Abstract
Aims
Carotid–femoral pulse wave velocity (PWV), a direct measure of aortic stiffness, has become increasingly important for total cardiovascular (CV) risk estimation. Its application as a routine tool for clinical patient evaluation has been hampered by the absence of reference values. The aim of the present study is to establish reference and normal values for PWV based on a large European population.
Methods and results
We gathered data from 16 867 subjects and patients from 13 different centres across eight European countries, in which PWV and basic clinical parameters were measured. Of these, 11 092 individuals were free from overt CV disease, non-diabetic and untreated by either anti-hypertensive or lipid-lowering drugs and constituted the reference value population, of which the subset with optimal/normal blood pressures (BPs) (n = 1455) is the normal value population. Prior to data pooling, PWV values were converted to a common standard using established conversion formulae. Subjects were categorized by age decade and further subdivided according to BP categories. Pulse wave velocity increased with age and BP category; the increase with age being more pronounced for higher BP categories and the increase with BP being more important for older subjects. The distribution of PWV with age and BP category is described and reference values for PWV are established. Normal values are proposed based on the PWV values observed in the non-hypertensive subpopulation who had no additional CV risk factors.
Conclusion
The present study is the first to establish reference and normal values for PWV, combining a sizeable European population after standardizing results for different methods of PWV measurement.
Keywords: Adult, Aged, Arteries, Arteriosclerosis, Blood pressure, Humans, Pulse, Pulse wave velocity, Stiffness
See page 2320 for the editorial comment on this article (doi:10.1093/eurheartj/ehq211)
Introduction
Among markers of arterial disease, arterial stiffness has proven to be an important parameter for the assessment of cardiovascular (CV) risk. From the different methods to assess arterial stiffness,1 carotid to femoral pulse wave velocity (PWV) has emerged as the gold standard method because of its relative ease in determination, its perceived reliability,2,3 and most importantly because of the large body of evidence demonstrating its association with incident CV disease (CVD), independently of traditional risk factors and in various populations.2,4–10 Increasingly, arterial stiffness measures, and PWV in particular, are included both in the routine clinical assessment of patients and within the framework of large-scale clinical studies, as illustrated by their inclusion in the 2007 ESH/ESC guidelines for the management of hypertension.3
In spite of its emergence as the gold standard method for the assessment of central arterial stiffness, a wider implementation of PWV into clinical practice is hampered by the lack of established reference values based on a large population and the absence of a standardization of methodology for PWV assessment. The fixed threshold value (12 m/s) proposed in the 2007 ESH/ESC hypertension guidelines3 was based on published epidemiological studies but could not take into account the multiple factors influencing PWV. Two groups have recently published normative data, but one related only to a specific elderly population11 and the other concerned only one methodology.12 It has been proven that important differences in absolute PWV values exist between methodologies13–15 and/or between populations.16 On the other hand, many risk factors have been shown to influence PWV in small-scale studies and may be confounded by differences in age and blood pressure (BP) level.17 Thus, establishment of reference value for PWV must standardize methodology and must be based on the wider possible population, taking into account the influence of major CV risk factors on PWV. Another problem with PWV is its strong dependence on age and BP.12,17 It is unclear now whether reference values must be (or not) determined as a function of age and BP, but it is important to take them into account. For example, subjects might be classified at higher risk than others in a certain age group, even when PWV does not reach the 12 m/s threshold. Similarly, knowing how PWV distributes along classes of BP would help to identify individuals with excessively high PWV or preserved PWV, which may be of importance for modalities of treatment.18 Establishing normality is not equivalent to providing the range of values observed in a general population, this is why we distinguish between normal values, in a population with no CV risk factor (apart from age and sex), and reference values, which are closer to a population-based distribution of PWV.
With financial support from the French Ministry of Research (Agence Nationale pour la Recherche) endorsed by the European Network for Non-Invasive Investigation of Large Arteries, the Reference Values for Arterial Stiffness' Collaboration aims at building a large database combining existing clinical and arterial stiffness data from participating centres across Europe.19 The objectives of the present study were to (i) establish ‘normal’ values of carotid-to-femoral PWV in a population with no CV risk factor and (ii) to propose ‘reference’ value in a population with various risk factors, according to age and BP categories. For this purpose, it was necessary to first identify the main determinants of PWV and to standardize the expression of PWV.
Methods
Study population
The population retained for the present analysis is a subgroup of the ‘Reference Values for Arterial Stiffness' Collaboration database’, described previously.19 Briefly, this database contains patients and subjects having had measurements of arterial stiffness (PWV or local stiffness measures obtained from ultrasound echotracking) and/or measurements of central pressure, together with a full medical history on record. These were provided by 13 centres distributed across eight European countries (see Appendix for the list of contributing centres). Inclusion criteria also included the availability of a full set of documentation regarding the protocol and measurement techniques used for the assessment of stiffness parameters.
Subjects were excluded from the present analysis if PWV measurement was unavailable, if they had an identified genetic cause of hypertension or secondary hypertension, or had overt CVD. Diabetic patients (either treated or untreated) and patients treated for hypertension or dyslipidaemia were also excluded.
Subject data included vital parameters, BPs, and the recording of any relevant CV risk factor, CVD, or treatment at the time of measurement. Ethnicity was not reported in all data sets; however, subjects other than Caucasians were a small minority. Subjects were further categorized (Figure 1) according to the presence of additional CV risk factors (gender, dyslipidaemia, or current smoking). Dyslipidaemia was defined as total cholesterol >5.0 mmol/L, HDL cholesterol <1.0 mmol/L for men and <1.2 mmol/L for women, LDL cholesterol >3.0 mmol/L, or triglycerides >1.7 mmol/L. Blood pressure was measured according to the procedures of each participating centre. The values of BP are those obtained during the measurement of PWV. Methods for BP measurement may vary with time and within centres. Automatic oscillometric devices were used in more than 80% of subjects. Mean BP (MBP) was calculated from systolic BP (SBP) and diastolic BP (DBP) as MBP = DBP + 0.4(SBP − DBP). Threshold values for CV risk factors were chosen according to the 2007 ESC/ESH hypertension guidelines.3
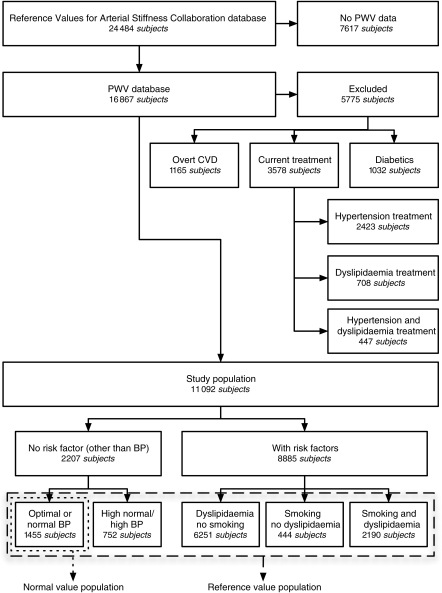
Figure 1.
Flowchart describing the selection and categorization of subjects from the reference value database for the present analysis. PWV, pulse wave velocity; CVD, cardiovascular disease; BP, blood pressure.
Study populations
A ‘normal’ population was defined as subjects having optimal or normal BP values and no additional CV risk factors. We further studied the influence of CV risk factors on PWV. On the basis of this analysis, we defined a ‘reference’ value population as subjects or patients of both sexes, presenting CV risk factors which had been shown to have no independent influence on PWV values.
For normal and reference values, the population was categorized according to the age decade (<30, 30–39, 40–49, 50–59, 60–69, and ≥70 years) and BP category3 (reference population only: optimal, i.e. <120/80; normal, i.e. ≥120/80 and <130/85; high normal, i.e. ≥130/85 and <140/90; Grade I hypertension, i.e. ≥140/90 and <160/100; and Grade II/III hypertension, i.e. ≥160/100 mmHg).
Pulse wave velocity measurement: methodological considerations
Pulse wave velocity values depend on both the algorithm used for detecting the so-called ‘foot of the wave’ at the measurement sites and path length measurement. An overview of the different techniques was recently published,20 and the list of techniques and devices applied in the different centres is provided in Appendix. We had to standardize the calculation of PWV.
Transit times are assessed as the time difference between two characteristic points on carotid and femoral waveforms. The characteristic points chosen are dependent on the type of waveform (flow, pressure, or diameter distension) and the algorithm used for its detection. The two most popular algorithms are (i) the intersecting tangent algorithm (Sphygmocor® system and for manual identification) and (ii) the point of maximal upstroke during systole (as used in the Complior® system). Different algorithms applied on the same waveforms can lead to differences in measured PWV values of 5–15%.13 Since the point of maximal upstroke has been shown to underestimate PWV, especially when the rise time of the waveform is low,13 we chose to standardize transit time on the intersecting tangent algorithm. To convert maximal upstroke transit times into the intersecting tangent algorithm, we used the relationship previously found by Millasseau et al.:13
| (1) |
Pulse wave velocity values are also markedly dependent on the carotid–femoral pathway measurement. This pathway can either be the direct distance measured between the carotid and femoral measurement sites, or the distance obtained by subtracting the carotid measurement site to sternal notch distance from the sternal notch to femoral measurement site distance. Differences in path length alone can lead to differences in PWV values of up to 30%.21,22 Equations to convert between these path length definitions with good precision were recently published:23
| (2) |
| (3) |
Because participating centres used different methods to measure PWV (see Table A1), path length values had to be standardized. The bulk of the data in the reference value database consists of PWV calculated using the direct path length. Subtracted path lengths were therefore standardized into direct path lengths using Eq. (2). However, as the use of direct distance (i.e. measured over the body surface) leads to overestimation of real PWV [using magnetic resonance imaging (MRI) or invasive measurements], we used a scaling factor of 0.8 derived from Sugawara et al.24 and Weber et al.15 to convert PWV obtained using direct distances to ‘real’ PWV.
| (4) |
In what follows, PWV is calculated using the intersecting tangent algorithm and the direct carotid to femoral path length, and then rescaled to real PWV using Eq. (4). Tables using PWV values based on the intersecting tangent algorithm and direct or subtracted distances are supplied as Supplementary material online.
Statistical analyses
All statistical analyses were performed using R version 2.9.1.25 Values reported are mean (standard deviation). The threshold for statistical significance was chosen to be P = 0.05 unless stated otherwise. The influence of CV risk factors and gender on PWV was examined by performing analysis of covariance, before and after adjustment for age and MBP. Pulse wave velocity values per category are represented as mean (standard deviation) and median (10th to 90th percentile). Correlations were assessed using regression analyses.
Results
Study population
A flowchart describing how subjects were selected from the complete reference value database and then further subdivided according to the presence of CV risk factors is presented in Figure 1. The complete reference value database contains data from 24 484 subjects and patients. The number of subjects included per centre is listed in Appendix. Pulse wave velocity and relevant clinical data were present in 16 867 subjects. Of those, 1165 subjects were excluded due to the presence of overt CVD. An additional 3578 subjects were excluded because of treatment for hypertension or dyslipidaemia and finally 1032 subjects were excluded for diabetes (treated or not), resulting in a study population of 11 092 subjects. This population represents the ‘reference value population’. Demographic parameters and clinical data of the ‘reference value’ and ‘normal value’ population are summarized in Table 1. The number of subjects included in each category of age and BP is listed in Table 2.
Table 1.
Description of general clinical parameters of the reference value and normal value populations
| Parameter | PWV database (16 867 subjects) | Reference value population (11 092 subjects) | Normal value population (1455 subjects) |
|---|---|---|---|
| Age (years) | 55 (17) | 50 (17) | 33 (16) |
| Age range (years) | (15–97) | (15–97) | (15–85) |
| Gender (M/F) | 8753/8114 | 5520/5572 | 600/855 |
| Weight (kg) | 74.4 (14.8) | 73.0 (14.3) | 66.8 (11.9) |
| Height (m) | 1.69 (0.09) | 1.70 (0.09) | 1.71 (0.09) |
| SBP (mmHg) | 135 (21) | 130 (19) | 114 (9) |
| DBP (mmHg) | 79 (12) | 78 (12) | 69 (7) |
| MBP (mmHg) | 102 (14) | 99 (13) | 87 (7) |
| PP (mmHg) | 56 (16) | 52 (14) | 45 (8) |
| Total cholesterol (mmol/L) | 5.5 (1.2) | 5.5 (1.2) | 4.1 (0.6) |
| HDL cholesterol (mmol/L) | 1.5 (0.4) | 1.5 (0.4) | 1.6 (0.3) |
| LDL cholesterol (mmol/L) | 3.3 (1.1) | 3.3 (1.1) | 2.2 (0.5) |
| Triglycerides (mmol/L) | 1.3 (0.9) | 1.2 (0.8) | 0.8 (0.3) |
| Glycaemia (mmol/L) | 5.4 (1.3) | 5.0 (0.6) | 4.8 (0.6) |
| Smoking [# (%)] | 3761 (22) | 2634 (24) | — |
| Dyslipidaemia [# (%)] | 13 284 (79) | 8441 (76) | — |
| Hypertension [# (%)] | 6,818 (40) | 3431 (31) | — |
| Diabetes [# (%)] | 1223 (7) | — | — |
| Treated hypertension [# (%)] | 4149 (25) | — | — |
| Treated lipids [# (%)] | 1879 (11) | — | — |
| Treated diabetes [# (%)] | 616 (4) | — | — |
SBP, DPB, PP, and MBP signify systolic, diastolic, pulse, and mean blood pressure, respectively. PWV, pulse wave velocity; HDL and LDL, mean high- and low-density lipoproteins, respectively.
Table 2.
Number of subjects included in each of the age and blood pressure categories in the reference values population (11 092 subjects)
| Age category (years) | Optimal | Normal | Blood pressure group |
||
|---|---|---|---|---|---|
| High normal | Grade I HT | Grade II/III HT | |||
| <30 | 896 | 417 | 220 | 96 | 40 |
| 30–39 | 315 | 245 | 184 | 189 | 109 |
| 40–49 | 822 | 562 | 385 | 325 | 167 |
| 50–59 | 514 | 519 | 434 | 490 | 238 |
| 60–69 | 414 | 509 | 485 | 648 | 289 |
| ≥70 | 163 | 244 | 333 | 535 | 305 |
HT, hypertension.
Cardiovascular risk factors and observed pulse wave velocity
Table 3 summarizes the effects of different CV risk factors on PWV values. Although PWV was markedly higher in males, dyslipidaemics, and smokers, the presence of these CV risk factors was also accompanied by marked differences in age and BP. After correction for quadratic age (a + b × age + c × age2) and MBP, there was no significant influence of smoking status or dyslipidaemia and negligible influence of gender on PWV (<0.1 m/s difference, P = 0.04). Although PWV was significantly dependent on heart rate, further adjustment on sex, quadratic age, and MBP reduced its residual influence on PWV which was very small (standardized coefficient for heart rate is 1/4th the one for MBP and 1/10th the one for age).
Table 3.
Influence of gender and major cardiovascular risk factors on pulse wave velocity, before and after adjustment on quadratic age and mean blood pressure
| Cardiovascular risk factor | # subjects | Mean age (years) | MBP (mmHg) | Pulse wave velocity before adjustment |
Pulse wave velocity after adjustment |
||
|---|---|---|---|---|---|---|---|
| Mean (m/s) | P-value | Mean (m/s) | P-value | ||||
| Gender | |||||||
| F | 1127 | 37 | 91 | 7.4 | <0.001 | 7.7 | 0.04 |
| M | 1080 | 39 | 97 | 8.2 | 7.8 | ||
| Dyslipidaemia | |||||||
| No | 2207 | 38 | 94 | 7.8 | <0.001 | 8.9 | 0.68 |
| Yes | 6251 | 54 | 101 | 9.3 | 8.9 | ||
| Current smoker | |||||||
| No | 2207 | 38 | 94 | 7.8 | 0.04 | 7.8 | 0.84 |
| Yes | 444 | 43 | 96 | 8.0 | 7.8 | ||
MBP, mean blood pressure.
Diabetic subjects and subjects treated for hypertension and dyslipidaemia had significantly elevated PWV values, compared with untreated patients, even after correction for age and MBP (data not shown). This is why we decided not to include these patients. Since gender, smoking, and lipid status have no independent influence on PWV after correction for quadratic age and BP differences, we defined the reference value population as including all untreated (i.e. with no anti-hypertensive or lipid-lowering agents) non-diabetic subjects.
Normal and reference values for pulse wave velocity
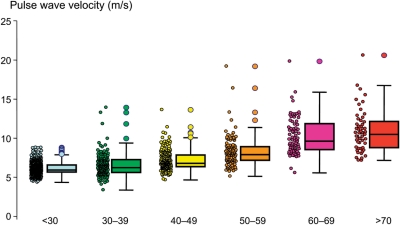
Normal values are presented in Table 4 and Figure 2. Subjects with optimal or normal BP and no additional CV risk factors had the lowest values of PWV and showed the smallest increase in PWV with age. It can be noted from Figure 3 and Table 5 that subjects with normal BP values had already elevated PWV values, compared with subjects with optimal BP, together with an increased age/PWV coefficient. The scattering of data is very small for the first age categories and increases with age. Outliers were more frequent at older ages (Figure 2).
Table 4.
Distribution of pulse wave velocity (m/s) according to the age category in the normal values population (1455 subjects)
| Age category (years) | Mean (±2 SD) | Median (10–90 pc) |
|---|---|---|
| <30 | 6.2 (4.7–7.6) | 6.1 (5.3–7.1) |
| 30–39 | 6.5 (3.8–9.2) | 6.4 (5.2–8.0) |
| 40–49 | 7.2 (4.6–9.8) | 6.9 (5.9–8.6) |
| 50–59 | 8.3 (4.5–12.1) | 8.1 (6.3–10.0) |
| 60–69 | 10.3 (5.5–15.0) | 9.7 (7.9–13.1) |
| ≥70 | 10.9 (5.5–16.3) | 10.6 (8.0–14.6) |
SD, standard deviation; 10 pc, the upper limit of the 10th percentile; 90 pc, the lower limit of the 90th percentile.
Figure 2.
Normal values for pulse wave velocity: average according to age (1455 subjects). Boxes contain 50% of the data and bars contain the remainder; horizontal lines indicate medians and the circle indicates outliers.
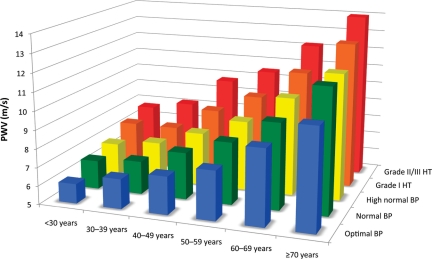
Figure 3.
Reference values for pulse wave velocity (PWV): mean values according to age and blood pressure (BP) categories (11 092 subjects). HT, hypertension.
Table 5.
Distribution of pulse wave velocity (PWV) values (m/s) in the reference value population (11 092 subjects) according to age and blood pressure category
| Age category (years) | Blood pressure category |
||||
|---|---|---|---|---|---|
| Optimal | Normal | High normal | Grade I HT | Grade II/III HT | |
| PWV as mean (±2 SD) | |||||
| <30 | 6.1 (4.6–7.5) | 6.6 (4.9–8.2) | 6.8 (5.1–8.5) | 7.4 (4.6–10.1) | 7.7 (4.4–11.0) |
| 30–39 | 6.6 (4.4–8.9) | 6.8 (4.2–9.4) | 7.1 (4.5–9.7) | 7.3 (4.0–10.7) | 8.2 (3.3–13.0) |
| 40–49 | 7.0 (4.5–9.6) | 7.5 (5.1–10.0) | 7.9 (5.2–10.7) | 8.6 (5.1–12.0) | 9.8 (3.8–15.7) |
| 50–59 | 7.6 (4.8–10.5) | 8.4 (5.1–11.7) | 8.8 (4.8–12.8) | 9.6 (4.9–14.3) | 10.5 (4.1–16.8) |
| 60–69 | 9.1 (5.2–12.9) | 9.7 (5.7–13.6) | 10.3 (5.5–15.1) | 11.1 (6.1–16.2) | 12.2 (5.7–18.6) |
| ≥70 | 10.4 (5.2–15.6) | 11.7 (6.0–17.5) | 11.8 (5.7–17.9) | 12.9 (6.9–18.9) | 14.0 (7.4–20.6) |
| PWV as median (10–90 pc) | |||||
| <30 | 6.0 (5.2–7.0) | 6.4 (5.7–7.5) | 6.7 (5.8–7.9) | 7.2 (5.7–9.3) | 7.6 (5.9–9.9) |
| 30–39 | 6.5 (5.4–7.9) | 6.7 (5.3–8.2) | 7.0 (5.5–8.8) | 7.2 (5.5–9.3) | 7.6 (5.8–11.2) |
| 40–49 | 6.8 (5.8–8.5) | 7.4 (6.2–9.0) | 7.7 (6.5–9.5) | 8.1 (6.8–10.8) | 9.2 (7.1–13.2) |
| 50–59 | 7.5 (6.2–9.2) | 8.1 (6.7–10.4) | 8.4 (7.0–11.3) | 9.2 (7.2–12.5) | 9.7 (7.4–14.9) |
| 60–69 | 8.7 (7.0–11.4) | 9.3 (7.6–12.2) | 9.8 (7.9–13.2) | 10.7 (8.4–14.1) | 12.0 (8.5–16.5) |
| ≥70 | 10.1 (7.6–13.8) | 11.1 (8.6–15.5) | 11.2 (8.6–15.8) | 12.7 (9.3–16.7) | 13.5 (10.3–18.2) |
SD, standard deviation, 10 pc, the upper limit of the 10th percentile, 90 pc, the lower limit of the 90th percentile; HT, hypertension.
Reference values for PWV are illustrated in Figure 3, showing a graphical representation of PWV values according to age and BP categories. Pulse wave velocity values and distributions in each of the categories are described in Table 5.
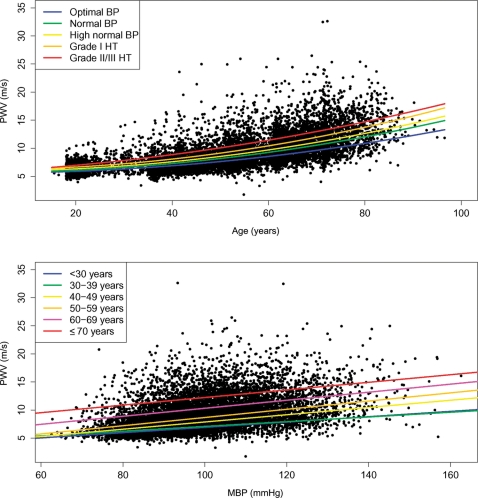
Pulse wave velocity increased both with increasing age and BP levels in the reference value population. Within all BP categories, quadratic regression offered the best fit to the observed data. Regression equations within each of the BP categories are summarized in Table 6. The increase in PWV with age was more pronounced when the levels of BP increased (Figure 4, top), the fit with a full quadratic equation (a + b × age + c × age2) providing better values of R2 than linear fit by 4–6%. The enhanced influence of ageing with high BP was gradual: the coefficient c for square-age increases by 1.5-fold (the linear coefficient being negligible), and the intercept increased by 0.6 m/s between optimal BP and Grade I hypertension. For Grade II/III hypertension, the linear term became obvious and accelerated effect of age was visible in Figure 4, top. Likewise, the increase in PWV with MBP was more pronounced as the subjects were older (Figure 4, bottom). Here, the best fit between MBP and PWV was linear (Table 6). The slope of the PWV/MBP relationship increased gradually with increasing categories of age, up to 1.5-fold higher in older subjects than in younger subjects. All correlations (PWV and age, PWV and MBP) were highly significant (P < 0.001). Models including all interaction terms show that MBP is only significantly associated with PWV through its interaction with age and square-age in the reference population, whereas it has a small, but significant independent association with PWV in the normal population (see Supplementary material online).
Table 6.
Regression equations for pulse wave velocity vs. age (top) and mean blood pressure (bottom) according to blood pressure category (top) and age category (bottom) in the reference value population (11 092 subjects)
| PWV ∼ age + age2 | R2 | |
|---|---|---|
| BP category | ||
| Optimal | PWV = 0.000 × age + 0.83 × 10−3 × age2 + 5.55 | 0.46 |
| Normal | PWV = 0.000 × age + 0.99 × 10−3 × age2 + 5.69 | 0.47 |
| High normal | PWV = 0.000 × age + 1.05 × 10−3 × age2 + 5.91 | 0.41 |
| Grade I HT | PWV = 0.000 × age + 1.18 × 10−3 × age2 + 6.17 | 0.39 |
| Grade II/III HT | PWV = 0.044 × age + 0.85 × 10−3 × age2 + 5.73 | 0.31 |
| PWV ∼ MBP | R2 | |
| Age category (years) | ||
| <30 | PWV = 0.0472 × MBP + 2.20 | 0.26 |
| 30–39 | PWV = 0.0423 × MBP + 2.20 | 0.13 |
| 40–49 | PWV = 0.0646 × MBP + 1.41 | 0.23 |
| 50–59 | PWV = 0.0731 × MBP + 1.35 | 0.17 |
| 60–69 | PWV = 0.0715 × MBP + 3.16 | 0.13 |
| ≥70 | PWV = 0.0676 × MBP + 5.46 | 0.07 |
Linear coefficient for age marked 0.00 means <10e–10. All R2 are highly significant (P < 0.001).
Figure 4.
(Top) Pulse wave velocity (PWV) vs. age in the reference value population (11 092 subjects). Regression lines denote the results of regression on age2 for different blood pressure (BP) categories. (Bottom) Pulse wave velocity vs. mean blood pressure (MBP) in the reference value population (11 092 subjects). Regression lines denote the results of linear regression on mean blood pressure for different age categories. HT, hypertension; BP, blood pressure.
Discussion
The main result of this study is the establishment of normal and reference values for PWV based on an extensive data set obtained from 13 centres distributed across Europe. This is the first study taking into account different methodological approaches for the determination of PWV by applying previously established conversion equations for path lengths and transit times. Reference values are presented per age decade and BP category. These normal and reference values represent a critical step in the implementation of PWV as a clinical tool for detecting subclinical organ damage in routine patient workup.
Despite the independent value of PWV as a measure of aortic stiffness and, vitally, of prognosis, its use has been limited without reliable reference values. Standardization is mandatory for generalizing any diagnostic test, be it biological26 or morphological.27 In the case of PWV, most of the necessity for standardization is purely methodological. Pulse wave velocity values obtained using different definitions, while yielding the same type of information for a group of patients, are not readily comparable between groups. This has certainly hampered establishing reference values, as this typically requires the pooling of data across different centres in order to acquire a sufficiently large set of data. Efforts at establishing reference values have previously been made, but are usually limited by the size and specificity of the population,11 yielding results that are only valid in these specific populations. More importantly, values previously reported have always been limited to a single PWV methodology,12 restricting their applicability to specific measurement protocols or devices.
The Reference Values for Arterial Stiffness' Collaboration's database contains data obtained with a range of techniques representative of the currently used systems. In the present paper, we used previously published conversion equations for distance and transit time measurements to display the data in a uniform fashion. A major aim here was to unify the methodology and propose an appropriate way to express PWV. For transit times, we chose detection of the foot of the waveform using an algorithm based on intersecting tangents. This choice was motivated by several reasons. Algorithms for transit time determination have previously been compared,13 and although all algorithms provide proportional results, the intersecting tangent algorithm was found to be the most reliable. For distance measurements, the ideal choice is less clear-cut. Physiologically, the subtracted distance more closely resembles invasive values,14,15,24,28,29 as it accounts for the time a wave takes to travel over arterial lengths from the heart to the carotid measurement site. Despite this, the subtracted distance still differs from the MRI gold standard.15,24 However, the subtracted distance requires two distance measurements, whereas only one measure is enough for direct measurement. Combining two measurements adds more complexity, with extra inaccuracies due to the imprecision of measurement (typically by tape measure or baby rod).23 The bulk of epidemiological PWV data available has used direct distance for PWV calculation. These arguments have recently been discussed in more depth.21 For these reasons, we chose to express PWV values here using direct distance and intersecting tangent, and further calibrated the values to direct ‘MRI’ values by using a scaling factor of 0.8, as established previously24 [Eq. (4)].
The PWV data in the reference value database can be represented in different ways. For practical use, the presentation chosen needs to take into account the pathophysiological features affecting PWV. Arterial stiffness increases with age and BP; as these are the major determinants of PWV, its values are presented by age and BP categories, providing a large number of subjects within each category allowing reliable estimates.
Pulse wave velocity at any age is linearly related to BPs and symetrically at any BP level is dependent on the quadratic age. The slope of the relation between age (and MBP) and PWV increases by 1.5-fold between the younger/lower BP and the elderly/higher BP. Although this relationship has been known for nearly a century,30 our results show that the increase in PWV with BP is not simply attributable to the increase in BP with age, confirming previous observations.12 The effect of age is emphasized in the presence of high BP. It is noteworthy that the inter-individual difference in PWV for a given age and BP value is much larger than the inter-class difference. This proves that PWV measurement includes additional information than the one provided by age or CV risk factors, as recently emphasized.17
Other classical risk factors potentially influencing PWV reference values were then evaluated in subsets of the whole population before and after adjustment on age and MBP. A comparison of the raw and adjusted mean PWV in Table 3 shows that apparent differences between men and women, smokers and non-smokers, and dyslipidaemic and normolipaemic subjects were virtually entirely accounted for by different age and BP levels. After correction for quadratic age and BP, smoking and lipid status did not significantly influence PWV, and gender differences were negligible (though still significant because of the size of the sample). The difference is <0.1 m/s, which is small regarding the precision of the technique. Anti-hypertensive and lipid-lowering treatments and diabetic status did however influence PWV beyond effects of age and BP, thus these patients were excluded from the final analysis.
It is important to discriminate between ‘normal’ and ‘reference’ values. The former supposedly supplies a normal (physiological) range. The latter expresses the range in a population selected to have no overt CVD. However, these two sets of values (normal and reference) are not super-imposable. Here, normal values were established in subjects with optimal or normal BP and no additional identified CV risk factors. The values of PWV were lower in this group, by comparison with any other, and the dependence on age was the weakest. The scattering of data increased with age, outliers with elevated PWV being more numerous at older age. The overlap of PWV values between younger and older people was large, perhaps because many CV risk factors were not quantifiable in the database (among them, psychological stress, family history of CVD, etc.). In the absence of any CV risk factors, the increase in PWV with age might even be smaller.
The role of age in presenting normal and reference values needs careful consideration. As for BP,3 it is not immediately clear whether normality should be defined according to age. For BP, it has proved to be wrong to accept that it should increase physiologically with age31,32 and fixed thresholds, regardless of age, have since been established. Once such thresholds were used, however, it became apparent that a large fraction of the elderly population had isolated systolic hypertension.26 Similarly, an important consequence of using a fixed threshold of PWV is that even if one ‘corrected’ for normal ageing, a majority of elderly or hypertensive subjects would have elevated PWV values. Examining Figure 3 (Table 5), a 9.6 m/s threshold value for PWV (12 m/s with directly measured distance), according to the ESC/ESH guidelines, would mean that on average, over half the population over 60 years old are at risk. This is similar to what is observed with the prevalence of systolic hypertension in the elderly. Blood pressure-lowering drugs improve outcome in hypertensive patients and some of these drugs also lower PWV. However, although the proposed fixed cut-off value of 9.6 m/s (12 m/s with directly measured distance) is supported by outcome data, evidence for successful intervention on PWV is not yet available so that its surrogate value has not yet been established. How the reference value data should be used to select and stratify patients (age/MBP adjusted or not, value of centile, etc.) needs to be addressed in future works.
On the basis of the distribution of PWV within each age and BP category, it is now possible to identify those people at higher risk than others in a certain age group and represent at which percentile of reference (or normal) population an individual subject stands.
Limitations
This study is cross-sectional. Although it provides accurate PWV values in a large aggregate European population, it provides no information about the evolution of PWV over time and we could not put in an evidence-tracking phenomenon. Secondly, at present, the reference value database does not contain outcome data, although they are available from many component studies. Whether the reference values should be used as cut-off values for treatment remains to be discussed. Thirdly, differences in techniques were encountered and compensated for. Even after full adjustment, differences between algorithm and path length were blunted, but not totally abolished. Because of the strong ‘centre/technique’ interaction, we could not solve the question whether this residual difference resulted from a centre effect or a suboptimal standardization. In the same line, it remains differences in PWV between centres even after full compensation and adjustment (mean difference 0 ± 0.59 m/s, range −0.9 to 0.8 m/s) which could not be compensated for. Whether these difference reflect geographical differences or unattended methodological issues remains to be determined. Together with the large time span of recruitment, this could increase the scattering of the reference values provided, but on the other hand, it also improves the external validity of our results.
In conclusion, the present study provides reference values for PWV based on a large European population, using standardized methodology. The data presented here allow identification of people in whom PWV is abnormal and who might warrant more intensive follow-up.
Supplementary material online
Supplementary material is available at European Heart Journal online.
Funding
This work was funded by the ANR program (ANR-05-PCOD-004-01) of the French Ministry of Research. Funding to pay the Open Access publication charges was provided by INSERM U970.
Funding of the included databases
| Centre | Origin of funding |
|---|---|
| Rotterdam (The Netherlands) | — |
| Cambridge/Cardiff (UK) | British Heart Foundation, National Institute for Health Research |
| HEGP, Paris (France) | Agence nationale de la recherche (ANR), INSERM, Fondation de Recherche en Hypertension |
| Copenhagen (Denmark) | Danish Heart Foundation (grant 01-2-9-9A-22914), the Lundbeck Fonden (grant R32-A2740). |
| Ghent (Belgium) | The Asklepios study was funded by FWO research grant G042703 |
| Athens (Greece) | — |
| Nancy (France) | Agence nationale de la recherche (ANR), INSERM, Fondation de Recherche en Hypertension |
| Amsterdam/Maastricht (The Netherlands) | Hoorn study: The Netherlands Heart Foundation (Grant no. 98154) Amsterdam Growth and Health Longitudinal Study (AGAHLS): Dutch Prevention Fund (ZON), and The Netherlands Heart Foundation (post-doc grant no. 2006T050—Dr I. Ferreira) |
| Paris XIII (France) | INSERM |
| Fleury-Mérogis (France) | Agence nationale de la recherche (ANR), INSERM, Fondation de Recherche en Hypertension |
| Manchester (UK) | British Heart Foundation |
| Brescia (Italy) | — |
| Plzen (Czech Republic) | 7th Framework Programme of the EU, grant agreement number 201550 |
Conflict of interest: none declared.
Supplementary Material
Appendix
Author list and participating centres
| Center | Authors | Affiliations |
|---|---|---|
| Rotterdam (The Netherlands) | Francesco U.S. Mattace-Raso,a,b Albert Hofman,a Germaine C. Verwoert,a,b Jacqueline C.M. Wittemana |
|
| Cambridge/Cardiff (UK) | Ian Wilkinson,a John Cockcroft,b Carmel McEniery,a Yasmina |
|
| HEGP, Paris (France) | Stéphane Laurent,a,b,c Pierre Boutouyrie,a,b,c Erwan Bozeca,b,c |
|
| Copenhagen (Denmark) | Tine Willum Hansen,a,b Christian Torp-Pedersen,c Hans Ibsen,d,e,f Jørgen Jeppesenb,g |
|
| Ghent (Belgium) | Sebastian J. Vermeersch,a Ernst Rietzschel,b Marc De Buyzere,b Thierry C. Gillebert,b Luc Van Bortel,c Patrick Segersa |
|
| Athens (Greece) | Charalambos Vlachopoulos,a Constantinos Aznaouridis,a Christodoulos Stefanadisa |
|
| Nancy (France) | Athanase Benetos,a,b,c Carlos Labat,a,b,c Patrick Lacolleya,b,c |
|
| Amsterdam/Maastricht (The Netherlands) | Hoorn Study: Coen D.A. Stehouwer,a,b Giel Nijpels,c,d Jacqueline M. Dekkerc,d; AGAHLS: Coen D.A. Stehouwer,a,b Isabel Ferreira,a,b,e,f Jos W.R. Twiskg,h |
|
| Paris XIII (France) | Sebastien Czernichow,a,b Pilar Galan,a Serge Hercberga,b |
|
| Fleury-Mérogis (France) | Bruno Pannier,a,b Alain Guérin,a,b Gérard Londona,b |
|
| Manchester (UK) | J. Kennedy Cruickshank,a,b Simon G. Andersona |
|
| Brescia (Italy) | Anna Paini,a Enrico Agabiti Rosei,a Maria Lorenza Muiesan,a Massimo Salvettia |
|
| Plzen (Czech Republic) | Jan Filipovsky,a Jitka Seidlerova,a Milena Dolejsovaa |
|
Contribution of the participating centres
Most of data management and statistical analysis have been performed by S.J.V., during his PhD thesis, under the supervision of P.B.
The contribution of the various centres participating to the ‘Reference Values for Arterial Stiffness' Collaboration’ was the following:
conception and design of the research: S.L. and P.B.
acquisition of the data: all
analysis and interpretation of the data: all
statistical analysis: S.J.V. and P.B.
funding and supervision: S.L. and P.B.
draft of the manuscript: S.J.V., P.B., and S.L.
important critical revision of the manuscript for important intellectual content: J.K.C., L.V.B., P.L., P.S., I.F., C.D.A.S., F.U.S.M.R., I.W., J.C., H.I., T.W.H., A.B., J.J., C.V., J.F., and S.C.
other (specify): all authors participated to two interim meetings during which the method for managing the project was finalized.
Table A1.
Number of subjects contributing to the Reference Values for Arterial Stiffness' Collaboration, ranked by decreasing number of inclusions
| Centre | Contributed subjects (ranked by increasing number) | Algorithm | Pathway measurement | Device |
|---|---|---|---|---|
| Rotterdam (The Netherlands) | 4729 | Max upstroke | Direct | Complior |
| Cambridge/Cardiff (UK) | 4687 | Intersecting tangent | Subtracted | Sphygmocor |
| HEGP, Paris (France) | 3400 | Max upstroke | Direct | Complior |
| Copenhagen (Denmark) | 2592 | Intersecting tangent | Direct | Dedicated device |
| Ghent (Belgium) | 2524 | Intersecting tangent | Subtracted | Dedicated device |
| Athens (Greece) | 1311 | Max upstroke | Subtracted | Complior |
| Nancy (France) | 1107 | Max upstroke | Direct | Complior and Pulsepen |
| Amsterdam/Maastricht (The Netherlands) | 1102 | Intersecting tangent | Direct | WallTrack |
| Paris XIII (France) | 937 | Max upstroke | Direct | Complior |
| Fleury-Mérogis (France) | 883 | Max upstroke | Direct | Complior |
| Manchester (UK) | 579 | Intersecting tangent | Direct | Dedicated device |
| Brescia (Italy) | 377 | Max upstroke | Direct | Complior |
| Plzen (Czech Republic) | 256 | Intersecting tangent | Subtracted | Dedicated device |
| Total | 24 484 |
References
- 1.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. doi:10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 2.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Struijker Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Manolis A, Nilsson PM, Redon J, Struijker-Boudier HA, Viigimaa M, Adamopoulos S, Bertomeu V, Clement D, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, O'Brien E, Ponikowski P, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B The task force for the management of arterial hypertension of the European Society of H, The task force for the management of arterial hypertension of the European Society of C. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 4.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 5.Choi CU, Park EB, Suh SY, Kim JW, Kim EJ, Rha SW, Seo HS, Oh DJ, Park CG. Impact of aortic stiffness on cardiovascular disease in patients with chest pain: assessment with direct intra-arterial measurement. Am J Hypertens. 2007;20:1163–1169. doi: 10.1016/j.amjhyper.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. doi:10.1161/01.CIR.0000033824.02722.F7. [DOI] [PubMed] [Google Scholar]
- 7.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. doi:10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 8.Shokawa T, Imazu M, Yamamoto H, Toyofuku M, Tasaki N, Okimoto T, Yamane K, Kohno N. Pulse wave velocity predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J. 2005;69:259–264. doi: 10.1253/circj.69.259. doi:10.1253/circj.69.259. [DOI] [PubMed] [Google Scholar]
- 9.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. doi:10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 10.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. doi:10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 11.Alecu C, Labat C, Kearney-Schwartz A, Fay R, Salvi P, Joly L, Lacolley P, Vespignani H, Benetos A. Reference values of aortic pulse wave velocity in the elderly. J Hypertens. 2008;26:2207–2212. doi: 10.1097/HJH.0b013e32830e4932. doi:10.1097/HJH.0b013e32830e4932. [DOI] [PubMed] [Google Scholar]
- 12.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46:1753–1760. doi: 10.1016/j.jacc.2005.07.037. doi:10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Millasseau SC, Stewart AD, Patel SJ, Redwood SR, Chowienczyk PJ. Evaluation of carotid-femoral pulse wave velocity: influence of timing algorithm and heart rate. Hypertension. 2005;45:222–226. doi: 10.1161/01.HYP.0000154229.97341.d2. doi:10.1161/01.HYP.0000154229.97341.d2. [DOI] [PubMed] [Google Scholar]
- 14.Sugawara J, Tanaka H. Arterial path length measurements required for the pulse wave velocity. J Hypertens. 2009;27:1102. doi: 10.1097/HJH.0b013e3283295d7f. author reply 1102–4 doi:10.1097/HJH.0b013e3283295d7f. [DOI] [PubMed] [Google Scholar]
- 15.Weber T, Ammer M, Rammer M, Adji A, O'Rourke MF, Wassertheurer S, Rosenkranz S, Eber B. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens. 2009;27:1624–1630. doi: 10.1097/HJH.0b013e32832cb04e. doi:10.1097/HJH.0b013e32832cb04e. [DOI] [PubMed] [Google Scholar]
- 16.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 17.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. doi:10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 18.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103:987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 19.Boutouyrie P, Vermeersch S, Laurent S, Briet M. Cardiovascular risk assessment through target organ damage: role of carotid to femoral pulse wave velocity. Clin Exp Pharmacol Physiol. 2008;35:530–533. doi: 10.1111/j.1440-1681.2008.04911.x. doi:10.1111/j.1440-1681.2008.04911.x. [DOI] [PubMed] [Google Scholar]
- 20.Boutouyrie P, Briet M, Collin C, Vermeersch S, Pannier B. Assessment of pulse wave velocity. Artery Research. 2009 doi:10.1016/j.artres.2008.11.002. [Google Scholar]
- 21.Rajzer MW, Wojciechowska W, Klocek M, Palka I, Brzozowska-Kiszka M, Kawecka-Jaszcz K. Comparison of aortic pulse wave velocity measured by three techniques: Complior, SphygmoCor and Arteriograph. J Hypertens. 2008;26:2001–2007. doi: 10.1097/HJH.0b013e32830a4a25. doi:10.1097/HJH.0b013e32830a4a25. [DOI] [PubMed] [Google Scholar]
- 22.Salvi P, Magnani E, Valbusa F, Agnoletti D, Alecu C, Joly L, Benetos A. Comparative study of methodologies for pulse wave velocity estimation. J Hum Hypertens. 2008;22:669–677. doi: 10.1038/jhh.2008.42. doi:10.1038/jhh.2008.42. [DOI] [PubMed] [Google Scholar]
- 23.Vermeersch SJ, Rietzschel ER, De Buyzere ML, Van Bortel LM, Gillebert TC, Verdonck PR, Laurent S, Segers P, Boutouyrie P. Distance measurements for the assessment of carotid to femoral pulse wave velocity. J Hypertens. 2009;27:2377–2385. doi: 10.1097/HJH.0b013e3283313a8a. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1:739–748. doi: 10.1016/j.jcmg.2008.06.010. doi:10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R foundation for Statistical Computing; 2008. [Google Scholar]
- 26.Loeliger EA, van den Besselaar AM, Lewis SM. Reliability and clinical impact of the normalization of the prothrombin times in oral anticoagulant control. Thromb Haemost. 1985;53:148–154. [PubMed] [Google Scholar]
- 27.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 28.Milnor WR. Hemodynamics. 2nd ed. Baltimore: Williams & Wilkins; 1989. [Google Scholar]
- 29.Nichols WW, O'Rourke MF, McDonald DA. McDonald's Blood flow in Arteries: Theoretic, Experimental and Clinical Principles. 5th ed. London: Hodder Arnold; 2005. [Google Scholar]
- 30.Bramwell JC, Hill AD. Velocity of transmission of the pulse wave and elasticity of arteries. Lancet. 1922;1:891–892. [Google Scholar]
- 31.SHEP Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. doi:10.1001/jama.265.24.3255. [PubMed] [Google Scholar]
- 32.Franklin SS, Gustin WT, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.