A genetic system to assess in vivo the functions of histones and histone modifications in higher eukaryotes
The study describes the development of a genetic tool for Drosophila melanogaster in which the canonical histone genes can be replaced by multiple copies of experimentally modified histone transgenes to directly assess histone function.
Keywords: histone deletion, histone transgenes, functional assay, Drosophila melanogaster
Abstract
Despite the fundamental role of canonical histones in nucleosome structure, there is no experimental system for higher eukaryotes in which basic questions about histone function can be directly addressed. We developed a new genetic tool for Drosophila melanogaster in which the canonical histone complement can be replaced with multiple copies of experimentally modified histone transgenes. This new histone-replacement system provides a well-defined and direct cellular assay system for histone function with which to critically test models in chromatin biology dealing with chromatin assembly, variant histone functions and the biological significance of distinct histone modifications in a multicellular organism.
Introduction
Eukaryotic genomes are packaged into arrays of nucleosomes composed of 147-base-pair DNA intervals wrapped around an octameric complex of the core histones H2A, H2B, H3 and H4 (Luger et al, 1997). Together with the H1-type linker histones, these proteins are termed canonical histones, as they constitute the vast majority of histone proteins in chromatin (Marzluff et al, 2008). Concomitant with genome duplication during S-phase of the cell cycle, disassembled parental nucleosomes and de novo synthesized histones are used as distinct sources for replication-coupled nucleosome assembly (Corpet & Almouzni, 2009). In a subset of nucleosomes, the canonical histones are subsequently replaced by histone variant proteins by replication-independent processes, which can specify functionally distinct chromatin regions (Henikoff & Ahmad, 2005). The complexity of chromatin diversification is further increased by the many post-translational modifications of histones, which are thought to constitute an epigenetic ‘histone code' (Jenuwein & Allis, 2001; Kouzarides, 2007). Despite the central role of histones in chromatin assembly and diversification, no experimental tools have been developed so far to directly address canonical histone divergence from replacement variants and the post-translational modifications in multicellular organisms. In fact, genetic analysis has been limited to lower eukaryotes, such as the yeast Saccharomyces cerevisiae, in which histone genes are encoded by tandem repeats (Marzluff et al, 2008). In all higher eukaryotes, however, canonical histones are encoded by multiple gene units of between 10 and 400 copies that are mostly distributed over several chromosomes (Marzluff et al, 2008). The high number of histone genes and their distribution within the genome prevent straightforward functional genetics and explains the correlative nature of many previous studies in the field (Kouzarides, 2007). As the yeast model system is clearly not suitable to address chromatin diversification and its function in the context of the complex multicellular development of higher organisms, we developed an experimental system for the model organism Drosophila melanogaster that serves as a tool to directly assess histone functions by molecular genetics and by transgene-dependent rescue.
Results and Discussion
Generation of a molecularly defined histone deficiency
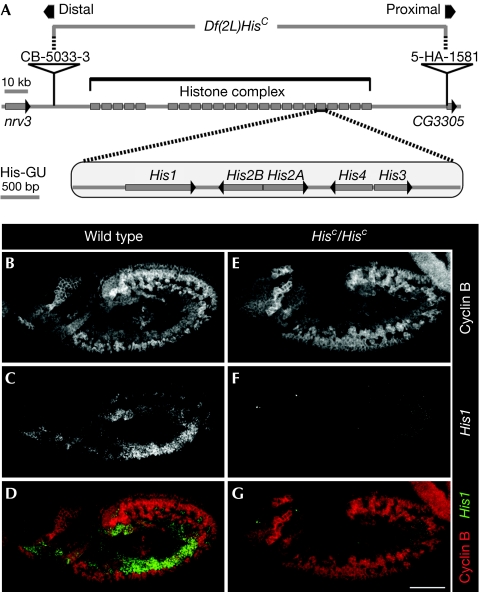
D. melanogaster is an ideal model organism in which to develop an experimental system to address fundamental questions regarding histone function for several reasons. The genetic toolset in D. melanogaster is highly advanced, the fly undergoes a complex but well-characterized development and, most importantly, all canonical histone genes are clustered in a single chromosomal locus. This arrangement is distinct from that in other multicellular organisms, including genetically accessible systems such as the mouse and nematodes, in which the histone genes are distributed throughout the genomes (Marzluff et al, 2008). According to the genome annotation, the histone gene complex of D. melanogaster is composed of 23 canonical histone gene repeat units (His-GUs), each bearing a gene array encoding the five canonical histones (for simplicity we refer to them as ‘histones'; for details of the histone complex see http://flybase.org; Fig 1A). Owing to this arrangement, we were able to use the DrosDel system (Ryder et al, 2004) to generate the deletion Df(2L)HisC, which precisely uncovers the entire histone complex and thus all histone genes in the genome. We call this molecularly defined histone null mutation ‘HisC' (Fig 1A).
Figure 1.
Df(2L)HisC deletes all canonical histone genes. (A) Schematic representation of the canonical histone gene organization in Drosophila melanogaster. A total of 23 histone gene repeat units, each containing a single His1, His2B, His2A, His4 and His3 gene (His-GU), are clustered in the histone complex. The deletion of the histone complex includes the region between the distal rearrangement screen element CB-5033-3 and the proximal rearrangement screen element 5-HA-1581. Neighbouring genes are nrv3 and CG3305. (B–D) Wild-type histone H1 (His1) expression in S-phase 15 (S15). Cyclin B labels cells in G214; cells in early S15 show low or absent Cyclin B staining (B). S15 cells expressed His1 mRNA as detected by in situ hybridization (C). Expression was absent from G2 cells (D, Cyclin B red, His1 green). (E–G) Corresponding staining showed that His1 expression was undetectable in homozygous HisC mutant embryos (HisC/HisC). Wild type refers to internal control. Scale bar, 100 μm; anterior, left. mRNA, messenger RNA.
Replacement of the Drosophila histone complement
Homozygous HisC mutant individuals die during embryogenesis. This allows validation of a transgene-based histone-replacement system by its ability to rescue the lethality associated with the HisC deletion. Our strategy included three important technical aspects. We used the genuine His-GUs for histone expression instead of an artificial expression system, thereby maintaining S-phase-specific histone expression and avoiding histone overexpression. We used the ‘Multisite Gateway' system (Invitrogen) for a modular transgene design from single His-GUs (supplementary Fig S1 online). Finally, we used the φC31 site-specific recombination system (Bischof et al, 2007), which allowed us to target transgene integrations to defined genomic locations (landing sites), an essential prerequisite for drawing conclusions from comparing the effects of modified and wild-type histone transgenes that could suffer from variable position effects if transgenes were randomly integrated.
Lethality of HisC was not rescued by two or six transgene-encoded His-GUs. The respective transgenes carried one or three His-GUs integrated into the same landing site on chromosome 3R and were assayed as homozygous individuals. Whereas HisC mutant embryos lacking His-GU transgenes did not develop a larval cuticle, individuals bearing two His-GU transgenes developed a defective cuticle, and individuals bearing six His-GU transgenes developed a wild-type cuticle pattern but failed to hatch (supplementary Fig S2 online). These results indicate a dose-dependent partial rescue with increasing copy numbers of His-GU transgenes. Finally, we increased the His-GU copy numbers by integrating three His-GUs into an additional landing site on chromosome 3L to generate flies that carried 12 transgene-encoded His-GUs in their genome by recombination. HisC mutant individuals with 12 His-GUs were rescued to become fertile adults with no visible morphological defects. Furthermore, when we compared the protein expression of transgene-encoded histones from rescued flies with endogenous histones from wild-type flies, we did not find a significant difference (supplementary Fig S3 online). The complete rescue of the HisC mutation by 12 His-GU transgenes indicates that the deletion does not affect any essential genes other than the canonical histones. The combination of histone transgenesis with the HisC mutation is, therefore, an effective system in which to replace endogenous histones with transgene-encoded histones in a multicellular organism.
Assay for cell cycle related histone function
To validate the system as a cellular assay for histone function, we characterized the embryonic lethal phenotype of the HisC mutation by studying cell cycle progression. For this, we used antibodies of the cyclin B protein as a cell cycle marker. Cyclin B reaches peak levels in G2-phase, is degraded during the metaphase-to-anaphase transition in mitosis and re-accumulates gradually during the next S-phase (Lehner & O'Farrell, 1990). Homozygous HisC mutant embryos lack zygotic histone synthesis, but as they derive from heterozygous females they receive maternal histone proteins and mRNAs (Lanzotti et al, 2002). These embryos complete the first 14 cell cycles of embryogenesis with no scorable defects and undergo mitosis 14 (M14) similarly to the wild type (Fig 1B,E). Thus, the maternal store of histone mRNA and histone protein is sufficient to drive the early embryonic cell cycles and development up to the blastoderm stage. Histone mRNA is unstable outside the S-phase of the cell cycle (Marzluff et al, 2008). Therefore, the maternal store of histone mRNA is degraded completely during the G2-phase of cell cycle 14 (G214) in both wild-type embryos and the HisC mutants (Lanzotti et al, 2002). Hence, newly synthesized histone mRNA was observed in S-phase 15 (S15) of wild-type cells (Fig 1B–D), whereas no histone mRNA was present in the corresponding HisC mutant cells (Fig 1E–G; supplementary Fig S4 online).
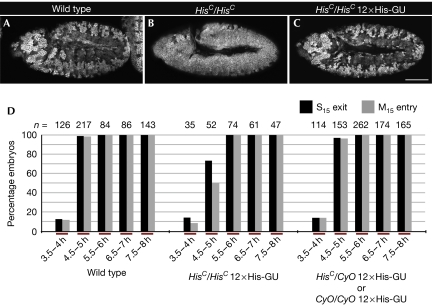
The pattern of epidermal cell divisions is stereotyped in wild-type fly embryos (supplementary Fig S5 online). Mitosis 15 (M15) first takes place in the dorsal epidermis about 4.5–5 h after egg laying, as marked by Cyclin B degradation in those cells (Fig 2A). By contrast, HisC mutants maintained uniformly high levels of Cyclin B in all parts of the epidermis, both at this stage (Fig 2B) and during the later stages of embryogenesis (supplementary Fig S6 online). These results indicate that loss of de novo synthesized histones abrogates the completion of cell cycle 15 in all epidermal cells. The HisC phenotype is fully penetrant and invariant. Thus, a possible rescue of the mutant phenotype into a wild-type pattern by transgene-derived histone synthesis can be reliably scored. In fact, the presence of 12 transgenic His-GUs in the genome of the HisC mutants causes a reversion of the mutant M15 pattern to that of wild-type embryos (Fig 2C; supplementary Fig S7 online). Quantitative estimates on rescued HisC mutant embryos confirmed that not only the pattern, but also the timing of M15 was fully restored (Fig 2D). This result shows that multiple His-GUs are required to ensure normal development of a multicellular organism, such as Drosophila, when expressed under the control of endogenous regulatory elements. This finding is consistent with the phenomenon that all multicellular organisms contain multiple histone gene units, ranging from about 10 up to several hundred copies per genome (Marzluff et al, 2008).
Figure 2.
Rescue of HisC by 12 His-GUs. (A–C) Representative embryos from time-matched collections 4.5–5 h after egg laying stained for Cyclin B. Wild-type embryos undergo M15 in the dorsal epidermis (A), whereas HisC mutant embryos are blocked with high levels of Cyclin B before M15 (B). In the presence of 12 transgene-based His-GUs, HisC mutant embryos display a wild-type M15 pattern (C); wild type refers to w− control. (D) The percentage of embryos that completed S15 (S15 exit) or progressed into M15 (M15 entry) in the dorsal epidermis was determined. Embryonic collections were time matched for the indicated time interval after egg laying. The left set of columns are wild type. The middle display HisC homozygotes rescued by 12 His-GUs and the right display embryos from the same collection, which were not homozygous HisC mutant. These embryos acted as internal controls to ensure reproducible timing of the collections. For details on classification of embryos see supplementary Fig S5 online. Scale bar, 100 μm (A–C); anterior left (A–C), His-GU; histone gene repeat unit; M15, mitosis 15; n, number of embryos.
Introduction of modified histone transgenes
Previous studies, which targeted histone synthesis in higher organisms by reducing histone translation and transcript stability, have yielded pleiotropic effects (Lanzotti et al, 2002; Pettitt et al, 2002; Zhao et al, 2004). In view of the clearly defined phenotype of the HisC mutation obtained here, these earlier observations might be due to incomplete deprivation of the newly synthesized histones or off-target effects, highlighting the value of the newly established mutation. In fact, the complete lack of de novo histone synthesis, similar to that in HisC mutants, is unprecedented for eukaryotes and will enable not only direct and detailed studies of chromatin assembly, but also the assessment of the function of histones in cell cycle progression.
The functional replacement of endogenous histones by histone transgenes offers a unique opportunity to directly address the relevance of specific histone modification sites in a multicellular organism. Such an approach requires that transgenes carrying histones with altered modification sites do not interfere with the development or viability of the HisC/+ heterozygous individuals bearing the modified His-GU transgenes. As a proof of principle, we targeted the methyl-modified Lys 27 residue in histone H3 (H3 K27), a well-characterized epigenetic modification site involved in Polycomb-dependent gene regulation (Müller & Verrijzer, 2009). We replaced a lysine with an arginine (H3 K27R) in a single His-GU to prevent lysine methylation, and subsequently generated transgenes containing three repeats of H3 K27R. The resulting transgenes were integrated into the same landing sites on chromosome 3L and R, which were used for the wild-type transgenes. After recombination and the necessary crosses to obtain HisC/+ heterozygous transgene-bearing flies, we investigated whether H3 K27R expression has any effects that would preclude the functional analysis of this specific modification site. No such effects were observed, indicating that the expression of H3 K27R would interfere in a dominant manner with fly development, viability and fertility. We note, however, that this result does not exclude the possibility that other histone modification mutations would not cause dominant effects. Therefore, each histone modification mutation to be characterized needs to be tested as shown for H3 K27R here. In a similar approach with the unicellular organism yeast, Dai et al (2008) used constitutive expression of mutated histones to study the relevance of individual histone modifications. Of the 486 mutations targeting histones H3 and H4, none caused a dominant effect against a background of reduced endogenous histone expression. Thus, it seems unlikely that the experimental system introduced here will be limited by dominant effects from transgene-encoded mutated histones. The experimental system described in this study should extend beyond yeast studies and allow examination of the effects of designed histone mutants on the development and homeostasis of an entire multicellular organism. It will also benefit from the advanced genetic methodology and tools for stage and tissue assessment of histone functions and cell clone analysis by mitotic recombination that are available for the fly (Ashburner, 1998). Biological questions of high relevance, such as histone-dependent chromatin assembly, the effect of histone deprivation on transcriptional regulation, functional differences between canonical and replacement histones, as well as concepts including the ‘histone code' hypothesis, can now be examined and put to a critical test in vivo.
Methods
Plasmid construction. Plasmid construction is schematically included in supplementary Fig S1 online. Using the oligonucleotides 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTACGGTACCGGACAATTGACACTGTCCCTTCAAACGCCTG-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTAACCGGTCGTACGACCTCTTCAATAATAACACTTTCTTCAGTTAACACATG-3′ as primers for PCR, the His-GU was amplified from w1118 genomic DNA, recombined into pDONR221 (Invitrogen) leading to the ‘Gateway' entry vector pENTR221-HisGU and verified by DNA sequencing. In addition, we generated pENTRL4R1-504bpseq and pENTRR2L3-504bpseq entry vectors by recombining a 504-bp noncoding PCR product into pDONRP4P1R and pDONRP2RP3, respectively. The oligonucleotides used as PCR primers were 5′-GGGGACAACTTTGTATAGAAAAGTTGACGGTACCGCATAGCTAACGAATGTAACTG-3′ and 5′-GGGGACTGCTTTTTTGTACAAACTTGCACCGGTGCACACGAATAAGACGGTC-3′ for pDONRP4P1R; 5′-GGGGACAGCTTTCTTGTACAAAGTGGACGGTACCGCATAGCTAACGAATGTAACTG-3′ and 5′-GGGGACAACTTTGTATAATAAAGTTGAACCGGTGCACACGAATAAGACGGTC-3′ for pDONRP2RP3.
pENTRL4R1-HisGU and pENTRR2L3-HisGU were generated by replacing the noncoding sequences in pENTRL4R1-504bpseq and pENTRR2L3-504bpseq with the His-GU from pENTR221-HisGU through Acc65I and AgeI. pENTR221-HisGU.H3K27R was generated by replacing an NcoI/SacI fragment in pENTR221-HisGU with a synthetic fragment (Mr Gene, Regensburg, Germany) containing an AAG into CGC codon exchange leading to the H3 K27R mutation. The pENTRL4R1-HisGU.H3K27R and pENTRR2L3-HisGU.H3K27R entry vectors were generated analogously to the wild-type variants. The pDESTR3R4-φC31attB destination vector was created from pwP-Ex2UASTattB (Bischof et al, 2007) by replacing a BamHI fragment (UAS elements, basal promotor, multiple cloning site and SV40) with a BglII fragment containing the attR4-ccdB-CmR-attR3 cassette for ‘MultiSite Gateway' that was amplified from pDESTR4-R3 using the oligonucleotides 5′-GTCAGATCTACCTAGGAGATACCAGCGGATAACAATTTCAC-3′ and 5′-ACCAGATCTATCTAGAGGCAAGGCGATTAAGTTGGGTAAC-3′ as primers for PCR. Recombination of pENTR221-HisGU, pENTRL4R1-HisGU and pENTRR2L3-HisGU with pDESTR3R4-φC31attB resulted in the transgene integration construct pφC31attB3xHisGU. Analogously, pENTR221-HisGU, pENTRL4R1-504bpseq and pENTRR2L3-504bpseq led to pφC31attB1xHisGU and pENTR221-HisGU.H3K27R. pENTRL4R1-HisGU.H3K27R and pENTRR2L3-HisGU.H3K27R led to pφC31attB3xHisGU.H3K27R.
Fly strains. Df(2L)HisC was constructed from w1118; P{FRT, w+mc}5-HA-1581 (Szeged Stock Center) and w1118; P{FRT, w+mc}CB5033-3 (provided by G. Reuter) following a previously described scheme (Ryder et al, 2004). Df(2L)HisC was kept heterozygous over CyO, P{ftz/lacB}E3 to identify mutant embryos by lacking β-galactosidase expression, or CyO, P{ActGFP}JMR1 to identify mutant embryos lacking green fluorescent protein expression. Wild-type controls were either nonmutant sibling embryos (internal control) or w1118 embryos (w− control) as indicated in the respective figure legends. φC31-mediated transgenesis using landing sites M{3xP3-RFP.attP}ZH-86Fb or M{3xP3-RFP.attP}ZH-68E (Bischof et al, 2007) was purchased from BestGene, Chino Hills, CA. pφC31attB1xHisGU was used to obtain M{1xHisGU.wt}ZH-86Fb; pφC31attB3xHisGU for M{3xHisGU.wt}ZH-86Fb and M{3xHisGU.wt}ZH-68E; pφC31attB3xHisGU.H3K27R for M{3xHisGU.H3K27R}ZH-86Fb and M{3xHisGU.H3K27R}ZH-68E. Transgenes were recombined and crossed into the Df(2L)HisC mutant background.
Embryo collection, classification, fixation and mounting. Time-matched embryonic collections were obtained by restricting egg deposition to 30 min with subsequent ageing at 25°C. Embryos were dechorionated with 50% bleach, fixed in 1:1 heptane/4% paraformaldehyde, 50 mM ethylene glycol tetraacetic acid (pH 7.4) for 20 min and devitellinized in a 1:1 heptane/methanol followed by washes and storage in methanol. After staining, embryos were mounted in ProlongGold (Invitrogen). Staining was visualized with TCS-SP2 AOBS or TCS-SP5 AOBSconfocal laser-scanning microscopes (Leica).
Fluorescence in situ hybridizations. Dioxigenin-labelled anti-sense RNA probes for His1, His2A, His2B, His3 and His4 were prepared using standard methods and hybridized to embryos at 57°C overnight as described previously (Lécuyer et al, 2008). Probes were detected by sheep anti-digoxigenin-POD Fab fragments (1:300; Roche) and signal amplification with TSA Cyanine3 reagent (Perkin Elmer) for 40 min. Horseradish peroxidase was inactivated for 10 min at 70°C for subsequent stainings.
BrdU incorporation. The incorporation of bromodeoxyuridine (BrdU) was carried out as described previously (Lehner et al, 1991). Embryos were air-dried for 5 min, permeabilized in octane for 6 min and incubated in Schneider's medium (Gibco) with 1 mg/ml BrdU (Sigma) for 15 min at 25°C followed by fixation. The incorporation of BrdU was included in the ageing period for time-matched embryonic collections. Embryos were re-hydrated in phosphate-buffered saline (PBS) with 0.1% Tween-20 (PBS-T), treated with 2 N HCl for 40 min, washed twice with 0.1 M NaBrO3 for 2 min and with PBS-T for 15 min. BrdU was detected by a mouse BrdU antibody (1:80; Becton Dickinson).
Antibody staining. Embryos were re-hydrated in PBS with 0.1% Triton X-100 (PBTx), blocked for 30 min at room temperature and labelled with primary antibodies at 4°C overnight in PBTx and 10% goat serum (Sigma). Secondary antibody incubation was in PBTx, 10% goat serum for 2 h at 25°C. Washes after antibody incubations were 20 min in PBTx, performed three times. Primary antibodies were rabbit Cyclin B (Jacobs et al, 1998; 1:3,000) and chicken β-galactosidase (1:1,000; Abcam). Secondary antibodies were goat anti-mouse IgG Alexa Fluor488 (1:400; Invitrogen), goat anti-rabbit IgG Alexa Fluor633 (1:400; Invitrogen) and goat anti-chicken IgY Cy3 (1:400; Jackson ImmunoResearch). For fluorescence in situ hybridizations and BrdU incorporations, chicken β-galactosidase antibody was detected by donkey anti-chicken IgY Biotin-SP (1:500; Jackson ImmunoResearch) followed by VectastainABC (Vector Laboratories) incubation and TSA (Perkin Elmer) detection for 5 min.
Western blot analyses. Pools of six male flies, aged 7 days, were frozen in liquid nitrogen, disrupted with a pestle in 75 μl PBS with 0.1% Tween-20 (PBS-T) containing Complete Protease Inhibitor (Roche) and lysed by adding 25 μl of 4 × sodium dodecyl sulphate sample buffer, 5-min incubation at 95°C and sonication for 5 min at low power in a Bioruptor (Diagenode). Samples were centrifuged for 15 min, at 14,000 g at 25°C and the supernatant was incubated for 10 min at 95°C before loading onto 15% polyacrylamide Tris/Glycin gels. After blotting, nitrocellulose membranes were blocked with 1% bovine serum albumin and 3% dry milk powder in PBS-T at 4°C overnight. Incubation with primary or secondary antibodies was in blocking buffer for 2 h or 1 h, respectively, at 25°C. Washes after incubations were three changes 10 min each in PBS-T. Primary antibodies were rabbit histone H4 (1:1,000; Abcam), rabbit histone H2A (1:1,000; upstate), rabbit histone H3 (1:20,000; Abcam), mouse histone H2B (1:1,000, Abcam) and mouse α-tubulin (DM1A, 1:2,000; Sigma). Secondary antibodies were goat anti-rabbit IgG horseradish peroxidase conjugated and goat anti-mouse IgG horseradish peroxidase conjugated (Thermo Scientific). Signals were detected using the ECL Western Blotting Substrate Kit (Thermo Scientific). Membranes were re-used after stripping with the Restore Western Blot Stripping Buffer (Thermo Scientific).
Cuticle preparations. Mutant embryos homozygous for Df(2L)HisC and carrying two or six transgene-encoded His-GUs were identified by lacking green fluorescent protein expression, selected from 22- to 24-h embryo collections, dechorionated, fixed for 10 min in 1:1 heptane/methanol and mounted in 1:1 Hoyers medium/lactic acid.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank U. Schäfer for critical reading of the paper, G. Reuter for flies and C. F. Lehner for antibodies, comments and suggestions. U.G. was supported by a fellowship from the Boehringer Ingelheim foundation. The work was supported by funds of the Max-Planck-Gesellschaft.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ashburner M (1998) Drosophila: A Laboratory Handbook, 2nd edn, Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci USA 104: 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet A, Almouzni G (2009) Making copies of chromatin: the challenge of nucleosomal organization and epigenetic information. Trends Cell Biol 19: 29–41 [DOI] [PubMed] [Google Scholar]
- Dai J, Hyland EM, Yuan DS, Huang H, Bader JS, Boeke JD (2008) Probing nucleosome function: a highly versatile library of synthetic histone H3 and H4 mutants. Cell 134: 1066–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Ahmad K (2005) Assembly of variant histones into chromatin. Annu Rev Cell Dev Biol 21: 133–153 [DOI] [PubMed] [Google Scholar]
- Jacobs HW, Knoblich JA, Lehner CF (1998) Drosophila cyclin B3 is required for female fertility and is dispensable for mitosis like cyclin B. Genes Dev 12: 3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD (2001) Translating the histone code. Science 293: 1074–1080 [DOI] [PubMed] [Google Scholar]
- Kouzarides T (2007) Chromatin modifications and their function. Cell 128: 693–705 [DOI] [PubMed] [Google Scholar]
- Lanzotti DJ, Kaygun H, Yang X, Duronio RJ, Marzluff WF (2002) Developmental control of histone mRNA and dSLBP synthesis during Drosophila embryogenesis and the role of dSLBP in histone mRNA 3′ end processing in vivo. Mol Cell Biol 22: 2267–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lécuyer E, Parthasarathy N, Krause HM (2008) Fluorescent in situ hybridization protocols in Drosophila embryos and tissues. Methods Mol Biol 420: 289–302 [DOI] [PubMed] [Google Scholar]
- Lehner CF, O'Farrell PH (1990) The roles of Drosophila cyclins A and B in mitotic control. Cell 61: 535–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner CF, Yakubovich N, O'Farrell PH (1991) Exploring the role of Drosophila cyclin A in the regulation of S phase. Cold Spring Harb Symp Quant Biol 56: 465–475 [DOI] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ (1997) Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389: 251–260 [DOI] [PubMed] [Google Scholar]
- Marzluff WF, Wagner EJ, Duronio RJ (2008) Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat Rev Genet 9: 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Verrijzer P (2009) Biochemical mechanisms of gene regulation by polycomb group protein complexes. Curr Opin Genet Dev 19: 150–158 [DOI] [PubMed] [Google Scholar]
- Pettitt J, Crombie C, Schümperli D, Müller B (2002) The Caenorhabditis elegans histone hairpin-binding protein is required for core histone gene expression and is essential for embryonic and postembryonic cell division. J Cell Sci 115: 857–866 [DOI] [PubMed] [Google Scholar]
- Ryder E et al. (2004) The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167: 797–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, McKillop-Smith S, Müller B (2004) The human histone gene expression regulator HBP/SLBP is required for histone and DNA synthesis, cell cycle progression and cell proliferation in mitotic cells. J Cell Sci 117: 6043–6051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.