Abstract
Background:
Evaluation of gastric physiology (gastric emptying and motility) is important for the diagnosis of disturbances such as functional dyspepsia. MRI is a non-invasive technique that allows simultaneous registration of gastric emptying and motility.
Aim:
To provide an overview of the literature of studies that used MRI as a tool for evaluation of gastric function in both research and clinical settings.
Materials and methods:
A MEDLINE search was performed (1990–2008) directed at the radiology and gastroenterology literature on gastric physiology. Key words that were used included: functional tests, gastric emptying, gastric motility, gastric physiology and MRI. Twenty-five articles fulfilled the inclusion criteria and were included in the analysis.
Results and conclusion:
Over the last decade, MRI has developed as a reliable, non-invasive method for detailed evaluation of gastric emptying and motility without the disadvantages of ionizing radiation and without the use of intragastric catheters that influence gastric physiology.
Keywords: MRI, Stomach, Emptying, Motility, Gastric physiology
Introduction
The function of the stomach comprises storage of ingested food, production of gastric secretion and mixing food with gastric secretion, grinding the ingested food (motility) and emptying of the stomach to the duodenum. This function can roughly be summed up by two main processes: gastric emptying and gastric motility. Disorders of gastric function such as diabetic gastroparesis, functional dyspepsia and disorders after gastrointestinal surgery are characterized by a variety of changes in gastric motility and emptying: hyper- or hypomotility, disturbed gastric accommodation and delayed emptying, all in the absence of pathology found at endoscopy or abnormal laboratory results [1–3]. Patients with disorders of gastric motility and gastric emptying experience a wide variety of complaints that are meal-related, such as nausea, bloating, gastric pain and/or gastric tension after meal consumption. Assessment of these disorders plays an important role in the differential diagnosis and subsequent treatment of these patients.
Several techniques are currently applied to study normal and pathological gastric motility and gastric emptying, but they measure or evaluate only a few of the various aspects. For example, the barostat technique is considered the gold standard for evaluation of proximal gastric motor function, including gastric accommodation [19]. Accommodation of the stomach wall is considered to be a vagally mediated reflex that causes the stomach wall to relax in response to a meal that has been consumed and acts as both a chemical and a mechanical stimulus. Gastric accomodation results in a reduction of gastric wall tone, which gives the stomach the opportunity to distend, providing a reservoir for the meal [7]. Disadvantages of the barostat technique are the invasive nature requiring oral intubation with intragastric positioning of a polyethylene bag. Moreover, questions have been raised about possible interference of the barostat with gastric physiology [11, 14, 17], since it could act as a mechanical stimulus itself. Recognition of these characteristics may contribute to the diagnosis of motility disorders.
Since the early 1990s, magnetic resonance imaging (MRI) has been employed experimentally to investigate gastric motility and emptying non-invasively [4–7]. Feinle et al. validated MRI as a tool for the analysis of gastric emptying [8]. Since that time, MRI has been used more often to study gastric pathophysiology [1, 2, 9–13].
This review summarizes the literature from the validation studies to the clinical studies that used MRI as a method for evaluation of gastric physiology.
Methods
A systematic search for relevant original publications was conducted on MEDLINE from 1990 to 2008 using the following search keys: functional tests, gastric physiology, gastric emptying, gastric motility and MRI. The articles were primarily selected on the basis of the title and the abstract; no specific exclusion criteria were used. Additional references were obtained from the bibliographies of the original articles. The full text of 15 relevant articles was retrieved. A summary of the studies mentioned in the article are listed in Table 1.
Table 1.
Summary of all studies mentioned in the article
| Modality | Gastric volume (ml) | ||
|---|---|---|---|
| Basal | After glucagon | After erythromycin | |
| Barostat | 225 ± 52 | 561 ± 58* | 149 ± 61 |
| MR imaging | 331 ± 56 | 720 ± 65* | 206 ± 66† |
Technical aspects of gastric MRI
The evaluation of gastric physiology requires the use of appropriate techniques to evaluate the various aspects of changes in volume and contraction. Based on the literature, we summarize how investigators perform MRI studies to assess gastric physiology. First, the preparation of the meal is reviewed; second, the preparation of the patient and MRI acquisition techniques used; third, the analysis of the data to assess both gastric emptying and gastric motility.
Preparation of the meal
To evaluate gastric emptying, positive labeling of a meal is neccessary to obtain reliable results [4, 8]. Since the exprimental studies of Schwizer and Kunz in the 1990s, most authors have used gadolinium tetraazacyclododecane tetraacetic acid (Gd-DOTA) to label the meal, since it has been shown that Gd-DOTA is the most stable contrast agent in the acidic gastric environment. Moreover, Gd-DOTA adheres well to both fluids and solids, and is not easily absorbed by the gastrointestinal tract. It thereby provides excellent positive contrast [14].
Patient positioning and MRI technique
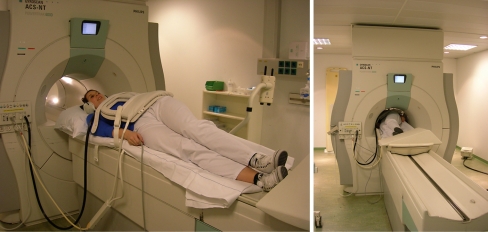
In most studies, each experiment starts after a period of fasting. Then, the subjects are positioned in a conventional MRI scanner (mostly 1.5-T field strength) in a right-sided semi-supine position (30–45°) (Fig. 1) [4, 5, 8, 10, 15–17]. Most studies use this semi-supine position, since MRI in the sitting position is not widely available [18].
Fig. 1.
Illustration of patient positioning for gastric MRI study. Patient is positioned in a right-sided semi-supine position to enhance gastric emptying. The surface coil is positioned anterior to the patient for improved image quality at 1.5 T
For the evaluation of gastric emptying, a three-dimensional (3D) volume scan is applied [4, 5, 8, 10, 15–17]. In this sequence, the entire stomach is included in the volume acquisition; from the volume data multiple slices with a slice thickness of 0.5 to 1 cm are reconstructed for further evaluation. To evaluate momentary gastric volume, the use of a multi-receive parallel body synergy coil has been advocated at 1.5 T. Sequence parameters include, for example, a turbo field echo (TFE) sequence, TE = 3.5 ms, TR = 10 ms, field of view 450 mm, rectangular field of view 55%, symmetric reduction 50%, flip angle 25°, 256 × 256 pixels, slice thickness 10 mm, with a total scan duration of 25 s, without breath-hold [15].
To assess gastric motility, a fast two-dimensional (2D) dynamic scan (such as turbo field echo, turbo spin echo, echo planar imaging, true FISP, balanced FFE, RARE) has been advocated [4, 5, 7, 15, 19, 20]. There is no particular preference for a specific acquisition technique, as the only goal of the technique is to be sufficiently fast to reach a temporal resolution that is high enough to analyze gastric motility with MRI. Some authors have advocated a single slice approach at the level of the stomach to assess dynamic gastric volume changes in a semi-coronal image orientation in which both parts of the antrum and part of the fundus along the longitudinal stomach axis are included (see Fig. 2) [4, 5, 15]. The following parameters have been proposed for a turbo field echo acquisition, including 300 images per scan over a time period of, for example, 5 min, a temporal resolution of 1 image per second, TE = 3.6 ms, TR = 10 ms, field of view 450 mm, rectangular field of view 55%, symmetric reduction 50%, flip angle 25°, 256/128 pixels, slice thickness 10 mm, without breath-hold.
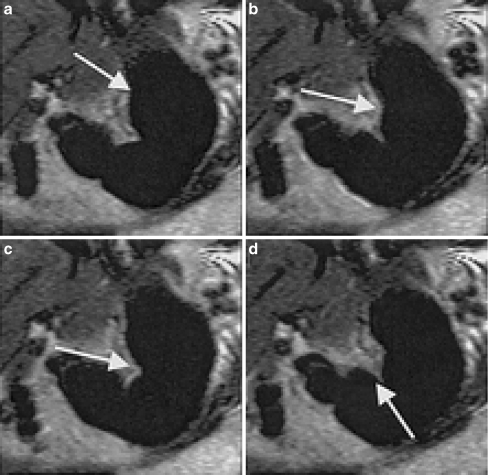
Fig. 2.
An example of a peristaltic contraction beginning at the level of the gastric fundus and propagating towards the antrum
Data analysis
Gastric emptying
As part of the post-processing, in most studies, the outline of the stomach is drawn manually and special software (such as MASS© [21]) is used. Slices encompassing the entire stomach are then summed up to measure momentary gastric volume. As a function in time, the percentage in decrease of the gastric volume represents gastric emptying. As gastric secretions mix with the meal and thereby influence gastric volume, Kunz et al. designed a robust method to correct gastric meal volume for progressive dilution due to gastric secretion [4]. First, the relationship between dilution of gastric content by gastric secretions and signal intensity for meals was experimentally determined by filling small bottles with defined quantities of the meal and mixing them with increasing quantities of hydrochloric acid. Then, the bottles were imaged simultaneously in a single plane. With liquid meals, an exponential correlation was found between the concentration of hydrochloric acid and the signal intensity. During gastric emptying studies, an external standard (containing the same meal that the subject had just ingested consisting of a mixture of fluid or solid food mixed with contrast medium) was placed in a polyethylene tube beside the subject and imaged simultaneously. The image intensity of the tube content served as a reference to correct for gastric secretion. An increase in gastric secretion resulted in a decrease in the signal intensity of the gastric contents [8].
-
(2)
Gastric motility
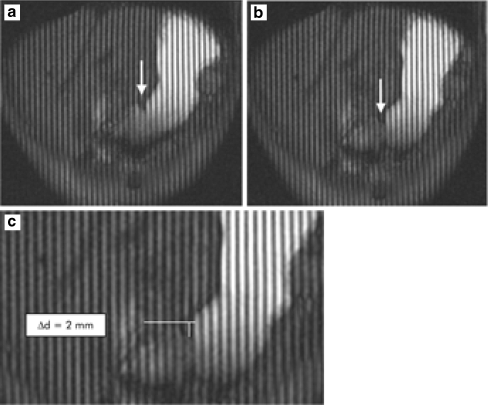
When gastric motility is studied, most attention is given to gastric peristalsis. In most studies, gastric diameters were measured at equally distributed points perpendicular to the stomach axis. On the basis of this diameter calculation, peristaltic contractions are detected, and their frequency is calculated [4, 15]. Other authors advocate the use of dark parallel tagging lines that can be applied in the images in the cranio-caudal direction in a manner similar to that described for cardiac MRI. The gap between two tagging lines can be measured, and the distance that a peristaltic wave had passed, by counting the number of tagging lines, can be quantified (Figs. 3 and 4) [3, 29].
Fig. 3.
For comparison purposes, T2-weighted and T1-weighted hydro-MR images of subject 1 are shown. a T2-weighted hydro-MR coronal projection image of subject 1: The stomach is loaded with 500 ml of 10% dextrose solution. b T1-weighted coronal projection image of subject 1 24 h later. The stomach is loaded with 500 ml of 10% dextrose solution and 1.0 mM of the contrast agent Gd-DOTA
Fig. 4.
a T2-weighted coronal slice-selective images of subject 1 depicting the stomach in its full J-shaped form. For dynamic study, respiratory gating determined data acquisition at times 0, 3.2, 6.5, 9.5 and 13.3 s. The contractions started in the proximal part of the body and moved aborally toward the antrum. The mean velocity was approximately 49.5 cm/min. b Transaxial scan through the middle part of the stomach. Nearly symmetrically and concentrically configured constrictor rings can be assessed. The in-plane resolution of 1.4 mm × 1.4 mm was sufficient to identify the longitudinal fissure of the gastric wall
Basic research for validation of MRI
Several initial studies have validated MRI techniques for studying gastric physiology.
In 1999, the applicability of MRI to assess gastric emptying and motility of liquid and solid meals was studied by Kunz et al. [5]. Gastric emptying and motility of a liquid and a solid meal were studied in eight volunteers. Gastric emptying of the liquid meal was faster than emptying of the solid meal when considering half-times of emptying. Hereby, this study showed that with MRI it was possible to evaluate gastric motility and gastric emptying, and at the same time it provided an important new insight into the physiology of gastric emptying of different gastric contents.
In 2002, MRI was validated for evaluation of gastric accomodation with the barostat method, which is considered the gold standard for evaluation of postprandial accommodation of the gastric wall. In the barostat method, the subject ingests an intragastric balloon that is connected to a device that maintains the pressure in the barostat balloon at a certain level. When the stomach wall relaxes by postprandial relaxation, the system starts to sufflate air into the balloon to maintain the pressure inside the balloon to the preselected level. Measurements of gastric volume and motility with MRI were compared with simultaneously performed measurements with a barostat. MR images and barostat measurements were obtained both at rest and after infusion of glucagon and erythromycin, which alter gastric volume and motility. Volume measurements with MRI followed volume changes of the barostat balloon. That study showed that MRI is as accurate as barostat measurement in determining changes in gastric volume [10]. The same study raised questions about the influence of the barostat balloon on gastric physiology. To address this issue, gastric accommodation, motility and emptying have been studied twice in 14 healthy subjects with MRI once in the presence of a barostat bag and once when the barostat bag was not present. Fasting and postprandial intragastric volumes were significantly higher in the experiment with a barostat vs. without a barostat. No significant differences were found in gastric emptying and contraction frequency between both experiments. The accommodation response observed in the presence of the barostat bag was not observed in the absence of the barostat bag. The presence of an intragastric barostat bag did not interfere with gastric emptying or motility, but the accommodation response measured with the barostat in situ is not observed without the barostat bag in situ [23]. Postprandial gastric accomodation shown as gastric wall distension is solely an effect caused by the barostat device. The intragastric meal alone does not cause the stomach to distend to the same proportions as the barostat balloon, which distends the stomach more pronouncedly than the intragastric meal volume.
Demonstrating that MRI is able to detect changes in gastric physiology is the basis of the validation of MRI for evaluation of gastric motility and emptying disorders. These changes in emptying and motility can, of course, be caused by the most natural way, i.e., food that acts as both a mechanical and a chemical stimulus to gastric function, but also more artificially by infusion of pharmacological substances. The evaluation of the effect of these pharmacological stimuli in itself forms the basis of development and evaluation of future pharmacological therapy for disturbances of gastric function disorders.
Lauenstein et al. assessed the effect of intravenously administered erythromycin on gastric emptying and subsequent small-bowel filling using three-dimensional (3D) MRI in both healthy subjects and patients with functional dyspepsia [17]. Six healthy volunteers and six patients with symptoms of functional dyspepsia ingested 500 ml of a gadolinium-labeled, fluid meal. In healthy volunteers, gastric volumes decreased significantly more after the administration of erythromycin. In three patients with functional dyspepsia, MRI revealed reduced rates of gastric emptying. The administration of erythromycin resulted in a significantly faster rate of gastric emptying in two of those three patients, indicating the possible therapeutic effect of this drug.
Ajaj et al. determined the practicality of MRI for the assessment of gastric motion, and tried to quantify the effects of metoclopramide and scopolamine [22]. The intravenous application of these substances resulted in significant changes in the motility index. The administration of metoclopramide resulted in an average increase of the index by a factor of 1.5, whereas the application of scopolamine led to a decrease of the index by a factor of 3.0 [22].
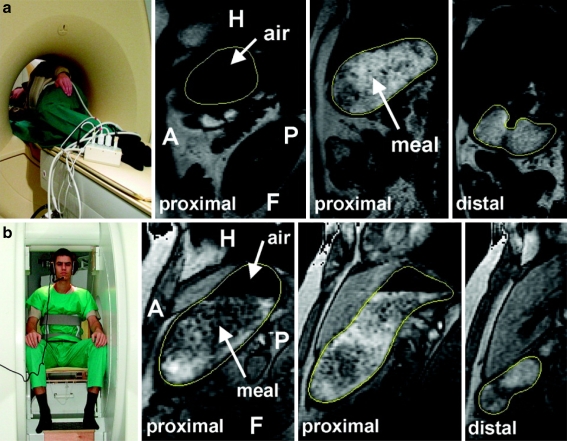
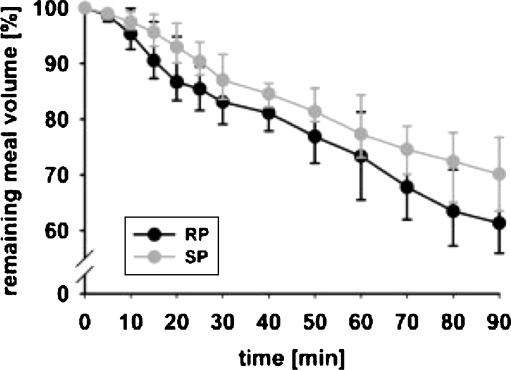
One could criticize MRI because in most MRI scanners, subjects are studied in a non-physiological body position, i.e., supine instead of upright or seated, which might cause a different meal distribution inside the stomach, which might influence gastric physiology (Fig. 5). Several studies were aimed at evaluation of these possible disadvantages of MRI as compared to other validated examination methods. Treier et al. determined the effect of the right decubitus lying body position on relevant parameters of human gastric motor function in healthy volunteers (see Fig. 6) [18]. In this study, postprandial gastric function after ingestion of a solid/liquid meal was assessed in volunteers in the right decubitus position and seated position. Stomach and intragastric air volume, intragastric meal distribution, gastric emptying and gastric peristalsis were compared between the right decubitus position and seated position. It was shown that body position did not affect gastric relaxation and initial gastric volumes. Postprandial stomach volume and gastric activity were also similar. Meal emptying showed different characteristics, resulting in a significant but small difference in meal volume (see Fig. 6) that could have been induced by posture-dependent vagal activity. This study is valuable because it confirmed that MRI in the right decubitus position is a reliable imaging technique for assessing gastrointestinal physiology and that results from MRI studies in the right decubitus position can be compared to studies performed in the sitting position. This is a solution to the fact that MRI in the sitting position is not widely possible to perform.
Fig. 5.
a Left: Volunteer in RP inside the 1.5-T whole-body MRI system. Rectangular surface coils were fixed around the abdomen. Right: Three sagittal MR image slices (two proximal and one distal) of a volume scan in RP with outlined stomach wall. Air and meal are indicated in the MR images by arrows (F: feet, H: head, A: anterior, P: posterior). b Left: Volunteer sitting inside the 0.5-T open-configuration MRI system. A send-receive coil was fixed around the abdomen. Right: Three sagittal MR image slices (two proximal and one distal) of a volume scan in SP with outlined stomach wall. Air and meal are indicated in the MR images by arrows (F: feet, H: head, A: anterior, P: posterior)
Fig. 6.
Normalized gastric emptying curves (percentage of remaining meal volume in the stomach) for RP (black) and SP (gray). Data are expressed as the median (interquartile range). A divergence of the emptying curves is observed, especially during the first 30 min after meal ingestion
Study of patients with functional dyspepsia
After validation of MRI for evaluation of gastric function, MRI was employed as a method for research and study of several gastropathological disorders, such as functional dyspepsia.
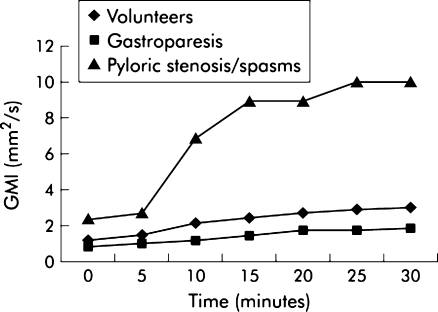
Ajaj et al. evaluated whether patients with increased or decreased gastric motility can be differentiated from healthy volunteers by means of real-time MRI [9]. In this study, ten healthy volunteers, ten patients with gastroparesis and ten patients with functional pylorospasm/peptic pyloric stenosis underwent real-time MRI. All patients were examined on two separate days: once prior to therapy and once after adequate therapy. Antral motility was quantified by calculating the gastric motility index. Patients with gastroparesis showed a lower motility index compared with the healthy volunteer group, while the mean motility index of the patient group with pylorospasm was more than three times higher than that of the reference value of the volunteer group. However, the gastric motility index in the patient group with gastroparesis increased, and in the group with functional pylorospasm/peptic pyloric stenosis, it decreased significantly after therapy. This study shows that MRI can clinically assess patients with suspected gastric pathophysiology as it is able to differentiate healthy subjects from patients with gastroparesis.
Borovicka et al. studied the effect of orally administrated cisapride on gastric emptying and motility in eight diabetic patients with previously demonstrated delayed gastric emptying. MRI studies were also performed in seven diabetic patients with normal emptying who served as disease controls. Gastric emptying was slower in the gastroparetic patients compared to patients with normal emptying. Cisapride accelerated gastric emptying in patients with gastroparesis. The contraction amplitudes in the proximal stomach of gastroparetic patients were increased during cisapride treatment, whereas antral contraction frequency, amplitude and velocity were unchanged. It was concluded that cisapride-induced acceleration of liquid gastric emptying in diabetic gastroparesis does not appear to result from changes in antral contractility, but may be related to changes in proximal gastric tone or gastric outlet resistance [10].
Discussion and conclusion
The prevalence of functional gastrointestinal disorders is high [12], but no single diagnostic test has been generally recommended for clinical use in patients with suspected functional dyspepsia. All available tests have certain disadvantages that prevent them from being used in the clinical environment. The barostat, for example, is an invasive test that comprises the use of an intragastric balloon and therefore has poor patient acceptance. Nuclear studies are associated with considerable exposure to ionizing radiation and lack spatial and temporal resolution [8], as does electro-gastrography, which gives no details about gastric contractions [24].
Therefore, since the early 1990s, researchers have searched for a non-invasive, non-radiation technique that has sufficient diagnostic accuracy, as well as patient acceptance. As MRI is becoming more and more widely available, involves no exposure to ionizing radiation and is accepted by patients in the clinical setting since it does not require any oral intubation, MRI has been proposed for evaluation of gastric functional disorders.
First attempts were hampered by long aquisition times that resulted in poor image quality and less robust results. But as experience with MRI has increased and aquisition times have become shorter, MRI has proved to be a promising new technique for evaluation of gastric function. Standard fast MRI techniques allow complete depiction of the stomach volume and gastric peristalsis in sufficient temporal and spatial resolution without exposure to ionizing radiation.
MRI has evolved from a technique that evaluates gastric function in healthy subjects to a technique that evaluates the effect of pharmaceutical products in patients with functional dyspepsia or other disorders of gastric function [15, 17].
In smaller research and clinical settings, MRI has been shown to be able to evaluate both gastric emptying and gastric motility. Now the technique should be evaluated in the clinical setting on a larger scale.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00330-010-1959-4
Contributor Information
Ingrid M. de Zwart, Phone: +31-71-5262993, FAX: +31-23-5312083, Email: i.m.de_zwart@lumc.nl
Albert de Roos, Email: a.de_roos@lumc.nl.
References
- 1.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/S0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 2.Vu MK, Straathof JWA, Van der Schaar PJ, Arndt JW, Ringers J, Lamers CBHW, et al. Motor and sensory function of the proximal stomach in reflux disease and after laparoscopic Nissen fundoplication. Am J Gastroenterol. 1999;94:1481–1489. doi: 10.1111/j.1572-0241.1999.1130_f.x. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead WE, Delvaux M. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. The working team of Glaxo-Wellcome research, UK. Dig Dis Sci. 1997;42:223–241. doi: 10.1023/A:1018885028501. [DOI] [PubMed] [Google Scholar]
- 4.Kunz P, Crelier GR, Schwizer W, Borovicka J, Kreiss C, Fried M, et al. Gastric emptying and motility: assessment with MR imaging—preliminary observations [see comments] Radiology. 1998;207:33–40. doi: 10.1148/radiology.207.1.9530296. [DOI] [PubMed] [Google Scholar]
- 5.Kunz P, Feinle C, Schwizer W, Fried M, Boesiger P. Assessment of gastric motor function during the emptying of solid and liquid meals in humans by MRI. J Magn Reson Imaging. 1999;9:75–80. doi: 10.1002/(SICI)1522-2586(199901)9:1<75::AID-JMRI10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 6.Boulby P, Moore R, Gowland P, Spiller RC. Fat delays emptying but increases forward and backward antral flow as assessed by flow-sensitive magnetic resonance imaging. Neurogastroenterol Motil. 1999;11:27–36. doi: 10.1046/j.1365-2982.1999.00133.x. [DOI] [PubMed] [Google Scholar]
- 7.Wright J, Evans D, Gowland P, Mansfield P. Validation of antroduodenal motility measurements made by echo-planar magnetic resonance imaging. Neurogastroenterol Motil. 1999;11:19–25. doi: 10.1046/j.1365-2982.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 8.Feinle C, Kunz P, Boesiger P, Fried M, Schwizer W. Scintigraphic validation of a magnetic resonance imaging method to study gastric emptying of a solid meal in humans. Gut. 1999;44:106–111. doi: 10.1136/gut.44.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ajaj W, Goehde SC, Papanikolaou N, Holtmann G, Ruehm SG, Debatin JF, et al. Real time high resolution magnetic resonance imaging for the assessment of gastric motility disorders. Gut. 2004;53:1256–1261. doi: 10.1136/gut.2003.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borovicka J, Lehmann R, Kunz P, Fraser R, Kreiss C, Crelier G, et al. Evaluation of gastric emptying and motility in diabetic gastroparesis with magnetic resonance imaging: effects of cisapride. Am J Gastroenterol. 1999;94:2866–2873. doi: 10.1111/j.1572-0241.1999.01392.x. [DOI] [PubMed] [Google Scholar]
- 11.Fruehauf H, Goetze O, Steingoetter A, Kwiatek M, Boesiger P, Thumshirn M, et al. Intersubject and intrasubject variability of gastric volumes in response to isocaloric liquid meals in functional dyspepsia and health. Neurogastroenterol Motil. 2007;19:553–561. doi: 10.1111/j.1365-2982.2007.00904.x. [DOI] [PubMed] [Google Scholar]
- 12.Richter J. Dyspepsia: organic causes and differential characteristics from functional dyspepsia. Scand J Gastroenterol Suppl. 1991;182:11–16. doi: 10.3109/00365529109109531. [DOI] [PubMed] [Google Scholar]
- 13.Tack J, Broeckaert D, Coulie B, Janssens J. The influence of cisapride on gastric tone and the perception of gastric distension. Aliment Pharmacol Ther. 1998;12:761–766. doi: 10.1046/j.1365-2036.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 14.Schwizer W, Fraser R, Maecke H, Siebold K, Funck R, Fried M. Gd-DOTA as a gastrointestinal contrast agent for gastric emptying measurements with MRI. Magn Reson Med. 1994;31:388–393. doi: 10.1002/mrm.1910310407. [DOI] [PubMed] [Google Scholar]
- 15.de Zwart IM, Mearadji B, Lamb HJ, Eilers PHC, Masclee AAM, de Roos A, et al. Gastric motility: comparison of assessment with real-time MR imaging or barostat measurement—initial experience. Radiology. 2002;224:592–597. doi: 10.1148/radiol.2242011412. [DOI] [PubMed] [Google Scholar]
- 16.Kunz P, Feinle-Bisset C, Faas H, Boesiger P, Fried M, Steingoetter A, et al. Effect of ingestion order of the fat component of a solid meal on intragastric fat distribution and gastric emptying assessed by MRI. J Magn Reson Imaging. 2005;21:383–390. doi: 10.1002/jmri.20295. [DOI] [PubMed] [Google Scholar]
- 17.Lauenstein TC, Vogt FM, Herborn CU, DeGreiff A, Debatin JF, Holtmann G. Time-resolved three-dimensional MR imaging of gastric emptying modified by IV administration of erythromycin. Am J Roentgenol. 2003;180:1305–1310. doi: 10.2214/ajr.180.5.1801305. [DOI] [PubMed] [Google Scholar]
- 18.Treier R, Steingoetter A, Weishaupt D, Goetze O, Boesiger P, Fried M, et al. Gastric motor function and emptying in the right decubitus and seated body position as assessed by magnetic resonance imaging. J Magn Reson Imaging. 2006;23:331–338. doi: 10.1002/jmri.20507. [DOI] [PubMed] [Google Scholar]
- 19.Schwizer W, Fraser R, Borovicka J, Asal K, Crelier G, Kunz P, et al. Measurement of proximal and distal gastric motility with magnetic resonance imaging. Am J Physiol. 1996;271(1 Pt 1):G217–G222. doi: 10.1152/ajpgi.1996.271.1.G217. [DOI] [PubMed] [Google Scholar]
- 20.Bilecen D, Scheffler K, Seifritz E, Bongartz G, Steinbrich W. Hydro-MRI for the visualization of gastric wall motility using RARE magnetic resonance imaging sequences. Abdom Imaging. 2000;25:30–34. doi: 10.1007/s002619910005. [DOI] [PubMed] [Google Scholar]
- 21.van der Geest RJ, Reiber JH. Quantification in cardiac MRI. J Magn Reson Imaging. 1999;10:602–608. doi: 10.1002/(SICI)1522-2586(199911)10:5<602::AID-JMRI3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 22.Ajaj W, Lauenstein T, Papanikolaou N, Holtmann G, Goehde SC, Ruehm SG, et al. Real-time high-resolution MRI for the assessment of gastric motility: pre- and postpharmacological stimuli. J Magn Reson Imaging. 2004;19:453–458. doi: 10.1002/jmri.20029. [DOI] [PubMed] [Google Scholar]
- 23.de Zwart IM, Haans JJ, Verbeek P, Eilers PH, de Roos A, Masclee AA. Gastric accomodation and motility are influenced by the barostat device: assessment with magnetic resonance imaging. Am J Physiol Gastrointest Liver Physiol. 2007;292:G208–G214. doi: 10.1152/ajpgi.00151.2006. [DOI] [PubMed] [Google Scholar]
- 24.Lee KJ, Kim JH, Cho SW. Dietary influence on electro-gastrography and association of alterations in gastric myoelectrical activity with symptoms in patients with functional dyspepsia. J Gastroenterol Hepatol. 2006;21(1 Pt 1):59–64. doi: 10.1111/j.1440-1746.2005.04088.x. [DOI] [PubMed] [Google Scholar]