Abstract
Purpose
In lung cancer patients with multiple lesions, the differentiation between metastases and second primary tumours has significant therapeutic and prognostic implications. The aim of this retrospective study was to investigate the potential of 18F-FDG PET to discriminate metastatic disease from second primary lung tumours.
Methods
Of 1,396 patients evaluated by the thoracic oncology group between January 2004 and April 2009 at the Radboud University Nijmegen Medical Centre, patients with a synchronous second primary lung cancer were selected. Patients with metastatic disease involving the lungs served as the control group. Maximum standardized uptake values (SUVs) measured with 18F-FDG PET were determined for two tumours in each patient. The relative difference between the SUVs of these tumours (∆SUV) was determined and compared between the second primary group and metastatic disease group. Receiver-operating characteristic (ROC) curve analysis was performed to determine the sensitivity and specificity of the ∆SUV for an optimal cut-off value.
Results
A total of 37 patients (21 metastatic disease, 16 second primary cancer) were included for analysis. The ∆SUV was significantly higher in patients with second primary cancer than in those with metastatic disease (58 vs 28%, respectively, p < 0.001). The area under the ROC curve was 0.81 and the odds ratio for the optimal cut-off was 18.4.
Conclusion
SUVs from 18F-FDG PET images can be helpful in differentiating metastatic disease from second primary tumours in patients with synchronous pulmonary lesions. Further studies are warranted to confirm the consistency of these results.
Keywords: FDG PET, Second primary tumour, Metastatic disease, Lung cancer, Standardized uptake value, CT
Introduction
Lung cancer is the leading cause of cancer-related mortality [1]. Although the incidence of lung cancer is decreasing [1], the number of patients presenting with a second primary cancer has dramatically increased in the last decades [2, 3]. A simultaneous second primary lung carcinoma occurs in 1–8% of lung cancer patients [4, 5]. The occurrence of multiple primary cancers may be attributed to shared aetiological factors [3, 6]. Specifically, 70% of second primary cancers presenting in lung cancer patients are tobacco related, the most common locations including the upper aerodigestive tract, the uroepithelium and the colorectum [7].
Second primary cancers can be divided into synchronous cancers, occurring simultaneously with the index tumour, and metachronous cancers, presenting more than 6 months after the index tumour [8].
For lung cancer patients 18F-fluorodeoxyglucose positron emission tomography (FDG PET) is recommended according to the American College of Chest Physicians (ACCP) guidelines as standard work-up in potentially curable lung cancer based on conventional imaging. The rate of detection of unanticipated metastasis by FDG PET has been reported as 1–18% in patients with clinical stage I or II disease [9]. When an FDG PET scan is made for lung cancer staging, both metastases as well as synchronous primary tumours can be visualized. While multiple lung nodules of varying sizes are usually classified as metastases, it is a much greater challenge to distinguish a lung metastasis from a second primary lung carcinoma when only one additional pulmonary lesion is detected [10].
Discriminating metastatic disease from second primary lung cancer is of great clinical interest because it has large therapeutic and prognostic implications. Metastatic lung cancer is considered incurable and is treated with palliative intent [1]. The survival of lung cancer patients presenting with multiple primary cancers has been found to be similar compared to patients with solitary primary lung cancer [7, 11], and an aggressive surgical approach has proven to be safe and justified in patients with synchronous multiple primary lung cancers and node-negative disease [12]. Therefore, multiple primary cancers should be staged separately and—in cases of early stage—treated with curative intent, including surgery when the tumours are resectable.
Obviously, when two tumours are histologically different they are easily recognized as separate primary tumours. However, when tumours share common histological features, it remains uncertain whether they should be classified as metastases or separate primary tumours. Immunohistochemistry and TP53 gene mutation analyses may be used to ascertain the clonality of synchronous tumours. The latter has been promoted as a gold standard for differentiation of second primary tumours from metastatic disease [13–15].
FDG uptake reflects metabolic activity of tumour lesions, which depends on a variety of tumour characteristics, such as degree of proliferation, hypoxia and tumour aggressiveness. FDG uptake can be quantified by calculating standardized uptake values (SUVs) on PET images. SUVs have been reported to correlate with histological subtypes and tumour stage [16–18] and have been shown to increase with poorer tumour differentiation [16, 19], a higher proportion of actively proliferating cells [19] and increased aggressiveness of the tumour [20].
Since tumours with a shared clonal origin often behave similarly and have common histological features, we hypothesized that the SUVs of clonally related tumours (i.e. metastases) would be more similar than those of tumours with a different clonal origin. Consequently, we hypothesized that the SUVs of metastases approach the SUV of the primary tumour they originate from and that the SUVs of two primary tumours differ to a greater extent.
This retrospective study evaluated the potential of SUVs measured with FDG PET for the characterization of synchronous pulmonary lesions.
Materials and methods
Patients
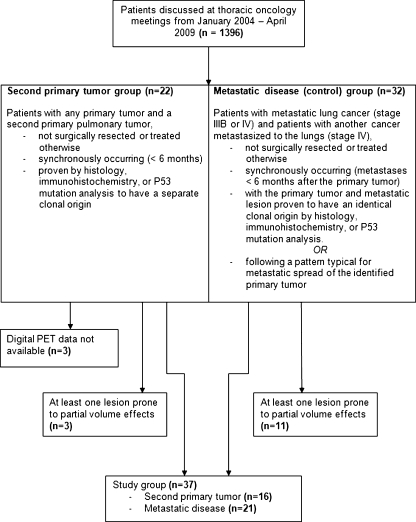
A total of 1,396 patients (536 women and 860 men) who had been evaluated by the multidisciplinary thoracic oncology group between January 2004 and April 2009 at the Radboud University Nijmegen Medical Centre were retrospectively screened. First, patients were included in the ‘second primary group’ when they presented with two primary tumours, including any index tumour and a synchronous pulmonary tumour, defined as a tumour diagnosed within 6 months of diagnosis of the index tumour [8]. Second, patients with lung cancer metastasized to the same lobe (stage IIIB) or to different lobes or other organs (stage IV) and patients with a primary cancer elsewhere in the body metastasized to the lungs (stage IV) were consecutively searched for and included to form the control group (or ‘metastatic disease group’), until a similar sample size as of the second primary group was reached. Patients with any cancer-related treatment prior to FDG PET were excluded from the study. In all patients, a diagnostic contrast-enhanced chest computed tomography (CT) scan was performed including CT scan of liver and adrenal glands prior to or directly following the FDG PET. An overview of the inclusion and exclusion criteria is presented in the flow chart of Fig. 1.
Fig. 1.
Flow chart of the included and excluded patients
A tumour was considered a second primary tumour when histopathological or immunohistochemical features differed from those of the index tumour. In cases of tumours with identical histological and immunohistochemical features, TP53 mutation analysis had to be performed demonstrating different clonal origins of the tumours. Patients without conclusive diagnosis of second primary cancer due to impossibility of gaining adequate tissue samples were excluded. Metastatic disease was concluded based on identical histopathological findings of multiple lesions. Additionally, tumours were considered metastases when multiple (more than two) tumours in a pattern typical for metastatic spread of the identified primary tumour had been localized on imaging modalities.
When FDG uptake is measured in small tumours, bias can be introduced by the partial volume effect resulting in underestimation of the tumour SUV [21]. To prevent bias by partial volume effects, patients with a tumour smaller than 15 mm were excluded from analysis.
Data on patient and tumour characteristics were extracted from patient charts and histopathology reports.
18F-FDG PET data acquisition and reconstruction
All patients underwent whole-body FDG PET as part of their routine preoperative staging procedure. Prior to FDG injection, patients fasted for at least 6 h. Intake of sugar-free liquids was permitted. Immediately prior to the procedure, patients were hydrated with 500 ml of water and 60 min after intravenous injection of approximately 250 MBq FDG (Covidien, Petten, The Netherlands) and 10 mg furosemide, images of the area between the proximal femora and the base of the skull were acquired.
Scans were acquired with a hybrid PET/CT scanner (Biograph Duo, Siemens Medical Solutions USA, Inc., Knoxville, TN, USA) containing a two-slice CT scanner. A low-dose CT scan for localization and attenuation correction purposes was acquired in the caudocranial direction. Scanning parameters included 40 mA s (50 mA s for patient weight >100 kg and 60 mA s for >120 kg), 130 kV, 5-mm slice collimation, 0.8-s rotation time and pitch of 1.5, reconstructed to 3-mm slices for smooth coronal representation. CT scans were acquired during timed unforced expiration breath-hold. No intravenous contrast was applied. For PET, a three-dimensional whole-body emission scan was acquired during free breathing; the acquisition time per bed position was 4 min for emission only. All images were iteratively reconstructed using 2 iterations and 8 subsets and a 5-mm 3-D Gaussian filter, resulting in an effective spatial resolution of 5 mm full-width at half-maximum (FWHM).
Standardized uptake values
The maximum SUV [SUVmax, the activity from the maximum-valued pixel within the tumour volume of interest (VOI); hereafter referred to as SUV] normalized to injected activity and patient body weight was calculated at approximately 60 min after tracer injection for each primary lesion and the chosen metastatic lesion with use of the following equation: SUV = maximum activity concentration in the VOI [kBq/ml]/(injected dose [MBq/ml]/patient body weight [kg]). In patients with multiple metastatic lesions, the lesion with the largest diameter was chosen to prevent partial volume effects. Subsequently, the relative difference between the SUV of the index tumour and the SUV of the synchronous tumour (the second primary tumour or metastatic lesion) was assessed (∆SUV) and expressed in percentages of the highest SUV. Examples of deduction of the ∆SUV from the SUVs of two tumours are given in Figs. 2 and 3. Image analyses were performed on the Inveon Research Workplace version 2.2 (IRW, Siemens/CT, Knoxville, TN, USA).
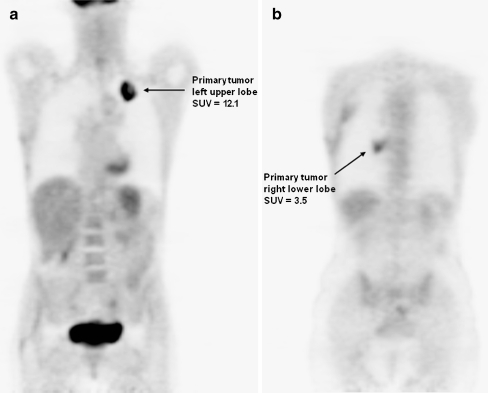
Fig. 2.
FDG PET of a patient with two primary lung tumours. FDG PET demonstrating a moderately differentiated adenocarcinoma (a) in the left upper lobe with an SUV of 12.1 and a synchronous second primary with similar histology but a different clonal origin demonstrated by TP53 mutation analysis (b) in the right lower lobe with an SUV of 3.5. The ∆SUV between the SUVs of the two primary tumours was 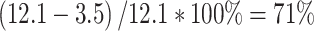
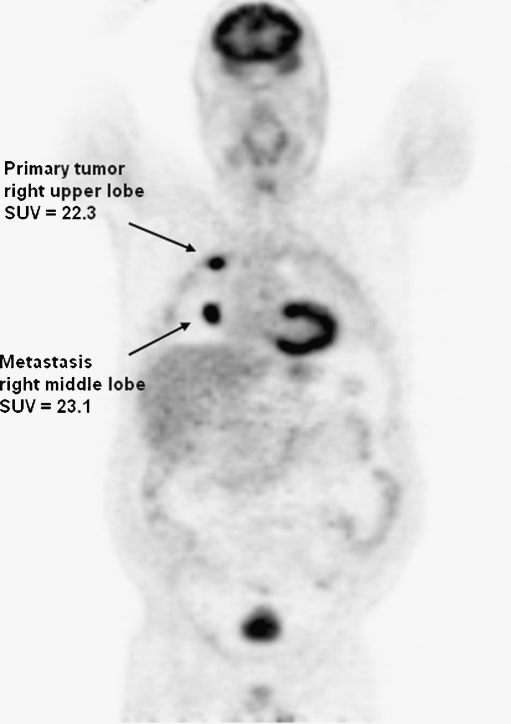
Fig. 3.
FDG PET of a patient with metastasized lung cancer (stage IV). FDG PET demonstrating a primary well-differentiated adenocarcinoma located in the right upper lobe of the lung with an SUV of 22.3 and a metastatic lesion in the right middle lobe of the lung with an SUV of 23.1. The ∆SUV in this patient was 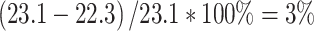
Area under the receiver-operating characteristic curve and cut-off value
After constructing a receiver-operating characteristic (ROC) curve of the ∆SUV, the area under the curve (AUC) was assessed, and the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and odds ratio (OR) were determined for an optimal cut-off of the ∆SUV.
Chest CT
In all patients, a diagnostic contrast-enhanced chest CT scan was performed. For each patient, CT tumour characteristics were assessed by a radiologist. Morphological features of pulmonary nodules suspicious for a primary cancer were speculation, cavitation, irregular margins and pleural or bronchial traction. Tumours were considered metastases if the following features were present: round lesions, sharp borders and homogeneous. In cases of more than two pulmonary nodules the same nodules used for the FDG PET evaluation were chosen.
Statistical analyses
Since the ∆SUV was not normally distributed in both groups (Shapiro-Wilk test, p < 0.05), an independent samples one-tailed Mann-Whitney U test was used to compare the mean ∆SUV between the second primary tumour and metastatic disease group. Mean age and number of pack years were compared using a two-sided t test. A chi-square test was used to compare the proportions of smokers between the groups. The level of significance was set at 0.05 for all analyses.
Results
Patient characteristics
A total of 54 eligible patients with synchronous malignancies (32 metastatic disease and 22 second primary cancer, respectively) were included. After exclusion of patients for whom the digital PET data were unavailable (n = 3), and after exclusion of patients with lesions <15 mm (n = 14), 37 patients remained for analysis. Of those patients, 21 were diagnosed with metastatic disease and 16 with two primary tumours (Fig. 1).
The mean age of the patients (23 men and 14 women) was 68 years (range: 47–85 years). Other patient characteristics, including smoking status, are presented in Table 1. Patient age, sex and smoking status were not significantly different between patients with metastatic disease and a second primary tumour (p > 0.05).
Table 1.
Patient and primary tumour characteristics
| Second primary group (n = 16) | Metastatic disease group (n = 21) | |||||
|---|---|---|---|---|---|---|
| Patient characteristics | p value | |||||
| Mean age (range) | 68 years (49–85 years) | 67 years (47–84 years) | 0.715 | |||
| Sex (m:v) | 11:5 | 12:9 | 0.471 | |||
| Smoking status | Smoker | 7 | 10 | 0.713 | ||
| Ex-smoker | 7 | 9 | ||||
| Non-smoker | 2 | 1 | ||||
| Missing | 0 | 1 | ||||
| Mean no. of pack years | 33.6 ± 15.9 | 35.0 ± 21.2 | 0.842 | |||
| Conclusiona based on | Histopathology | 13 | 5 | |||
| P53 mutation analysis | 1 | 0 | ||||
| Immunohistochemistry | 2 | 0 | ||||
| Clinical features | 0 | 16 | ||||
| Curabilityb | Potentially curable | 12 | 0 | |||
| Incurable | 4 | 21 | ||||
| Characteristics of primary tumours | Index tumour | Synchronous pulmonary tumour | ||||
| Site | Lung | 7 | 16 | 21 | ||
| Colorectum | 5 | 0 | 0 | |||
| Head and neck | 4 | 0 | 0 | |||
| Histopathology | SCLC | 0 | 0 | 3 | ||
| NSCLC | AC | 9 | 9 | 13 | ||
| BAC | 0 | 1 | 0 | |||
| SCC | 7 | 2 | 1 | |||
| LCC | 0 | 2 | 2 | |||
| Undifferentiated | 0 | 0 | 2 | |||
| Other | 0 | 2 | 0 | |||
| Differentiation | Very poor | 0 | 0 | 3 | ||
| Poor | 6 | 8 | 7 | |||
| Intermediate | 8 | 2 | 1 | |||
| Well | 0 | 2 | 1 | |||
| Unspecified | 2 | 4 | 9 | |||
| Stagec | IA | 4 | 5 | 0 | ||
| IB | 2 | 1 | 0 | |||
| IIA | 2 | 2 | 0 | |||
| IIB | 2 | 3 | 0 | |||
| IIIA | 4 | 2 | 0 | |||
| IIIB | 0 | 1 | 2 | |||
| IV | 2 | 1 | 19 | |||
| Missing | 0 | 1 | 0 | |||
SCLC small cell lung carcinoma, NSCLC non-small cell lung carcinoma, AC adenocarcinoma, BAC bronchioloalveolar carcinoma, SCC squamous cell carcinoma, LCC large cell carcinoma
aThe conclusion second primary tumour or metastatic disease was based on histopathological or immunohistochemical features, TP53 mutation analysis results or clinical features. The latter included localization of multiple (more than two) malignancies in a pattern typical for metastatic spread of the identified primary tumour
bPatients with two tumours of stage I–IIIA were considered potentially curable, whereas patients with at least one tumour staged IIIB/IV were considered incurable (TNM staging system version 6)
cAccording to the sixth edition of the TNM classification for lung cancer developed by the International Association for the Study of Lung Cancer [42]
Sites of the index tumour in patients with second primary lung cancer were the lung (n = 7), the colorectum (n = 5) and the head and neck (n = 4). Of 16 second primary cancer patients, 12 (75%) had two tumours of early stage (stage I–IIIA) and were considered potentially curable.
In the majority of cases (76%), metastatic disease was diagnosed based on the clinical pattern (multiple lesions spread in a manner consistent with metastatic pulmonary cancer). The diagnosis of second primary cancer was primarily (81%)—as can be expected—based on histopathological differences. Only in three cases was further immunohistochemistry (two patients) or TP53 mutation (one patient) analysis required for a definite conclusion.
Tumour characteristics
Tumour characteristics are presented in Table 1. All tumours were carcinomas, except for one tumour in the second primary group which was classified as a mesothelioma. Adenocarcinomas were the most commonly diagnosed tumours in both groups. Differentiation of the tumours was better in the second primary group than in the metastatic disease group, although in both groups tumours were most frequently poorly differentiated.
Relative differences between standardized uptake values
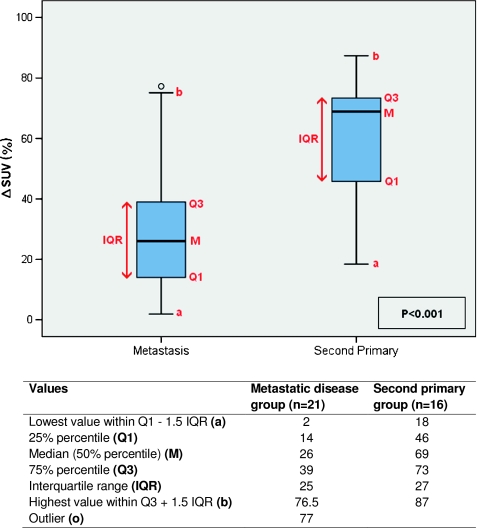
The mean intra-individual ∆SUV between lesions was significantly higher in patients with a second primary tumour [58%, 95% confidence interval (CI): 46–70%] as compared to those with metastatic disease (28%, 95% CI: 19–37%) (p < 0.001). Figure 4 shows box and whisker plots of the ∆SUV for both groups. Although the ranges of the groups show overlap, the majority (75%) of patients with metastatic disease has a ∆SUV below 39%, whereas the ∆SUV exceeds 46% for the majority of the second primary group (Fig. 4). The individual patient data on SUVmax and ∆SUV for the metastasis and second primary group are outlined in Tables 2 and 3.
Fig. 4.
Box and whisker plots showing the distribution of the ∆SUV for both groups. Q1 first quartile, M median, Q3 third quartile, IQR interquartile range
Table 2.
Individual results of metastasis group
| Patient no. | Location tumour 1 | SUVmax tumour 1 | Location tumour 2 | SUVmax tumour 2 | ΔSUVa | ΔSUV%b |
|---|---|---|---|---|---|---|
| 1 | Lung, left hilum | 7.90 | Left 5th rib | 7.30 | 0.60 | 8 |
| 2 | Lung, RUL | 6.30 | Lung, RUL | 4.30 | 2.00 | 32 |
| 3 | Lung, RUL | 16.2 | Liver | 14.3 | 1.90 | 12 |
| 4 | Lung, LLL | 8.00 | Larynx | 9.30 | 1.30 | 14 |
| 5 | Lung, right hilum | 18.2 | Lung, left | 24.6 | 6.40 | 26 |
| 6 | Lung, RML | 34.8 | Th 12 | 26.6 | 10.2 | 29 |
| 7 | Lung, RLL | 27.6 | Lung, LUL | 13.4 | 14.2 | 51 |
| 8 | Lung, RLL | 2.50 | Lung, RLL | 2.20 | 0.30 | 12 |
| 9 | Lung, LLL | 10.5 | Left iliac bone | 10.7 | 0.20 | 2 |
| 10 | Lung, RUL | 5.90 | Lung, right hilum | 3.60 | 2.30 | 29 |
| 11 | Lung, LUL | 16.5 | Right 6th rib | 4.10 | 12.4 | 75 |
| 12 | Lung, RUL | 22.3 | Lung, RML | 23.1 | 0.80 | 3 |
| 13 | Lung, RUL | 6.20 | Th 11 | 4.90 | 1.30 | 21 |
| 14 | Lung, right | 12.4 | Lung, LUL | 7.10 | 5.30 | 43 |
| 15 | Lung, RUL | 9.60 | Right adrenal gland | 5.70 | 3.90 | 41 |
| 16 | Lung, RUL | 13.2 | Right iliac crest | 3.00 | 10.2 | 77 |
| 17 | Lung, RLL | 7.20 | Right 5th rib | 5.30 | 1.90 | 26 |
| 18 | Lung, RML | 12.5 | Lung, RLL | 16.4 | 3.90 | 24 |
| 19 | Lung, RUL | 13.5 | Right PSIS | 11.4 | 2.10 | 16 |
| 20 | Lung, RLL | 5.40 | L1 | 6.40 | 1.00 | 16 |
| 21 | Lung, RML | 5.90 | Lung, RML | 4.10 | 1.80 | 31 |
| Mean ± SD | 4.00 ± 4.22 | 28 ± 21 | ||||
SUV max maximum standardized uptake value, RUL right upper lobe, RLL right lower lobe, LUL left upper lobe, RML right middle lobe, LLL left lower lobe, Th thoracic vertebra, L lumbar vertebra, PSIS posterior superior iliac spine, SD standard deviation of the mean
aThe absolute difference between the SUVmax of tumour 1 and SUVmax of tumour 2
bThe relative difference between the SUVmax of tumour 1 and the SUVmax of tumour 2, expressed in percentages of the highest SUVmax
Table 3.
Individual results of second primary group
| Patient no. | Location tumour 1 | SUVmax tumour 1 | Location tumour 2 | SUVmax tumour 2 | ΔSUVa | ΔSUV%b |
|---|---|---|---|---|---|---|
| 1 | Lung, RUL | 4.50 | Cecum | 7.70 | 3.20 | 42 |
| 2 | Lung, RLL | 8.00 | Lung, LLL | 3.00 | 5.00 | 63 |
| 3 | Lung, RML | 20.7 | Lung, RLL | 5.00 | 15.7 | 76 |
| 4 | Lung, RUL | 10.3 | Lung, LUL | 8.40 | 1.90 | 18 |
| 5 | Rectum | 16.3 | Lung, RUL | 4.83 | 11.47 | 70 |
| 6 | Colon | 9.90 | Lung, LLL | 2.40 | 7.50 | 76 |
| 7 | Lung, LUL | 8.70 | Lung, LLL | 7.10 | 1.60 | 18 |
| 8 | Soft palate | 2.70 | Lung, LUL | 17.6 | 14.9 | 85 |
| 9 | Lung, LUL | 13.4 | Lung, RML | 6.70 | 6.70 | 50 |
| 10 | Right oro/hypopharynx | 18.0 | Lung, LLL | 5.20 | 12.8 | 71 |
| 11 | Colon | 19.4 | Lung, LUL | 5.70 | 13.7 | 71 |
| 12 | Left oropharynx | 23.4 | Lung, RUL | 7.60 | 15.8 | 68 |
| 13 | Larynx | 3.90 | Lung, RLL | 4.80 | 0.90 | 19 |
| 14 | Lung, LUL | 12.12 | Lung, RLL | 3.54 | 8.58 | 71 |
| 15 | Colon | 5.40 | Lung, RUL | 11.1 | 5.70 | 51 |
| 16 | Left distal main bronchus | 13.5 | Lung, RUL | 1.70 | 11.8 | 87 |
| Mean ± SD | 8.58 ± 5.24 | 58 ± 23 | ||||
SUV max maximum standardized uptake value, RUL right upper lobe, RLL right lower lobe, RML right middle lobe, LUL left upper lobe, LLL left lower lobe, SD standard deviation of the mean
aThe absolute difference between the SUVmax of tumour 1 and SUVmax of tumour 2
bThe relative difference between the SUVmax of tumour 1 and the SUVmax of tumour 2, expressed in percentages of the highest SUVmax
Area under the ROC and cut-off value
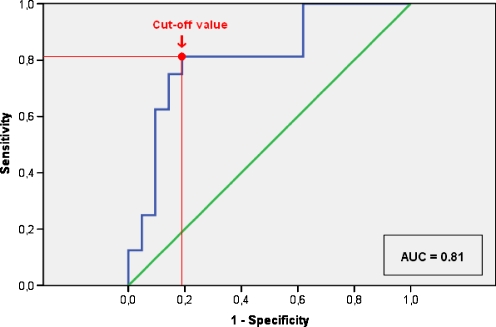
The AUC for ∆SUV was 0.81 (95% CI: 0.67–0.96, p = 0.001) to predict a second primary tumour (Fig. 5), which represents a moderately high discriminative ability of the ∆SUV [22]. The left upper corner of the ROC curve was chosen as the optimal cut-off, which corresponds with a ∆SUV of 41%. This cut-off was associated with a sensitivity, specificity, PPV, NPV and OR of 81, 81, 76, 85 and 18.4%, respectively.
Fig. 5.
ROC curve and corresponding AUC statistics for the ∆SUV. For the optimal cut-off of ∆SUV = 41%, both the sensitivity and specificity are 81%
Chest CT
Individual data of CT characteristics are outlined in Table 4. Of 21 patients in the metastasis group, 19 (90%) had at least one tumour containing morphological features of a metastasis. In the second primary group, a definite diagnosis based on CT morphological features could be made in only eight patients (50%). Of these, three patients had a tumour suspicious for a metastasis, and five patients had two tumours both suspected of being a second primary tumour.
Table 4.
Individual data of CT characteristics
| Patient no. | Tumour 1 | Tumour 2 | ∆SUV% | |
|---|---|---|---|---|
| Metastasis group | 1 | Metastasisa | Metastasis | 8 |
| 2 | Metastasis | Unsureb | 32 | |
| 3 | Metastasis | Unsure | 12 | |
| 4 | Metastasis | Unsure | 14 | |
| 5 | Metastasis | Unsure | 26 | |
| 6 | Metastasis | Metastasis | 29 | |
| 7 | Metastasis | Unsure | 51 | |
| 8 | Primaryc | Unsure | 12 | |
| 9 | Metastasis | Unsure | 2 | |
| 10 | Metastasis | Metastasis | 29 | |
| 11 | Metastasis | Metastasis | 75 | |
| 12 | Unsure | Unsure | 3 | |
| 13 | Metastasis | Unsure | 21 | |
| 14 | Primary | Metastasis | 43 | |
| 15 | Metastasis | Unsure | 41 | |
| 16 | Metastasis | Unsure | 77 | |
| 17 | Metastasis | Unsure | 26 | |
| 18 | Metastasis | Unsure | 24 | |
| 19 | Metastasis | Unsure | 16 | |
| 20 | Metastasis | Unsure | 16 | |
| 21 | Metastasis | Metastasis | 31 | |
| Second primary group | 1 | Primary | Unsure | 42 |
| 2 | Primary | Unsure | 63 | |
| 3 | Metastasis | Unsure | 76 | |
| 4 | Primary | Primary | 18 | |
| 5 | Unsure | Primary | 70 | |
| 6 | Unsure | Primary | 76 | |
| 7 | Primary | Primary | 18 | |
| 8 | Unsure | Primary | 85 | |
| 9 | Primary | Primary | 50 | |
| 10 | Unsure | Metastasis | 71 | |
| 11 | Unsure | Unsure | 71 | |
| 12 | Unsure | Metastasis | 68 | |
| 13 | Unsure | Primary | 19 | |
| 14 | Primary | Primary | 71 | |
| 15 | Unsure | Primary | 51 | |
| 16 | Primary | Primary | 87 |
∆SUV% the relative difference between the SUVmax of tumour 1 and the SUVmax of tumour 2, expressed in percentages of the highest SUVmax
aTumours were considered metastases if the following features were present: round lesions, sharp borders and homogeneous
bBased on CT characteristics, no definite diagnosis of a primary tumour or metastasis could be made
cTumours were considered primary tumours if the following features were present: speculation, cavitation, irregular margins, pleural or bronchial traction
Discussion
To our knowledge, this is the first study investigating the role of quantitative FDG PET in discriminating metastases from second primary tumours in cases of synchronously presenting lesions. A significantly larger ∆SUV between two tumours was found in patients presenting with two primary tumours as compared to patients with metastatic disease involving the lungs. The moderately high accuracy, as measured with the AUC, as well as the good sensitivity and specificity of the ∆SUV support the use of FDG PET as a modality for discriminating second primary lung tumours from metastases. The OR for the optimal cut-off of 41% was 18.4, indicating that the odds of a second primary tumour was 18.4 times higher in patients with a ∆SUV > 41% than in those with a ∆SUV < 41%. Most patients with second primary cancer (75%) had two early stage tumours (I–IIIA), meaning they were potentially curable.
A definite diagnosis of metastatic disease or second primary tumour based on CT scan characteristics could be made in 90% of patients with metastases and in only eight patients (50%) with second primary cancer. Furthermore, in only five of eight patients was the diagnosis right.
Previously, multiple case reports and studies have been published presenting cases of unexpected synchronous primary lung tumours detected by FDG PET [23–26]. On the contrary, only few reports exist in which FDG PET contributes in determining the clonal origin of synchronous tumours [27, 28]. The current available literature further supports our hypothesis that SUVs can differentiate tumours of common origin and with common biological behaviour (i.e. metastases) from those of separate clonal origin (i.e. multiple primary tumours). That is, FDG uptake has been reported to relate to several tumour characteristics, including histological subtype [16–19, 29] and tumour aggressiveness [16, 19, 20].
FDG PET imaging is already extensively being used in patients with lung cancer for several purposes, including the diagnosis of recurrent disease, staging, prognostic stratification and radiotherapy planning [30–33]. Also, it has been shown to be an accurate modality to differentiate benign from malignant solitary pulmonary lesions [30, 34]. Furthermore, FDG PET can be used to monitor the response of non-small cell lung cancer to chemotherapy [35], radiotherapy and potentially to targeting of cell signalling pathways [36]. The results presented implicate that the use of FDG PET might be expanded to the identification of early stage second primary tumours in patients with synchronous pulmonary lesions.
Currently, elaborate and invasive diagnostic procedures are required for the diagnosis of second primary cancer. FDG PET may be a cost-effective modality as it may identify second primary lung tumours at an early and curable stage (stage I–IIIA) in a non-invasive way. Large differences in SUVs between lung tumours in a single patient should urge physicians to consider a second primary lung cancer rather than metastatic disease, resulting in fewer patients wrongfully being withheld a curative treatment. Since our study also included patients with second primary cancer of the colorectum and head and neck, FDG PET might also be useful in patients with synchronous cancers of other organs in whom a diagnosis of metastasis or second primary cancer has yet to be made.
The population studied was carefully defined by stringent inclusion criteria. By including only those patients for whom sufficient data for a definite diagnosis of second primary cancer were available, the validity of this study was strengthened. Additionally, conditions between the patient groups studied were equalized as much as possible by choosing one reconstruction method for all PET images, since this is known to affect the SUV [37].
Several limitations to this study should be noted. First, this study has a small sample size. Because of the retrospective nature of the study, TP53 mutation analysis was missing in many patients with histologically identical lesions and suspicion of a second primary tumour. After exclusion of patients with missing digital PET data and lesions prone to partial volume effects, only one patient in whom TP53 analysis was performed was left for inclusion.
Second, diagnosis was made without histological confirmation in most cases of metastatic disease. In these patients, histopathology of the metastatic lesion was lacking, because the clinical presence of multiple lesions in a pattern typical for metastatic spread was considered sufficient for diagnosis of metastatic disease. If this study had been prospectively conducted, however, tissue for immunohistochemical and mutation analyses could have been sampled for all tumours, thereby assuring validity of diagnoses of both patient groups studied.
Full kinetic analysis of the metabolic rate of FDG has several advantages over the use of SUVs [38]. However, dynamic scans are required for kinetic analysis, which are not readily available in clinical settings [34]. Therefore, the SUV is the most commonly used measure to quantify tumour glucose metabolism on static PET images and its use is further supported by studies showing high reproducibility [39, 40].
To secure comparability of ∆SUVs between patients, only patients with FDG PET images reconstructed at the same resolution (2 iterations and 8 subsets) were included. However, reconstruction with a higher amount of iterations may be preferred to obtain sufficient convergence, which makes the SUV less dependent on surrounding activity [37]. Also, the SUV was normalized to body weight and not to the more preferable body surface area or plasma glucose values [41]. However, these limitations affect the absolute values of SUVs and do not have a large impact on intra-individual differences in SUVs (∆SUVs), as used in this study.
Conclusions
The results of this study suggest that measurement of the SUV using FDG PET images can be useful in differentiating metastatic disease from second primary cancer in patients presenting with synchronous pulmonary lesions. This non-invasive technique, which is standardly available in pre-surgically staged lung cancer patients, may increase cost-effectiveness due to less cumbersome diagnostic procedures and more efficient identification of potentially curable second primary cancer patients. However, larger and prospectively conducted studies are warranted to confirm the consistency of these results and to test the accuracy of the ∆SUV at the cut-off value proposed in this study.
Acknowledgments
Authors’ contributions
All authors have contributed to the preparation of the manuscript. BGD, OCJS, DV, HFMH, and LFGO led the study design. BGD, DV, and LFGO evaluated the PET images. MMS evaluated the CT images. MLS provided tumour samples. DV contributed to the statistical evaluation of the study. BGD, OCJS, HFMH, and LFGO drafted the report.
Funding
No source offunding has been received for this study.
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Bernadette G. Dijkman, Email: bernadijkman@hotmail.com
Olga C. J. Schuurbiers, Email: o.schuurbiers@long.umcn.nl
References
- 1.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15:2020–2026. doi: 10.1158/1055-9965.EPI-06-0414. [DOI] [PubMed] [Google Scholar]
- 3.Soerjomataram I, Coebergh JW. Epidemiology of multiple primary cancers. Methods Mol Biol. 2009;471:85–105. doi: 10.1007/978-1-59745-416-2_5. [DOI] [PubMed] [Google Scholar]
- 4.van Rens MT, Zanen P, Brutel de La Rivière A, Elbers HR, van Swieten HA, van Den Bosch JM. Survival in synchronous vs. single lung cancer: upstaging better reflects prognosis. Chest. 2000;118:952–958. doi: 10.1378/chest.118.4.952. [DOI] [PubMed] [Google Scholar]
- 5.Battafarano RJ, Meyers BF, Guthrie TJ, Cooper JD, Patterson GA. Surgical resection of multifocal non-small cell lung cancer is associated with prolonged survival. Ann Thorac Surg. 2002;74:988–993. doi: 10.1016/S0003-4975(02)03878-X. [DOI] [PubMed] [Google Scholar]
- 6.Ng AK, Travis LB. Subsequent malignant neoplasms in cancer survivors. Cancer J. 2008;14:429–434. doi: 10.1097/PPO.0b013e31818d8779. [DOI] [PubMed] [Google Scholar]
- 7.Aguiló R, Macià F, Porta M, Casamitjana M, Minguella J, Novoa AM. Multiple independent primary cancers do not adversely affect survival of the lung cancer patient. Eur J Cardiothorac Surg. 2008;34:1075–1080. doi: 10.1016/j.ejcts.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Douglas WG, Rigual NR, Loree TR, Wiseman SM, Al-Rawi S, Hicks WL., Jr Current concepts in the management of a second malignancy of the lung in patients with head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2003;11:85–88. doi: 10.1097/00020840-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, et al. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132:178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 10.Leong PP, Rezai B, Koch WM, Reed A, Eisele D, Lee DJ, et al. Distinguishing second primary tumors from lung metastases in patients with head and neck squamous cell carcinoma. J Natl Cancer Inst. 1998;90:972–977. doi: 10.1093/jnci/90.13.972. [DOI] [PubMed] [Google Scholar]
- 11.Koppe MJ, Zoetmulder FA, van Zandwijk N, Hart AA, Baas P, Rutgers EJ. The prognostic significance of a previous malignancy in operable non-small cell lung cancer. Lung Cancer. 2001;32:47–53. doi: 10.1016/S0169-5002(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 12.Chang YL, Wu CT, Lee YC. Surgical treatment of synchronous multiple primary lung cancers: experience of 92 patients. J Thorac Cardiovasc Surg. 2007;134:630–637. doi: 10.1016/j.jtcvs.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Chhieng DC, Cangiarella JF, Zakowski MF, Goswami S, Cohen JM, Yee HT. Use of thyroid transcription factor 1, PE-10, and cytokeratins 7 and 20 in discriminating between primary lung carcinomas and metastatic lesions in fine-needle aspiration biopsy specimens. Cancer. 2001;93:330–336. doi: 10.1002/cncr.9048. [DOI] [PubMed] [Google Scholar]
- 14.van Oijen MG, Leppers Vd Straat FG, Tilanus MG, Slootweg PJ. The origins of multiple squamous cell carcinomas in the aerodigestive tract. Cancer. 2000;88:884–893. doi: 10.1002/(SICI)1097-0142(20000215)88:4<884::AID-CNCR20>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.van Rens MT, Eijken EJ, Elbers JR, Lammers JW, Tilanus MG, Slootweg PJ. p53 mutation analysis for definite diagnosis of multiple primary lung carcinoma. Cancer. 2002;94:188–196. doi: 10.1002/cncr.10001. [DOI] [PubMed] [Google Scholar]
- 16.de Geus-Oei LF, van Krieken JH, Aliredjo RP, Krabbe PF, Frielink C, Verhagen AF, et al. Biological correlates of FDG uptake in non-small cell lung cancer. Lung Cancer. 2007;55:79–87. doi: 10.1016/j.lungcan.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA. The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg. 2005;130:151–159. doi: 10.1016/j.jtcvs.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Jeong HJ, Min JJ, Park JM, Chung JK, Kim BT, Jeong JM, et al. Determination of the prognostic value of [(18)F]fluorodeoxyglucose uptake by using positron emission tomography in patients with non-small cell lung cancer. Nucl Med Commun. 2002;23:865–870. doi: 10.1097/00006231-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Vesselle H, Salskov A, Turcotte E, Wiens L, Schmidt R, Jordan CD, et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol. 2008;3:971–978. doi: 10.1097/JTO.0b013e31818307a7. [DOI] [PubMed] [Google Scholar]
- 20.Higashi K, Ueda Y, Ayabe K, Sakurai A, Seki H, Nambu Y, et al. FDG PET in the evaluation of the aggressiveness of pulmonary adenocarcinoma: correlation with histopathological features. Nucl Med Commun. 2000;21:707–714. doi: 10.1097/00006231-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007;48:932–945. doi: 10.2967/jnumed.106.035774. [DOI] [PubMed] [Google Scholar]
- 22.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 23.Jeon SY, Ahn SH, Kim CH, Lim SM, Koh JS, Lee JC. Esophageal and laryngeal cancer incidentally found on [18F]fluorodeoxyglucose positron emission tomography/computed tomography during the staging workup for lung cancer. Clin Lung Cancer. 2008;9:230–231. doi: 10.3816/CLC.2008.n.035. [DOI] [PubMed] [Google Scholar]
- 24.Mittra E, Vasanawala M, Niederkohr R, Rodriguez C, Segall G. A case of three synchronous primary tumors demonstrated by F-18 FDG PET. Clin Nucl Med. 2007;32:666–667. doi: 10.1097/RLU.0b013e3180a1ad48. [DOI] [PubMed] [Google Scholar]
- 25.van Westreenen HL, Westerterp M, Jager PL, van Dullemen HM, Sloof GW, Comans EF, et al. Synchronous primary neoplasms detected on 18F-FDG PET in staging of patients with esophageal cancer. J Nucl Med. 2005;46:1321–1325. [PubMed] [Google Scholar]
- 26.Adriaensen M, Schijf L, de Haas M, Huijbregts J, Baarslag HJ, Staaks G, et al. Six synchronous primary neoplasms detected by FDG-PET/CT. Eur J Nucl Med Mol Imaging. 2008;35:1931. doi: 10.1007/s00259-008-0821-2. [DOI] [PubMed] [Google Scholar]
- 27.Obando JA, Samii JM, Yasrebi M. A case of two synchronous primary lung tumors demonstrated by FDG positron emission tomography. Clin Nucl Med. 2008;33:775–777. doi: 10.1097/RLU.0b013e318187ef85. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson MD, Fulham MJ, McCaughan BC, Constable CJ. Differentiation of synchronous tumors using FDG positron emission tomography. Clin Nucl Med. 2003;28:489–491. doi: 10.1097/00003072-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Aquino SL, Halpern EF, Kuester LB, Fischman AJ. FDG-PET and CT features of non-small cell lung cancer based on tumor type. Int J Mol Med. 2007;19:495–499. [PubMed] [Google Scholar]
- 30.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001;285:914–924. doi: 10.1001/jama.285.7.914. [DOI] [PubMed] [Google Scholar]
- 31.Gould MK, Kuschner WG, Rydzak CE, Maclean CC, Demas AN, Shigemitsu H, et al. Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med. 2003;139:879–892. doi: 10.7326/0003-4819-139-11-200311180-00013. [DOI] [PubMed] [Google Scholar]
- 32.de Geus-Oei LF, van der Heijden HF, Corstens FH, Oyen WH. Predictive and prognostic value of FDG-PET in nonsmall-cell lung cancer: a systematic review. Cancer. 2007;110:1654–1664. doi: 10.1002/cncr.22979. [DOI] [PubMed] [Google Scholar]
- 33.MacManus M, Nestle U, Rosenzweig KE, Carrio I, Messa C, Belohlavek O, et al. Use of PET and PET/CT for radiation therapy planning: IAEA expert report 2006–2007. Radiother Oncol. 2009;91:85–94. doi: 10.1016/j.radonc.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Degirmenci B, Wilson D, Laymon CM, Becker C, Mason NS, Bencherif B, et al. Standardized uptake value-based evaluations of solitary pulmonary nodules using F-18 fluorodeoxyglucose-PET/computed tomography. Nucl Med Commun. 2008;29:614–622. doi: 10.1097/MNM.0b013e3282f9b5a0. [DOI] [PubMed] [Google Scholar]
- 35.de Geus-Oei LF, van der Heijden HF, Visser EP, Hermsen R, van Hoorn BA, Timmer-Bonte JN, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med. 2007;48:1592–1598. doi: 10.2967/jnumed.107.043414. [DOI] [PubMed] [Google Scholar]
- 36.Schuurbiers OC, Kaanders JH, van der Heijden HF, Dekhuijzen RP, Oyen WJ, Bussink J. The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J Thorac Oncol. 2009;4:761–767. doi: 10.1097/JTO.0b013e3181a1084f. [DOI] [PubMed] [Google Scholar]
- 37.Jaskowiak CJ, Bianco JA, Perlman SJ, Fine JP. Influence of reconstruction iterations on 18F-FDG PET/CT standardized uptake values. J Nucl Med. 2005;46:424–428. [PubMed] [Google Scholar]
- 38.Lammertsma AA, Hoekstra CJ, Giaccone G, Hoekstra OS. How should we analyse FDG PET studies for monitoring tumour response? Eur J Nucl Med Mol Imaging. 2006;33(Suppl 1):16–21. doi: 10.1007/s00259-006-0131-5. [DOI] [PubMed] [Google Scholar]
- 39.Nakamoto Y, Zasadny KR, Minn H, Wahl RL. Reproducibility of common semi-quantitative parameters for evaluating lung cancer glucose metabolism with positron emission tomography using 2-deoxy-2-[18F]fluoro-D-glucose. Mol Imaging Biol. 2002;4:171–178. doi: 10.1016/S1536-1632(01)00004-X. [DOI] [PubMed] [Google Scholar]
- 40.Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804–1808. doi: 10.2967/jnumed.108.054239. [DOI] [PubMed] [Google Scholar]
- 41.Kim CK, Gupta NC, Chandramouli B, Alavi A. Standardized uptake values of FDG: body surface area correction is preferable to body weight correction. J Nucl Med. 1994;35:164–167. [PubMed] [Google Scholar]
- 42.Sobin LH, Wittekind C, editors. TNM classification of malignant tumours (UICC). 6th ed. New York: Wiley; 2002.