Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion
Pharmacological inhibition of HtrA uncovers a novel mechanism by which Helicobacter pylori disrupts adherence junctions in epithelial layers.
Keywords: H. pylori, HtrA, E-cadherin
Abstract
Mammalian and prokaryotic high-temperature requirement A (HtrA) proteins are chaperones and serine proteases with important roles in protein quality control. Here, we describe an entirely new function of HtrA and identify it as a new secreted virulence factor from Helicobacter pylori, which cleaves the ectodomain of the cell-adhesion protein E-cadherin. E-cadherin shedding disrupts epithelial barrier functions allowing H. pylori designed to access the intercellular space. We then designed a small-molecule inhibitor that efficiently blocks HtrA activity, E-cadherin cleavage and intercellular entry of H. pylori.
Introduction
High-temperature requirement A (HtrA) and its homologues are widely expressed as proteases and chaperones in prokaryotes and eukaryotes with crucial roles in protein quality control. Escherichia coli expresses the three proteases DegP, DegQ and DegS, the activities of which are differentially regulated. DegP is well characterized in its function as an ATP-independent chaperone protease that is converted to active forms on substrate binding (Jiang et al, 2008; Krojer et al, 2008). In contrast to the regulatory protease DegS that processes the anti-sigma factor RseA, DegP degrades unfolded proteins into small peptides. HtrAs have a highly conserved trypsin-like serine protease domain and at least one PSD-95/Discs-Large/ZO-1 domain. Furthermore, human HtrA1 contains an insulin-like growth-factor-binding protein domain and a serine protease inhibitor motif (Zumbrunn & Trueb, 1996). The bacterial proteases are mainly involved in eliminating misfolded proteins, whereas HtrA proteins are implicated in severe disorders, including whereas Alzheimer diseases, arthritis and cancer (Meltzer et al, 2009).
We have previously demonstrated that Helicobacter pylori secretes HtrA (HpHtrA) into the extracellular space, but the function of this is as yet unknown (Löwer et al, 2008). H. pylori is an important pathogen that colonizes the gastric epithelium, where it disrupts mucosal integrity and induces severe inflammatory responses or gastric cancer (Peek & Blaser, 2002; Cover & Blanke, 2005). Several studies have shown that the soluble H. pylori factors vacuolating cytotoxin A (VacA) or urease can selectively reduce the transepithelial electrical resistance (TER) of gastric epithelial cells, which is a measurable indicator of epithelial integrity (Papini et al, 1998; Wroblewski et al, 2009). Other studies have indicated that disruption of cell junctions requires another injected bacterial factor, cytotoxin-associated gene A, which might target tight and adherence junctions intracellularly (Amieva et al, 2003; Murata-Kamiya et al, 2007). Thus, the mechanism by which H. pylori disrupts the gastric epithelial barrier remains unclear.
We demonstrated recently that H. pylori induces ectodomain cleavage of E-cadherin in polarized cells by an unknown mechanism (Weydig et al, 2007). E-cadherin represents a mammalian cell surface protein that has essential functions in cell adhesion and tumour suppression. In gastric cancer, proteolytic release of the E-cadherin ectodomain is an important prognostic marker (Chan, 2006). The matrix metalloproteases MMP3, MMP7 and a disintegrin and metallopeptidase 10 (ADAM10) were identified to cleave E-cadherin extracellularly, generating a soluble fragment that impairs cell adhesions (Noe et al, 2001; Maretzky et al, 2005). In fact, some of these proteases are induced by H. pylori infection and might contribute to the disruption of epithelial integrity (Ogden et al, 2008; Schirrmeister et al, 2009).
Here, we provide the first direct evidence that HpHtrA acts as a specific E-cadherin protease, which effectively destroys adherence junctions in polarized epithelial cells. We also describe the identification of a pharmacological lead compound that prevents HtrA-mediated E-cadherin cleavage and loss of adherence junctions. Our results reveal a new mechanism that explains how H. pylori disrupts the epithelial barrier as an important step in bacterial pathogenesis.
Results And Discussion
H. pylori-secreted HtrA cleaves E-cadherin
Full-length 125-kDa E-cadherin (E-Cad-FL) can be cleaved in the extracellular domain amino-terminal fragment (NTF), generating a 40-kDa carboxy-terminal fragment (CTF1), which can be further processed by intracellular activities into a soluble 33-kDa CTF2 fragment (Fig 1A). After colonizing MKN-28 gastric epithelial cells with H. pylori, we detected ectodomain cleavage of E-cadherin, as monitored by the extracellular NTF in cell supernatants. Conversely, the amount of E-Cad-FL decreased in cell lysates, and intracellular CTF1 and CTF2 levels increased, as expected (Fig 1B). Comparison between the amount of soluble NTF and the total amount of E-Cad-FL indicated that NTF supernatant levels increased from 0.3±0.4% to 38.2±11.8% 24 h after infection (supplementary Fig S1A online).
Figure 1.
Helicobacter pylori induces ectodomain E-cadherin shedding independently of host proteases. (A) E-cadherin has five extracellular domains (EC1–EC5), a transmembrane domain and an intracellular domain. Cleavage by host proteases generates an extracellular N-terminal fragment (NTF) and two C-terminal fragments (CTF1 and CTF2). (B) MKN-28 cells were infected with H. pylori as indicated and the soluble 85-kDa E-cadherin fragment (NTF) was detected in the supernatant of host cells by using western blots with the E-cadherin ectodomain antibody. Loss of E-Cad-FL in whole-cell lysates was confirmed using an antibody detecting intracellular CTF1 and CTF2. GAPDH is shown as a control. (C) MKN-28 cells were preincubated with 150 nM MMP inhibitor II (MMP-Inh.; 5 × IC50) or treated with ADAM10 siRNA followed by infection with H. pylori. Soluble NTF in supernatants and E-Cad-FL in whole-cell lysates were detected. Mature ADAM10 (60 kDa) and GAPDH are shown as controls. ADAM10, a disintegrin and metallopeptidase 10; E-Cad-FL, full-length 125-kDa E-cadherin; MMP, matrix metalloprotease; siRNA, short interfering RNA.
Ectodomain E-cadherin cleavage occurs frequently and is an important step in the pathogenesis of inflammatory responses or neoplastic transformation (Chan, 2006), a phenomenon that was attributed to MMPs and ADAM10 (Fig 1A). Hence, we focused on shedding of the E-cadherin ectodomain and tested whether MMP or ADAM10 activity is involved in H. pylori-mediated E-cadherin cleavage. In agreement with previous studies, MMP7 was upregulated in H. pylori-infected MKN-28 cells (supplementary Fig S1C online). Recombinant E-cadherin containing the extracellular domain was efficiently cleaved by using recombinant MMP7, which was inhibited by increasing concentrations of MMP inhibitor II (supplementary Fig S1B online). Independently of mRNA induction (supplementary Fig S1C online), we observed an increase in mature ADAM10 protein in response to H. pylori (Schirrmeister et al, 2009), which was strongly downregulated using ADAM10-specific short interfering RNA (siRNA; Fig 1C). However, neither inhibition of MMPs nor further downregulation of ADAM10 efficiently blocked H. pylori-mediated E-cadherin cleavage on host cells (Fig 1C). We also used several inhibitors to block a wide range of proteases (data not shown), suggesting another major protease in H. pylori-induced E-cadherin fragmentation.
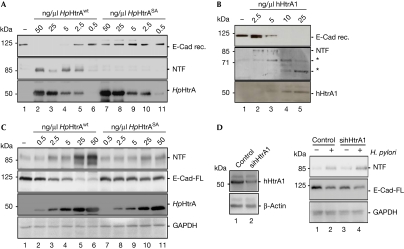
Previously, we observed that a secreted H. pylori factor induced ectodomain E-cadherin shedding (Weydig et al, 2007) and identified H. pylori-secreted HtrA as an active caseinolytic protease with unknown functions (Löwer et al, 2008). To analyse whether HpHtrA acts as an E-cadherin protease, we incubated increasing amounts of HpHtrAwt and an inactive variant HpHtrASA with recombinant E-cadherin. Low concentrations of HtrAwt, but not HtrASA efficiently degraded E-cadherin, as monitored by the loss of E-Cad-FL and increase of the cleaved 85-kDa NTF (Fig 2A).
Figure 2.
HpHtrA cleaves E-cadherin on the cell surface. (A) Recombinant E-cadherin was incubated with decreasing amounts of purified HpHtrAwt or inactive HpHtrASA. E-Cad rec., NTF and HpHtrA were detected. (B) E-Cad rec. was incubated with increasing amounts of hHtrA1. E-Cad rec., NTF and HtrA were detected by western blots. Putative NTF fragments are indicated by asterisks (*). (C) Detached MKN-28 cells were incubated in culture medium for 3 h for re-expression of E-Cad-FL, followed by treatment with HtrAwt or HtrASA for 16 h. Formation of NTF, loss of E-Cad-FL, HpHtrA and equal GAPDH expression were detected. (D) MKN-28 cells were treated with control or hHtrA1-specific siRNA for 48 h. Downregulation of hHtrA1 was demonstrated by western blot using an hHtrA1-specific antibody. β-Actin is shown as a control (left panel). Transfected cells were then infected with H. pylori for 16 h. NTF was detected in the supernatants of cells; loss of E-Cad-FL and GAPDH are shown in the lysates of transfected cells (right panel). E-Cad rec., recombinant E-cadherin containing the extracellular domain; E-Cad-FL, full-length 125-kDa E-cadherin; Hp, Helicobacter pylori; HtrA, high-temperature requirement A; siRNA, short interfering RNA.
HtrA proteins are widely distributed in the mammalian and prokaryotic kingdoms, suggesting that they have functional similarities. Sequence alignment of HpHtrA with E. coli DegP is significant (BLAST (Altschul et al, 1990): e-value=10−76, sequence identity=42%), although the putative active sites around Ser221 differ with regard to their polarity and subpocket shape (supplementary Fig S2C online). Despite having similar overall folds, the human HtrA1 contains different and more polar residues in parts of the active site, which might result in different target specificity (supplementary Fig S2A,B online).
HtrA1 has been described to degrade fibronectin that might be involved in cartilage catabolism (Grau et al, 2006). On the basis of the observed differences between HpHtrA and hHtrA1 (supplementary Fig S2B online), we asked whether HpHtrA can also cleave fibronectin. We found that HpHtrA efficiently cleaved fibronectin, which was not observed for inactive HpHtrASA (supplementary Fig S3A online), indicating that they might share biological functions. As E-cadherin is a new target of HpHtrA, we next analysed whether hHtrA1 can cleave E-cadherin. Interestingly, E-cadherin was efficiently cleaved by 10 ng/μl hHtrA1 in vitro (Fig 2B). In contrast to HpHtrA, we observed further protein bands, which probably result from multiple cutting events by hHtrA1 (Fig 2B, asterisks), which also reflect different substrate specificity as described for DegS and DegP (Meltzer et al, 2009). These data suggest that both HtrAs have E-cadherin as target, but cleave at different sequences.
As observed in vitro, HpHtrA also mediated E-cadherin ectodomain release from cultured epithelial cells. Low HpHtrAwt concentrations were sufficient to cleave E-cadherin in situ, as reflected by the increase of NTF fragment in the supernatant and loss of E-Cad-FL in cellular lysates (Fig 2C), supporting our hypothesis that H. pylori-secreted HtrA directly targets E-cadherin on the surface of host cells. An implication of hHtrA1 in E-cadherin ectodomain shedding was excluded in siRNA experiments. Expression of hHtrA1 was efficiently downregulated by 60%, but H. pylori still induced formation of NTF and loss of E-Cad-FL (Fig 2D).
To demonstrate the specificity of HpHtrA in an unbiased approach, we also tested other putative H. pylori protease candidates (Löwer et al, 2008). HP0657, HP1012 and HP0506 were analysed for E-cadherin cleavage, caseinase and IgAse activity in vitro. As expected, HpHtrA degraded casein as an artificial substrate, whereas the other tested putative H. pylori proteases were inactive (supplementary Fig S3B online). As a control, HpHtrA did not cleave recombinant IgA (supplementary Fig S3B online), EGFR or JAM (supplementary Fig S3C online), indicating that E-cadherin and fibronectin represent selective substrates for HpHtrA.
HHI is a pharmacological lead compound blocking HtrA
We were unable to generate ΔhtrA knockout mutants, confirming previous reports that HtrA is an essential H. pylori factor, which cannot be deleted (Salama et al, 2004). We therefore focused on pharmacological inhibition and performed structure-based virtual screening for small-molecule inhibitors of HpHtrA. A comparative protein model of HtrA (Fig 3A) was used to create a pharmacophore model of the presumed protease active site (Fig 3B). After virtual screening of compound databases and biochemical tests, we identified HpHtrA inhibitor (HHI; supplementary Fig S4A online) as a small-molecule inhibitor that efficiently blocked E-cadherin cleavage (Fig 3C). HHI was docked into the active site of HpHtrA to determine a potential binding mode (Fig 3B). When HpHtrA was incubated with recombinant E-cadherin and increasing amounts of inhibitor, 10 μM HHI was sufficient to partly block HtrA-mediated E-cadherin cleavage, whereas 30 μM HHI completely blocked the cleavage (Fig 3C). We determined an IC50 value of 26±12 μM (supplementary Fig S4B online).
Figure 3.
Computer-assisted identification of a functional small-molecule inhibitor targeting HtrA. (A) Ribbon representation of the HtrA homology model identified from DegP from Escherichia coli with annotated domain organization. Blue spheres indicate the predicted ligand-binding sites, shaded by the predicted buried state. The solvent accessible surface is shown; the surface patch contributed by the active site Ser221 is highlighted in red. (B) Proposed binding mode of HHI (cyan). Interacting side chains and backbone atoms are shown in light grey, and the active site (His 116, Asp 147, Ser 221) and the partly shown protein backbone in dark grey. The loops L1 (including Pro 218 and Gly 219), L2 and L3 (including Phe 209) are shown in red. Secondary structure elements are named according to Protein Data Bank crystal structure 3cs0. Atoms have Corey, Pauling, Koltun colouring scheme. Green spheres: predicted lipophilic interactions; red spheres: predicted H-bond acceptors. (C) Recombinant E-cadherin was incubated with HpHtrAwt and indicated concentrations of HHI. Loss of E-cadherin (E-Cad rec.), the extracellular E-cadherin fragment (NTF) and HtrA are shown. (D) MKN-28 cells were infected with H. pylori for 16 and 24 h. Where indicated, cells were co-treated with 100 μM HHI. NTF, loss of E-Cad-FL and secreted HtrA were detected. (E) Confluent MKN-28 cells were left untreated (mock) or were infected with H. pylori for 16 h. As indicated, cells were co-treated with 100 μM HHI. Loss of E-cadherin (red) was demonstrated using an antibody detecting the extracellular domain. Nuclei (blue) were stained using DAPI. Scale bar, 20 μm. E-Cad-FL, full-length 125-kDa E-cadherin; E-Cad rec., recombinant E-cadherin containing the extracellular domain; HHI, HpHtrA inhibitor; Hp, Helicobacter pylori; HtrA, high-temperature requirement A; NTF, N-terminal fragment; PDZ, PSD-95/Discs-Large/ZO-1.
In a biological context, H. pylori-induced ectodomain E-cadherin cleavage in MKN-28 cells was clearly inhibited in the presence of HHI (Fig 3D). We can exclude that this effect was due to HHI hindering secretion of HtrA into cell supernatants (Fig 3D), or interfering with H. pylori adherence and viability (Fig 4B). Although not investigated, we assume that HHI blocks HtrA extracellularly, but not periplasmically. We further demonstrated that HHI did not affect MMP7 activity and that MMP inhibitor II did not block HpHtrA (supplementary Fig S5A online). Notably, in comparison with HpHtrA, HHI does not inhibit other serine proteases, such as thrombin or chymotrypsin, and a slight effect on trypsin was observed. However, all tested proteases were efficiently targeted by phenylmethyl-sulphonylfluorid (supplementary Fig S5B online). Furthermore, the E-cadherin cleavage activity of hHtrA1 was not blocked using HHI (supplementary Fig S5C online). In immunofluorescence studies it was demonstrated that H. pylori induced a loss of the E-cadherin ectodomain staining, which was blocked by addition of HHI (Fig 3E). Thus, we developed HHI as a first-in-class lead structure for efficiently blocking HtrA-mediated E-cadherin cleavage and disruption of intercellular adhesions. However, it is evident that future research should be devoted to designing and optimizing a more potent, selective and specific inhibitor of HpHtrA. HHI represents a first point of reference for virtual screening studies, which aim at selectivity with regard to other serine proteases and an improved potency. Ideally, new ‘drug-like' scaffold structures will be retrieved in future experiments, such as those now being performed in our laboratory.
Figure 4.
HpHtrA disrupts intercellular adhesions to enter the intercellular space. (A) Confluent MKN-28 cells were infected with Hp-expressing GFP for 24 h and stained for F-actin (red) along the xy-axis. Scale bar, 20 μm. Scanning along the xz-axis revealed Hp (green) in the intercellular space between MKN-28 cells (white arrows). (B) MKN-28 cells treated with 100 μM HHI or left untreated were infected with Hp for 16 and 24 h. Determination of the relative colony-forming units (CFU) showed no significant difference. (C) Hp's translocating across confluent epithelial monolayers were quantified by growing MKN-28 cells on filters. Cells were treated with 100 μM HHI before infection with Hp for 48 h. Bacteria migrating across the monolayer were harvested and CFUs were determined. HHI had a significant effect on preventing Hp translocation through the intercellular space (**P=0.01). GFP, green fluorescent protein; HHI, HpHtrA inhibitor; Hp, Helicobacter pylori; HtrA, high-temperature requirement A.
Our observation that secreted HpHtrA directly cleaves E-cadherin to disrupt adherence junctions adds an important new aspect to the understanding of H. pylori pathogenesis. Soluble H. pylori factors, such as VacA or urease, are still the subject of controversal discussions about whether they directly target intercellular adhesions (Papini et al, 1998; Wroblewski et al, 2009). To investigate whether HpHtrA contributes to the loss of intercellular adhesions and barrier function, we verified that MKN-28 can establish an impermeable cell monolayer. MKN-28 cells were stained for ZO-1 and E-cadherin to visualize lateral tight junctions and adherence junctions. As expected, tight junctions were localized at the apical end of lateral membranes, with E-cadherin-containing adherence junctions immediately below (supplementary Fig S6A online). As reported before (Wroblewski et al, 2009), MKN-28 cells formed intact tight junctions in a confluent monolayer that could be monitored by the measurement of TER. Infection with H. pylori significantly decreased TER, which was not prevented by HHI treatment of cells (supplementary Fig S6B online). Together with the observation that purified HpHtrA did not influence TER (supplementary Fig S6C online), we suggest that HtrAs selectively target E-cadherin-mediated adherence junctions and assume that tight junctions are disrupted by other bacterial factors (for example, VacA or urease), as proposed previously (Papini et al, 1998; Wroblewski et al, 2009).
On analysing intercellular adhesions, individual H. pylori were seen to actively invade the intercellular space between polarized epithelial cells. We clearly detected H. pylori in the intercellular spaces of confluent MKN-28 cells that were counterstained for the actin cytoskeleton by confocal laser scanning microscopy (Fig 4A). Importantly, HHI did not interfere with H. pylori adherence and viability (Fig 4B). However, HHI significantly inhibited H. pylori entering the intercellular space of confluent MKN-28 cells grown on filters (Fig 4C), indicating that secreted HtrA allows H. pylori to access the intercellular space between polarized cells in monolayers.
Here, we discovered that HtrA enables H. pylori to invade an intact epithelium between cells, supporting recent electron microscopy analyses of biopsy samples from cancer patients in which H. pylori was found in the intercellular space and at the basal lamina (Necchi et al, 2007). The identification of H. pylori-secreted HtrA as an E-cadherin protease is therefore an important finding because it reveals how H. pylori uses HtrA as a bacterial protease in a coordinated manner with the injected effector protein, cytotoxin-associated gene A. This leads to the destruction of the epithelial barrier function, allowing persistent H. pylori colonization, nutrition and pathogenesis (Wessler & Backert, 2008).
In general, secretion of bacterial-derived enzymes represents an efficient way for pathogens to cause pathology. In many pathogens (for example, Campylobacter jejuni and Salmonella typhimurium), HtrA contributes to virulence (Ingmer & Brondsted, 2009). However, the detailed mechanism for this in bacterial pathogenesis is unknown. Our finding that H. pylori-secreted HtrA cleaves E-cadherin might uncover a general bacterial strategy in persistent colonization and pathogenesis, and HtrA could therefore, be a promising candidate for therapeutic intervention.
Importantly, we identified a first-in-class lead compound directed against HpHtrA, which exhibits pronounced cell-protective activity without affecting hHtrA1. Owing to the growing spread of antibiotic resistance among bacterial pathogens, developing new therapeutic targets is a pressing issue worldwide. We propose that pharmacological inhibition of bacterial HtrA might act as an effective alternative strategy to combat H. pylori, and also, probably, other bacterial infections.
Methods
Detailed experimental procedures are described in the supplementary information online.
Cell culture and infection. Cells were seeded in tissue culture plates and grown to confluence. Before infection, the medium was replaced by fresh serum-free media. Where indicated, cells were treated with inhibitors or siRNAs. For infection, bacteria were added to the host cells at a multiplicity of infection of 50 or 100.
Western blots and immunofluorescence. Cells were harvested in lysis buffer (Weydig et al, 2007) and separated by SDS–PAGE. Proteins were blotted and probed with α-E-cadherin cl. 36 (BD Transduction Laboratories) to detect the intracellular E-cadherin domain. Monoclonal antibodies HecD1 and H-108 against the extracellular domain of E-cadherin (NTF) were obtained from Calbiochem and Santa Cruz, respectively. Expression of hHtrA1, ADAM10 (Santa Cruz), GAPDH (Abcam) and β-actin (Sigma) was analysed using specific antibodies. HpHtrA antiserum was raised against the N-terminal peptide (C-DKIKVTIPGSNKEY) of HpHtrA (Biogenes GmbH). For immunofluorescence, cells were stained using α-E-cadherin (HecD1 detects the extracellular domain). Filamentous actin was detected with Alexa Fluor 546-conjugated phalloidin (Invitrogen) and nuclei were stained using DAPI (Roche).
HtrA modelling and in vitro cleavage experiments. HtrA modelling and inhibitor screen are described in the supplementary information online. For in vitro cleavage studies, 100 ng recombinant E-cadherin (R&D Systems) was incubated with purified HpHtrAwt, HpHtrASA, hHtrA1 or MMP7 in HEPES, pH 7.4 at 37°C for 16 h. Where indicated, HHI was added.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank A. Friedrich for purification of H. pylori proteases.
Footnotes
The authors declare that they have no conflict of interest.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S (2003) Disruption of the epithelial apical–junctional complex by Helicobacter pylori CagA. Science 300: 1430–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AO (2006) E-cadherin in gastric cancer. World J Gastroenterol 12: 199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover TL, Blanke SR (2005) Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol 3: 320–332 [DOI] [PubMed] [Google Scholar]
- Grau S, Richards PJ, Kerr B, Hughes C, Caterson B, Williams AS, Junker U, Jones SA, Clausen T, Ehrmann M (2006) The role of human HtrA1 in arthritic disease. J Biol Chem 281: 6124–6129 [DOI] [PubMed] [Google Scholar]
- Ingmer H, Brondsted L (2009) Proteases in bacterial pathogenesis. Res Microbiol 160: 704–710 [DOI] [PubMed] [Google Scholar]
- Jiang J, Zhang X, Chen Y, Wu Y, Zhou ZH, Chang Z, Sui SF (2008) Activation of DegP chaperone-protease via formation of large cage-like oligomers upon binding to substrate proteins. Proc Natl Acad Sci USA 105: 11939–11944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krojer T, Sawa J, Schafer E, Saibil HR, Ehrmann M, Clausen T (2008) Structural basis for the regulated protease and chaperone function of DegP. Nature 453: 885–890 [DOI] [PubMed] [Google Scholar]
- Löwer M, Weydig C, Metzler D, Reuter A, Starzinski-Powitz A, Wessler S, Schneider G (2008) Prediction of extracellular proteases of the human pathogen Helicobacter pylori reveals proteolytic activity of the Hp1018/19 protein HtrA. PLoS ONE 3: e3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P (2005) ADAM10 mediates E-cadherin shedding and regulates epithelial cell–cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA 102: 9182–9187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M et al. (2009) Structure, function and regulation of the conserved serine proteases DegP and DegS of Escherichia coli. Res Microbiol 160: 660–666 [DOI] [PubMed] [Google Scholar]
- Murata-Kamiya N et al. (2007) Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene 26: 4617–4626 [DOI] [PubMed] [Google Scholar]
- Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, Ricci V, Solcia E (2007) Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology 132: 1009–1023 [DOI] [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M (2001) Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114: 111–118 [DOI] [PubMed] [Google Scholar]
- Ogden SR et al. (2008) p120 and Kaiso regulate Helicobacter pylori-induced expression of matrix metalloproteinase-7. Mol Biol Cell 19: 4110–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini E, Satin B, Norais N, de Bernard M, Telford JL, Rappuoli R, Montecucco C (1998) Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J Clin Invest 102: 813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek RM Jr, Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2: 28–37 [DOI] [PubMed] [Google Scholar]
- Salama NR, Shepherd B, Falkow S (2004) Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. J Bacteriol 186: 7926–7935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirrmeister W, Gnad T, Wex T, Higashiyama S, Wolke C, Naumann M, Lendeckel U (2009) Ectodomain shedding of E-cadherin and c-Met is induced by Helicobacter pylori infection. Exp Cell Res 315: 3500–3508 [DOI] [PubMed] [Google Scholar]
- Wessler S, Backert S (2008) Molecular mechanisms of epithelial-barrier disruption by Helicobacter pylori. Trends Microbiol 16: 397–405 [DOI] [PubMed] [Google Scholar]
- Weydig C, Starzinski-Powitz A, Carra G, Lower J, Wessler S (2007) CagA-independent disruption of adherence junction complexes involves E-cadherin shedding and implies multiple steps in Helicobacter pylori pathogenicity. Exp Cell Res 313: 3459–3471 [DOI] [PubMed] [Google Scholar]
- Wroblewski LE, Shen L, Ogden S, Romero-Gallo J, Lapierre LA, Israel DA, Turner JR, Peek RM Jr (2009) Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology 136: 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumbrunn J, Trueb B (1996) Primary structure of a putative serine protease specific for IGF-binding proteins. FEBS Lett 398: 187–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.