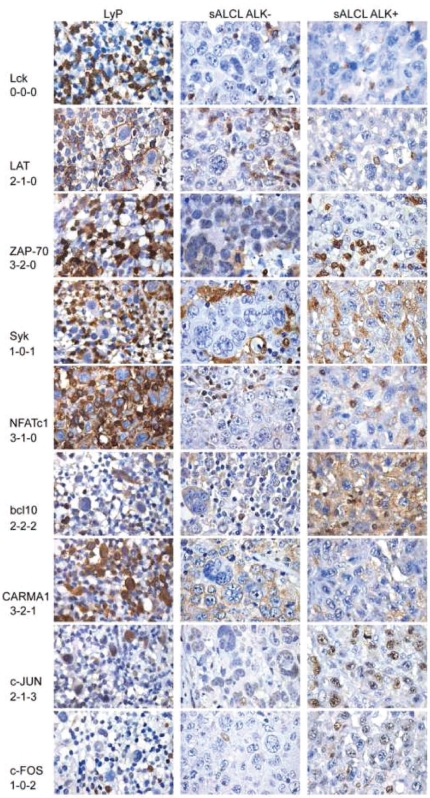
The most striking difference between the CD30+ lymphoproliferations and PTCL-NOS was found for Lck: this marker was not found in 35/38 ALK− systemic ALCL, 33/33 ALK+ systemic ALCL, 7/10 cutaneous ALCL, and 4/8 lymphomatoid papulosis, but was clearly expressed in all 20 PTCL-NOS. Moreover, we detected a slight but significant difference in Lck expression between the cases of cutaneous and systemic CD30+ T-cell lymphoproliferations, with the former having a higher percentage of Lck-positive cases. As for the transcription factors c-Jun and c-Fos, there was an increased expression in the whole group of CD30+ T-cell lymphoproliferations compared to PTCL-NOS. Online Supplementary Table S2 summarizes these results and characteristic immunohistochemical stains are depicted in Figure 4. The complete raw data including the results of all stains in all cases are provided in Online Supplementary Table S3.
Figure 4.
Immunostaining of TCR/CD3 complex-associated signal transduction molecules. The left column shows characteristic staining results for lymphomatoid papulosis, the middle column shows results for an ALK− systemic ALCL, and the right column shows results for an ALK+ systemic ALCL. Scoring results are provided below the respective molecule (from left to right).
Discussion
The results of our study add more details on the disturbance of the TCR/CD3 complex and associated signaling molecules in systemic ALCL and expand these observations to the group of primary cutaneous CD30+ T-cell lymphoproliferative disorders.
A functional TCR/CD3 complex is crucial for T-cell development, as well as for the activation and differentiation of mature T cells. It is well known, that the TCRαβ heterodimer is non-covalently associated with three dimers formed by the four different CD3 chains (δɛ, γɛ, ζζ). There is obligatory co-expression of the TCR with the CD3 molecules, and the absence of any single subunit results in a loss of surface expression of the whole complex, and the expressed chains are retained in the endoplasmic reticulum or Golgi complex and degraded.12–14
We were surprised that our immunohistochemical stains for all four different CD3-chains and the TCRβ-chain revealed entirely random losses of various combinations of the five molecules in most systemic and cutaneous CD30+ T-cell lymphoproliferations. Usually, there is rapid degradation of unassembled subunits, unless formation of more stable heterodimers or even hexamers occurs, rendering these subunits detectable by immunohistochemical approaches. Since CDζ forms a homodimer, its half-life is longer than that of the other CD3 chains when expressed alone, well in line with our observation that positivity for only one component was most often detected for CD3ζ. Only very few cases showed cytoplasmic but not surface positivity for all five molecules: these cases included two ALK- systemic ALCL, three cutaneous ALCL and two lymphomatoid papulosis. Since an immunohistochemical analysis of the TCRα chain is technically not feasible, we cannot rule out that the lack of the subunit in these few cases was responsible for the failure of surface expression.
Given that our data suggest an expression defect of at least one subunit of the TCR/CD3 complex in virtually all systemic and cutaneous CD30+ T-cell lymphoproliferations, we wondered whether this defect may be due to a dysfunction of transcription factors regulating TCR/CD3 gene expression.15 Among the molecules for which immunohistochemical staining results were reliable (ATF-1, ATF-2, Ets-1, TCF-1, TCF-1α/LEF-1), only TCF-1 and TCF-1α/LEF-1, two structurally related members of the TCF family of HMG-box DNA-binding factors with largely overlapping expression patterns,16,17 showed significant differences in expression among the CD30+ lymphoproliferations themselves, as well as compared to PTCL-NOS. Specifically, both proteins were significantly down-regulated in the whole group of CD30+ lymphoproliferations in comparison to PTCL-NOS cases; moreover, both transcription factors were almost absent from ALK+ ALCL, although detectable in a subset of ALK− ALCL. This finding is in slight contrast with that of Dorfman et al., who found complete TCF-1 and TCF-1α/LEF-1 negativity in 30/30 cases of ALCL irrespective of their ALK status.18
It is well known that ATF-1 and ATF-2 regulate TCRα and TCRβ gene transcription and that TCF-1 and TCF-1α/LEF-1 regulate TCRα, β and TCRδ gene transcription, while Ets-1 targets all four TCR chains, namely the TCRα, TCRβ, TCRγ and TCRδ genes. In addition, CD3ɛ, CD4 and interleukin-2 genes are presumed target genes of TCF-1, CD4 and Lck of Ets-1 and CD4 and CD3ɛ of TCF-1α/LEF-1.15,19 Since we could not detect a clear-cut correlation between expression of these transcription factors and that of their target genes, it is unlikely that a transcriptional malfunction is the primary cause of disturbed expression of the TCR/CD3 complex.
We next investigated whether the expression of key molecules of the TCR/CD3 complex associated intracellular signaling cascades20–22 is also altered. As a major finding in our study, confirming a briefly mentioned result from a previous study on a small number of T-cell lymphomas,23 Lck protein expression was almost completely abrogated in systemic ALCL and in more than half the cases of primary cutaneous CD30+ T-cell lymphoproliferative disorders, whereas all cases of PTCL-NOS expressed Lck. In normal T cells, Lck, a src kinase, is the first intracellular signaling molecule that is activated following TCR ligation.22,24 Why Lck is not expressed in systemic ALCL tumor cells that lack expression of the TCR/CD3 complex anyway is unclear. Perhaps, loss of Lck expression is just a bystander phenomenon or represents ‘collateral damage’ in a tumor cell in which TCR/CD3 complex-associated signaling is malfunctioning. Alternatively, loss of Lck expression might provide an additional growth advantage to the tumor cells independently of TCR signaling, e.g. by abrogating the pro-apoptotic properties of Lck under specific circumstances.25
In a previous study, we had already demonstrated a loss of ZAP-7022,26 expression in the majority of systemic ALCL.1 Interestingly, the primary cutaneous CD30+ T-cell lymphoproliferative disorders in this study showed a different picture. Only 16% of primary cutaneous CD30+ T-cell lymphoproliferative disorders, but more than half of the systemic ALCL displayed no or only very weak ZAP-70 expression. Interestingly, Syk, a protein kinase molecularly and functionally highly related to ZAP-70 and normally expressed in B cells,26–31 was aberrantly expressed in 29% of ALK− systemic ALCL, 64% ALK+ systemic ALCL, and 32% of primary cutaneous CD30+ T-cell lymphoproliferative disorders, while all 20 PTCL-NOS remained negative. Thus, for unknown reasons, we were unable to reproduce the results of Feldmann et al.32 who detected Syk-positivity in nearly 100% of CD30+ T-cell lymphoproliferations and the vast majority of PTCL-NOS. Whether Syk might constitute a promising new drug target in PTCL will have to await larger, future studies. Given that Syk and ZAP-70 may partially substitute each other in normal and neoplastic B and T cells26,30,31 we investigated whether Syk and ZAP-70 expression was mutually exclusive in CD30+ T-cell lymphoproliferations. However, this turned out not to be the case.
While most of the molecules discussed so far were expressed in PTCL-NOS cases and significantly down-regulated in CD30+ T-cell lymphoproliferations, c-Jun and c-Fos, forming the AP1 transcription complex,33 had increased expression particularly in the group of ALK+ ALCL. Since the activation of both proteins cannot be the consequence of TCR/CD3 complex signaling, other potentially oncogenic mechanisms may account for the increased expression of c-Jun and c-Fos in these cases. Our results parallel data from another group comparing c-Jun expression in CD30+ and CD30− lymphoproliferations.34 They reported c-Jun expression in more than 70% of systemic ALCL and in around 50% of primary cutaneous CD30+ T-cell lymphoproliferative disorders, while all CD30− lymphomas (including 40 cases of PTCL-NOS) remained negative. There are currently no data available in the literature about c-Fos expression in systemic ALCL, although Mao et al. have investigated the expression of c-Fos and c-Jun in various cutaneous lymphomas. They described that the two transcription factors are expressed in only a small percentage of cutaneous ALCL and lymphomatoid papulosis.35
NF-κB transcription factors are thought to play important roles in T-cell activation and development and variable expression of NF-κB target genes has been reported in PTCL. While published data on the potential role of NF-κB in the various subgroups of PTCL remain – for the most part – inconclusive, there is some evidence that NF-κB expression may be lower in ALCL than in PTCL-NOS.36,37 Since in our hands none of the commercially available antibodies directed against the NF-κB subunits led to reliable staining results, we decided to investigate the expression of the upstream located molecules Carma1/Card11 and Bcl-10 which are part of the trimolecular CBM-complex that is assembled after TCR stimulation.38 Given that there appeared to be no significant differences in expression in Bcl-10 and Carma1 when comparing the whole group of systemic and primary cutaneous CD30+ lymphoproliferations with PTCL-NOS, these proteins are unlikely to be responsible for the varying activity of NF-κB and expression of its target genes between PTCL subgroups.
One goal of our study was to compare the expression of the TCR/CD3 complex and its associated signaling molecules and transcription factors between systemic ALCL and primary cutaneous CD30+ T-cell lymphoproliferative disorders. In general, the expression of these proteins in primary cutaneous CD30+ T-cell lymphoproliferative disorders paralleled that in systemic ALCL. However, it seems that there may be a ‘gradient’, with the lowest disturbance in primary cutaneous CD30+ T-cell lymphoproliferative disorders, an intermediate disturbance in ALK− systemic ALCL and the highest degree of disturbance in ALK+ systemic ALCL. The minor differences we found between expression in primary cutaneous CD30+ T-cell lymphoproliferative disorders and systemic ALCL are surprising, given the dramatic discrepancy in the clinical presentation and prognosis between these entities. However, a recent gene expression profiling study also revealed highly related expression signatures between primary cutaneous ALCL and systemic ALCL,36 in concordance with our results. Nevertheless, statistical analysis demonstrated some significant differences between primary cutaneous CD30+ T-cell lymphoproliferative disorders and systematic ALCL for the expression of ATF-1, Ets-1, ZAP-70, NFATc1, c-Fos, bcl10, Carma1, and Lck, while no obvious divergent expression profile was evident between cases of lymphomatoid papulosis and cutaneous ALCL. However, the utility of these markers is of limited value in routine diagnosis of cutaneous infiltrates, since no single marker or a panel of markers can reliably differentiate between a primary or secondary cutaneous CD30+ lymphoproliferation.
In contrast, several markers may be of diagnostic value in distinguishing between ALK− systemic ALCL and PTCL-NOS. This differential diagnosis is sometimes difficult, especially in highly pleomorphic CD30+ lymphomas. Our series contained two consistently CD30+ and four partially CD30+ PTCL-NOS cases that did not differ in their immunohistochemical expression profile from the remaining PTCL-NOS. We would argue that a loss of any of the CD3 subunits in the tumor cells would strongly favor the diagnosis of systemic ALCL or a primary cutaneous CD30+ T-cell lymphoproliferative disorder. Moreover, a panel of antibodies including NFATc1, TCF-1, TCF-1α/LEF-1, LAT, Syk, Lck, c-Jun, and c-Fos might be of help in cases in which staining for the CD3 subunits is inconclusive, although no single marker is able to make a robust distinction.
In conclusion, our results shed further light on the disturbance of the TCR/CD3 complex, associated signaling cascades and transcription factors in systemic ALCL, which appears to parallel the situation in classical Hodgkin’s lymphoma in which there is usually a lack of expression of B-cell receptor and other characteristic B-cell markers.39,40 Furthermore, primary cutaneous CD30+ T-cell lymphoproliferative disorders are highly similar to systemic ALCL with regards to their expression profile of TCR-associated molecules, even though their clinical behaviors differ significantly. In contrast, PTCL-NOS retain the full expression program of proteins required for a functioning TCR signaling. This biological feature might be of diagnostic utility in distinguishing PTCL-NOS from the entire group of CD30+ T-cell lymphoproliferations.
Footnotes
Funding: this work was supported by a grant from the Deutsche Forschungsgemeinschaft (RU814/2-1).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Bonzheim I, Geissinger E, Roth S, Zettl A, Marx A, Rosenwald A, et al. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood. 2004;104(10):3358–60. doi: 10.1182/blood-2004-03-1037. [DOI] [PubMed] [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SHCE, Harris NL, Jaffe ES, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008. [Google Scholar]
- 4.Yu JB, Blitzblau RC, Decker RH, Housman DM, Wilson LD. Analysis of primary CD30+ cutaneous lymphoproliferative disease and survival from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2008;26(9):1483–8. doi: 10.1200/JCO.2007.14.1374. [DOI] [PubMed] [Google Scholar]
- 5.Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33 (Suppl 1):58–70. doi: 10.1111/j.0303-6987.2006.00548.x. [DOI] [PubMed] [Google Scholar]
- 6.Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8(1):11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 7.Amin HM, Lai R. Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood. 2007;110(7):2259–67. doi: 10.1182/blood-2007-04-060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch B, Hummel M, Bentink S, Fouladi F, Spang R, Zollinger R, et al. CD30-induced signaling is absent in Hodgkin’s cells but present in anaplastic large cell lymphoma cells. Am J Pathol. 2008;172(2):510–20. doi: 10.2353/ajpath.2008.070858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staber PB, Noehammer C, Durkop H, Schauer S, Kenner L, Linkesch W, et al. mRNA expression patterns indicate CD30 mediated activation of different apoptosis pathways in anaplastic large cell lymphoma but not in Hodgkin’s lymphoma. Leuk Res. 2006;30(3):343–8. doi: 10.1016/j.leukres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Al-Shamkhani A. The role of CD30 in the pathogenesis of haematopoietic malignancies. Curr Opin Pharmacol. 2004;4(4):355–9. doi: 10.1016/j.coph.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Wright CW, Rumble JM, Duckett CS. CD30 activates both the canonical and alternative NF-kappaB pathways in anaplastic large cell lymphoma cells. J Biol Chem. 2007;282 (14):10252–62. doi: 10.1074/jbc.M608817200. [DOI] [PubMed] [Google Scholar]
- 12.Call ME, Wucherpfennig KW. Molecular mechanisms for the assembly of the T cell receptor-CD3 complex. Mol Immunol. 2004;40(18):1295–305. doi: 10.1016/j.molimm.2003.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Call ME, Wucherpfennig KW. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu Rev Immunol. 2005;23:101–25. doi: 10.1146/annurev.immunol.23.021704.115625. [DOI] [PubMed] [Google Scholar]
- 14.Kuhns MS, Davis MM, Garcia KC. Deconstructing the form and function of the TCR/CD3 complex. Immunity. 2006;24(2):133–9. doi: 10.1016/j.immuni.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Leiden JM. Transcriptional regulation of T cell receptor genes. Annu Rev Immunol. 1993;11:539–70. doi: 10.1146/annurev.iy.11.040193.002543. [DOI] [PubMed] [Google Scholar]
- 16.Castrop J, van Wichen D, Koomans-Bitter M, van de Wetering M, de Weger R, van Dongen J, et al. The human TCF-1 gene encodes a nuclear DNA-binding protein uniquely expressed in normal and neoplastic T-lineage lymphocytes. Blood. 1995;86(8):3050–9. [PubMed] [Google Scholar]
- 17.Oosterwegel M, van de Wetering M, Timmerman J, Kruisbeek A, Destree O, Meijlink F, et al. Differential expression of the HMG box factors TCF-1 and LEF-1 during murine embryogenesis. Development. 1993;118(2):439–48. doi: 10.1242/dev.118.2.439. [DOI] [PubMed] [Google Scholar]
- 18.Dorfman DM, Greisman HA, Shahsafaei A. Loss of expression of the WNT/beta-catenin-signaling pathway transcription factors lymphoid enhancer factor-1 (LEF-1) and T cell factor-1 (TCF-1) in a subset of peripheral T cell lymphomas. Am J Pathol. 2003;162(5):1539–44. doi: 10.1016/s0002-9440(10)64287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo CT, Leiden JM. Transcriptional regulation of T lymphocyte development and function. Annu Rev Immunol. 1999;17:149–87. doi: 10.1146/annurev.immunol.17.1.149. [DOI] [PubMed] [Google Scholar]
- 20.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitcher LA, van Oers NS. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003;24(10):554–60. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23(48):7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 23.Paterson JC, Tedoldi S, Craxton A, Jones M, Hansmann ML, Collins G, et al. The differential expression of LCK and BAFF-receptor and their role in apoptosis in human lymphomas. Haematologica. 2006;91(6):772–80. [PubMed] [Google Scholar]
- 24.van Oers NS, Killeen N, Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J Exp Med. 1996;183 (3):1053–62. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samraj AK, Stroh C, Fischer U, Schulze-Osthoff K. The tyrosine kinase Lck is a positive regulator of the mitochondrial apoptosis pathway by controlling Bak expression. Oncogene. 2006;25(2):186–97. doi: 10.1038/sj.onc.1209034. [DOI] [PubMed] [Google Scholar]
- 26.Au-Yeung BB, Deindl S, Hsu LY, Palacios EH, Levin SE, Kuriyan J, et al. The structure, regulation, and function of ZAP-70. Immunol Rev. 2009;228(1):41–57. doi: 10.1111/j.1600-065X.2008.00753.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan AC, van Oers NS, Tran A, Turka L, Law CL, Ryan JC, et al. Differential expression of ZAP-70 and Syk protein tyrosine kinases, and the role of this family of protein tyrosine kinases in TCR signaling. J Immunol. 1994;152(10):4758–66. [PubMed] [Google Scholar]
- 28.Canetti C, Aronoff DM, Choe M, Flamand N, Wettlaufer S, Toews GB, et al. Differential regulation by leukotrienes and calcium of Fc gamma receptor-induced phagocytosis and Syk activation in dendritic cells versus macrophages. J Leukoc Biol. 2006;79(6):1234–41. doi: 10.1189/jlb.0705374. [DOI] [PubMed] [Google Scholar]
- 29.Brumbaugh KM, Binstadt BA, Billadeau DD, Schoon RA, Dick CJ, Ten RM, et al. Functional role for Syk tyrosine kinase in natural killer cell-mediated natural cytotoxicity. J Exp Med. 1997;186(12):1965–74. doi: 10.1084/jem.186.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu DH, Morita CT, Weiss A. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol Rev. 1998;165:167–80. doi: 10.1111/j.1600-065x.1998.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 31.Chu DH, van Oers NS, Malissen M, Harris J, Elder M, Weiss A. Pre-T cell receptor signals are responsible for the down-regulation of Syk protein tyrosine kinase expression. J Immunol. 1999;163(5):2610–20. [PubMed] [Google Scholar]
- 32.Feldman AL, Sun DX, Law ME, Novak AJ, Attygalle AD, Thorland EC, et al. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia. 2008;22(6):1139–43. doi: 10.1038/leu.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4(5):E131–6. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 34.Drakos E, Leventaki V, Schlette EJ, Jones D, Lin P, Medeiros LJ, et al. c-Jun expression and activation are restricted to CD30+ lymphoproliferative disorders. Am J Surg Pathol. 2007;31(3):447–53. doi: 10.1097/01.pas.0000213412.25935.e4. [DOI] [PubMed] [Google Scholar]
- 35.Mao X, Orchard G, Russell-Jones R, Whittaker S. Abnormal activator protein 1 transcription factor expression in CD30-positive cutaneous large-cell lymphomas. Br J Dermatol. 2007;157(5):914–21. doi: 10.1111/j.1365-2133.2007.08150.x. [DOI] [PubMed] [Google Scholar]
- 36.Eckerle S, Brune V, Doring C, Tiacci E, Bohle V, Sundstrom C, et al. Gene expression profiling of isolated tumour cells from anaplastic large cell lymphomas: insights into its cellular origin, pathogenesis and relation to Hodgkin lymphoma. Leukemia. 2009;23(11):2129–38. doi: 10.1038/leu.2009.161. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Delgado B, Cuadros M, Honrado E, Ruiz de la Parte A, Roncador G, Alves J, et al. Differential expression of NF-kappaB pathway genes among peripheral T-cell lymphomas. Leukemia. 2005;19(12):2254–63. doi: 10.1038/sj.leu.2403960. [DOI] [PubMed] [Google Scholar]
- 38.Weil R, Israel A. Deciphering the pathway from the TCR to NF-kappaB. Cell Death Differ. 2006;13(5):826–33. doi: 10.1038/sj.cdd.4401856. [DOI] [PubMed] [Google Scholar]
- 39.Kuppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9(1):15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz R, Stanelle J, Hansmann ML, Kuppers R. Pathogenesis of classical and lymphocyte-predominant Hodgkin lymphoma. Annu Rev Pathol. 2009;4:151–74. doi: 10.1146/annurev.pathol.4.110807.092209. [DOI] [PubMed] [Google Scholar]