Abstract
Background
Allogeneic stem cell transplantation is usually considered the only curative treatment option for patients with advanced or transformed myelodysplastic syndromes in complete remission, but post-remission chemotherapy and autologous stem cell transplantation are potential alternatives, especially in patients over 45 years old.
Design and Methods
We evaluated, after intensive anti-leukemic remission-induction chemotherapy, the impact of the availability of an HLA-identical sibling donor on an intention-to treat basis. Additionally, all patients without a sibling donor in complete remission after the first consolidation course were randomized to either autologous peripheral blood stem cell transplantation or a second consolidation course consisting of high-dose cytarabine.
Results
The 4-year survival of the 341 evaluable patients was 28%. After achieving complete remission, the 4-year survival rates of patients under 55 years old with or without a donor were 54% and 41%, respectively, with an adjusted hazard ratio of 0.81 (95% confidence interval [95% CI], 0.49–1.35) for survival and of 0.67 (95% CI, 0.42–1.06) for disease-free survival. In patients with intermediate/high risk cytogenetic abnormalities the hazard ratio in multivariate analysis was 0.58 (99% CI, 0.22–1.50) (P=0.14) for survival and 0.46 (99% CI, 0.22–1.50) for disease-free survival (P=0.03). In contrast, in patients with low risk cytogenetic characteristics the hazard ratio for survival was 1.17 (99% CI, 0.40–3.42) and that for disease-free survival was 1.02 (99% CI, 0.40–2.56). The 4-year survival of the 65 patients randomized to autologous peripheral blood stem cell transplantation or a second consolidation course of high-dose cytarabine was 37% and 27%, respectively. The hazard ratio in multivariate analysis was 1.22 (95% CI, 0.65–2.27) for survival and 1.02 (95% CI, 0.56–1.85) for disease-free survival.
Conclusions
Patients with a donor and candidates for allogeneic stem cell transplantation in first complete remission may have a better disease-free survival than those without a donor in case of myelodysplastic syndromes with intermediate/high-risk cytogenetics. Autologous peripheral blood stem cell transplantation does not provide longer survival than intensive chemotherapy. (Eudract number: NCT00002926; http://www.cancer.gov/clinicaltrials/EORTC-06961)
Keywords: myelodysplastic syndromes, secondary acute myeloid leukemia, cytogenetic characteristics, allogeneic stem cell transplantation, autologous stem cell transplantation, intensive chemotherapy
Introduction
The evolution of the spectrum of myelodysplastic syndromes (MDS) varies from an indolent course over several years to rapid progression to acute myeloid leukemia (AML).1 Since 1982 MDS have been classified according to French-American-British criteria.2 The International Prognostic Scoring System (IPSS)3 distinguishes four risk groups for survival and AML evolution based on cytogenetic subgroups, percentage of bone marrow blasts and number of cytopenias.4 The World Health Organization proposed a new classification system for MDS in 1997.5
For the minority of patients younger than 60 years with advanced stages of MDS, allogeneic stem cell transplantation (SCT) is usually considered the treatment of choice.6–8 However, the outcome of autologous peripheral blood SCT for patients lacking a suitable donor, appeared, in some studies, comparable to that of allogeneic SCT.6,9,10 Moreover, a retrospective study comparing intensive chemotherapy alone against chemotherapy followed by transplantation did not show a clear benefit for chemotherapy followed by SCT.11
This study addressed the issue of whether the existence of an HLA-identical sibling donor and the intention to perform an allogeneic SCT after the first consolidation course of chemotherapy results in a favorable outcome. The main aim of this study was to compare prospectively the value of autologous peripheral blood SCT with a second consolidation course. Finally the study assessed the impact of cytogenetic characteristics, including those determined by fluorescence in situ hybridization (FISH) studies, on outcome.
Design and Methods
Patients and investigations
Patients seen between November 1996 and September 2003 were included in this study if they met the following selection criteria: MDS with more than 10% bone marrow blasts, other forms of MDS with multiple chromosomal abnormalities and/or profound cytopenias (defined as a neutrophil count <0.5×109/L and/or platelet count <20×109/L), chronic myelomonocytic leukemia with more than 5% bone marrow blasts, chronic myelomonocytic leukemia with more than 16×109/L neutrophils or 2.6×109/L monocytes in the blood, and MDS transformed to AML after a documented MDS lasting 6 months or longer. Patients who had already received intensive chemotherapy and/or radiotherapy for their MDS or AML were not eligible. Patients treated with biological response modifiers and/or low-dose cytarabine in the 4 weeks preceding potential inclusion in this study were not eligible for the study (further details of inclusion and exclusion criteria and laboratory investigations, including FISH analyses, are given in the Online Supplementary Appendix).
This study was approved by the ethics committees of the participating institutions and was conducted in accordance with the Declaration of Helsinki. All participants gave their informed consent.
Study design
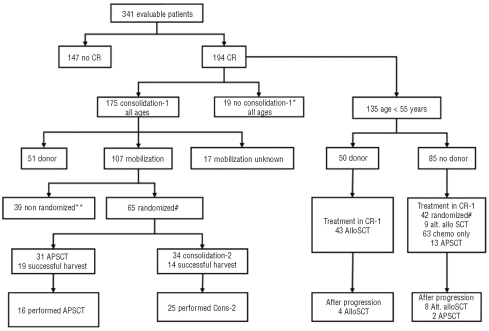
The Criant study was a randomized, phase 3 intergroup study carried out by the European Organization for Research and Treatment of Cancer (EORTC) Leukemia Group, the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA), the Dutch-Belgian Haemato-Oncology Cooperative group (HOVON), the Swiss Group for Clinical Cancer Research (SAKK), the European Blood and Marrow Transplantation (EBMT) group and the Nordic MDS group in 39 European centers. The design of the study and the disposition of patients are illustrated in Figure 1.
Figure 1.
Overview of treatment allocation of all 341 eligible patients.*Reasons for not administering the scheduled consolidation course were: early relapse (n=2), protocol violation (n=4), toxicity (n=10), refusal (n=1), and other reasons (n=2). Seven of these patients had an HLA-identical sibling and five of them received an allograft in first complete remission without the consolidation course. **Reasons for not randomizing the patients were: early relapse (n=11), toxicity (n=11), refusal (n=3), protocol violation (n=3), allograft (n=1), autograft (n=3), ineligibility for randomization (n=2), other (n=5) #: In total 68 patients were randomized (including 42 patients younger than 55 years who participated in the donor versus no donor comparison), but three patients were considered ineligible because they had relapsed before randomization. CR: complete remission; CR-1: first complete remission; AlloSCT: allogeneic stem cell transplantation; APSCT: autologous peripheral blood stem cell transplantation.
Remission-induction chemotherapy consisted of idarubicin 10 mg/m2 on days 1, 3, and 5, cytarabine 100 mg/m2, as a continuous intravenous infusion, on days 1–10, and etoposide 100 mg/m2, intravenously, on days 1–5 (ICE), followed by a second identical remission-induction course in the case of a partial remission. The availability of histocompatible sibling donors was evaluated for patients who achieved a complete remission.
After achieving complete remission, a course of consolidation therapy was given which consisted of idarubicin 10 mg/m2 administered intravenously on days 4–6 in combination with cytarabine 500 mg/m2 12-hourly in a 2-hour intravenous infusion on days 1–6 (IDIA). Patients aged 55 years or less (but also some who were older, according to the policy of each center) with an identified HLA-A, -B, -DR identical sibling, non-reactive mixed lymphocyte culture and confirmed complete remission after the course of consolidation chemotherapy were offered allografting. Patients without a donor received 300 μg granulocyte colony-stimulating factor subcutaneously daily from day 20 after the start of the consolidation course until completion of mobilization. Patients without a donor and in complete remission after the consolidation course were randomized between a second consolidation course consisting of cytarabine 1 g/m2 intravenously, every 12 h, on days 1–6 and autologous peripheral blood SCT. The randomization did not depend on the quality of the stem cell harvest during the first mobilization procedure. Patients with an insufficient harvest and randomized for autologous SCT were candidates for a second attempt at stem cell mobilization with granulocyte colony-stimulating factor only. Details of the transplantation regimens and definitions are given in the Online Supplementary Appendix.
Statistical analysis
All patients were registered prospectively at the EORTC Data Center in Brussels. Randomization (autologous peripheral blood SCT versus high-dose cytarabine) was stratified for center and various other factors (for details, see the Online Supplementary Appendix. The duration of survival was calculated from the date of the start of treatment until death, irrespective of the cause. For patients who achieved complete remission after induction, the disease-free survival was calculated from the date of first complete remission until the date of first relapse or until death in complete remission. The duration of survival of patients who achieved a complete remission was taken to be the time from the first complete remission to the date of death. For patients randomized, the starting point for disease-free survival and other survival analyses was the date of randomization. The Kaplan-Meier technique was used to estimate survival-type distributions and the standard errors (SE) of the estimates were obtained using Greenwood’s formula. The estimates of the incidence of relapse and of death in complete remission were obtained using the competing risk method. A Cox proportional hazard model was used to determine the prognostic importance of several factors and to obtain estimates of the hazard ratio (HR) and the corresponding 95% confidence interval (CI) or 99% CI, in the case of subgroup analysis. Analyses were performed according to the intention-to-treat principle. However, patients who did not reach complete remission or who relapsed before randomization were excluded from the disease-free survival and survival from randomization analyses. For the comparisons of disease-free survival and overall survival in patients younger than 55 years who did or did not have a donor, the survival times were measured from the time at which the patient achieved complete remission. This means that patients who did not receive a consolidation course were included in this analysis as well.
Power calculations
In the initial protocol it was planned to randomize a total of 100 patients, of whom 80 were required to be followed until relapse or death, in order to detect a 20% difference (10% versus 30%) in the 2-year disease-free survival rates between the two treatment groups, corresponding to a hazard ratio of 0.52 (logrank 2 tailed test, alpha error=0.05) with an 80% statistical power. During the study period, only 65 patients could be randomized, of whom 49 relapsed or died in complete remission, providing a 60% statistical power for the detection of a treatment difference regarding disease-free survival.
Results
Patients’ characteristics
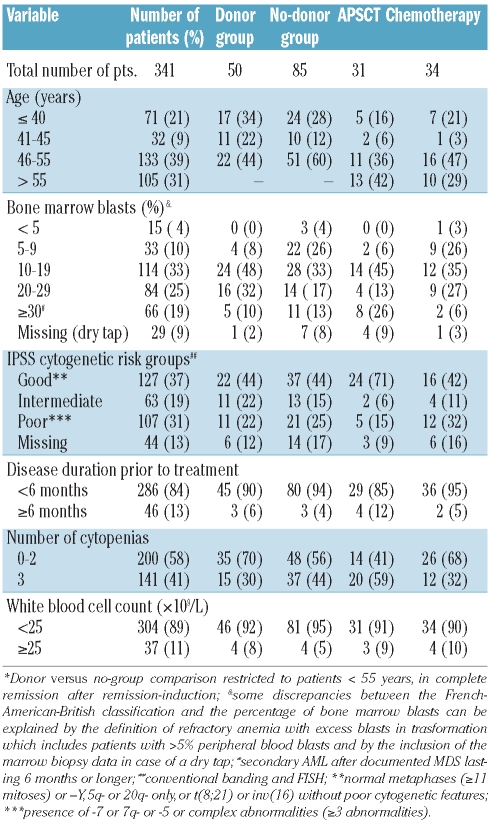
A total of 345 patients were registered, of whom 341 were evaluable for response (Figure 1). Reasons for exclusion were treatment refusal, wrong diagnosis, World Health Organization performance status greater than 2, and missing data. The characteristics of the 341 evaluable patients are summarized in Table 1. Their median age was 51 years (range, 16–67 years). According to the French-American-British criteria 7 patients had refractory anemia, 2 patients had refractory anemia with ring sideroblasts, 104 patients had refractory anemia with excess of blasts, 131 patients had refractory anemia with excess of blasts in transformation, 20 patients had chronic monomyelocytic leukemia, and 77 patients had secondary AML. Twenty-two patients were classified in the intermediate-1 risk group according to IPSS; the remaining patients had a higher risk score reflecting the advanced disease stage of most patients. Reviewed cytogenetic data were available for 295 (86%) patients and FISH data for 158 patients (46%).
Table 1.
Characteristics of total study group, of the donor and no-donor groups* and of the groups randomized between autologous peripheral blood stem cell transplantation (APSCT) and chemotherapy.
Remission-induction and consolidation therapy
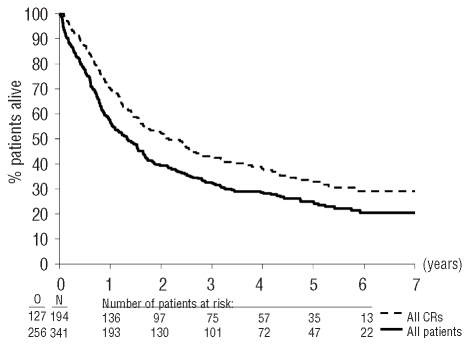
The median follow-up of the 341 evaluable patients was 5.3 years and 256 patients have died. Complete remission was achieved in 173 patients after one course of treatment and in 194 patients (57%) after one or two courses. The remaining patients had either resistant disease, persistent hyperplasia or died before hematopoietic recovery (Figure 1). The first consolidation course was administered to 175 of the 194 patients who achieved a complete remission (Figure 1). The median survival in these patients was 1.3 years (95% CI, 1.0 – 1.7 years) and the 4-year survival rate was 28% (SE=2.5%) (Figure 2). The median disease-free survival was 1.0 year and the 4-year disease-free survival rate was 29%. The 4-year cumulative incidence of relapse was 60% and that of death in complete remission, 11%. Fifty-three patients (27%) continued to be alive in continuous complete remission.
Figure 2.
Survival from registration in study, for all patients, and survival from complete remission for all patients who reached complete remission.
Prognostic factors influencing survival
Age influenced the treatment outcome significantly (P=0.0001): the 4-year survival rate was 43% for patients younger than 46 years, 25% for those 46 to 55 years old and 19% for those older than 55 years. The percentage of bone marrow blasts did not influence outcome (Online Supplementary Table S1). The number of cytopenias was of prognostic importance for survival (P=0.02) mainly due to the poor outcome of patients with trilineage cytopenia. Disease duration longer than 6 months prior to inclusion in the study was associated with a significantly (P=0.009) lower 4-year survival rate: 13% (SE=5%) versus 30% (SE=3%) for patients whose disease had been present for a shorter time. The white blood cell count also influenced survival (overall P=0.02) mainly due to a negative impact of counts higher than 25×109/L.
Cytogenetic characteristics were the most significant (P<0.0001) disease-associated prognostic factor. The 4-year survival rate of the 127 patients with good-risk cytogenetic features was 44%, while the survival rates of the intermediate- and high-risk groups were 28% and 9%, respectively. The IPSS risk group was not of prognostic importance for survival, mainly due to the high weight of bone marrow blast percentage in this model (Online Supplementary Table S1).
Multivariate analysis showed that age (> 45 years versus ≤ 45 years: HR, 1.8; 95% CI, 1.3 to 2.4) and IPSS cytogenetic risk group (intermediate versus good: HR, 1.8; 95% CI, 1.2–2.6; poor versus good: HR, 3.2; 95% CI, 2.4–4.5) were the most important independent prognostic variables for duration of survival. Prolonged disease duration (≥ 6 months), high white blood cell count (> 25×109/L) and trilineage cytopenia were additional independent features associated with a shorter survival, with P values of 0.004, 0.005, and 0.02, respectively.
Post-remission therapy: donor versus no donor
The policy of performing HLA-typing of patients and their siblings was age-dependent. An HLA-identical sibling was identified in 50 of the 135 patients younger than 56 years (37%) compared to only 8 of the 55 patients older than 55 years (15%). The comparison of the treatment outcome of patients with or without a donor was, therefore, restricted to patients younger than 56 years (Figure 1). Among the 50 patients with a donor, 47 received the planned allogeneic SCT, including four patients who received the transplant after progression of their disease. The median time from complete remission to allogeneic SCT in the 44 patients who received the allogeneic SCT in first complete remission was 3 months (range, 0 to 8 months). Details of the transplant procedures are presented in the Online Supplementary Appendix.
The 85 patients without a donor received autologous peripheral blood SCT (13 patients), high-dose chemotherapy only (63 patients) or allogeneic SCT from alternative donors (9 patients). The median time from complete remission to autologous SCT or high-dose cytarabine chemotherapy was 3 months (range, 2 to 19 months).
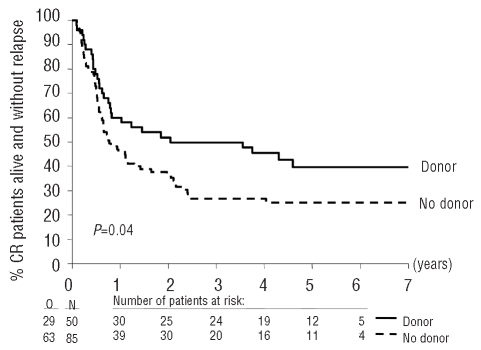
Eight patients underwent allogeneic SCT from an alternative donor after progression of their disease. The 4-year disease-free survival rates of patients with or without a donor were 46% and 27%, respectively (HR, 0.63; 95% CI, 0.41 – 0.99) (Table 2; Figure 3). The 4-year relapse incidence in patients with a donor was 41% whereas it was 64% in the group without donors (P=0.008); the cumulative incidences of death in complete remission were 14% and 10%, respectively (P=0.38). The 4-year survival rates in the two groups were 54% and 41% respectively (HR, 0.75, 95% CI, 0.46 to 1.20) (Table 2). The median time from complete remission to transplantation of the nine patients transplanted with grafts from alternative donors was 3.8 months (range, 2.5–6.2 months). Three patients died in complete remission and six remained in complete remission (range, 2.6–6.6 years). By censoring their follow-up at the time of the allogeneic SCT, the comparison of disease-free survival in patients with or without donors, adjusted for age, cytogenetics/FISH and number of cytopenias, yielded similar results (HR, 0.62; 95% CI, 0.39–0.99).
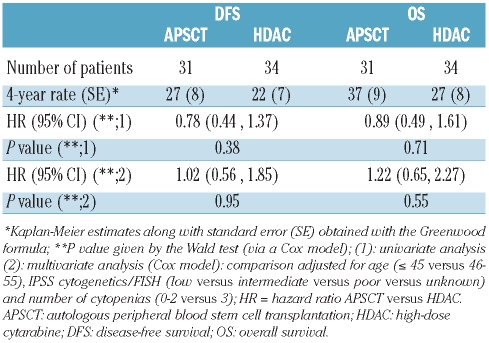
Table 2.
Comparison of patients with and without a donor who reached complete remission and were ≤ 55 years old, regarding disease-free survival (DFS) and overall survival (OS) from complete remission.
Figure 3.
Survival from complete remission without relapse according to the availability of a HLA identical sibling in patients ≤55 years old.
According to a multivariate Cox model (Table 2), the existence of a donor resulted in a better disease-free survival: the ‘donor’ versus ‘no donor’ comparison yielded a HR of 0.67 (95% CI, 0.42–1.06). The HR ratio for survival was, however, close to 1 (0.81; 95% CI, 0.49–1.35). In this model, the test for an interaction ‘donor availability’ –‘cytogenetic risk group’ was of borderline statistical significance (P=0.10) with regards to disease-free survival, indicating that some heterogeneity may exist according to the cytogenetic group regarding the differences in outcome between patients with or without a donor. In the low risk cytogenetic group (59 patients) the ‘donor’ versus ‘no donor’ comparison yielded a HR of 1.02 (99% CI, 0.40–2.56). In contrast, in the group with intermediate or poor cytogenetic characteristics (56 patients), the ‘donor’ versus ‘no donor’ comparison yielded a HR for disease-free survival of 0.46 (99% CI, 0.19–1.13) (Table 2) and for duration of survival a HR of 0.58 (99% CI, 0.22–1.50).
Mobilization of stem cells, stem cell harvest and post-consolidation randomization
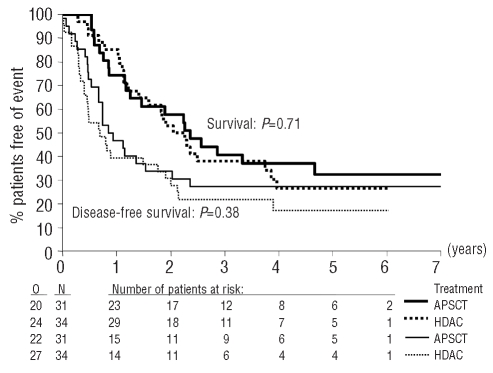
Mobilization of stem cells during the recovery phase after the first consolidation course was adequate in 48 patients and failed in 61 patients. In total 65 patients in complete remission were randomized: 31 to autologous peripheral blood SCT and 34 to a second consolidation course (high-dose cytarabine) (Figure 1). Forty-two of the randomized patients were younger than 56 years and 23 patients were older than 55 years. The relevant characteristics are presented in Table 1. The reasons for not randomizing the 39 patients are indicated in Figure 1. The normal treatment completion rate was 52% (16/31) and 74% (25/34) in the autologous and high-dose cytarabine groups, respectively. In the autologous SCT arm 12 patients did not receive the planned transplant because of an inadequate harvest of stem cells and four patients because of toxicity. In the high-dose cytarabine group two patients did not receive the second consolidation course due to toxicity, two patients due to protocol violation (one underwent allogeneic SCT from an alternative donor and one underwent autologous SCT) and five patients due to early relapse. Out of the 65 randomized patients, 47 relapsed (20 autologous SCT, 27 high-dose cytarabine) and two patients died in complete remission after autologous SCT. The 4-year disease-free survival rates in the autologous SCT and high-dose cytarabine groups were 29% and 19%, respectively (Table 3 and Figure 4). Several differences were identified between the two groups: in the autologous SCT group more patients were older than 55 years and fewer patients had poor-risk cytogenetic characteristics (Table 1). The estimated HR for autologous SCT versus high-dose cytarabine was 0.98 (95% CI, 0.56–1.86) after adjustment of disease-free survival for these two factors. The 4-year survival rate of the two groups was 37% and 27%, respectively (P=0.71) (Table 3 and Figure 4). The treatment comparison adjusted for age and cytogenetics yielded a HR of 1.22 (95% CI, 0.65 to 2.29).
Table 3.
Comparison of patients randomized between autologous peripheral blood stem cell transplantation and a second consolidation course with high-dose cytarabine, regarding disease-free survival and overall survival from randomization.
Figure 4.
Survival (two upper curves) and disease-free survival (two lower curves) from randomization according to the randomization group. N: number of patients; O: observed number of events (death –for survival analysis –, or relapse or death without relapse); P value given by the logrank test. APSCT: autologous peripheral blood stem cell transplantation; HDAC: high-dose cytarabine.
Discussion
The results of intensive chemotherapy in patients with advanced stages of MDS have improved with complete remission rates now ranging between 44% and 64%,12–17 similar to the 57% complete remission rate observed in this study. Remission after chemotherapy usually lasts less than 12 months.14,17 Chromosomal characteristics are important prognostic factors influencing remission duration.13 The percentage of patients with poor-risk cytogenetic characteristics in this study was high (31%) compared to the observed 10% poor-risk patients in large AML studies.18,19 The 9% 4-year survival rate of patients with poor-risk cytogenetic characteristics in this study was low compared to 4-year survival rates of patients with good risk (44%) or intermediate risk characteristics (28%). The IPSS did not influence the outcome in this study due to the lack of impact of the percentage of bone marrow blasts which constitutes an important factor in this score.3 The patient’s age influenced the outcome significantly, irrespective of the post-remission therapy modality. The overall 4-year survival rate was 43% in patients younger than 45 years compared to only 19% for those older than 55 years.
In view of the high relapse rate after chemotherapy alone, transplantation with autologous stem cells has been applied with the aim of intensifying post-remission therapy.20–22 In a previous study by our group, we analyzed 100 patients in first complete remission who were candidates for SCT.9,23 The 4-year disease-free survival rates in the donor versus no-donor groups were similar: 31% and 27%, respectively. This outcome suggests that patients with advanced stages of MDS may benefit both from allogeneic and from autologous SCT. In our present study the 46% 4-year disease-free survival rate in the group of patients with donors was considerably higher than that in the previous study. This may reflect the generally better transplant results in more recently performed SCT.6 In the present study the non-relapse mortality was only 14% in the donor-group compared to 27% in the previous study.9,23 Subgroup analysis of this study showed that the advantage of the presence of a donor was only apparent in the group of patients with intermediate and high risk cytogenetics (table 3). This observation is in line with the previous study9 which showed only long-term survivors after autologous SCT in patients with good-risk characteristics.24 The overall survival in the donor group was not significantly superior to that in the no-donor group, although in patients with intermediate and high-risk cytogenetic characteristics a trend to a superior survival in the donor group was observed (HR, 0.58; 99% CI, 0.22–1.50; P=0.14). However, the disease-free survival was significantly superior in the donor-group with intermediate and poor risk characteristics (HR, 0.46; 99% CI, 0.19–1.13; P=0.03). Salvage therapy after relapse may have contributed to the improved overall survival from complete remission in the no-donor group. Thirteen patients underwent allogeneic SCT after relapse and five patients in the no-donor group are still alive with a median follow-up of 4.1 years after relapse. Several large studies of patients with de novo AML have addressed the prognostic impact of a histocompatible sibling donor.19,25,26 In these studies the advantage of the existence of such a donor was mainly restricted to patients younger than 40 years. In our study group the average age was considerably higher and only 28% (38/135) of the patients who reached complete remission and were 55 years or younger, were actually under 40 years old. This small number precludes a separate analysis in the young age group. The results in de novo AML are in line with our own observations. Usually patients with unfavorable or intermediate cytogenetic characteristics appear to benefit from allogeneic SCT.
Polyclonal primitive hematopoietic progenitors can be mobilized in patients with MDS after treatment with intensive chemotherapy.27 The present study shows that stem cell mobilization was feasible in a minority (45%) of the patients. This relatively low yield of a sufficient number of stem cells may reflect the low number of residual normal stem cells after chemotherapy or damage to the bone marrow stroma caused by pro-apoptotic cytokines produced by the MDS clone.28 The prolonged hypoplasia after the first consolidation course interfered with the number of randomized patients since the randomization was planned after hematopoietic recovery from this consolidation course. Only 65 of the 107 candidates were randomized between autologous SCT and the second consolidation course. The outcome of the two groups after adjustment for the most important confounding factors was identical. It is clear that better mobilization schedules should be developed before autologous peripheral blood SCT can be recommended as part of the intensive post-remission treatment protocols of MDS patients. New prospective studies will be necessary when better mobilizing schedules for MDS have been identified.
Whether patients with advanced stages of MDS should receive remission-induction chemotherapy prior to the transplant conditioning remains a point for discussion. Retrospective analyses provided conflicting data and interpretation of the data is hampered by various selection biases in the two treatment approaches and by a lack of detail on the chemotherapy administered.6,29 This study shows that the great majority of patients with an identified donor received the transplant (94%). The 4-year disease-free survival rate of 46% among the group with a donor is encouraging compared to results from a large registry.30
In conclusion, our data suggest that allogeneic SCT may be the treatment of choice for the young patients (age ≤55 years) with MDS, characterized by poor risk or intermediate risk cytogenetics, who have a histocompatible donor. For MDS patients lacking an HLA-compatible sibling donor, but with good-risk cytogenetic characteristics, autologous SCT or chemotherapy may be good alternatives.
Appendix
The following investigators participated in this study: Institution Name/Centre (Country) Pr. Bron, Institut J. Bordet, Brussels (BE); Dr. Selleslag, A.Z. St Jan, Bruges (BE); Dr. De Bock, A.Z. Middelheim, Antwerpen (BE) 117 Pr. Berneman, U.Z. Antwerpen, Antwerpen (BE); Pr. Ferrant, Clin. Univ. St. Luc, Brussels (BE); Dr. Bosly, C.U. De Mont Godinne, Yvoir (BE); Pr. Feremans, University Hospital Erasme, Brussels (BE); Drs. Delforge, U.Z. Gasthuisberg, Leuven (BE); Pr. Thyss, Centre A. Lacassagne, Nice (FR); Drs. Michallet, Thomas, Belhabri, Hopital E.Herriot, Lyon (FR); Pr. Marie, Dr. Vekhoff, Hotel-Dieu, Paris (FR); Pr. Varet, H. Necker, Paris (FR); Pr. Lowenberg, U.Z. Rotterdam (NL); Pr. De Witte, Dr. Muus, University Medical Centre Nijmegen, Nijmegen (NL); Dr. Ossenkoppele, VU Medical Centrum Amsterdam (NL); Dr. De Valk, Olv Gasthuis Amsterdam, Amsterdam (NL); Pr. Willemze, University Medical Centre Leiden, Leiden (NL); Pr. Schouten, AZ, Maastricht (NL); Dr. Van Marwijk Kooi, Sophia Ziekenhuis, Zwolle (NL); Dr. Wijermans, Hague Ziekenhuis, Hague (NL); Pr. Sonneveld, U.Z., Rotterdam (NL); Dr. Van Der Lelie, AMC, Amsterdam (NL); Dr. Starobinski, HU, Genève (CH); Dr. Fey, Inselspital, Bern (CH); Dr. Gratwohl, Kantonsspital, Basel (CH); Dr. Hess, Kantonsspital, St Gallen (CH); Dr. Kovacsovics, CHU Vaudois (CH); Dr. Ghielmini, S. Giovanni Hospital, Bellinzona (CH); Pr. Ho, Ruprecht Karls, Heidelberg (DE); Drs. Kobbe, Germing and Avaido, Heinrich-Heine Clinic, Dusseldorf (DE); Dr. Biersack, University Hospital Essen (DE); Pr. Jehn, Klinik Grosshadern, Munchen (DE); Pr. Amadori, San Eugenio Hospital, Roma (IT); Dr. Nilsson-Ehle, Sahlgrenska Goteborg (SE); Dr. Juliusson, Linkoping University Hospital (SE); Dr. Hellstrom-Lindberg, Huddinge University Hosp. (SE); Pr. Labar, University Hospital Rebro, Zagreb (HR); Pr. Cermak, Institute Hematology, Prague (CZ); Dr. Linder, Orebro University Hospital (SE)
Footnotes
Funding: this study was supported by Ankerbestrijding/Koningin Wilhelmina Fonds, the Netherlands, Biomed-2 EU grant PL 95-0357, Fonds Cancer (FOCA) from Belgium, and also by grants from the US National Cancer Institute (grant numbers 5U10 CA11488-26 through 5U10 CA11488-38). Its contents are solely the responsibility of the authors and do not represent the official views of the National Cancer Institute (Bethesda, Maryland, USA). Pharmacia-Upjohn provided an educational grant.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Mufti GJ, Stevens JR, Oscier DG, Hamblin TJ, Machin D. Myelodysplastic syndromes: a scoring system with prognostic significance. Br J Haematol. 1985;59(3):425–33. doi: 10.1111/j.1365-2141.1985.tb07329.x. [DOI] [PubMed] [Google Scholar]
- 2.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–99. [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 4.Maes B, Meeus P, Michaux L, Bijnens L, Boogaerts M, Hagemeijer A, et al. Application of the International Prognostic Scoring System for myelodysplastic syndromes. Ann Oncol. 1999;10(7):825–9. doi: 10.1023/a:1008335814674. [DOI] [PubMed] [Google Scholar]
- 5.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Mod Pathol. 2000;13(2):193–207. doi: 10.1038/modpathol.3880035. [DOI] [PubMed] [Google Scholar]
- 6.de Witte T, Hermans J, Vossen J, Bacigalupo A, Meloni G, Jacobsen N, et al. Haematopoietic stem cell transplantation for patients with myelodysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2000;110(3):620–30. doi: 10.1046/j.1365-2141.2000.02200.x. [DOI] [PubMed] [Google Scholar]
- 7.Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA, et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood. 2002;100(4):1201–7. doi: 10.1182/blood-2002-02-0527. [DOI] [PubMed] [Google Scholar]
- 8.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 9.Oosterveld M, Suciu S, Verhoef G, Labar B, Belhabri A, Aul C, et al. The presence of an HLA-identical sibling donor has no impact on outcome of patients with high-risk MDS or secondary AML (sAML) treated with intensive chemotherapy followed by transplantation: results of a prospective study of the EORTC, EBMT, SAKK and GIMEMA Leukemia Groups (EORTC study 06921) Leukemia. 2003;17(5):859–68. doi: 10.1038/sj.leu.2402897. [DOI] [PubMed] [Google Scholar]
- 10.Al-Ali HK, Brand R, van BA, Finke J, Boogaerts M, Fauser AA, et al. A retrospective comparison of autologous and unrelated donor hematopoietic cell transplantation in myelodysplastic syndrome and secondary acute myeloid leukemia: a report on behalf of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Leukemia. 2007;21(9):1945–51. doi: 10.1038/sj.leu.2404774. [DOI] [PubMed] [Google Scholar]
- 11.Oosterveld M, Muus P, Suciu S, Koller C, Verhoef G, Labar B, et al. Chemotherapy only compared to chemotherapy followed by transplantation in high risk myelodys-plastic syndrome and secondary acute myeloid leukemia; two parallel studies adjusted for various prognostic factors. Leukemia. 2002;16(9):1615–21. doi: 10.1038/sj.leu.2402591. [DOI] [PubMed] [Google Scholar]
- 12.Wattel E, Solary E, Hecquet B, Caillot D, Ifrah N, Brion A, et al. Quinine improves the results of intensive chemotherapy in myelodysplastic syndromes expressing P glycoprotein: results of a randomized study. Br J Haematol. 1998;102(4):1015–24. doi: 10.1046/j.1365-2141.1998.00870.x. [DOI] [PubMed] [Google Scholar]
- 13.Fenaux P, Morel P, Rose C, Lai JL, Jouet JP, Bauters F. Prognostic factors in adult de novo myelodysplastic syndromes treated by intensive chemotherapy. Br J Haematol. 1991;77(4):497–501. doi: 10.1111/j.1365-2141.1991.tb08616.x. [DOI] [PubMed] [Google Scholar]
- 14.de Witte T, Suciu S, Peetermans M, Fenaux P, Strijckmans P, Hayat M, et al. Intensive chemotherapy for poor prognosis myelodysplasia (MDS) and secondary acute myeloid leukemia (sAML) following MDS of more than 6 months duration. A pilot study by the Leukemia Cooperative Group of the European Organisation for Research and Treatment in Cancer (EORTC-LCG) Leukemia. 1995;9(11):1805–11. [PubMed] [Google Scholar]
- 15.Ossenkoppele GJ, Graveland WJ, Sonneveld P, Daenen SM, Biesma DH, Verdonck LF, et al. The value of fludarabine in addition to ARA-C and G-CSF in the treatment of patients with high-risk myelodysplastic syndromes and AML in elderly patients. Blood. 2004;103(8):2908–13. doi: 10.1182/blood-2003-07-2195. [DOI] [PubMed] [Google Scholar]
- 16.Parker JE, Pagliuca A, Mijovic A, Cullis JO, Czepulkowski B, Rassam SM, et al. Fludarabine, cytarabine, G-CSF and idarubicin (FLAG-IDA) for the treatment of poor-risk myelodysplastic syndromes and acute myeloid leukaemia. Br J Haematol. 1997;99(4):939–44. doi: 10.1046/j.1365-2141.1997.4763281.x. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann WK, Heil G, Zander C, Wiebe S, Ottmann OG, Bergmann L, et al. Intensive chemotherapy with idarubicin, cytarabine, etoposide, and G-CSF priming in patients with advanced myelodysplastic syndrome and high-risk acute myeloid leukemia. Ann Hematol. 2004;83(8):498–503. doi: 10.1007/s00277-004-0889-0. [DOI] [PubMed] [Google Scholar]
- 18.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 19.Suciu S, Mandelli F, de Witte T, Zittoun R, Gallo E, Labar B, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIME MA AML-10 trial. Blood. 2003;102(4):1232–40. doi: 10.1182/blood-2002-12-3714. [DOI] [PubMed] [Google Scholar]
- 20.Wattel E, Solary E, Leleu X, Dreyfus F, Brion A, Jouet JP, et al. A prospective study of autologous bone marrow or peripheral blood stem cell transplantation after intensive chemotherapy in myelodysplastic syndromes. Groupe Francais des Myelodysplasies. Group Ouest-Est d’etude des Leucemies aigues myeloides. Leukemia. 1999;13(4):524–9. doi: 10.1038/sj.leu.2401387. [DOI] [PubMed] [Google Scholar]
- 21.de Witte T, van Biezen A, Hermans J, Labopin M, Runde V, Or R, et al. Autologous bone marrow transplantation for patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia following MDS. Chronic and Acute Leukemia Working Parties of the European Group for Blood and Marrow Transplantation. Blood. 1997;90(10):3853–7. [PubMed] [Google Scholar]
- 22.Kroger N, Brand R, van Biezen A, Cahn JY, Slavin S, Blaise D, et al. Autologous stem cell transplantation for therapy-related acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2006;37(2):183–9. doi: 10.1038/sj.bmt.1705226. [DOI] [PubMed] [Google Scholar]
- 23.de Witte T, Suciu S, Verhoef G, Labar B, Archimbaud E, Aul C, et al. Intensive chemotherapy followed by allogeneic or autologous stem cell transplantation for patients with myelodysplastic syndromes (MDSs) and acute myeloid leukemia following MDS. Blood. 2001;98(8):2326–31. doi: 10.1182/blood.v98.8.2326. [DOI] [PubMed] [Google Scholar]
- 24.Ducastelle S, Ades L, Gardin C, Dombret H, Prebet T, Deconinck E, et al. Long-term follow-up of autologous stem cell transplantation after intensive chemotherapy in patients with myelodysplastic syndrome or secondary acute myeloid leukemia. Haematologica. 2006;91(3):373–6. [PubMed] [Google Scholar]
- 25.Cornelissen JJ, van Putten WL, Verdonck LF, Theobald M, Jacky E, Daenen SM, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109(9):3658–66. doi: 10.1182/blood-2006-06-025627. [DOI] [PubMed] [Google Scholar]
- 26.Burnett AK, Wheatley K, Goldstone AH, Stevens RF, Hann IM, Rees JH, et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br J Haematol. 2002;118(2):385–400. doi: 10.1046/j.1365-2141.2002.03724.x. [DOI] [PubMed] [Google Scholar]
- 27.Delforge M, Demuynck H, Vandenberghe P, Verhoef G, Zachee P, van DV, et al. Polyclonal primitive hematopoietic progenitors can be detected in mobilized peripheral blood from patients with high-risk myelodysplastic syndromes. Blood. 1995;86(10):3660–7. [PubMed] [Google Scholar]
- 28.Raza A, Mundle S, Shetty V, Alvi S, Chopra H, Span L, et al. A paradigm shift in myelodysplastic syndromes. Leukemia. 1996;10(10):1648–52. [PubMed] [Google Scholar]
- 29.Nakai K, Kanda Y, Fukuhara S, Sakamaki H, Okamoto S, Kodera Y, et al. Value of chemotherapy before allogeneic hematopoietic stem cell transplantation from an HLA-identical sibling donor for myelodysplastic syndrome. Leukemia. 2005;19(3):396–401. doi: 10.1038/sj.leu.2403640. [DOI] [PubMed] [Google Scholar]
- 30.Sierra J, Perez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100(6):1997–2004. [PubMed] [Google Scholar]