Abstract
Background
MDV3100 is a rationally-designed androgen receptor antagonist that blocks androgen receptor (AR) binding, nuclear translocation, and co-activator recruitment more effectively than the androgen receptor antagonists currently in use. MDV3100 is also unique in that it prevents DNA binding, induces apoptosis, and has no agonist activity when AR is overexpressed. Because growth of castration-resistant prostate cancer (CRPC) appears to depend upon continued androgen receptor signaling, we hypothesized that MDV3100 could be effective therapy for men with CRPC. Antitumor activity and safety were assessed in a phase 1-2 trial.
Methods
Eligible patients with progressive metastatic CRPC were enrolled in cohorts of 3-6 patients. Once the safety of a dose was established, cohorts were expanded to include at least 12 chemotherapy-naïve and 12 post-chemotherapy treated patients.
Findings
140 patients were treated with doses ranging from 30 to 600 mg daily. Positron emission tomography (PET) imaging to assess androgen receptor blockade showed decreased 18-fluorodihydrotestosterone binding at dosages of 60 mg/day and above. Antitumor effects were observed at all dosages including declines in serum PSA of 50% or more in 56% of patients, responses in soft tissue, stabilized bone disease, and conversion from unfavourable to favourable circulating tumour cell counts. The median time to progression was 47 weeks for radiological progression. The maximal tolerated dose for sustained treatment (>28 days) was 240 mg and the most common adverse event was dose-dependent fatigue, which generally resolved following dose reduction.
Interpretation
Encouraging antitumor activity on all outcomes assessed was observed for MDV3100 in both chemotherapy-naïve and post-chemotherapy patients with CRPC, establishing that patients with CRPC are not uniformly hormone-refractory. A phase 3 trial in patients with progressive disease after docetaxel treatment is underway.
Background
Significant progress in the treatment of cancer with targeted therapy has been achieved through elucidating secondary changes that contribute to treatment resistance. These include Ras mutations that predict for resistance to epidermal growth factor receptor (EGFR) inhibitors,1-2 and second site mutations in BCR-ABL,3-4 Kit or EGFR associated with acquired resistance.5 6 Strategies to target acquired resistance mutations have proven clinically useful in chronic myeloid leukemia and gastrointestinal stromal tumors.7-9 Here we apply this principle to the development of a hormone antagonist for the treatment of castration-resistant prostate cancer (CRPC), which for most patients is invariably fatal.
Virtually all CRPCs continue to produce prostate specific antigen (PSA) and many respond to 2nd and 3rd line hormonal therapies. The response to second line anti-androgens is typically of short duration, and none have shown sufficient activity in CRPC to warrant more definitive testing.10-12 These observations still suggest that a proportion of these tumors remain dependent on androgen receptor (AR) signaling for growth, even when the measured levels of testosterone in the blood are in the castrate range (<1.7 nmol/L).13 Molecular profiling studies have identified and validated a series of progressive changes in AR signaling in CRPC relative to newly diagnosed untreated primary tumors; the most frequent is AR overexpression.14-16 In laboratory models, AR overexpression is sufficient to shorten tumor latency in castrate mice and confer resistance to bicalutamide, the most widely used androgen receptor antagonist.17
MDV3100 is a novel AR antagonist selected for activity in prostate cancer model systems with overexpressed AR.18 The drug was designed to overcome deficiencies of the currently available AR antagonists including low AR binding affinity and partial agonism, which explain in part the clinical responses seen upon discontinuation of these agents.19 In contrast to bicalutamide, MDV3100 has a higher affinity for the receptor; impairs nuclear translocation, DNA binding, and coactivator recruitment; and induces apoptosis Also in contrast to bicalutamide, MDV3100 is a pure antagonist, with no detectable agonist effects in LNCaP/AR cells which overexpress AR. The drug also induces regression of established LNCaP/AR xenograft tumors growing in castrate male mice, a model where bicalutamide treatment only slows growth. The regression is associated with continued evidence of apoptosis as long as 25 days after the start of treatment.18
Based on these promising preclinical results, MDV3100 was selected for clinical development in the Prostate Cancer Clinical Trials Consortium.20 The primary objectives of this first-in-man study of MDV3100 were to assess pharmacokinetics (PK), safety and tolerability, and to define a maximal tolerated dose. Secondary objectives were to assess antitumor effects based on changes in PSA, imaging of soft-tissue and osseous disease, circulating tumor cell (CTC) number, and time to disease progression. Selected patients underwent positron emission tomography (PET) with the testosterone analog, 18-fluorodihydrotesterone (FDHT), to demonstrate AR blockade by MDV3100 and 18-fluorodeoxyglucose (FDG) to assess tumor response.
Methods
This study was conducted at Memorial Sloan-Kettering Cancer Center (MSKCC), Oregon Health and Science University Knight Cancer Institute, the University of Washington, Dana-Farber Cancer Institute, and M.D. Anderson Cancer Center, following institutional review board (IRB) approval from each institution. All patients signed an IRB-approved written informed consent prior to the conduct of any study procedures and after a full explanation of the study to the patient by a study investigator. Eligible patients had a diagnosis of prostate cancer that was histologically confirmed at their treating institution and progressive castration-resistant disease, defined by the combination of castrate levels of testosterone (<1.7 nmol/L), and a rising PSA with or without detectable metastases. The criteria for rising PSA required a minimum of 3 measurements obtained more than 2 weeks apart showing a 50% or greater increase, with the last value above 2 ng/ml.
The pre-study evaluation also included a history and physical examination, complete blood and platelet count, chemistry panel, creatinine, PSA, CTC number and electrocardiogram. Imaging for metastatic disease included a radionuclide bone scan and either computed tomography (CT) or magnetic resonance imaging of the chest, abdomen and pelvis. Enrollment required adequate hematologic, hepatic and renal function. Patients were enrolled from July, 2007 through December, 2008.
MDV3100 was administered orally at a starting dosage of 30 mg/day in cohorts of 3 to 6 patients. Patients in the dose-escalation cohorts were given a single dose, observed for six days, and then treated continuously. After a minimum of 3 patients in each cohort had completed 28 days of continuous treatment, a safety review occurred before moving to the next dose level. However, after PSA declines were observed in all of the first six patients treated, enrollment was expanded by an additional 24 patients (12 chemotherapy-naive and 12 post-chemotherapy) at each dose from 60 mg to 360 mg daily. The final dosages studied were 30, 60, 150, 240, 360, 480 and 600 mg/day. All patients treated at 480 and 600 mg per day levels were post-chemotherapy and received the drug in two divided doses of 240 and 300 mg each. Doses were reduced for significant adverse events.
Patient Evaluation
Patients were examined and assessed for adverse events at a minimum of monthly intervals, and toxicities graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE Version 3.0). A complete blood and platelet count, serum chemistry panel, PSA and creatinine were repeated monthly. CTC number was assessed at baseline, week 4 and week 12. Imaging studies were repeated at 3-month intervals for patients who had metastatic disease at baseline, and at 6-month intervals for those who did not. MDV3100 concentrations in plasma were measured using a validated, Good Laboratory Practice-compliant liquid chromatography tandem mass spectrometry assay.
Twelve-lead electrocardiograms were obtained on days 1, 3, 7, 21, 28, 42, 84, 85, and 112 and every 28 days thereafter. Patients in the dose escalation cohorts also had multiple electrocardiograms performed on the day of single dose treatment and on the following day. The electrocardiograms were evaluated for changes in heart rate, PR and QRS duration, and QTc from baseline to each time point for each dose group (time point analysis), to the mean of all on-treatment electrocardiographic values for a given patient for each interval (time averaged analysis).
PET scans
Patients treated at MSKCC were offered participation in a study evaluating 16 beta[18F]-fluoro-5 alpha-dihydrotestosterone (FDHT) and 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) PET/CT scans under a separate protocol approved by the institutional review board at MSKCC. Enrollment was limited by the availability of the FDHT tracer, which was prepared at MSKCC. Scans were performed pre-therapy, and 4- and 12-weeks post-therapy. Tracer uptake was scored using the sum of the total standard uptake value (SUV) for each of up to 5 index lesions at baseline, and 4 and 12 weeks post-treatment as described.21
Antitumor effects
Antitumor effects were assessed using the Prostate Cancer Clinical Trials Working Group 2 (PCWG2) criteria22 except as noted. Specifically, post-therapy PSA changes are reported as change from baseline as specified in the protocol, and also as change from nadir as suggested by PCWG2. PSA change was reported using waterfall plots, and osseous disease on radionuclide bone scan was recorded as improved, progressed or no change. Soft-tissue disease was evaluated by the Response Evaluation Criteria in Solid Tumors (RECIST),23 except the criterion for index lesions in lymph nodes was a greatest diameter of > 2 cm (per PCWG2), rather than >1 cm (per RECIST). Time to progression was reported separately based on imaging and PSA; time to PSA progression was defined as the interval from the start of therapy to the documentation of a 25% or greater rise from baseline that represents a 5 μg/mL or greater increase. Every effort was made to keep patients on MDV3100 therapy until the time of radiological progression.
CTC enumeration
CTC counts were determined with CellSearch (Veridex, LLC, Huntingdon Valley, PA), an analytically valid assay cleared by the US Food and Drug Administration24 and reported as the number of CTCs per 7.5 ml of blood as previously described.25 Samples were collected at baseline, 4 weeks, and 12 weeks post-treatment. The post-treatment value was considered to be the 12-week CTC count, except for patients lacking a 12-week sample, for whom the 4-week value was used. Samples collected from outside centers were shipped to MSKCC overnight and processed in the MSKCC Clinical Chemistry laboratory, which is certified under the Clinical Laboratory Improvement Act.
All clinical data were collected by electronic data capture (Synteract, Carlsbad, CA). Critical safety and efficacy variables were monitored with 100% source data verification. Medivation, Inc. (San Francisco, CA, USA) provided funding and the study drug. The corresponding author had full access to the data, independently confirmed the efficacy data, and made the decision where to submit the manuscript for publication. The trial is registered with Clinicaltrials.gov, number NCT00510718.
Statistics
The Kaplan-Meier method was used to estimate the rates of progression over time and median times to progression. Unstratified log rank tests were used for comparisons of median times to progression and Fisher's Exact tests for comparison of proportions between groups. All testing was two-sided at the 5% level of significance. Confidence intervals for proportions are of the Clopper Pearson type for exact binomial proportions with 95% confidence. Statistical inferences and confidence intervals were derived using SAS version 9.1.3. The cutoff date for the data included in this report was 1 April, 2009.
Findings
Patients
The characteristics of the 140 patients enrolled are summarized in Table 1. The mean age was 68 years; 9 patients were greater than 80 years old. All patients had CRPC but differed in terms of prior treatment of the primary tumor and in number and type of prior hormonal therapies and chemotherapies. Sites of disease included bone metastases in 109 (78%), lymph nodes in 75 (54%), and visceral in 13 (17%). A rising PSA was the only evidence of disease in 7 patients. Overall 59 (64%) of the 92 soft tissue lesions met the size criterion defined by the PCWG2 for an index lesion. Enrollment by dose level and prior chemotherapy exposure is shown in Table 2. Ninety-eight patients (70%) patients discontinued treatment. Of those, 47 (33%) were discontinued due to radiological progression, 17 (12.1%) d ue to PSA progression, 16 (11.4%) due to clinical disease progression, 8 (5.7%) due to an adverse event, 7 (5%) for other reasons, and 3 (2.1%) withdrew consent. The median time on study drug was 23 weeks.
Table 1. Patient characteristics.
| Characteristic | No. (%) or Median (range) |
|
|---|---|---|
| Number of patients | 140 | |
| Age, years | 68 (44–93) | |
| PSA, ng/mL | 50 (2–2159) | |
| Treatment of the primary tumour | ||
| Surgery | 42 (30%) | |
| Radiation | 37 (26%) | |
| No primary therapy | 61 (44%) | |
| Prior hormone therapy | 140 (100%) | |
| 1 line | 32 (23%) | |
| 2 lines | 42 (30%) | |
| 3 lines | 37 (25%) | |
| ≥ 4 lines | 29 (21%) | |
| Ketoconazole | 63 (45%) | |
| Prior chemotherapy | ||
| None | 65 | |
| Any | 75 | |
| 1 line | 50 | |
| 2 or more lines | 25 | |
| Chemotherapy-Naive CTCs (n=60) | ||
| <5 | 44 | |
| ≥5 | 16 | |
| Post-chemotherapy CTCs (n=68) | ||
| <5 | 33 | |
| ≥5 | 35 | |
| Sites of disease | Total | Evaluable by PCWG2 |
| Bone disease | 109 | 109a |
| Bone only | 41 | 41a |
| Bone and soft tissue: | 68 | 68a |
| Soft tissue disease | 92 | 59b |
| Lymph node only | 21 | 17b |
| Lymph node and bone | 54 | 31b |
| Visceral and bone | 12 | 8b |
| Visceral only | 1 | 1b |
| Other (pelvis/bladder) and bone | 2 | qb |
| Other (pelvis/bladder) alone | 2 | 0b |
| No evidence of metastases | 7 | N/A |
Number with bone disease evaluable by PCWG2
Number with soft tissue disease evaluable by PCWG2
Table 2. Enrollment by dosage.
| Dosage (mg/day) |
Total Patients | Chemotherapy Naive | Prior Chemotherapy |
|---|---|---|---|
| 30 | 3 | 3 | 0 |
| 60 | 27 | 15 | 12 |
| 150 | 28 | 15 | 13 |
| 240 | 29 | 17 | 12 |
| 360 | 28 | 15 | 13 |
| 480 | 22 | 0 | 22 |
| 600 | 3 | 0 | 3 |
| TOTAL | 140 | 65 | 75 |
Pharmacokinetics
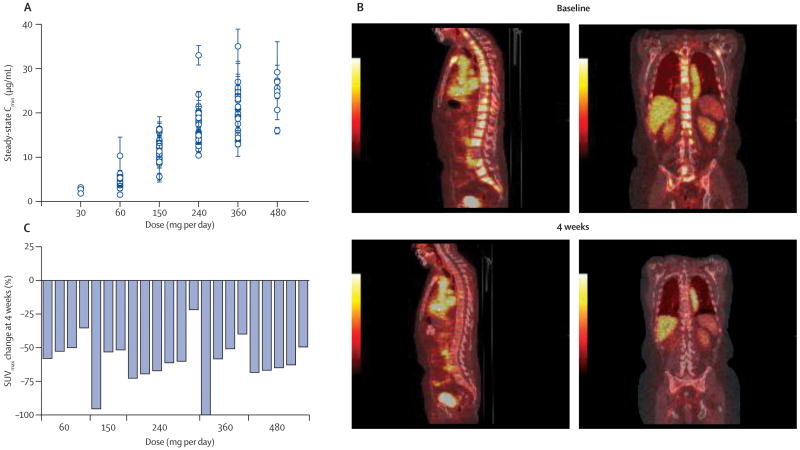
Following administration of a single dose, the drug was absorbed rapidly, and Cmax was between 30 min and 4 hours. The t1/2 was approximately one week (3 to 13 days in individual patients) and not affected by the dose. Full PK profiles were linear and consistent over the dosage range studied (N=137). Levels reached steady state after one month of daily treatment. Importantly, the steady-state serum concentrations observed in the 150 mg cohort (approximately 10 μg/mL) (Fig. 1a) were comparable to those found to be effective in xenograft models of CRPC.18
Figure 1.
Pharmacokinetics and pharmacodynamics of MDV3100.
A, Steady-state serum concentrations by dose level.
B. Sagittal and coronal views of PET scans at baseline and 4 weeks post-treatment. The sagittal and coronal images 1 hour after FDHT administration at baseline and 4 weeks after beginning MV3100 therapy show a reduction in FDHT accumulation in tumor within the vertebrae, in comparison to the cardiac and aortic blood pool, where FDHT metabolites circulate bound to serum proteins.
C. Percentage change in FDHT SUVmax average from baseline to 4 weeks by dosage. At baseline, all 22 patients had at least 1 FDHT-avid lesion that could serve as an index lesion, with the number of index lesions per patient as follows: 5 index lesions, 17 patients; 3 index lesions, 3 patients; 1 index lesion, 2 patients. At baseline the median FDHT SUVmax average was 7.81. Quantitatively, all 22 patients showed a clear-cut reduction of SUV, with a greater reduction at higher doses. This is evidence that MDV3100 is binding its target, ie the androgen receptor, and that changes are persistent with therapy.
Pharmacodynamics
AR binding by MDV3100 was evaluated in 22 patients receiving dosages ranging from 60 mg to 480 mg per day using FDHT PET scans to measure the change in FDHT uptake before and after starting treatment. A representative scan is shown in Figure 1b. All patients showed a clear reduction in FDHT uptake (range approximately ∼20-100%) (Figure 1c). We saw some indication that those receiving 60 mg/day had a smaller reduction (mean decrease <50%) than those receiving higher dosages (mean decrease ≥50%), with no appreciable differences among the higher dosages, despite substantial differences in serum levels of MDV3100 (e.g., steady-state levels approximately 12 μg/ml at 150 mg/day and >20 μg/ml at 360 and 480 mg/day).
Antitumor Effects
PSA
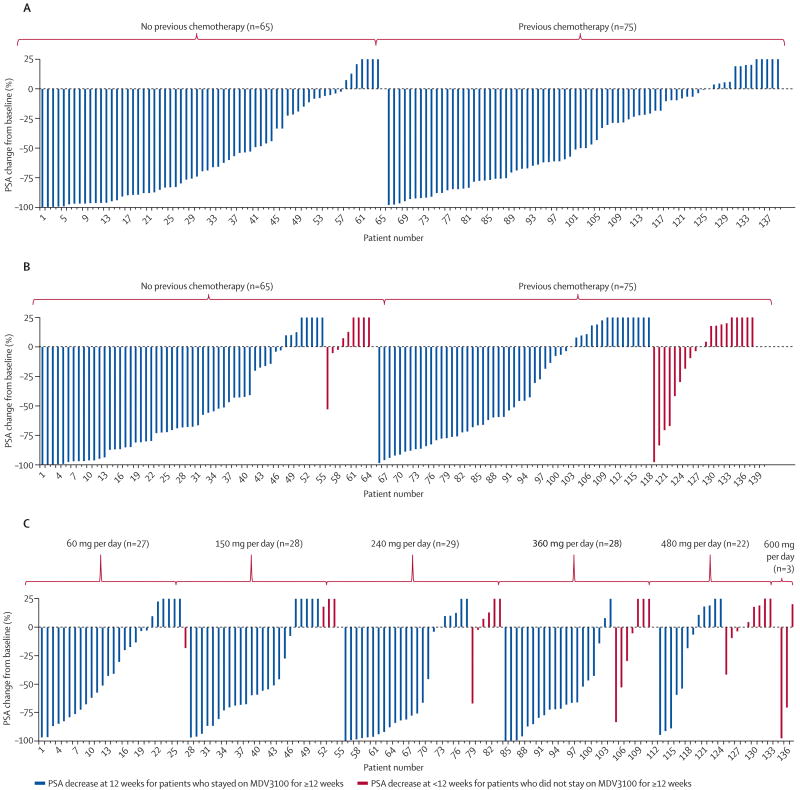
Since PSA expression is AR-dependent, short-term changes in serum PSA level serve as an additional pharmacodynamic marker of AR inhibition, but if sustained can also reflect antitumor activity. Waterfall plots of the maximal and 12-week post-therapy changes (Figures 2a and 2b) showed substantial declines at all dosages, and in both chemotherapy-naïve and chemotherapy treated patients. The proportion of patients with a maximal PSA decline (Figure 2a) in excess of 50% was not different (p=0.23) among the chemotherapy naïve patients (62%; 95% confidence interval 47% to 73%) compared to the chemotherapy treated patients (51%; 95% confidence interval (39% to 62%). At 12 weeks, the proportion of patients with a PSA decline in excess of 50% (Figure 2b) was statistically higher (p=0.02) among the chemotherapy naïve patients (57%; 95% confidence interval 44% to 69%) relative to the chemotherapy treated patients (36%; 95% confidence interval 25% to 48%). Among the patients who discontinued the study before week 12 due to toxicity or progressive disease (indicated by the red lines in Figure 2b), 3 of 9 chemotherapy-naïve patients and 9 of 18 chemo-exposed patients had PSA levels that were below baseline at the time of study discontinuation. The degree of decline and proportion of patients showing a decline was dose-dependent up to 150 mg/day, with no obvious additional benefit to higher dosages seen (Figure 2c). There was no important difference in PSA decline rates (>50% decline from baseline) for patients who received 2 or less vs. 3 or more prior hormone treatments, or 1 vs. 2 chemotherapy regimens, but the rate was lower (p<0.001) among those with prior ketoconazole treatment (40%; 95% confidence interval 28 to 53) vs. those without prior ketoconazole (69%; 95% confidence interval 57 to 69) (Table 3).
Figure 2.
Waterfall plot of percent change in PSA from baseline.
A, maximal decline from baseline;
B, decline at 12 weeks from baseline (red lines show patients who discontinued treatment before 12 weeks for an adverse event or disease progression);
C, 12-week declines by dose level.
Table 3. Changes in PSA by prior treatment.
| Maximal Decline from Baseline | Prior Chemotherapy | Prior Hormones | Prior Ketoconazole | |||
|---|---|---|---|---|---|---|
| No (n=65) | Yes (n=75) | 2 or fewer (n=74) | 3 or more (n=66) | No (n=77) | Yes (n=63) | |
| > 50% | 40 (62%) | 38 (51%) | 45 (61%) | 33(50%) | 53 (68%) | 25 (40%) |
| ≥ 30-50% | 6 (9%) | 8 (11%) | 4 (5%) | 10 (15%) | 4 (5%) | 10 (15%) |
| < 30% or None | 19 (29%) | 29 (38%) | 25 (34%) | 23 (35%) | 18 (23%) | 30 (48%) |
Radiological findings
Imaging studies showed that treatment with MDV3100 was associated with tumor regressions (22% overall) and stable disease (49% overall) in soft tissue and stable disease in bone (56%) in both the chemotherapy naive and post-chemotherapy patients (Table 4).
Table 4. Imaging results and circulating tumour cell numbers.
| Total | No Prior Chemotherapy | Prior Chemotherapy | |
|---|---|---|---|
| Soft tissue | 59 | 25 | 34 |
| Partial response | 13 (22%) | 9 (36%) | 4 (12%) |
| Stable disease | 29 (49%) | 11 (44%) | 18 (53%) |
| Bone scan (week 12) | 109 | 41 | 68 |
| Stable disease | 61 (56%) | 26 (63%) | 35 (51%) |
| FDG PET (week 12)a | 22 | 11 | 11 |
| >25% decline from baseline | 10 (45%) | 6 (55%) | 4 (45%) |
| <25% decline | 12 (55%0 | 7 (64%) | 5 (45%) |
| Circulating tumour cell numberb | 128 | 60 | 68 |
| <5 at baseline | 77 | 44 | 33 |
| < 5 on treatment | 70 (91%) | 40 (91%) | 30 (91%) |
| ≥ 5 on treatment | 7 (9%) | 4 (9%) | 3 (9%) |
| ≥ 5 at baseline | 51 | 16 | 35 |
| < 5 on treatment | 25 (49%) | 12 (75%) | 13 (37%) |
| ≥ 5 on treatment | 26 (51%) | 4 (25%) | 22 (63%) |
All 22 patients had at least one FDG-avid lesion that could be used as an index lesion, and the breakdown in terms of patients was 5 index lesions: 16; 4 index lesions: 3; 2 index lesions: 1; 1 index lesion: 1. The range of SUVmax average varied from 2.72 to 16.6 in the 22 patients, and the median SUVmax average was 5.025.
<5 CTCs per 7.5 ml is considered favourable; ≥5 is considered unfavourable.
Time to progression
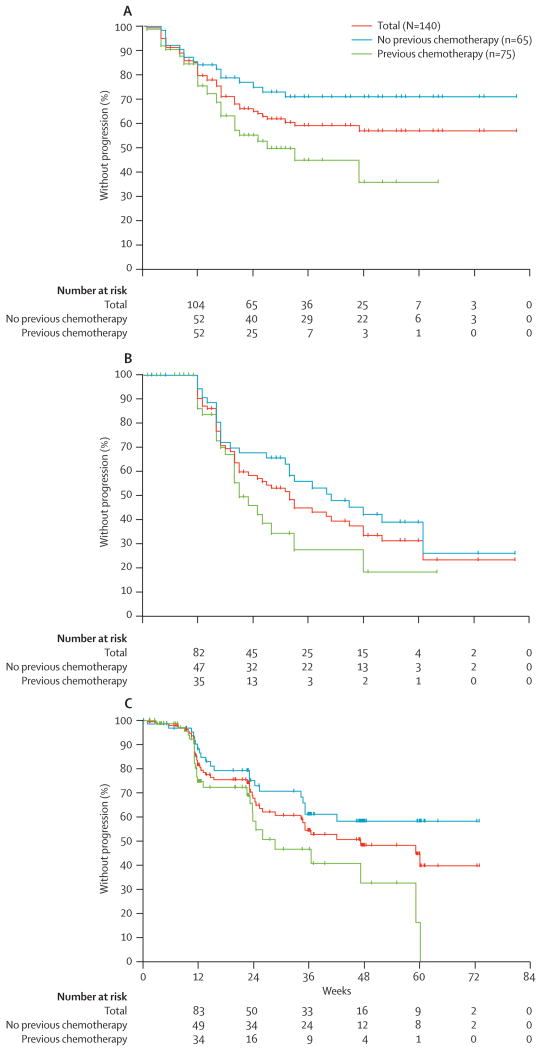
Kaplan-Meier median time to PSA progression, defined as a 25% or greater increase in PSA from baseline that represents a 5 μg/mL or greater increase, was not reached for all patients combined or the chemotherapy naïve patients, and was 27 weeks (95% confidence interval 20 to not reached) for the post-chemotherapy patients (chemotherapy-naïve vs. post-chemotherapy; p = 0.01) (Figure 3a). Using the PCWG2 criteria of a 25% or greater increase in PSA from the nadir), the median time to PSA progression was 41 weeks for chemotherapy-naïve patients, 32 weeks for all patients and 21 weeks for post-chemotherapy patients (Figures 3b). The median time to radiological progression was 47 weeks (95% confidence interval 34 to not reached) in all patients combined, not reached for chemotherapy-naïve patients, and 29 wks (95% confidence interval 24 to 59) for the post-chemotherapy patients (chemotherapy-naïve vs. post-chemotherapy; p = 0.01) (Figures 3c).
Figure 3.
Time to progression.
A, PSA progression using the protocol definition of a 25% or greater rise from baseline;
B, PSA progression using the PCWG criterion of a 25% or greater rise from nadir;
C, Imaging.
Exploratory biomarkers
FDG PET Imaging
The same 22 patients who had FDHT scans to evaluate MDV3100-AR binding also had FDG scans to assess tumor glucose uptake (based on FDG-avid disease at baseline), of whom 10 (46%) had 25% or greater declines in SUVmax after 12 weeks of treatment(Table 4).
CTC enumeration
Samples for CTC enumeration were received from 128 of the 140 patients enrolled (91%), of whom 51 (40%) had baseline counts that were unfavorable (≥ 5 cells/7.5 ml of blood). Following treatment, 25 of these patients (49%), converted from unfavorable to favorable counts (75% [95% confidence interval 48% to 93%] of the chemotherapy-naive and 37% [95% confidence interval 21% to 55%] of the post-chemotherapy groups; p = 0.02) (Table 4). Nineteen of these 25 patients (76%) had a maximal PSA decline of 50% or greater and 16 (64%) had a 12-week PSA decline of 50% or greater. Of those patients who initiated therapy with favorable counts, 91% retained favorable counts during treatment.
Safety
Table 5 summarizes adverse events rated by the investigator as Grade 3 or 4 (by the NCI CTCAE) and those that resulted in treatment discontinuation. The most common adverse event was fatigue, which generally developed after the 30-day safety assessment used to determine dose escalations. At dosages of 240 mg and above, an increasing proportion of patients required dose reductions for fatigue. Dose reductions were required in 1 of 29 patients (3.5%) treated at 240 mg/day; 3 of 28 patients (10.7%) at 360 mg/day; and 5 of 22 patients (22.7%) treated at 480 mg/day but none of the 58 patients treated at doses of 30, 60 and 150 mg/day. After dose reductions, the symptoms resolved. The most common mild adverse events (Grade 2 or less) were nausea, constipation, diarrhoea, and anorexia. None of the Grade 2 events required dose modification or the discontinuation of treatment, with the exception of one patient treated at 480 mg/day, who had nausea at baseline and stopped therapy after 7 weeks.
Table 5. Grade 3-4 adverse events and patients who discontinued treatment due to an adverse event, by dose cohort.
| Adverse Event | Dosage, mg/day | |||||||
|---|---|---|---|---|---|---|---|---|
| 30 (n = 3) |
60 (n = 27) |
150 (n = 28) |
240 (n = 29) |
360 (n = 28) |
480 (n = 22) |
600 (n = 3) |
Total (n = 140) |
|
| Grade 3-4 Adverse Events Occurring in More Than 2 Patients | ||||||||
| Fatigue | - | - | - | 5 (17%) | 6 (21%) | 5 (23%) | - | 16 (11%) |
| Anaemia | - | 2 (7%) | 1 (4%) | - | 1 (4%) | - | - | 4 (3%) |
| Arthralgia | - | 2 (7%) | - | - | - | 1 (5%) | - | 3 (2%) |
| Asthenia | - | - | - | - | 2 (7%) | 1 (5%) | - | 3 (2%) |
| Convulsions | - | - | - | - | 2 (9%)a | 1 (33%) | 3 (2%) | |
| Adverse Events Leading to Discontinuation of Treatment | ||||||||
| Seizure | - | - | - | - | 1 (4%) | 1 (5%) | 1 (33%) | 3 (2%) |
| Rash | - | - | - | - | - | 1 (5%) | 1 (33%) | 2 (1%) |
| Nausea/Vomiting | - | - | - | - | - | 1 (5%) | - | 1 (1%) |
| Fatigue | - | - | - | 1 (3%) | - | - | - | 1 (1%) |
| Myocardial infarction | - | - | - | - | 1 (4%) | - | - | 1 (1%) |
One patient had convulsion after dose reduction to 360 mg/day
There were two witnessed seizures at 600 mg/day and 360 mg/day dosages, and one possible seizure at 480 mg/day. Whether MDV3100 was responsible for these seizures is unclear as both patients experiencing witnessed seizures were concurrently taking medications that could contribute to a lowered seizure threshold. Both patients also had complicated medical problems that may have contributed to their seizures, including hypocalcaemia requiring intravenous calcium, anaemia requiring red cell transfusions, and skull metastases requiring skull radiation. Other causes of treatment discontinuation included a rash in two patients treated at 480 and 600 mg after 10 and 3 days respectively, and a myocardial infarction after 15 weeks of therapy in a patient with a history of diabetes, hypertension, and hypercholesterolemia. All recovered without sequelae. No effects on heart rate, cardiac conduction (PR and QRS duration) or on cardiac repolarization (QTc) were identified.
Only one of the 87 patients (1%; 95% confidence interval 0% to 6%) treated at 240 mg or below discontinued treatment for an adverse event, compared to seven of 53 (13%; 95% confidence interval 5% to 25%) of those treated at 360 mg or higher.
Because of the seizure at 360 mg/day, and the increasing frequency Grade 3 fatigue at 360 and 480 mg/day, the maximum tolerated dose was defined as 240 mg/day. All patients receiving higher dosages were instructed to reduce their daily dose to 240 mg.
Interpretation
MDV3100 was selected for clinical development based on potent anti-tumour effects in castration-resistant and bicalutamide-resistant xenograft models of prostate cancer. The clinical results reported here establish that MDV3100 has significant antitumor activity in men with chemotherapy-naive and chemotherapy-treated CRPC, thereby validating the preclinical models.
Antitumor effects were observed at all dosages studied, beginning with the first six patients treated at the lowest dosages, one of whom has remained on treatment for more than 2 years. In addition to substantial and sustained PSA declines, many patients had regressions of soft-tissue disease, no progression in bone disease, and prolonged times to PSA and radiographic progression. Overall, two-thirds of patients had partial remissions or stable disease in radiographically-evident soft tissue and bone lesions. The degree and proportion of patients showing PSA declines were dose-dependent from 30 mg to 150 mg/day, but reached a plateau between 150 and 240 mg/day, above which no additional antitumor effects were seen. The presentation of post-therapy PSA change data with waterfall plots (as suggested by the PCWG2 guidelines22) rather than with discrete percent PSA decline cutoff values was particularly helpful in recognizing the plateau in the dose-response relationship.
The time to PSA progression is presented using both the protocol specified definition of a 25% rise from baseline as well as the PCWG2 definition of a confirmed 25% or greater rise from nadir.22 Use of the PCWG2 criteria is important because it provides consistency in reporting outcomes that allows for relative comparisons between different treatment approaches. As expected, the time to PSA progression using the less conservative definition allows patients a longer exposure to the drug. The role of using PSA progression criteria alone to determine the need to discontinue treatment of an anti-androgen in CRPC remains controversial, as it known that that the relationship between PSA progression and survival is modest in this setting.26 Whether continued suppression of androgen signalling with an anti-androgen that has no agonist activity is warranted in patients who have responded and are now progressing solely on the basis of a rising PSA (versus discontinuing the treatment) is unknown and will require prospective testing. The approach is consistent with standard practice to continue androgen suppression and a new therapy for men with progressive CRPC,27 and to continue trastuzumab and add a new therapy for women with progressive breast tumors.28
In addition to conventional endpoints, we explored experimental biomarkers of AR inhibition and tumour response in subsets of the patients. Notably, FDHT PET scans revealed that MDV3100 substantially displaced FDHT binding at all dosages evaluated, with an apparent maximal effect seen at 150 mg despite the higher serum MDV3100 levels achieved at higher dosages. This suggests that AR binding by MDV3100 may be saturated at serum levels of ∼5-15 μg/mL, which were achieved consistently in patients receiving 150 mg/day but not at lower dosages. FDG-PET scans performed on the same 22 patients to assess early treatment response revealed declines of SUVmax of 25% or more in 45% of cases, showing that FDHT response (which occurred in essentially all patients) does not predict for FDG response, as would be expected for a pharmacodynamic biomarker.
A second early indicator of treatment efficacy was CTC number. Recent retrospective data from phase 2 clinical trials of prostate,29 breast,30 and colorectal cancer31 have shown that baseline CTC number is prognostic in patients about to start a new line of chemotherapy. In a recent trial, changes in CTC counts after treatment were more predictive of survival than were changes in PSA, with a favourable CTC count post-treatment associated with a 21-month median survival.29 In this study, unfavourable counts were observed in 40% of patients at baseline including 27% of the chemotherapy-naive and 51% of the post-chemotherapy patients. Early post-treatment conversions from unfavourable to favourable were observed in 75% of chemotherapy-naïve and 37% of post-chemotherapy patients. Declines in PSA were generally associated with parallel declines (or lack of progression) in CTCs, but not universally so, suggesting that these measures assess different aspects of the malignant process. PSA declines may in some cases reflect the mechanism of action of MDV3100 as an AR antagonist rather than an actual anti-tumor effect. However, the benefit of MDV3100 on multiple assessments, including CTCs and radiologic time to progression, suggest that in fact MDV3100 does have a true anti-tumor effect.
The predominant adverse event attributable to MDV3100 was fatigue that represented a clear change from the patient's baseline. In most patients, the fatigue appeared after 4 weeks, matching the time when drug concentrations reached steady state and resolved within two to four weeks of dose reduction. Although there was no other obvious cause to the fatigue such as depression, adrenal insufficiency was not formally excluded, although the mechanism of action of the drug does not suggest adrenal insufficiency as a possible side effect. Although dosages up to 480 mg/day were tolerated for 4 weeks, we determined the maximal tolerated dose to be 240 mg/day based on the frequency of treatment discontinuations required at higher doses.
Based on the declines in PSA, regressions of soft-tissue disease, CTC conversion rates, time to progression, and the safety profile demonstrated in this phase 1-2 study, a phase 3 randomized trial has been initiated to examine MDV3100 vs. placebo in men with progressive advanced prostate cancer, with a primary endpoint of overall survival. The results of the present trial validate in man preclinical studies implicating sustained AR signaling as a driver in CRPC and establish that a substantial fraction of tumours that progress despite multiple hormonal treatments and are currently treated with chemotherapy remain dependent on AR signaling for growth. MDV3100 may have the potential to significantly alter treatment options in metastatic disease, even in the post-chemotherapy setting where hormonal manipulations may have more utility than previously thought.
Acknowledgments
Support for this research came from Medivation; the Prostate Cancer Foundation, P50-CA92629 Specialized Program in Research Excellence in Prostate Cancer grant from the National Cancer Institute, P50-CA086438 MSKCC Center for Molecular Imaging in Cancer from the National Cancer Institute, the Howard Hughes Medical Institute, Doris Duke Charitable Foundation and Department of Defense Prostate Cancer Research Program Clinical Consortium (Memorial Sloan-Kettering Cancer Center: W81XWH-06-2-0011; Oregon Health and Science University Knight Cancer Institute: W81XWH-06-1-0237; The University of Washington: W81XWH-07-1-0097; Dana-Farber Cancer Institute: W81XWH-06-1-0261; MD Anderson Cancer Center: W81XWH-06-1-0203). We thank Janet Novak of Helix Editing for editing of the manuscript. Dr. Novak was paid for her work by Memorial Sloan-Kettering Cancer Center.
Footnotes
Authors' Contributions: HIS, TMB, DH, MH, LS and CS were involved in the study design.
HIS, TMB, CH, MET, DR EYY, JL and MJM were involved in patient recruitment, HIS, TMB, CH, AA, MET, LS, SL, MF and CS were involved with data analysis.
HIS, TMB, CH, MET, DR, DH, LS, MJM were involved in data interpretation.
HIS, TMB, MET, DCD and CS were involved in writing the manuscript.
EE, JS, and DCD were involved in data collection.
AA and DCD were involved in preparing the figures, DH identified MDV3100, licensed it into Medivation, performed all preclinical IND enabling work to enable entry into clinical trials and participated in the design and conduct of the clinical trials.
JH performed all of the actual FDHT PET scans for the pts on this trial.
CS is the co-discoverer of MDV3100 and leader of the preclinical development.
All authors contributed to the final approved version.
Conflict of Interest Statement: HIS, TMB and CH have research funding from Medivation. HIS and MH have travel support from Medivation. HIS has uncompensated consultancy from Medivation, DH, MH and LS are employees of Medivation. DH, MH and LS have stock options with Medivation. CS owns stock in Medivation and is co-inventor of patents filed by UCLA that covers MDV3100 and is entitled royalties from UCLA that could result from commercial success of MDV3100. All other authors declared no conflicts of interest.
References
- 1.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, Zanon C, Moroni M, Veronese S, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res. 2007 Mar 15;67(6):2643–8. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 2.Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008 Oct;9(10):962–72. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 3.Cortes J, Jabbour E, Kantarjian H, Yin CC, Shan J, O'Brien S, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. 2007 Dec 1;110(12):4005–11. doi: 10.1182/blood-2007-03-080838. [DOI] [PubMed] [Google Scholar]
- 4.Shah NP, Skaggs BJ, Branford S, Hughes TP, Nicoll JM, Paquette RL, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. J Clin Invest. 2007 Sep;117(9):2562–9. doi: 10.1172/JCI30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domainPLoS Med. PLoS Med. 2005 Mar;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005 Jun 1;11(11):4182–90. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 7.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006 Jun 15;354(24):2531–41. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 8.Gajiwala KS, Wu JC, Christensen J, Deshmukh GD, Diehl W, DiNitto JP, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009 Feb 3;106(5):1542–7. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demetri GD, Heinrich MC, Fletcher JA, Fletcher CD, Van den Abbeele AD, Corless CL, et al. Molecular target modulation, imaging, and clinical evaluation of gastrointestinal stromal tumor patients treated with sunitinib malate after imatinib failure. Clin Cancer Res. 2009 Sep 15;15(18):5902–9. doi: 10.1158/1078-0432.CCR-09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scher HI, Liebertz C, Kelly WK, Mazumdar M, Brett C, Schwartz L, et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol. 1997 Aug;15(8):2928–38. doi: 10.1200/JCO.1997.15.8.2928. [DOI] [PubMed] [Google Scholar]
- 11.Pomerantz M, Kantoff P. Advances in the treatment of prostate cancer. Annu Rev Med. 2007;58:205–20. doi: 10.1146/annurev.med.58.101505.115650. [DOI] [PubMed] [Google Scholar]
- 12.Small EJ, Vogelzang NJ. Second-line hormonal therapy for advanced prostate cancer: a shifting paradigm. J Clin Oncol. 1997 Jan;15(1):382–8. doi: 10.1200/JCO.1997.15.1.382. [DOI] [PubMed] [Google Scholar]
- 13.Scher HI, Sawyers C. Biology of progressive castration resistant prostate cancer: directed therapies targeting the androgen receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 14.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–5. [PubMed] [Google Scholar]
- 15.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004 Jan;164(1):217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006 Mar 1;66(5):2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 17.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004 Jan;10(1):33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 18.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009 May 8;324(5928):787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher HI, Kolvenbag GJ. The antiandrogen withdrawal syndrome in relapsed prostate cancer. Eur Urol. 1997;31 2:3–7. doi: 10.1159/000474540. discussion 24-7. [DOI] [PubMed] [Google Scholar]
- 20.Morris MJ, Basch EM, Wilding G, Hussain M, Carducci MA, Higano C, et al. Department of Defense prostate cancer clinical trials consortium: a new instrument for prostate cancer clinical research. Clin Genitourin Cancer. 2009 Jan;7(1):51–7. doi: 10.3816/CGC.2009.n.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris MJ, Akhurst T, Larson SM, Ditulio M, Chu E, Siedlecki K, et al. Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with chemotherapy. Clin Cancer Res. 2005;11:3210–16. doi: 10.1158/1078-0432.CCR-04-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008 Mar 1;26(7):1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 24.Veridex L. CellSearch circulating tumor cell kit premarket notification - expanded indications of use - metastatic prostate cancer [Google Scholar]
- 25.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007 Dec 1;13(23):7053–8. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 26.Scher HI, Warren M, Heller G. The Association Between Measures of Progression and Survival in Castrate-Metastatic Prostate Cancer. Clin Cancer Res. 2007 Mar 1;13(5):1488–92. doi: 10.1158/1078-0432.CCR-06-1885. [DOI] [PubMed] [Google Scholar]
- 27.Basch EM, Somerfield MR, Beer TM, Carducci MA, Higano CS, Hussain MH, et al. American Society of Clinical Oncology endorsement of the Cancer Care Ontario Practice Guideline on nonhormonal therapy for men with metastatic hormone-refractory (castration-resistant) prostate cancer. J Clin Oncol. 2007 Nov 20;25(33):5313–8. doi: 10.1200/JCO.2007.13.4536. [DOI] [PubMed] [Google Scholar]
- 28.von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol. 2009 Apr 20;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 29.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008 Oct 1;14(19):6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 30.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004 Aug 19;351(8):781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 31.Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. The relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal carcinoma. J Clin Oncol. 2008;26 doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]