Abstract
Biventricular pacing or cardiac resynchronization therapy (CRT) has been a considerable advance in the therapy of chronic heart failure. However, it is clear that not all patients benefit either in terms of symptoms or cardiac function, and some may be worsened by CRT. In this review, we consider the arguments, both clinical and economical, in favour of improved selection of patients for CRT other than those in current guidelines. It also seems clear that the fundamental mechanism of CRT is correction of dyssynchrony, and we review the various methodologies available to detect dyssynchrony. Other factors are probably also important in determining outcomes such as lead position, the extent and form of myocardial damage, optimizing pacemaker performance, and clinical expertise. The potential costs of inappropriate CRT implantation are high to our patients and to the health economy, and it behoves the cardiology community to develop better selection criteria. The current guidelines can and should be improved.
Keywords: Cardiac resynchronization therapy, Dyssynchrony, Echocardiography
The treatment of chronic heart failure took a major step forward when biventricular pacing or cardiac resynchronization therapy (CRT) was introduced. Meta-analyses of the five major clinical trials have shown that CRT significantly reduces mortality and the level of heart failure hospitalizations.1,2 It also improves the quality of life in patients with New York Heart Association (NYHA) class III and IV heart failure and evidence of dyssynchrony (currently reflected by a QRS duration >120 ms) who were also receiving optimal medical therapy. On average, implanting a CRT device in 13 patients should result in the saving of one additional life over a 3-year period when compared with medical therapy, and CRT is considered to be a cost-effective treatment.2 However, a number of adverse events are associated with CRT.1 The overall complication rate in the major trials was 14%, mostly related to the leads. The perioperative death rate was 0.8%, and there was a 9% failure to implant the device. In addition, about 11–46% apparently failed to benefit from CRT with lower rates, if theclinical parameters are used, and higher rates with echocardiographic measurements.3 Thus, it has been recognized from the start that not all patients benefit clinically, and in the recent Predictors of Response to CRT (PROSPECT) trial, the clinical composite response was unchanged in 50% and actually worsened in 16%, and while 35% had a 15% reduction in left ventricular (LV) end-systolic volume, in 9%, LV volumes actually increased by more than 15%.3 Thus, considerable effort has been directed at trying to identify these so-called non-responders. However, others have suggested that this is the wrong approach that the concept of ‘responders’ is a ‘false dichotomy’4 and that since overall there are benefits it is a wasted effort to attempt to identify these patients as it is difficult if not impossible to predict who will receive the mortality benefit. However, is this rather nihilistic approach justified?5
The concept of ‘non-response’
An essential element of this discussion is a precise definition of what constitutes a response. A number of criteria can be used to define a good response ranging from simple clinical measurements to echocardiographic functional assessments and volume changes, such as LV reverse remodelling. Clinical response as judged by NYHA functional class, quality-of-life score, exercise capacity as 6 min hall-walk distance tend to be more favourable (the data from the 15 largest studies gives a weighted mean response rate of 66.9% for clinical endpoints) compared with echocardiographic parameters (56.9% response rate), and this may likely relate in part to the placebo effect of device implantation.6 Reductions in end-systolic volumes and improvements in ejection fraction are more objective criteria. Furthermore, the absence of an obvious favourable response does not necessarily equate with harm as an unchanged situation is better than deterioration. Also, in some patients, clinical and echocardiographic improvement is slow, and many published studies are often short term. Mullens et al.7 found in a small group of patients that despite progressive cardiac remodelling (and with good lead positions and continuous biventricular pacing), there was a significant worsening of haemodynamics when the pacemaker was switched off, implying that the device was still giving a therapeutic benefit. However, this coincided with the reappearance of intraventricular mechanical dyssynchrony, therefore, implying that therapeutic benefit is related to mechanical dyssynchrony (see later). Notwithstanding these observations, it does seem that in some patients, CRT is associated with significant worsening of the clinical condition as in the PROSPECT trial probably by inducing dyssynchrony where none existed before or by having no effect on the progressive natural history of severe heart failure in some patients. Since CRT is expensive and is not without hazard, it seems sensible to try to identify more precisely those who will derive the best benefit and those least likely to, as in this latter group the cost-effective equation will be dramatically different. But a precise definition of what constitutes a good or poor response is still controversial. Nevertheless, the majority of successful medical therapies in heart failure that have reduced mortality have also shown some degree of improvement in symptoms, LV volumes, and reverse remodelling, as seen with beta-blockers, angiotensin-converting enzyme (ACE)-inhibitors, and aldosterone antagonists.8 The same seems to be true of CRT.9 It seems obvious that a composite definition, which includes both clinical and functional measures, would be most sensible.
Is the concept of non-responders flawed?
It has been suggested that the concept of response is a ‘flawed dichotomy as all medical therapies present a continuous spectrum ranging from harm to benefit’ and ‘since we prescribe ACE-inhibitors for all patients with heart failure we should apply the same approach equally to drugs and devices’.4 However, this reasoning is itself flawed on at least two counts. First, devices are different from drugs: the up-front costs are higher for the device and the implantation procedure, the initial complications are potentially greater (infection, coronary sinus dissection or perforation, and even death), and a device is much more difficult to remove than to stop a drug. Second, current pharmacotherapy and pharmacogenomics are trying to develop techniques to do exactly the opposite by identifying those patients who are most likely to benefit and least likely to have side effects introducing ‘tailored or individualized therapy’. Also, in most major drug trials in heart failure, patients were excluded from entry if they had already experienced an adverse effect with the study drug, which cannot be done with devices.
Cost of non-response
Guidelines recommend CRT for symptomatic heart failure with LVEF <35% and a prolonged QRS duration >120 ms, which is used as a basic measure of dyssynchrony. Prolonged QRS duration occurs in about 25% of the general heart failure population,10 and in 35% of patients with more severe LV systolic dysfunction, typical of patients who might be considered for CRT. Currently, it is estimated that up to 14 million people in Europe have heart failure.11 Therefore, possibly there are 5 million people with heart failure and prolonged QRS duration in Europe. Of these potential candidates for CRT in Europe, 30% may well turn out to be non-responders, which is about 1.5 million people. At a conservative estimate of €5000 per device (assuming they are receiving CRT pacemakers), this equals €7.5 billion spent on these individuals who will derive no clinical benefit and possible harm with a risk of death at time of implantation of 0.5%, which of 1.5 million non-responders approximates to 7500 people. In addition, there are risks of infection, removal of the system, and coronary dissection complications, which occurred in 2.4% of all patients in the CARE-HF study. Thus, the human and financial costs of implanting a device in a potential non-responder are considerable.
Dyssynchrony: does it matter and how to measure?
Some have argued that correction of dyssynchrony may not be the fundamental mode of action of CRT, and therefore identifying it is unnecessary. Dyssynchrony can occur between the atria and ventricles, between the ventricles (interventricular), or within the LV (intraventricular). Both inter- and intraventricular dyssynchrony appears to be more important, and the latter more so than the former.
To assess intraventricular dyssynchrony, septal-to-posterior wall delay >130 ms by M-mode has been proposed.12 Although it is a relatively simple tool of dyssynchrony, it was limited by the fact that dyssynchronous septal might have a flat motion or even paradoxical movement rendering a high practical difficulty in selecting the sampling point and therefore a high interobserver variability. Its ability to predict favourable CRT response was also questioned.13 On the other hand, the presence of early ‘septal flash’ during isovolumic period predicts LV reverse remodelling with a high specificity, although with a low sensitivity.14
Tissue Doppler imaging
Tissue Doppler imaging ( (TDI) can measure regional myocardial velocity and hence naturally evolved as a tool to assess regional myocardial function, as well as global function of chambers. Many earlier studies had suggested the useful role for assessing pre-pacing regional systolic dyssynchrony as predictor of a favourable response to CRT, in particular LV reverse remodelling, an increase in systolic function, and also long-term clinical outcome.15–19 Many of these studies measured LV long-axis function, the major axis of the heart, and the generated global indices of dyssynchrony that assessed the time to the peak or onset of systolic velocity at the myocardial velocity curve (Figure 1). Technical details of these parameters and their cut-off values have been described elsewhere.20 Commonly used parameters include septal-to-lateral wall delay in peak systolic velocity of 65 ms,16,17 maximal delay in peak systolic velocity of 12 LV segments of 100 ms, and standard deviation of time to peak systolic velocity of 12 LV segments (Ts-SD) of 33 ms.15,16 A recent review of the subject found that among the 24 studies using echocardiography to predict CRT response, two studies demonstrated some value of interventricular dyssynchrony, whereas all 24 studies showed some predictive value of LV intraventricular dyssynchrony (Figure 2).6 Recently, van Bommel et al.21 found in a sub-analysis of the PROSPECT study that ‘super-responders’ were significantly more dyssynchronous than the ‘negative’ responders with both a larger interventricular and septal-to-lateral delay by TDI assessment. This result also confirms that CRT is treating mechanical dyssynchrony (although there may still be a need to distinguish contractile disparity with normal activation from what is considered to be dyssynchrony due to delayed activation22). Non-responders were also more likely to have an ischaemic aetiology and a history of ventricular tachycardia suggesting the presence of more scar tissue, and clearly this is an important modifying factor as lead placement needs to be taken into account in any predictive algorithm. Furthermore, although the current guideline recommends CRT for any QRS morphology that causes a prolonged QRS duration, which typically includes left bundle branch block, right bundle branch block, and intraventricular conduction delay, there has been increasing evidence suggesting that patients with the latter two QRS morphologies do not or only minimally respond to CRT, which is attributable to the lack of intraventricular dyssynchrony.23 However, the concept of mechanical dyssynchrony has been heavily criticized, since the publication of the PROSPECT trial.3
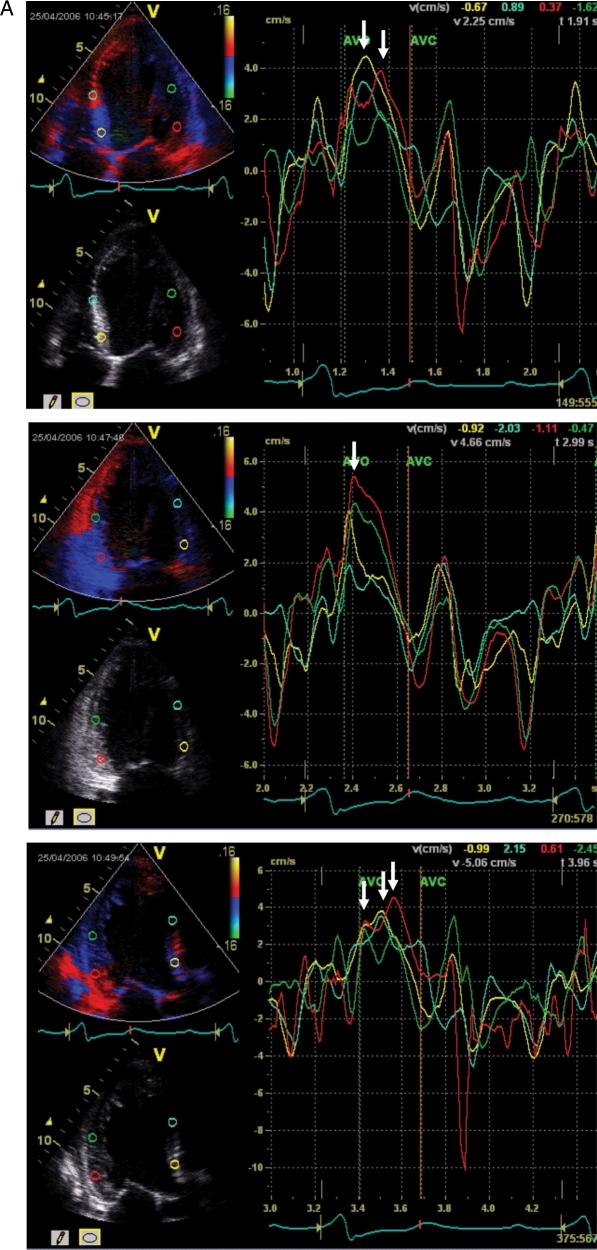
Figure 1.
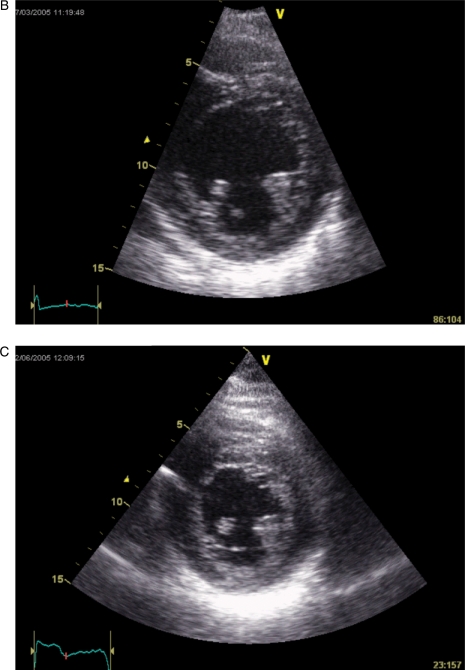
A patient fulfilling the criteria for cardiac resynchronization therapy with significant systolic dyssynchrony by tissue Doppler imaging. The measured baseline dyssynchrony index from three apical views was 52.6 ms (normal cut-off value is 33 ms) (A) and LV end-systolic volume decreased from 131 (B) to 67 mL (C) after CRT (reduced by 49%).
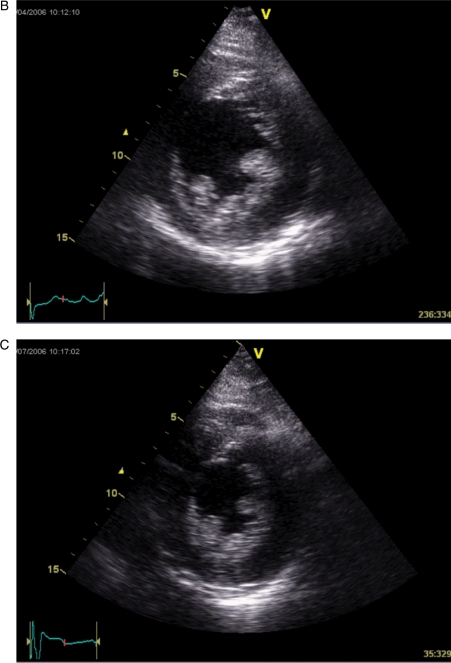
Figure 2.
A patient fulfilling the criteria for cardiac resynchronization therapy but without systolic dyssynchrony by tissue Doppler imaging. The measured baseline dyssynchrony index from three apical views was 18.8 ms (normal cut-off value is 33 ms) (A) and LV end-systolic volume decreased only from 94 (B) to 90 mL (C) after CRT (reduced by 4%).
Speckle tracking
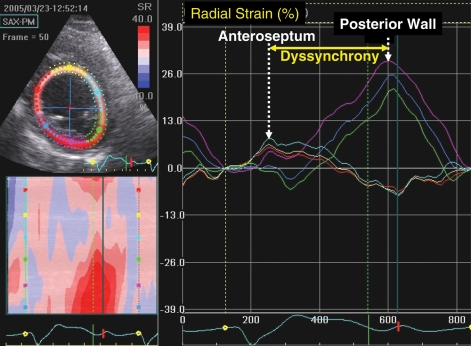
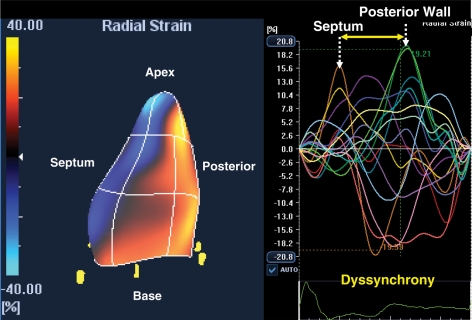
Advances in quantitative echocardiographic technology continue and may be applied to dyssynchrony analysis. Speckle tracking is one notable advance that utilizes routine grey scale digital imaging to determine myocardial strain. Theoretically, the major advantage is because strain can isolate active thickening from passive motion in a manner unaffected by Doppler angle of incidence such as TDI. Speckle-tracking strain analysis has been primarily applied to assess radial strain from parasternal short-axis views and can accordingly assess wall thickening (Figure 3). The time delay from anteroseptal to posterior wall maximal thickening with a cut-off of 130 ms has been shown to predict increase in ejection fraction after CRT. Furthermore, speckle tracking and TDI data when combined have been shown to have additive value to predict ejection fraction response.24 Other speckle-tracking approaches include longitudinal and circumferential strain, however, the radial method appears to be comparatively useful.25 The success of radial strain for dyssynchrony analysis appears to be related to the success of speckle tracking for detecting wall thickening. Both longitudinal and circumferential strain dyssynchrony exist in these patients, but often this appears to be too subtle for the current speckle-tracking software to detect reliably. Several ultrasound manufactures have developed speckle-tracking software, and a close agreement for determining the presence or absence of significant dyssynchrony was observed among three recently tested.26 Of clinical practical significance, radial dyssynchrony by speckle-tracking strain was recently associated with ejection fraction and reverse remodelling response to CRT in patients with borderline QRS duration of 100–130 ms.27 Of 78 borderline QRS duration patients implanted with echocardiographic dyssynchrony, a lower number of patients had significant dyssynchrony by routine pulsed Doppler markers of pre-ejection delay, interventricular mechanical delay, and filling time/RR interval when compared with 123 CRT patients with wide QRS duration. Speckle-tracking radial strain appeared to be more predictive of response than tissue Doppler or routine pulsed Doppler in this study and has potential to be used as an adjunct for these patients who may be considered borderline candidates.27 However, this technology is very dependent on the quality of two-dimensional images, and even more training is needed for off-line analysis compared with TDI. Furthermore, the generalizability of this technique has yet to be testified by multicentre studies.
Figure 3.
An echocardiographic mid-ventricular short-axis view used for speckle-tracking radial strain. The right panel shows time strain curves from six representative segments in a patient with left bundle branch block before cardiac resynchronization therapy. The yellow line is the anterospetum, which demonstrates early peak strain (arrow), and the purple line is the posterior wall, which demonstrates delayed peak strain (arrow), consistent with significant dyssynchrony.
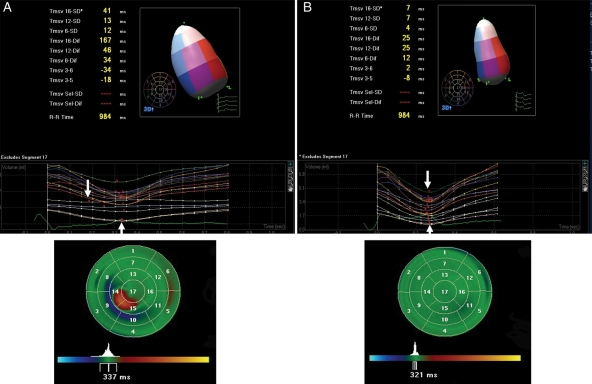
A recent technological advance in echocardiography dyssynchrony analysis has been three-dimensional speckle-tracking strain. The three-dimensional speckle-tracking system can determine radial strain using a 16-segment model from a pyramidal data set that includes the whole LV and can determine the site of latest mechanical activation (Figure 4). Initial experience has shown that three-dimensional mechanical mapping correlates with the two-dimensional approach to quantify dyssynchrony but provides far greater detail.28 The study compared 81 right ventricular-paced heart failure patients for upgrade to CRT with 227 patients with native left bundle branch block.28 Interestingly, only slight differences were in site of earliest activation, but the distribution and prevalence of sites of latest activation were very similar when either two-dimensional or three-dimensional speckle-tracking strain approaches were used. Furthermore, the degree of mechanical dyssynchrony in both right ventricular-paced patients and left bundle branch patients was similar before CRT, and the overall ejection fraction response was similar after CRT. These data using speckle-tracking technology help support the candidacy of CRT upgrade for patients with depressed ejection fraction who received right ventricular pacing for bradycardia.
Figure 4.
An echocardiographic image from a patient with left bundle branch block of three-dimensional speckle tracking strain acquired from a pyramid of data with colour coding of peak strain as orange–yellow. The right panel shows 16 corresponding time-strain curves from standard left ventricular segments demonstrating septal curves with early activation, and free-wall curves with late activation, consistent with significant mechanical dyssynchrony.
Real-time three-dimensional echocardiography
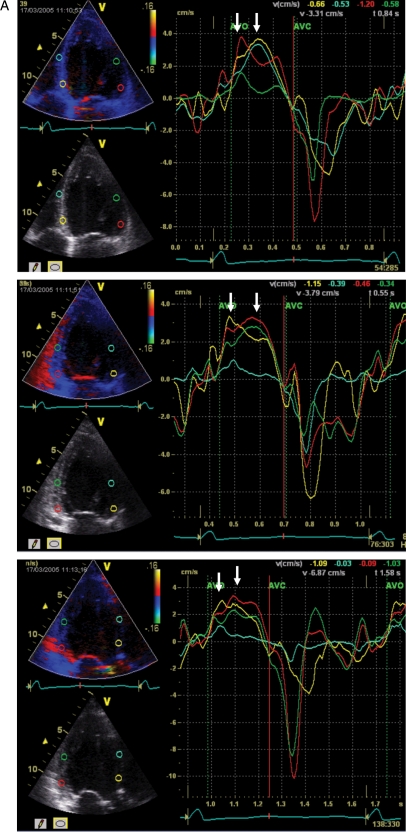
Real-time three-dimensional echocardiography (RT3DE) can provide a full volume-rendered LV cast in four to seven cardiac cycles. This can then be used to measure changes in LV volume and ejection fraction as well as systolic dyssynchrony based on 16 or 17 segments (depending on the software used). Early studies suggest that dyssynchrony can be measured from the scattering of the time to minimal regional volume of the LV segments (Figure 5).29 Cut-off values of 8.3% (normalized for R–R interval) by Tomtec software and 5.6% by Q-Lab (Philips) have been suggested in single-centre studies that predict LV reverse remodelling and improvement of ejection fraction.30,31 Technical challenges for RT3DE remain, as in very dilated ventricles of the failing heart stitching of more cardiac cycles is needed. Also, extensive training is needed to develop the necessary skill for image acquisition and off-line analysis so as to avoid suboptimal image and editing artefacts. Similar to speckle-tracking technology, the application of RT3DE in CRT is yet to be evaluated by multicentre studies.
Figure 5.
A heart failure patient had dyssynchrony measured by real-time 3D echocardiography before (A) and after (B) receiving cardiac resynchronization therapy (CRT). Before CRT, there was variation in the time to minimal regional volume (arrows), and parametric imaging confirms paradoxical movement over inferior septum and posterior wall. These changes were normalized after CRT.
Prospect trial
In retrospect, this study was blighted with major problems ranging from patient selection, methodology, technical expertise, ageing echocardiographic equipment from three different vendors, which were not standardized for frame rates with three different software programmes for offline analysis. In addition, three different core laboratories were used for echocardiography dyssynchrony analyses that were not calibrated for dyssynchrony analysis, which introduced the possibility of variability in analysis. These limitations have been highlighted elsewhere but are serious enough to undermine the conclusions.5,6,32 For example, 20.2% of patients had a LVEF more than 35% suggesting inclusion of less severe heart failure patients who did not meet the entry criteria or guideline recommendations for CRT. Furthermore, 37.8% had an LV end-diastolic dimension <65 mm, which raises the question how reverse remodelling, which was one of the primary endpoints, can be expected to occur in a non-dilated ventricle. In addition, interobserver variability was high. The study used a reduction of LV end-systolic volume >15% to define volumetric responders, although interobserver variability for this parameter was 14.5%, which was unacceptably high and may not be reliable to detect true responders.3 The mediocre image quality acquired from ageing echocardiographic machines also preclude competent off-line analysis of colour-coded TDI images, and in fact only 39% of enrolled patients had dyssynchrony analysis from the 12 LV segments. Proficiency with any new technology takes time to acquire. The PROSPECT trial was started in 2003 when the implantation technique of CRT devices was already mature, it was still the beginning of dyssynchrony analysis, in which technical training and knowledge transfer had yet to develop. With the recent advancement of echocardiographic technologies for dyssynchrony assessment and importantly mastering of skills for dyssynchrony assessment in many echocardiographic centres worldwide, now will be good time to conduct another multicentre trial to readdress whether baseline dyssynchrony can predict favourable response and clinical outcome to CRT. Therefore, the PROSPECT trial should not be a reason for discarding the whole concept of mechanical dyssynchrony, which is still the most likely target of CRT or the need for identifying non-responders to save them the hazards of a treatment that has risks and will provide them with no benefit and possibly worsen their condition.
Dyssynchrony assessment in the post-prospect era
The current challenge remains of how to reconcile the PROSPECT study results from many smaller studies from centres with expertise in dyssynchrony assessment. To date, there is no randomized study to assess whether dyssynchrony-guide implantation will result in a higher responder rate and even better clinical outcome. Although such a study design may provide a definite answer, it is unlikely to be conducted as the concept of dyssynchrony evolved after CRT guidelines that used QRS duration as a surrogate marker of dyssynchrony. Another major issue remaining is whether dyssynchrony assessment performed from experienced centres will provide better predictive values than novice centres, i.e. whether the acquisition of dyssynchrony assessment skill has a learning curve, a situation similar to the training required for optimal CRT implantation.
Does cardiac resynchronization therapy have a role in heart failure with a narrow QRS?
Several studies using TDI have demonstrated that mechanical dyssynchrony is apparent in about 30–40% of heart failure patients with a normal QRS.33–35 A few small studies have suggested that CRT may be of benefit to these patients as well, in particular when dyssynchrony is present (Figure 6).36,37 However, in the RethinQ study, there was no apparent benefit of CRT in patients with severe heart failure with QRS duration <130 ms and echocardiographic evidence of mechanical dyssnchrony.38 This study used peak O2 consumption as the primary endpoint, which may be a relatively insensitive marker of clinical response. However, it is clear that there are other causes of mechanical dyssynchrony or delayed contraction other than delayed electrical conduction, such as regional impairment of function due to ischaemia, myocardial disease and scarring, and disparate loading conditions or wall stress, which occur with differences in LV wall thickness. Furthermore, a recent study suggests that CRT produces an acute haemodynamic benefit in patients with a narrow QRS and with no or minor mechanical dyssynchrony by TDI.39 They postulate that this is due to reduction in external pericardial constraint with right ventricular volume reduction that may occur with pacing, i.e. less ventricular interaction allowing an increased LV volume and hence cardiac output via the Frank–Starling mechanism. Another possibility is that these patients had ‘occult’ dyssynchrony, as the usual measures of dyssynchrony are based on longitudinal function rather than radial motion. Efforts are underway to assess the potential benefits of CRT in heart failure patients with narrow QRS and echocardiographic dyssynchrony in a large randomized trial. Assessment of both longitudinal dyssynchrony by TDI and radial dyssynchrony by speckle tracking may improve the prediction of outcome as shown in patients with prolonged QRS duration received CRT,39 and this may even be better with real-time three-dimensional speckle tracking in the near future, when technical hurdles are overcome.40,41
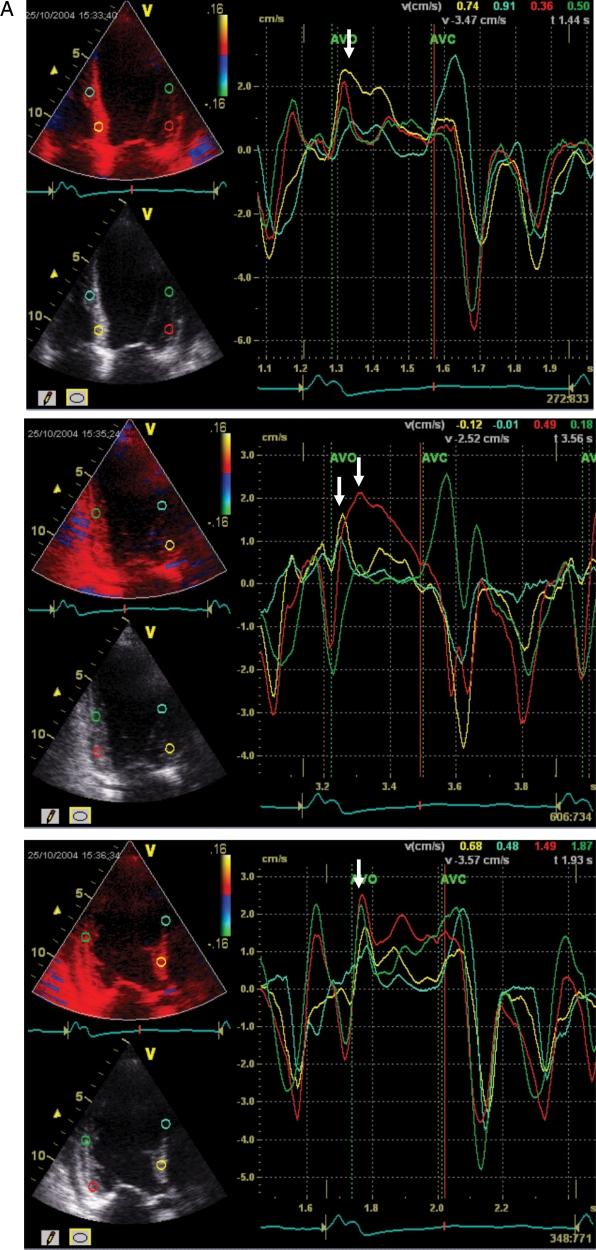
Figure 6.
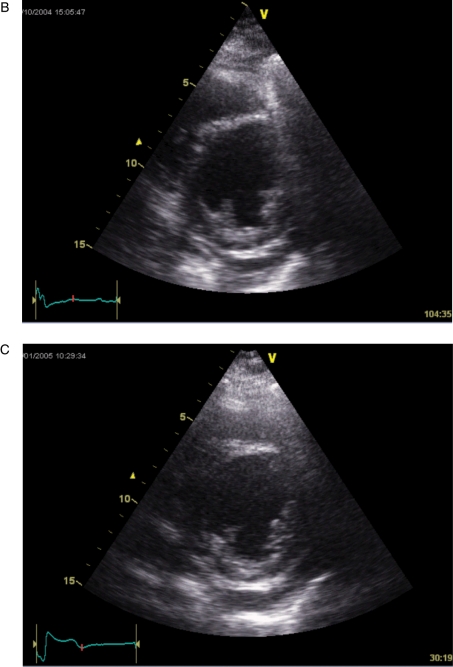
A heart failure patient with normal QRS duration and significant systolic dyssynchrony by tissue Doppler imaging. The measured baseline dyssynchrony index from three apical views was 43.1 ms (normal cut-off value is 33 ms) (A) and LV end-systolic volume decreased from 106 (B) to 62 mL (C) after cardiac resynchronization therapy (reduced by 41%).
Future of echocardiographic assessment of dyssynchrony
Parameters of dyssynchrony assessment by echocardiography continue to develop over time. Conceptually, electromechanical coupling delay causes, systolic dyssynchrony, and hence if one can accurately estimate its severity, this should translate into better response to CRT and favourable cardiovascular outcome. However, this link has not been firmly established due to a number of reasons. First, a variety of parameters reflecting mechanical motion have been suggested, which may quantify dyssynchrony at different times of the cardiac cycles and to different extents, such as time to onset and peak velocity as well as time to minimal strain/displacement/regional volume. This is further complicated by the complex LV geometry, which also changes from normal to disease stage. Currently, there is no single parameter, which is able to encompass all these factors. Secondly, while echocardiographic technologies are undergoing rapid evolution, these new skills might not be easy to master and yet continuous improvement is necessary to increase its accuracy, signal-to-noise ratio as well as intrinsic reproducibility. Thirdly, training and hands on workshops are necessary for knowledge and skill transfer and to overcome the learning curve. Fourthly, concerted efforts from echocardiographic vendors are important to arrive common consensus on the principles of algorithms for quantitative dyssynchrony assessment by new technologies, such as TDI velocities and strain. For example, simple echocardiographic parameters such as LV ejection fraction can be reproducibly measured by different machines, but the value of strain is entirely different when measured by different echocardiographic vendors, or even different off-line software with the same image. It will be extremely difficult and impractical to have vendor-specific parameter(s) (for both online and offline) to be recommended for dyssynchrony assessment, in particular as many of them are only available from state-of-the-art echocardiographic equipments. Lastly, without a collaborative approach between echocardiographic experts, electrophysiologists, heart failure specialists as well as active participations of both echocardiographic, and device vendors to properly conduct further multicentre trials, it will remain unknown whether echocardiographic assessment of dyssynchrony [and if so, which parameter(s)] can predict a favourable CRT response in a global perspective. Although this approach has the potential to reduce up to one-third of CRT device implantation in the ‘wide’ QRS heart failure population, a correct dyssynchrony-targeted approach may help identify those who might benefit from CRT in the ‘narrow’ QRS population.
Although systolic dyssynchrony plays a pivotal role in heart failure with systolic dysfunction leading to the evolution of CRT, it cannot be overemphasized that dyssynchrony does not only occur exclusively in systolic dysfunction. In fact, systolic dyssynchrony has also been shown to be present in other cardiac conditions, such as in patients with LV hypertrophy,42 diastolic heart failure,43 or even during dynamic stimulation of the heart.44 Conversely, in patients with right ventricular pacing-induced left bundle branch block and preserved ejection fraction, only about half of patients will exhibit systolic dyssynchrony.45 The pathophysiological role of dyssynchrony in these conditions needs further exploration.
Conclusions
The correction of mechanical dyssynchrony appears to be the major mechanism for CRT, and its detection, therefore, should be of value in predicting the clinical outcome. Other factors are also important in determining whether the patient will have a clinical or mortality benefit and will have to be included in any algorithm, such as LV lead position, myocardial contractile reserve, impact of mitral regurgitation, percentage of biventricular pacing, factors leading to loss of biventricular pacing post-device implantation, atrial arrhythmia, as well as optimizing atrioventricular and interventricular intervals; but lack of mechanical dyssynchrony, an ischaemic aetiology with a large myocardial scar burden and very low-ejection fraction as well as inappropriate lead position, are all likely to be associated with a poor clinical response.46,47 Current guidelines should of course be followed at the moment, but there is always room for improvement that may substantially benefit individual patients. Maseri48 recently put the issue succinctly: ‘Following guidelines makes it easy for cardiologists and protects them, while the very broad spectrum of patients included in clinical trials is good for the industry. But this approach provides no incentive to search for the multiple causal components of each cardiovascular syndrome in specific subgroups of patients. You only learn by following up cases that do not fit the paradigm. In clinical research, looking at “the average” does not help—looking at outliers is the way to try and understand something that is still unknown’. This process is already well advanced in cancer therapy with increasing development of targeted therapies. Cardiovascular medicine needs to move in this direction, and the selection of patients who stand to gain the most benefit from CRT would be a start.
Funding
J.G. received grants from NIH award K24 HL04503-01, and from GE Healthcare, Medtronic, St Jude Medical and Biotronik.
Conflict of interest: C.-M.Y. is speaker for St Jude Medical and Philips; and consultant for Philips.
References
- 1.McAlister FA, Ezekowitz JA, Wiebe N, Rowe B, Spooner C, Crumley E, Hartling L, Klassen T, Abraham W. Systematic review: cardiac resynchronization in patients with symptomatic heart failure. Ann Intern Med. 2004;141:381–390. doi: 10.7326/0003-4819-141-5-200409070-00101. [DOI] [PubMed] [Google Scholar]
- 2.Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, Taylor RS. The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model. Health Technol Assess. 2007;11:iii-248. doi: 10.3310/hta11470. [DOI] [PubMed] [Google Scholar]
- 3.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, III, St John SM, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. doi:10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins NM, Petrie MC, Burgess MI, McMurray JJ. Selecting patients for cardiac resynchronization therapy: the fallacy of echocardiographic dyssynchrony. J Am Coll Cardiol. 2009;53:1944–1959. doi: 10.1016/j.jacc.2008.11.062. doi:10.1016/j.jacc.2008.11.062. [DOI] [PubMed] [Google Scholar]
- 5.Sanderson JE. Echocardiography for cardiac resynchronization therapy selection: fatally flawed or misjudged? J Am Coll Cardiol. 2009;53:1960–1964. doi: 10.1016/j.jacc.2008.12.071. doi:10.1016/j.jacc.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 6.Bax JJ, Gorcsan J., III Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol. 2009;53:1933–1943. doi: 10.1016/j.jacc.2008.11.061. doi:10.1016/j.jacc.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 7.Mullens W, Verga T, Grimm RA, Starling RC, Wilkoff BL, Tang WH. Persistent hemodynamic benefits of cardiac resynchronization therapy with disease progression in advanced heart failure. J Am Coll Cardiol. 2009;53:600–607. doi: 10.1016/j.jacc.2008.08.079. doi:10.1016/j.jacc.2008.08.079. [DOI] [PubMed] [Google Scholar]
- 8.Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 9.Ypenburg C, van Bommel RJ, Borleffs CJ, Bleeker GB, Boersma E, Schalij MJ, Bax JJ. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J Am Coll Cardiol. 2009;53:483–490. doi: 10.1016/j.jacc.2008.10.032. doi:10.1016/j.jacc.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Baldasseroni S, Opasich C, Gorini M, Marchionni N, Campana C, Deorsola A, Masotti G, Tavazzi L, Maggioni AP. Complete left bundle-branch block (LBBB) is associated with increased 1-year mortality in patients with congestive heart failure: data from IN-CHF Registry. J Am Coll Cardiol. 2001;37:156A. [Google Scholar]
- 11.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, Van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 12.Pitzalis MV, Iacoviello M, Romito R, Massari F, Rizzon B, Luzzi G, Guida P, Andriani A, Mastropasqua F, Rizzon P. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol. 2002;40:1615–1622. doi: 10.1016/s0735-1097(02)02337-9. doi:10.1016/S0735-1097(02)02337-9. [DOI] [PubMed] [Google Scholar]
- 13.Marcus GM, Rose E, Viloria EM, Schafer J, De Marco T, Saxon LA, Foster E. Septal to posterior wall motion delay fails to predict reverse remodeling or clinical improvement in patients undergoing cardiac resynchronization therapy. J Am Coll Cardiol. 2005;46:2208–2214. doi: 10.1016/j.jacc.2005.05.095. doi:10.1016/j.jacc.2005.05.095. [DOI] [PubMed] [Google Scholar]
- 14.Parsai C, Bijnens B, Sutherland GR, Baltabaeva A, Claus P, Marciniak M, Paul V, Scheffer M, Donal E, Derumeaux G, Anderson L. Toward understanding response to cardiac resynchronization therapy: left ventricular dyssynchrony is only one of multiple mechanisms. Eur Heart J. 2009;30:940–949. doi: 10.1093/eurheartj/ehn481. doi:10.1093/eurheartj/ehn481. [DOI] [PubMed] [Google Scholar]
- 15.Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, Kum LC, Kong SL, Zhang Y, Sanderson JE. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66–73. doi: 10.1161/01.CIR.0000133276.45198.A5. doi:10.1161/01.CIR.0000133276.45198.A5. [DOI] [PubMed] [Google Scholar]
- 16.Yu CM, Gorcsan J, III, Bleeker GB, Zhang Q, Schalij MJ, Suffoletto MS, Fung JW, Schwartzman D, Chan YS, Tanabe M, Bax JJ. Usefulness of tissue Doppler velocity and strain dyssynchrony for predicting left ventricular reverse remodeling response after cardiac resynchronization therapy. Am J Cardiol. 2007;100:1263–1270. doi: 10.1016/j.amjcard.2007.05.060. doi:10.1016/j.amjcard.2007.05.060. [DOI] [PubMed] [Google Scholar]
- 17.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. doi:10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–1586. doi: 10.1161/CIRCULATIONAHA.105.538272. doi:10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q, van Bommel RJ, Fung JW, Chan JY, Bleeker GB, Ypenburg C, Yip G, Liang YJ, Schalij MJ, Bax JJ, Yu CM. Tissue Doppler velocity is superior to strain imaging in predicting long-term cardiovascular events after cardiac resynchronisation therapy. Heart. 2009;95:1085–1090. doi: 10.1136/hrt.2008.161653. doi:10.1136/hrt.2008.161653. [DOI] [PubMed] [Google Scholar]
- 20.Gorcsan J, III, Abraham T, Agler DA, Bax JJ, Derumeaux G, Grimm RA, Martin R, Steinberg JS, Sutton MS, Yu CM. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting—a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 21.van Bommel RJ, Bax JJ, Abraham WT, Chung ES, Pires LA, Tavazzi L, Zimetbaum PJ, Gerritse B, Kristiansen N, Ghio S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009;30:2470–2477. doi: 10.1093/eurheartj/ehp368. doi:10.1093/eurheartj/ehp368. [DOI] [PubMed] [Google Scholar]
- 22.Kass DA. An epidemic of dyssynchrony: but what does it mean? J Am Coll Cardiol. 2008;51:12–17. doi: 10.1016/j.jacc.2007.09.027. doi:10.1016/j.jacc.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 23.Wokhlu A, Rea RF, Asirvatham SJ, Webster T, Brooke K, Hodge DO, Wiste HJ, Dong Y, Hayes DL, Cha YM. Upgrade and de novo cardiac resynchronization therapy: impact of paced or intrinsic QRS morphology on outcomes and survival. Heart Rhythm. 2009;6:1439–1447. doi: 10.1016/j.hrthm.2009.07.009. doi:10.1016/j.hrthm.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Gorcsan J, III, Tanabe M, Bleeker GB, Suffoletto MS, Thomas NC, Saba S, Tops LF, Schalij MJ, Bax JJ. Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. J Am Coll Cardiol. 2007;50:1476–1483. doi: 10.1016/j.jacc.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 25.Delgado V, Ypenburg C, van Bommel RJ, Tops LF, Mollema SA, Marsan NA, Bleeker GB, Schalij MJ, Bax JJ. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol. 2008;51:1944–1952. doi: 10.1016/j.jacc.2008.02.040. doi:10.1016/j.jacc.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka H, Hara H, Saba S, Gorcsan J., III Prediction of response to cardiac resynchronization therapy by speckle tracking echocardiography using different software approaches. J Am Soc Echocardiogr. 2009;22:677–684. doi: 10.1016/j.echo.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Oyenuga O, Hara H, Tanaka H, Kim HN, Adelstein EC, Saba S, Gorcsan J., III Usefulness of echocardiographic dyssynchrony in patients with borderline QRS duration to assist with selection for cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2010;3:132–140. doi: 10.1016/j.jcmg.2009.09.020. doi:10.1016/j.jcmg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka H, Hara H, Adelstein EC, Schwartzman D, Saba S, Gorcsan J., III Comparative mechanical activation mapping of RV pacing to LBBB by 2D and 3D speckle tracking and association with response to resynchronization therapy. J Am Coll Cardiol Cardiovasc Imaging. 2010 doi: 10.1016/j.jcmg.2009.12.014. 3:461–471. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Yu CM, Fung JW, Zhang Y, Chan YS, Chan HC, Yip GW, Sanderson JE. Assessment of the effect of cardiac resynchronization therapy on intraventricular mechanical synchronicity by regional volumetric changes. Am J Cardiol. 2005;95:126–129. doi: 10.1016/j.amjcard.2004.08.078. doi:10.1016/j.amjcard.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 30.Kapetanakis S, Kearney MT, Siva A, Gall N, Cooklin M, Monaghan MJ. Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation. 2005;112:992–1000. doi: 10.1161/CIRCULATIONAHA.104.474445. doi:10.1161/CIRCULATIONAHA.104.474445. [DOI] [PubMed] [Google Scholar]
- 31.Marsan NA, Bleeker GB, Ypenburg C, Ghio S, van de Veire NR, Holman ER, van der Wall EE, Tavazzi L, Schalij MJ, Bax JJ. Real-time three-dimensional echocardiography permits quantification of left ventricular mechanical dyssynchrony and predicts acute response to cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2008;19:392–399. doi: 10.1111/j.1540-8167.2007.01056.x. doi:10.1111/j.1540-8167.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 32.Yu CM, Bax JJ, Gorcsan J., III Critical appraisal of methods to assess mechanical dyssynchrony. Curr Opin Cardiol. 2009;24:18–28. doi: 10.1097/hco.0b013e32831bc34e. doi:10.1097/HCO.0b013e32831bc34e. [DOI] [PubMed] [Google Scholar]
- 33.Yu CM, Lin H, Zhang Q, Sanderson JE. High prevalence of left ventricular systolic and diastolic asynchrony in patients with congestive heart failure and normal QRS duration. Heart. 2003;89:54–60. doi: 10.1136/heart.89.1.54. doi:10.1136/heart.89.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bleeker GB, Schalij MJ, Molhoek SG, Holman ER, Verwey HF, Steendijk P, van der Wall EE, Bax JJ. Frequency of left ventricular dyssynchrony in patients with heart failure and a narrow QRS complex. Am J Cardiol. 2005;95:140–142. doi: 10.1016/j.amjcard.2004.08.082. doi:10.1016/j.amjcard.2004.08.082. [DOI] [PubMed] [Google Scholar]
- 35.Haghjoo M, Bagherzadeh A, Fazelifar AF, Haghighi ZO, Esmaielzadeh M, Alizadeh A, Emkanjoo Z, Sadeghpour A, Samiei N, Farahani MM, Sadr-Ameli MA, Maleki M, Noohi F. Prevalence of mechanical dyssynchrony in heart failure patients with different QRS durations. Pacing Clin Electrophysiol. 2007;30:616–622. doi: 10.1111/j.1540-8159.2007.00722.x. doi:10.1111/j.1540-8159.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 36.Bleeker GB, Holman ER, Steendijk P, Boersma E, van der Wall EE, Schalij MJ, Bax JJ. Cardiac resynchronization therapy in patients with a narrow QRS complex. J Am Coll Cardiol. 2006;48:2243–2250. doi: 10.1016/j.jacc.2006.07.067. doi:10.1016/j.jacc.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 37.Yu CM, Chan YS, Zhang Q, Yip GW, Chan CK, Kum LC, Wu L, Lee AP, Lam YY, Fung JW. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48:2251–2257. doi: 10.1016/j.jacc.2006.07.054. doi:10.1016/j.jacc.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 38.Beshai JF, Grimm RA, Nagueh SF, Baker JH, Beau SL, Greenberg SM, Pires LA, Tchou PJ. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–2471. doi: 10.1056/NEJMoa0706695. doi:10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 39.Williams LK, Ellery S, Patel K, Leyva F, Bleasdale RA, Phan TT, Stegemann B, Paul V, Steendijk P, Frenneaux M. Short-term hemodynamic effects of cardiac resynchronization therapy in patients with heart failure, a narrow QRS duration, and no dyssynchrony. Circulation. 2009;120:1687–1694. doi: 10.1161/CIRCULATIONAHA.108.799395. doi:10.1161/CIRCULATIONAHA.108.799395. [DOI] [PubMed] [Google Scholar]
- 40.Miyazaki C, Powell BD, Bruce CJ, Espinosa RE, Redfield MM, Miller FA, Hayes DL, Cha YM, Oh JK. Comparison of echocardiographic dyssynchrony assessment by tissue velocity and strain imaging in subjects with or without systolic dysfunction and with or without left bundle-branch block. Circulation. 2008;117:2617–2625. doi: 10.1161/CIRCULATIONAHA.107.733675. doi:10.1161/CIRCULATIONAHA.107.733675. [DOI] [PubMed] [Google Scholar]
- 41.Koch J, Pedersen HD, Jensen AL, Flagstad A, Poulsen K, Bie P. Short term effects of acute inhibition of the angiotensin-converting enzyme on the renin-angiotensin system and plasma atrial natriuretic peptide in healthy dogs fed a low-sodium diet versus a normal-sodium diet. Zentralbl Veterinarmed A. 1994;41:121–127. doi: 10.1111/j.1439-0442.1994.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 42.Pai RG, Gill KS. Amplitudes durations timings of apically directed left ventricular myocardial velocities: II. Systolic and diastolic asynchrony in patients with left ventricular hypertrophy. J Am Soc Echocardiogr. 1998;11:112–118. doi: 10.1016/s0894-7317(98)70068-9. [DOI] [PubMed] [Google Scholar]
- 43.Yu CM, Zhang Q, Yip GW, Lee PW, Kum LC, Lam YY, Fung JW. Diastolic and systolic asynchrony in patients with diastolic heart failure: a common but ignored condition. J Am Coll Cardiol. 2007;49:97–105. doi: 10.1016/j.jacc.2006.10.022. doi:10.1016/j.jacc.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Lafitte S, Bordachar P, Lafitte M, Garrigue S, Reuter S, Reant P, Serri K, Lebouffos V, Berrhouet M, Jais P, Haissaguerre M, Clementy J, Roudaut R, DeMaria AN. Dynamic ventricular dyssynchrony: an exercise-echocardiography study. J Am Coll Cardiol. 2006;47:2253–2259. doi: 10.1016/j.jacc.2005.11.087. doi:10.1016/j.jacc.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q, Fang F, Yip GW, Chan JY, Shang Q, Fung JW, Chan AK, Liang YJ, Yu CM. Difference in prevalence and pattern of mechanical dyssynchrony in left bundle branch block occurring in right ventricular apical pacing versus systolic heart failure. Am Heart J. 2008;156:989–995. doi: 10.1016/j.ahj.2008.06.027. doi:10.1016/j.ahj.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 46.Santaularia-Tomas M, Abraham TP. Criteria predicting response to CRT: is more better? Eur Heart J. 2009;30:2835–2837. doi: 10.1093/eurheartj/ehp378. doi:10.1093/eurheartj/ehp378. [DOI] [PubMed] [Google Scholar]
- 47.Leyva F, Foley PW, Stegemann B, Ward JA, Ng LL, Frenneaux MP, Regoli F, Smith RE, Auricchio A. Development and validation of a clinical index to predict survival after cardiac resynchronisation therapy. Heart. 2009;95:1619–1625. doi: 10.1136/hrt.2009.173880. doi:10.1136/hrt.2009.173880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maseri A. European Perspectives: Pioneer in cardiology. Circulation. 2009;119:f1–f6. [Google Scholar]