Abstract
Engagement of the high affinity receptor for IgE (FcεRI) causes its phosphorylation by Lyn kinase. Two alternatively spliced variants, Lyn A and B are expressed in mast cells and both isoforms interact with FcεRI. Unlike Lyn A, Lyn B lacks a 21 amino acid region in the NH2-terminal unique domain. Here we investigated the role of Lyn A and B isoforms in mast cell signaling and responses. Lyn B was found to be a poor inducer of mast cell degranulation and was less potent in both IP3 production and calcium responses. Expression of Lyn B alone showed reduced phosphorylation of both phospholipase Cγ-1 and 2 and decreased interaction of phospholipase Cγ-1 with the phosphorylated linker for activation of T cells. Lyn B also showed increased binding of tyrosine phosphorylated proteins, which included the negative regulatory lipid phosphatase SHIP-1. In contrast, both Lyn A and B caused similar total cellular tyrosine phosphorylation and FcεRI phosphorylation and neither Lyn A nor Lyn B alone could completely restore mast cell degranulation or dampen the excessive cytokine production seen in the absence of Lyn. However, expression of both isoforms showed complementation and normalized responses. These findings demonstrate that Lyn B differs from Lyn A in its association with SHIP-1 and in the regulation of calcium responses. However, complementation of both isoforms is required in mast cell activation.
Introduction
Mast cells are important innate immune cells that can amplify the adaptive immune response (1). They are also known as the central effector cell in IgE-mediated allergic and inflammatory disorders. In an allergic reaction, mast cell activation is initiated through the recognition of an antigen (Ag) by antigen-specific IgE bound to the α subunit of the high affinity IgE receptor (FcεRI), which is expressed on the cell surface. The Src family protein tyrosine kinase (Src PTK) Lyn provides the key recognition signal that inteprets receptor engagement into intracelluar events by transphosphorylating the FcεRI β and γ subunits (2). Efficient phosphorylation of the FcεRI requires specialized regions of the cell membrane that are enriched in cholesterol and sphingolipids (commonly termed lipid rafts) as both Lyn and FcεRI can be concentrated in these domains upon receptor engagement (3). Phosphorylation occurs within the cytoplasmic tails of the β and γ subunits in a domain that encodes the immunoreceptor tyrosine-based activation motif (ITAM), which is characterized by a YXXL-X7-YXXL amino acid sequence (4). Once phosphorylated, phospho-ITAMs constitute a novel docking site for the binding and subsequent activation of Src homology 2 (SH2)-domain containing molecules, such as the spleen tyrosine kinase (Syk), a tyrosine kinase that is crucial for mast cell activation (5). The activation of Syk results in the phosphorylation of multiple substrates among which the membrane-localized linker for activation of T cells (LAT) coordinates the assembly of a molecular complex that includes proteins like phospholipase C (PLC)-γ. PLCγ catalyzes the hydrolysis of phosphatidylinositol-4,5-biphosphate to inositol 1,4,5-triphosphate (IP3) and diacylglycerol (DAG). IP3 binds to its receptors on the endoplasmic reticulum promoting calcium release from the intracellular stores, which upon emptying trigger calcium influx from the extracellular environment via store-operated calcium channels like Orai1/CRACM (6, 7). DAG binds the C1 domain of a number of proteins (like protein kinase C (PKC)) promoting their membrane localization and activity. Both the calcium influx and PKC activation are essential for the release of preformed granule-stored allergic mediators and the de novo synthesis of cytokines and eicosanoids from mast cells (8, 9).
As the key initiating kinase the therapeutic targeting of Lyn is of interest, since intervening at this step should presumably abrogate mast cell activation. However, recent studies suggest that Lyn has both positive and negative regulatory roles (10–12)and, in the context of a particular genetic background, Lyn-deficiency could result in either reduced or increased mast cell degranulation and anaphylactic responses (13, 14). In mast cells as well as in other cell types (15), Lyn kinase exists as two isoforms, Lyn A and Lyn B of 56 and 53 kDa, respectively. These isoforms are generated by alternative splicing and differ by a 21 amino acid insert found in the NH2-terminal unique domain of Lyn A (Figure 1A) (16). Prior studies have shown that both Lyn A and Lyn B co-immunoprecipitate with FcεRI (17). In addition, both isoforms can be found in lipid rafts (18). In mast cells derived from a mouse model of Smith-Lemli-Opitz Syndrome (an inborn error of cholesterol metabolism leading to loss of cholesterol from lipid rafts) both isoforms of Lyn are lost from lipid rafts and these mast cells showed a hyperresponsive phenotype (19). While distinguishing the individual roles of Lyn A and Lyn B (or whether one isoform has a more dominant negative function) is of considerable interest, this was not feasible by means of silencing (si)RNA or genetic deletion strategies, as both isoforms arise from a single gene and differ in a relatively short stretch of nucleotide sequence.
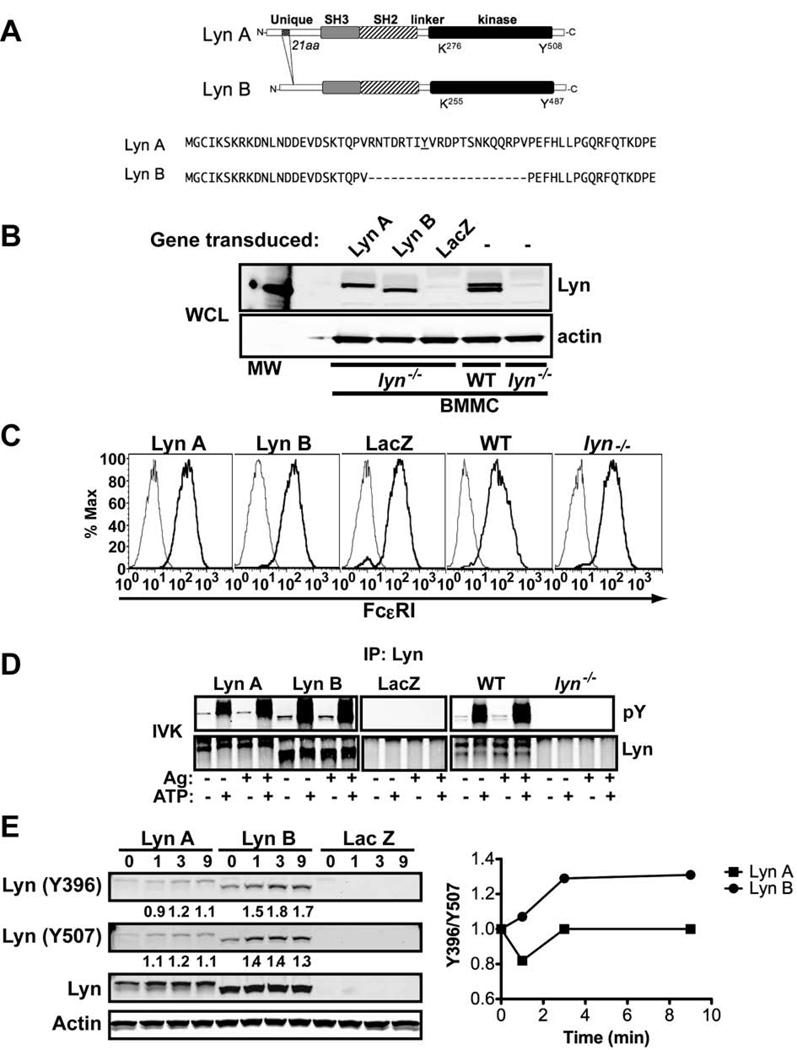
Figure 1. Lyn A and Lyn B isoforms and their expression in Lyn-null mast cells.
(A) Schematic representation of Lyn isoforms with the sequence of the 21 amino acid insert found in Lyn A, but not in Lyn B, indicated. (B) Protein expression of Lyn A and Lyn B relative to wild type (WT) mast cells. (C) Analysis of FcεRI expression on cells transduced with lentiviral constructs of Lyn A and Lyn B after blasticidin selection and cell differentiation (5 weeks) in IL-3 and SCF. (D) Activity of Lyn A and Lyn B as measured by in vitro kinase assays (autophosphorylation). The activity of Lyn A and Lyn B isoforms is not impaired by their individual expression and is regulated by ATP. (E) Immunoblot of the phosphorylation status of the activation loop Y396 (using an antibody to human Lyn Y416) and of inhibitory Y507 on Lyn A and Lyn B isoforms, upon FcεRI stimulation. Graph shows the ratio of Y396/Y507 for both isoforms. (B–E) A representative experiment is shown.
Here, we investigate the roles of the two Lyn isoforms by expressing each individually or together in mast cells derived from lyn−/− mice. Our results show unique properties as well as redundant roles for the Lyn A and B isoforms. Lyn B was found to dominantly associate with the negative regulatory lipid phosphatase SHIP-1 and was less effective in eliciting calcium responses and mast cell degranulation. However, Lyn B was equivalent to Lyn A in total cellular tyrosine phosphorylation and FcεRI phosphorylation. Lyn A showed stronger calcium responses and degranulation than Lyn B and was better in promoting the interaction of PLCγ with phospho-LAT. Importantly, expression of each individual isoform did not fully restore calcium fluxes or degranulation nor controlled hyper-cytokine production, but expression of both isoforms fully normalized these responses. The findings demonstrate differences in the role of these two Lyn kinase isoforms in mast cell signaling and responses. However, they also demonstrate a requirement for both Lyn A and Lyn B in generating normal mast cell responses.
Materials and Methods
Animals
Mice used in the present study were wild type (WT) and lyn−/− (129/Sv, or 129/Sv x C57BL/6 (N8)) which were bred in-house. Animals were maintained and used according to National Institutes of Health (NIH) guidelines and a National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) approved animal study proposal.
Antibodies
Mouse anti-DNP mAb was purified from culture supernatants of the H1-DNP-e-26.82 hybridoma (20). Mouse monoclonal antibody to FcεRIβ was previously described (21). Rabbit polyclonal antibody to GST or Lyn were purchased from Invitrogen (Carlsbad, CA) or Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Anti-GST, anti-SHIP-1, anti-PLCγ1 and PLCγ2, anti-phospho PLCγ1 (Y783), anti-phospho Syk (Y352), anti-phospho-LAT (Y191) antibodies were purchased from Cell Signaling Technology (Boston MA). Anti-phosphotyrosine 4G10 mAb was from Upstate Biotechnology, Inc. (Lake Placid, NY). Anti-mouse Ig linked to infrared fluorescent dye IR800 (Rockland Immunochemicals, Philadelphia, PA) and anti-rabbit Ig linked to Alexa Flour 680 (Invitrogen, Carlsbad, CA) was used as secondary antibodies for western blotting.
Cell cultures
Total bone marrow from the femurs of lyn−/− and wt 6-week old female mice was extracted and used to obtain cultures of bone marrow-derived mast cells (BMMC). Cells were cultured for 4 weeks in the presence of IL-3 and SCF (PeproTech Inc., Rocky Hill, NJ). Cultures were checked periodically for mast cell differentiation and purity by FcεRI and c-Kit expression. Cells were used when cultures achieved ≥ 95% of the population expressing both markers.
Lentiviral expression constructs
To generate expression construct expressing Lyn A, murine cDNA was amplified using the specific primers: LynA sense: 5’ATG GGA TGT ATT AAA TCA AAA AGG AAA G 3’, Lyn A antisense: 5’ CTA CGG TTG CTG CTA TAC TG 3’. Lyn B was subsequently generated by deleting the 63 nucleotides encoding the 21 amino acid insert using the primers; Lyn B sense: 5’ AAG ACT CAA CCA GTA CCT GAA TTT CAT CTT 3’, Lyn B antisense: 5’ AAG ATG AAA TTC AGG TAC TGG TTG AGT CTT 3’. Both Lyn A and Lyn B were then cloned into pLenti6/V5-TOPO vector using the Gateaway technology kit following manufacturer's instructions (Invitrogen).
Lentiviral gene transduction
Supernatants containing virus were harvested after a 48h transfection of 293FT cells with Lyn A or B constructs (Cell Biolabs) as previously described (22). Viral particles were concentrated 25X by centrifugation for 2h at 20,000 × g. BMMCs (10 × 106) were incubated with the viral preparations, medium was changed after 24h, and selection was started 48h later with 8µg/ml blasticidin (Invitrogen). Expression of Lyn A or Lyn B was analyzed by western blot 2 weeks after selection.
Ag stimulation, immunoprecipitation and western blotting
For Ag stimulation, BMMCs were sensitized at 37°C for 3h with 1µg/ml IgE in RPMI without cytokines. Cells were washed and stimulated at 37°C with 100ng/ml Ag (DNP-BSA), or with the indicated concentration, in Tyrode’s buffer (135mM NaCl, 5mM KCl, 20mM Hepes, 5.6mM glucose, 1mM MgCl2, 1.8mM CaCl2, 0.05% BSA, pH 7.4). Stimulation time varied with the type of experiment as indicated. Cell lysates were prepared by incubation of 20 × 106 cells in 1ml of BSS buffer containing 0.5% Triton X-100 and 20 mM Octyl β-D-glucopyranoside (Sigma-Aldrich St. Louis, MO) on ice for 15 min. Lysates were clarified by centrifugation for 15 min at maximun speed at 4°C, and supernatants collected. Immunoprecipitation was performed with Protein A (GE Healthcare) and the indicated antibodies by incubation of cell lysates for 3h at 4°C in rotation. Proteins were resolved in 10% NuPAGE Bis-Tris gels and transferred to nitrocellulose membranes (Invitrogen). For western blotting, the membranes were blocked for 1h at room temperature with Odyssey blocking buffer (Li-COR Biosciences, Lincoln, NE), diluted 2X in PBS. Membrane were then incubated with the indicated primary antibodies, followed by secondary antibodies linked to infrared fluorescence dyes. After washings with PBS-0.1% Tween-20 (Sigma), blots were analyzed in the Odyssey Infrared Imaging system (Li-COR).
Measurement of IP3 production and calcium fluxes
Intracellular IP3 was extracted with 20% cold perchloric acid from IgE-sensitized cells (10 × 106/sample) challenged with 25 ng/ml Ag. The acidified samples were centrifuged at 2000 × g for 15 min at 4°C, and the supernatants were neutralized with a 1:1 (v/v) mixture of 1,1, 2-trichlorotrifluoroethane:tri-noctylamine. The concentration of IP3 in each sample was measured with a competitive binding assay (IP3-[3H] Biotrak Assay System, Amersham Biosciences).
To measure calcium mobilization two methods were used. In some experiments, cells were sensitized overnight and loaded with 3 µM FURA-2AM for 20 min, washed, and aliquoted in 96-well plates (9 × 104 cells/well). Cells were then challenged with Ag (25 to 50 ng/ml), and changes in intracellular calcium were monitored with a microplate fluorescence reader (Opti-Fluor; BMG Labtechnologies) as previously described (23). In some experiments, single cell calcium imaging was performed using a Zeiss LSM510 META confocal microscope (Carl Zeiss MicroImaging, Inc.) The procedure was essentially described (24). Briefly, 5 × 106 BMMCs were sensitized with 0.5 mg/ml IgE for 2h at 37°C in Hepes-Tyrode’s buffer. Cells were loaded with 1.5µM Fluo-4 AM and 5µM Fura-Red AM (Invitrogen), plated on coverslips and incubated for 30 min at 37°C in the dark. Ag was added after capture of the first ten images. A total of seventy-five images at 5 s intervals were captured. The mean of the fluorescent signal of all cells from a field was obtained with the Zeiss LSM510 META software. Data was plotted as a Fluo-4/ Fura Red ratio.
β-Hexosaminidase release assays
Mast cells degranulation was measure by the release of the enzyme β-hexosaminidase as described elsewhere (25). Briefly, cells were sensitized for 2h with 1µg/ml anti-DNP IgE in medium without growth cytokines. Cells were stimulated for 30 min with varying concentrations of Ag (ranging from 0.1 to 1000ng/ml) in Tyrode’s buffer followed by supernatant collection. β-hexosaminidase released into supernatants was measured by incubation with substrate and quantified colorimetrically as described (Blank and Rivera).
Cytokine production
For cytokine secretion analysis, 1 × 106 BMMCs were incubated with anti-DNP IgE as described above and stimulated with 100ng/ml DNP for 4 h. Supernatants were collected and the amounts of IL-6, IL-13 and TNF-α were measured by Millipore Cytokine Multiplex Panel (Millipore Corporation, Billerica, MA).
Statistical analysis
Statistical significance of observed differences (P value) was assessed by two tailed Student's t- test using the Prism software (Graph Pad, CA).
Results
Expression of the individual isoforms, Lyn A or Lyn B, does not alter FcεRI and Syk expression or Lyn-dependent phosphorylation
Lyn A and Lyn B isoforms (Figure 1A) were expressed in BMMCs derived from lyn−/− mice. Stable expression was achieved after two weeks of blasticidin selection, as decribed in Materials and Methods, with the protein levels being comparable to those found in WT mast cells (Figure 1B). Expression of Lyn A or Lyn B alone caused no significant change in mast cell differentiation and FcεRI expression (Figure 1C). Given the extensive regulatory crosstalk among SrcPTKs (26), we verified that each individual isoform was active when expressed individually. The kinase activity, after immunoprecipitation of each isoform from cells expressing them individually, was determined in an in vitro kinase assay and compared to the activity in WT cells. As shown in Figure 1D, each individual isoform was active only in the presence of ATP. Ag stimulation caused only a minimal increase in Lyn activity for both isoforms as measured in vitro, consistent with previously published reports (13), suggesting that large fractions of both Lyn A and B can be readily activated by the presence of ATP in the absence of FcεRI engagement. No kinase activity was found in the immunoprecipitates from Lyn-null mast cells whether transfected to express LacZ or not (Figure 1D). Thus, expression of the individual Lyn isoforms did not appear to alter their intrinsic kinase activity. To assess the relative activation of Lyn A and B in cells, we measured the phosphorylation of the activation loop Y396 (as recognized by an antibody to Y416 in human Lyn) versus the inhibitory Y507 prior to and after FcεRI-stimulation. Figure 1E shows that, when normalized for the amount of protein expressed, the FcεRI-dependent phosphorylation of Lyn B at Y396 is more prominent than that of Lyn A. Thus, the ratio of Y396/Y507 increased after FcεRI stimulation whereas the fraction of Lyn A phosphorylated at Y396 showed an initial decrease and a subsequent return to baseline levels. This suggested that Lyn B may be more active in mast cells, but the fraction of Lyn A and Lyn B that is involved in FcεRI stimulated mast cell responses is unknown and this would likely determine their relative activities in vivo.
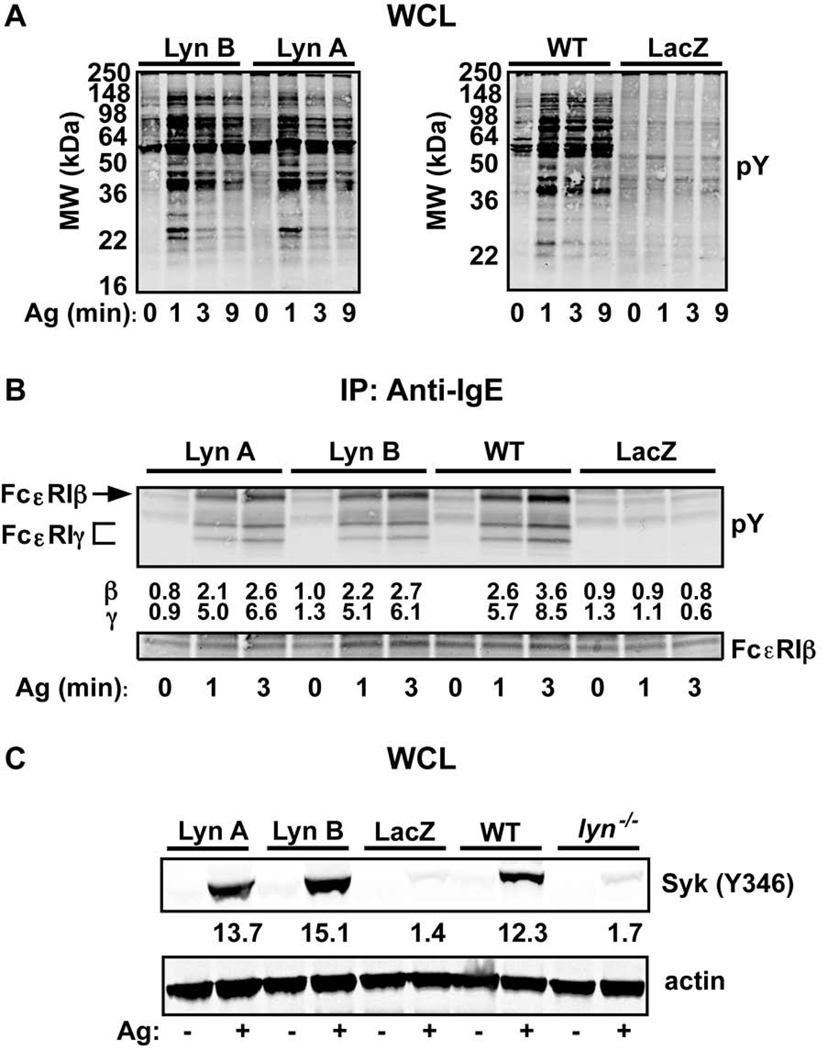
As shown in the control LacZ-transduced lyn−/− BMMC (Figure 2A), and also as previously reported (10), the absence of Lyn kinase caused a marked reduction in the total cellular tyrosine phosphorylation in resting and FcεRI-stimulated mast cells. This demonstrates that the dectable tyrosine phosphorylation seen in mast cells is highly dependent on Lyn activity. Therefore, it was of interest to determine if the profile of tyrosine phosphorylated proteins might differ considerably in cells individually expressing Lyn A or Lyn B. As shown in Figure 2A, the total cellular tyrosine phosphorylation did not differ markedly between the two isforms and the gross pattern of phosphorylation observed was similar (albeit not identical) to that of WT mast cells. Since Lyn is the kinase responsible for ITAM phosphorylation of β and γ chains of FcεRI, we evaluated the relative contribution of the Lyn isoforms to FcεRI tyrosine phosphorylation, following the stimulation of this receptor. As shown in Figure 2B, both Lyn A and Lyn B were equally effective in the phosphorylation of the FcεRI β and γ chains, albeit at levels that appeared to be slightly less than that of WT mast cells. Quantitation of the phosphorylation of the FcεRI β and γ chains for all experiments showed that a 3 min post-stimulation the β chain in WT mast cells had a significantly higher phosphorylation (30%) than in Lyn A or Lyn B expressing cells. However, although a trend for increased phosphorylation of the FcεRI γ chain at 3 min was observed, no significant difference was found for the phosphorylation of the FcεRI γ chain and the kinetics of FcεRI phosphorylation were unaltered upon expression of either isoform when compared to WT cells. Thus, Lyn A and Lyn B were equally efficient in the phosphorylation of FcεRI and showed similar ability in inducing total cellular tyrosine phosphorylation.
Figure 2. Protein tyrosine-, FcεRI-, and Syk (Y346) phosphorylation is similar in Lyn A and Lyn B expressing cells.
(A) Total protein tyrosine phosphorylation (pY) in whole cell lysates (WCL) of cells expressing Lyn A, Lyn B, LacZ (negative control), and from wild type (WT) cells. Kinetic analysis following FcεRI stimulation for the indicated times. (B) Tyrosine phosphorylation (pY) kinetics of stimulated (for the indicated time) FcεRI immunoprecipitated from cells expressing Lyn A or Lyn B and compared to WT or LacZ controls. Fold induction (for β and γ) normalized to the 0 min time point for WT cells (=1.0) is shown. (C) Phosphorylation of Syk at Y346 prior to or after FcεRI stimulation (3 min) in cells expressing Lyn A, Lyn B, LacZ as compared to Lyn-null (lyn−/−) or WT cells. An antibody that detects phosphorylation of the Y352 of human Syk was used to determine the phosphorylation status of the equivalent mouse residue (Y346). Quantitation of all experiments was by densitometry and data is shown in panel C. One representative of a minimum of four experiments (A–C) is shown.
Given that FcεRI phosphorylation is essential for the activation of Syk kinase (5), and that phosphorylation of mouse Syk on Y346 (which is recognized by an antibody to human Syk Y352, the equivalent site on human Syk) requires Lyn kinase (13), we investigated the effect of Lyn A and Lyn B on phosphorylation of Syk. As shown in Figure 2C, phosphorylation of Syk at 3 min post-stimulation occurred efficiently in cells expressing Lyn A or Lyn B and appeared to be comparable to that seen in WT cells. Varying doses of Ag did not reveal any difference in Syk Y346 phosphorylation between Lyn A and Lyn B expressing cells (data not shown). Thus, it is clear that the extent of Syk phosphorylation at Y346 did not differ significantly between Lyn A and Lyn B (Figure 2C), arguing that both isoforms can promote Lyn-dependent phosphorylation of this site. This was verified, since cells expressing LacZ or Lyn-null cells showed minimal phosphorylation of Y346, consistent with our previous results (13). Thus, both Lyn A and Lyn B are capable of causing Syk phosphorylation at the Y346 site in a manner similar to that of WT cells, consistent with the ability of each isoform to similarly elicit FcεRI phosphorylation.
Lyn A and Lyn B differ in promoting mast cell degranulation but not cytokine production
We assayed cells expressing the individual Lyn isoforms for their ability to promote FcεRI-mediated degranulation, as measured by release of granule-localized enzyme β-hexosaminidase. Our past studies (11, 13) demonstrated that Lyn-null mast cells derived from 129/Sv mice or from early crosses to C57BL/6 mice showed hyperresponsive degranulation. In contrast, mast cells from Lyn-null C57BL/6 mice or mice backcrossed extensively (N8) into this background showed hyporesponsive degranulation when compared to WT mice. This difference was due, in part, to the relative levels of Fyn expression, as C57BL/6 mice showed lower levels of Fyn kinase than and hyperdegranulation was restored by overexpression of this kinase (13).
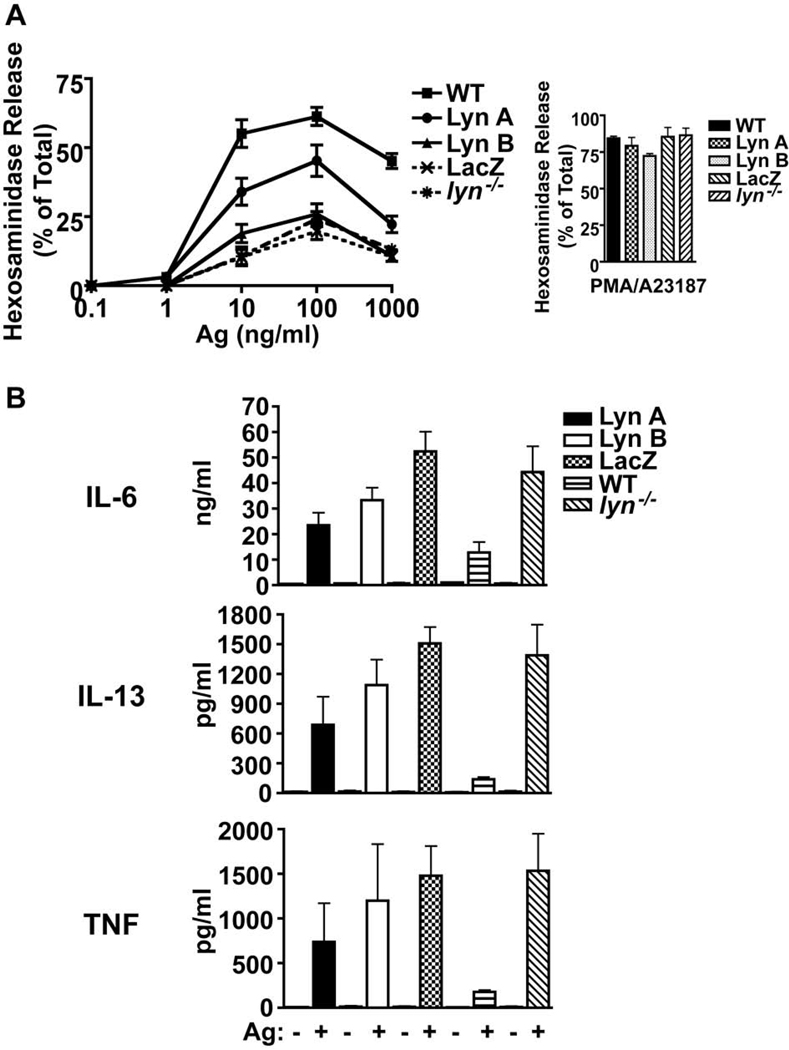
At the onset of these studies, we decided to use lyn−/− BMMC derived from mice backcrossed extensively (N8) into C57BL/6 background. These cells showed a marked loss of FcεRI-induced degranulation (Figure 3A), thus allowing us to determine if Lyn A or Lyn B or both isoforms were needed to promote degranulation. Cells expressing Lyn A or Lyn B showed reduced degranulation response when compared to WT cells (Figure 3A), albeit the impairment observed in cells expressing Lyn B was more marked. While Lyn A-expressing cells also showed a reduction in their degranulation response, in some experiments these cells approached the degranulation response of WT cells. Moreover, Lyn A-expressing cells consistently showed a degranulation response that was considerably higher than Lyn B-expressing cells. The impaired degranulation of Lyn B-expressing cells was not due to a deficiency in granule content or to a developmental defect in the granule fusion apparatus since PMA and calcium ionophore (A23187) elicited a similar and potent degranulation from all cells, regardless of whether they expressed the individual Lyn isoforms or none at all (Figure 3A).
Figure 3. Lyn A and Lyn B expressing mast cells differ in the extent of degranulation and each individual isoform fails to dampen cytokine production.
(A) Mast cell degranulation (as measured by release of hexosaminidase) is impaired in both Lyn A and Lyn B expressing cells relative to wild type (WT) cells. However, Lyn A elicits a more robust degranulation response at all antigen (Ag) concentrations tested than Lyn B. PMA and calcium ionophore (A23187) elicit degranulation of similar magnitude from the indicated genotypes. Data shown is a compilation of four experiments from individual cultures. More than ten experiments were performed. (C) Cytokine production (as measured by secretion into the medium) is hyperresponsive to FcεRI stimulation (4 hrs) in all genotypes (Lyn A, Lyn B, LacZ, and lyn−/−) relative to WT cells. The amount produced is normalized to 106 cells. Data shown (mean ± SD) is compiled from 3 individual experiments.
Previous work (11) demonstrated that the loss of Lyn kinase led to increased cytokine responses in mast cells and that this phenotype was independent of the genetic background of the mice from which the cells were derived (13). Thus, we explored if the different isoforms of Lyn had differing roles in suppressing cytokine responses. In these experiments we used BMMC’s derived from lyn−/− 129/Sv mice because of their potent cytokine responses relative to BMMC’s from lyn−/− C57BL/6 mice. We reasoned that a more potent cytokine response might allow us to more easily ascertain the relative contributions of Lyn A and Lyn B to this response. As shown in Figure 3B, both Lyn A and Lyn B showed a similar loss in the ability to suppress the hyper-production of IL-6, IL-13 or TNF, similar to Lyn-null mast cells but markedly differing from WT cells. Neither isoform alone was able to completely restore the negative control of cytokine production to the levels seen in WT mast cells. However, cells expressing Lyn A showed a trend (albeit not significant) towards dampening of the hyper-cytokine response than those expressing Lyn B. Thus, the findings show that Lyn A more effectively promotes mast cell degranulation and is seemingly better in dampening cytokine responses.
Lyn B is less effective than Lyn A in evoking Ca2+ responses and optimal IP3 production following FcεRI engagement
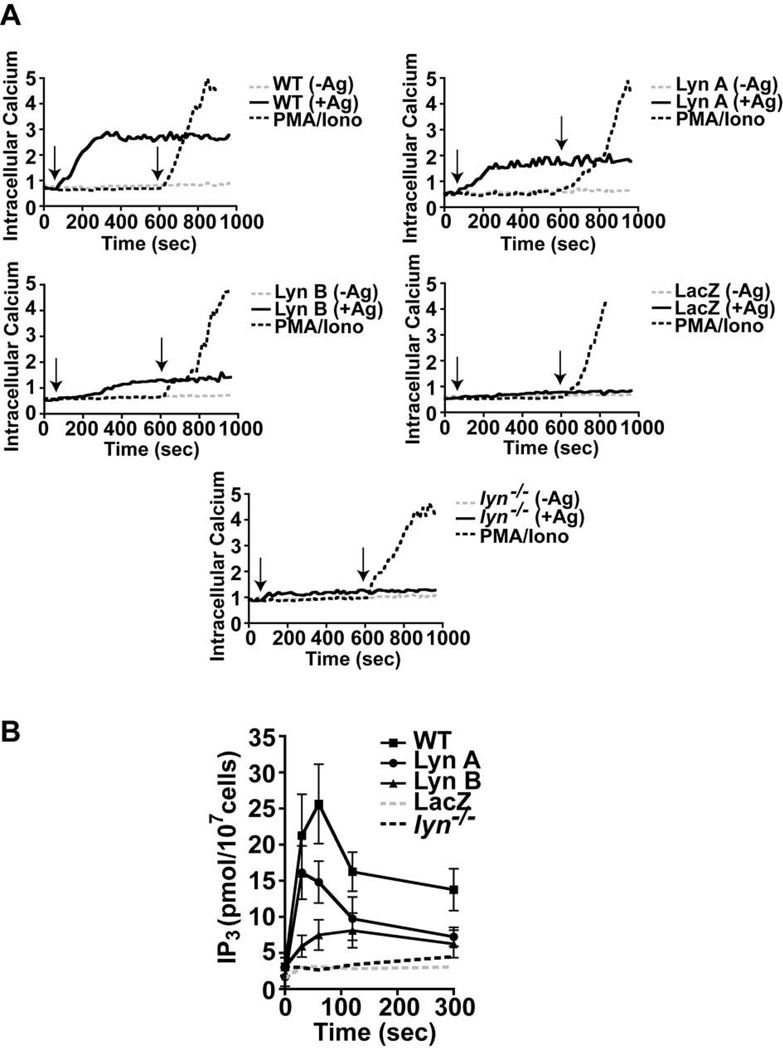
To explore the underlying mechanism for the observed difference between Lyn A and Lyn B in inducing mast cell degranulation, we investigated whether the two isoforms were similarly able to induce intracellular Ca2+ mobilization upon FcεRI stimulation. A rise in the intracellular Ca2+ concentration that is coupled to the influx of Ca2+ from the extracellular medium is essential for the degranulation response (9). As shown in Figure 4A, both Lyn A and Lyn B showed altered Ca2+ fluxes when compared to WT cells. For both isoforms the onset and extent of the calcium response was impaired. Lyn B-expressing cells showed the most marked delay in Ca2+ fluxes relative to Lyn A expressing or WT cells. Nonetheless, in many experiments the Ca2+ flux of Lyn A-expressing cells was also delayed when compared to WT cells and the extent of the response appeared to be diminished for both Lyn A- and Lyn B-expressing cells. Relative to the Ca2+ flux of LacZ or Lyn-null mast cells, both Lyn A and B elicited a significant rise in intracellular Ca2+ concentration and all the analyzed transductants showed a marked increase in intracellular Ca2+ concentrations when stimulated by PMA and the calcium ionophore A23187 (Figure 4A).
Figure 4. Lyn A and Lyn B differ in generating inositol 3, 4, 5-trisphosphate (IP3) and inducing Ca2+ fluxes following FcεRI engagement.
(A) Relative induction (340/380 ratio) of intracellular Ca2+ fluxes in cells expressing Lyn A, Lyn B, and LacZ as well as wild type (WT) and Lyn-null (lyn−/−) mast cells stimulated (+) or not (−) with antigen (Ag). Time of addition of Ag is indicated by the left arrow whereas the time of stimulation with PMA and calcium ionophore (A23187) is indicated by the right arrow. One representative of seven experiments from 4 individual cultures is shown. (B) Measurement of IP3 production after FcεRI stimulation in cells expressing Lyn A, Lyn B, LacZ compared to WT or lyn−/− mast cells. Data is from three experiments using individual cell cultures.
Since the production of IP3 is key for the mobilization of Ca2+ from intracellular stores, which subsequently leads to extracellular Ca2+ influx, the role of Lyn A and Lyn B in IP3 production was also explored. These experiments showed that both Lyn A or Lyn B alone were unable to generate IP3 at levels comparable to that of WT cells, following FcεRI stimulation (Figure 4B). For Lyn B, there was a marked impairment in the onset and extent of IP3 production relative to WT cells. In contrast, it was found that WT and Lyn A-expressing cells showed a similar early rise in IP3 production, although the extent of the response appeared to be diminished in Lyn A-expressing cells. Thus, the findings demonstrate that Lyn A and Lyn B differ in their ability to induce IP3 production and Ca2+ fluxes, consistent with their observed ability to elicit mast cell degranulation.
Lyn A and Lyn B differ in their ability to associate with phosphoproteins and LAT
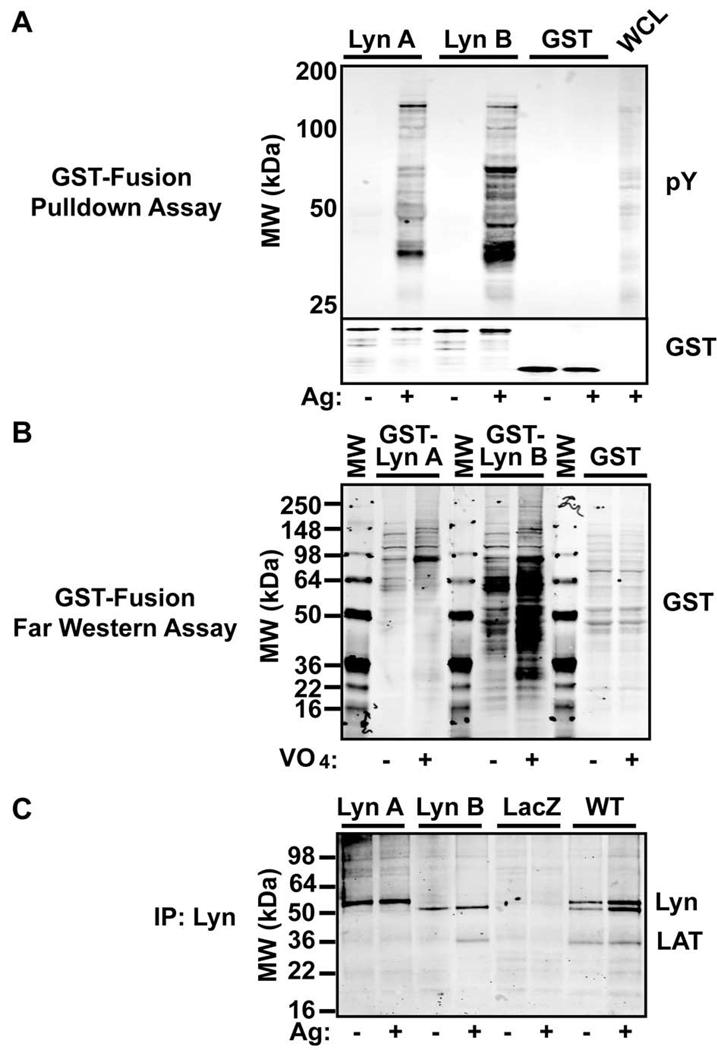
The difference of Lyn A and Lyn B in promoting IP3 production and Ca2+ fluxes suggested that these isoforms might differ in protein-protein or protein-lipid interactions required for these events. To explore such possibilities, we first assessed whether the two isoforms could co-immunoprecipate similar profiles of tyrosine phosphorylated proteins. Figure 5A shows that GST-fusion proteins of Lyn A and Lyn B differed in their binding to tyrosine phosphorylated proteins from FcεRI stimulated cell lysates. Relative to Lyn A, Lyn B showed enhanced binding to tyrosine phosphorylated proteins, including a prominent band with the molecular mass of LAT. This was further demonstrated by Far Western. Cells were stimulated with pervanadate (to maximize the abundance of phosphorylated proteins) and the total cellular proteins were transferred to nitrocellulose and probed with GST-fusion proteins encoding Lyn A and Lyn B without their catalytic domains. As shown in Figure 5B, GST-Lyn B bound more effectively than Lyn A to tyrosine phosphorylated proteins in cell lysates from both non-stimulated and stimulated cells. Collectively, the results show that Lyn B forms stronger or more abundant interactions with tyrosine phosphorylated proteins than Lyn A. To further explore whether these differences might be seen in cells, we immunoprecipitated Lyn A or Lyn B or both from cells expressing them individually or from WT cells, respectively. Figure 5C shows that a dominant band identified as LAT was found to co-immunoprecipiate with Lyn B and not Lyn A (albeit weak interactions with Lyn A could be seen in some experiments). LAT was also found to co-immunoprecipitate with Lyn kinase from WT cells and was present in resting cells, though at reduced levels when all experiments were analyzed.
Figure 5. Lyn B shows increased binding of phosphoproteins, like LAT, when compared to Lyn A.
(A) GST-fusion proteins (10 µgs) encoding Lyn A and Lyn B (without the catalytic domain) were used in pull down assays from lysates derived from mast cells stimulated (+) or not (−) with antigen (Ag). Interacting phosphoproteins were resolved on SDS-PAGE and immunoblotted with an antibody to phosphotyrosine (pY). GST alone was used as a control and a sample of whole cell lysate (WCL) from stimulated cells was included as a control for testing anti-phosphotyrosine antibody recognition. (B) Proteins in the cell lysates (75 µg/lane) from cells stimulated (+) or not (−) with pervanadate (VO4) were resolved by SDS-PAGE and transferred to nitrocellulose membranes. GST-Lyn A, GST-Lyn B, or GST were used to probe the membranes (Far Western) and binding was detected by use of an antibody to GST. (C) Immunoprecipitation of Lyn isoforms from Ag stimulated (+) or resting (−) mast cells expressing Lyn A, Lyn B, LacZ, and from wild type (WT) cells. Bands corresponding to the Lyn isoforms and LAT were detected with the respective antibodies.
Lyn B forms a stable complex with SHIP-1 and decreases the interaction of PLCγ with phosphorylated LAT
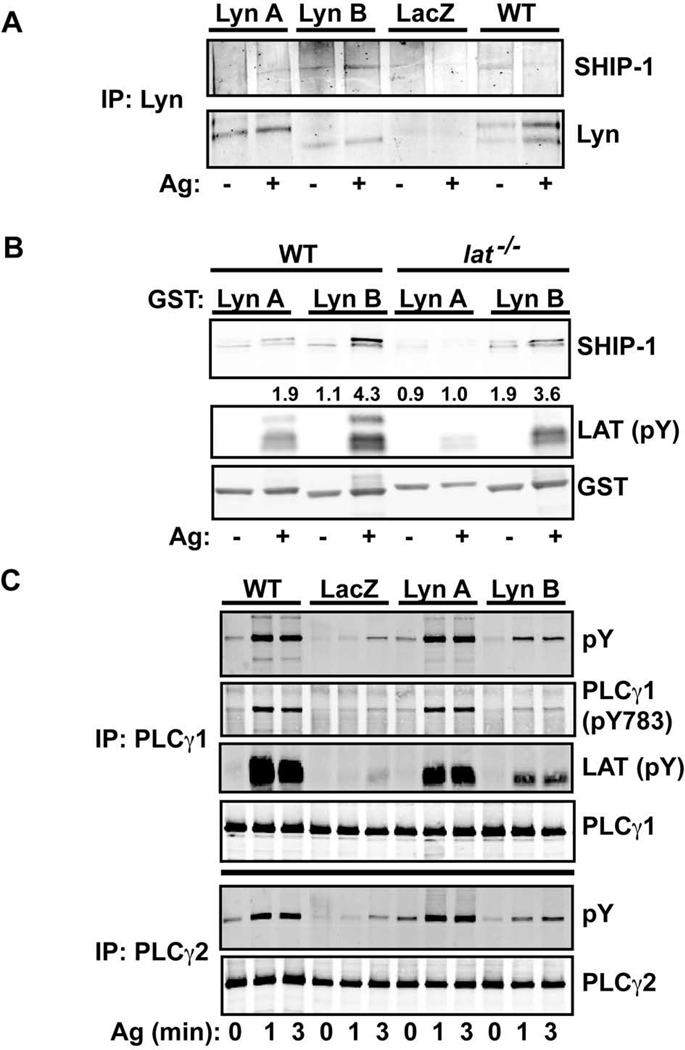
Among the many proteins described to interact with Lyn kinase, the lipid phosphatase SHIP-1 is key in controlling the levels of PIP3 following FcεRI stimulation (27). SHIP-1 has been shown to interact with PLCγ1 and modulate its activation (28). Lyn is also required for the phosphorylation of the Tec family kinase BTK (29), which was shown to be required for full phosphorylation of PLCγ and increased activity (29, 30). Since both BTK and PLCγ require PIP3 for their membrane localization and activation, we explored if the Lyn isoforms showed differences in co-immunoprecipitation of SHIP-1, which might explain differences in promoting calcium responses. Figure 6A shows that Lyn B binds SHIP-1 more efficiently than Lyn A following FcεRI stimulation of mast cells expressing the respective kinases. Tyrosine phosphorylation of SHIP-1 could be seen in the Lyn B co-immunoprecipitates whereas it was difficult to detect in Lyn A co-immunoprecipitates (data not shown). This interaction was confirmed in pulldown assays where a GST-Lyn B fusion protein showed the increased presence of SHIP-1 relative to a GST-Lyn A fusion protein (Figure 6B). Moreover, a GST-Lyn SH2 domain construct showed similar efficiency in its interaction with SHIP-1 (data not shown), demonstrating that this domain is key in promoting this interaction. Because of these findings and the observation that Lyn B associates more prominently with LAT than Lyn A, we explored if LAT was required for the interaction of Lyn B with SHIP-1. Figure 6B shows that the absence of LAT had only a minor effect on Lyn B-SHIP-1 interactions. A more marked effect was seen for Lyn A, particularly after FcεRI stimulation. Moreover, a phosphoprotein of the molecular mass of NTAL/LAB/LAT2 (the antibody recognizing Y191 of LAT also recognizes an equivalent site on LAT2) was also more prominently associated with Lyn B. Regardless, the association of Lyn B with SHIP-1 was not significantly altered by the absence of LAT, showing that this interaction was not LAT-dependent.
Figure 6. SHIP-1 association with Lyn B is prominent and PLCγ phosphorylation and its association with LAT1 is impaired in Lyn B-expressing mast cells.
(A) Immunoprecipitation of Lyn from mast cells, stimulated (+) or not (−) with antigen (Ag), that were expressing Lyn A, Lyn B, or LacZ in comparison to wild type (WT) cells. Resolved proteins were immunoblotted with antibodies to SHIP-1 or Lyn. One of three representative experiments is shown. (B) GST-fusion proteins encoding Lyn A and Lyn B (without the catalytic domain) were used in pull down experiments to detect interacting proteins derived from lysates of Ag stimulated (+) or not (−) WT or lat−/− mast cells.
SHIP-1 was recognized by antibody to this protein. Phospho-LAT1 antibody (Y191) and LAT1-deficiency was used to recognize LAT1. This antibody also reacts with phospho-LAT2 (NTAL/LAB), which is the remaining band in the immunoblot from lat−/− mast cell lysates. Antibody to GST was used as a control for the amount of GST-fusion proteins used in this assay. One representative of four experiments is shown. Fold increase of SHIP-1 interaction with Lyn A or Lyn B is shown and is normalized to the interaction of SHIP-1 with Lyn A from non-stimulated cells. (C) Immunoprecipitation of PLCγ1 and 2 from mast cells expressing Lyn A, Lyn B, LacZ, in comparison to WT cells.
Phosphorylation of PLCγ1 and 2 was assessed with an antibody to phosphotyrosine (pY) and also (for PLCγ1) by phosphorylation at Y783 (whose phosphorylation is required for PLCγ activity) of this protein. Association of phosphorylated LAT with PLCγ1 was determined by the antibody to LAT (Y191). One representative of five experiments from three individual cultures is shown.
Since Lyn B was less effective than Lyn A in inducing Ca2+ fluxes, we further explored if Lyn B-expressing cells might be less efficient in causing PLCγ phosphorylation, which would be consistent with the marked reduction in IP3 production (Figure 4B). As shown in Figure 6C, tyrosine phosphorylation of PLCγ1 and PLCγ2 was markedly diminished (albeit not completely absent) in Lyn B-expressing cells when compared to Lyn A or WT cells. In general, cells expressing Lyn A showed were also slightly impaired in PLCγ1 phosphorylation though this was not apparent in all experiments. Strikingly, the amount of phosphorylated LAT co-immunoprecipitating with PLCγ1 was reduced in cells expressing Lyn B relative to WT cells (Figure 6C). Lyn A-expressing cells also showed less binding of phospho-LAT to PLCγ but this interaction was less affected relative to Lyn B-expressing cells. Thus, the findings demonstrate that Lyn B-expressing cells have an impaired phosphorylation of PLCγ1 and 2 and a reduced association with phosphorylated LAT relative to Lyn A or WT cells. Since Lyn kinase has been reported to phosphorylate the protein tyrosine phosphatase SHP-1, we explored the possibility of differential control of this phosphatase by Lyn A and Lyn B, which might explain the differences in the level of LAT and PLCγ phosphorylation. Co-immunoprecipitation experiments revealed no significant association of SHP-1 with Lyn A or B, under conditions where SHIP-1 was detected, and no difference in the phosphorylation of Y536 on SHP-1 (the site phosphorylated by Lyn) was observed (data not shown).
This demonstrates that the mechanism underlying the the ability of the two Lyn isoforms to evoke a Ca2+ response lies in their relative capacity to activate PLCγ and IP3 production and this appears to be independent of a role in SHP-1 phosphorylation.
Complementation of Lyn A and Lyn B activity is required for normal mast cell activation and responses
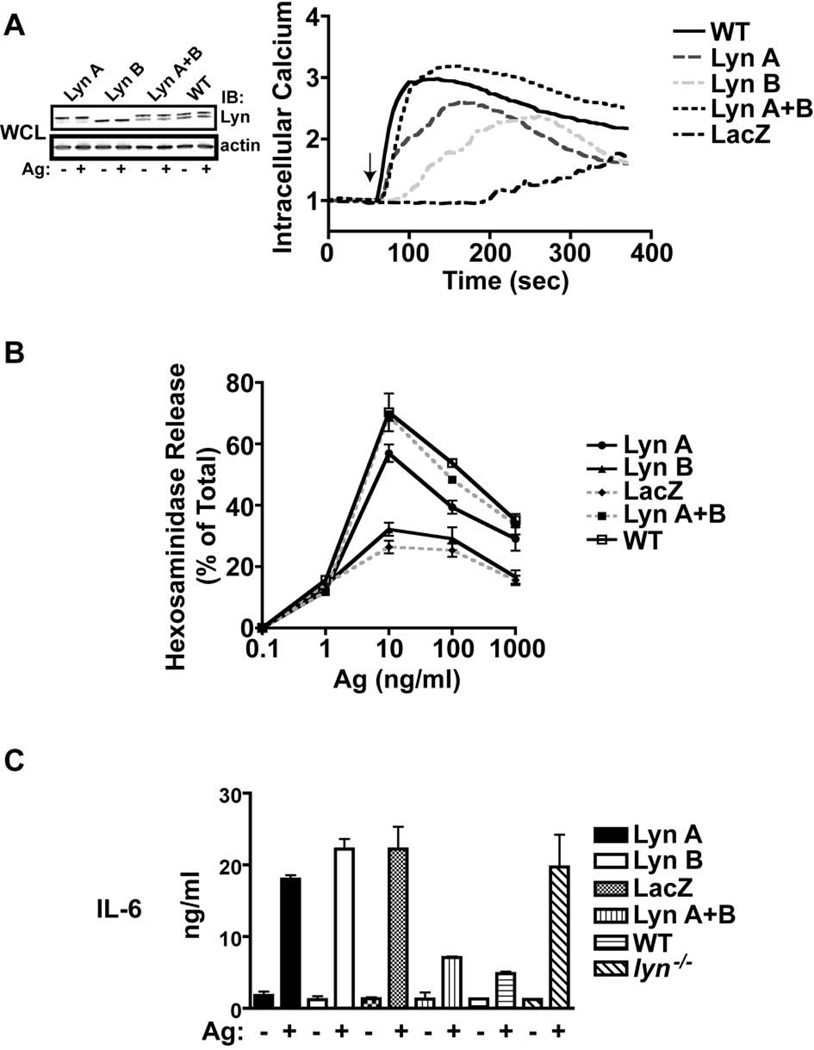
The presence of both Lyn A and Lyn B isoforms in mast cells, and their association with FcεRI, suggests that both isoforms are likely to complement for maximal FcεRI-induced mast cell activation. As noted in the above experiments, expression of Lyn A or Lyn B alone did not fully restore many of the cellular responses to those seen WT cells. Here we set out to investigate whether expression of both Lyn isoforms in lyn−/− BMMCs complemented to normalize some of the responses, such as calcium responses, degranulation, and cytokine production, to the levels observed in WT cells. As shown in Figure 7A, left panel, the ectopic expression of both Lyn A and Lyn B together was similar to the levels of these two isoforms in WT cells and FcεRI stimulation did not alter this expression. Analysis of the effect on Ca2+ responses when both isoforms are restored showed that the isoforms complemented in restoring this response to WT levels (Figure 7A, right panel). Interestingly, in most experiments the rise in intracellular Ca2+ was still slightly delayed in cells expressing both Lyn A and Lyn B suggesting that the rate may be determined by factors independent of Lyn or that minor differences in the expression levels of each isoform may affect the rate of this response. As shown in Figure 7B, the degranulation of cells reconstituted with both Lyn A and Lyn B isoforms was now similar to that of WT cells and consistently the response was greater than that of cells expressing Lyn A or Lyn B alone. Thus, the presence of both isoforms caused a full degranulation response in an apparently additive manner (Figure 7B). Finally, analysis of whether expression of both Lyn A and B would restore control (dampen) on cytokine responses revealed a marked inhibition of IL-6 production (which was similar to the levels of IL-6 production in WT cells) when both isoforms were expressed (Figure 7C). Collectively, the findings demonstrate that, although Lyn A and Lyn B differ in the efficacy of PLC-γ activation and Ca2+ fluxes following FcεRI stimulation, both isoforms complement to achieve a full Ca2+ response, normal degranulation, and to dampen cytokine responses.
Figure 7. Lyn A and Lyn B complement to elicit full Ca2+ mobilization, mast cell degranulation, and to suppress cytokine production.
(A) Mast cells expressing Lyn A or Lyn B individually or jointly were analyzed for Ca2+ responses and compared to wild type (WT) or control LacZ expressing cells. Lyn expression levels were determined from whole cell lysates (WCL) of each genotype prior to (−) or after (+) stimulation of cells with antigen (Ag). Antibody to actin was used to assess similar protein loading. Relative induction (340/380 ratio) of intracellular Ca2+ fluxes was measured by single cell analysis (see Materials and Methods). One representative of six experiments is shown. (B) Degranulation as measured by the release of hexosaminidase from Ag-stimulated cells (at the indicated concentrations) expressing the individual or both Lyn isoforms, in comparison to WT or LacZ expressing cells. One representative of six experiments is shown. (C) Cytokine secretion by mast cells expressing the indicated Lyn isoforms and appropriate controls. IL-6 was measured using an ELISA assay. One representative of three experiments is shown.
Discussion
The presence of two Lyn kinase isoforms in hematopoietic cells where this kinase is expressed has long been recognized (16). However, how these two isoforms function and whether both isoforms are required for normal cellular responses has long remained a mystery. In the present study we explored this unknown by reconstituting Lyn A and Lyn B isoforms individually or together in Lyn-null mast cells. This investigation was not amenable to RNA silencing strategies or gene deletion since the two Lyn isoforms are alternatively spliced products from a single gene and differ only in a 21 amino acid NH2-terminal insert found in Lyn A, and not in Lyn B (16). Moreover, the requirements for the alternative splicing that generates the Lyn B isoform are not understood hampering a genetic knock-in approach. How the 21 amino acid difference in Lyn A and B can cause the marked changes in functional responses analyzed herein is not yet completely clear. However, some clues may be derived from their relative ability to co-immunoprecipitate tyrosine phosphorylated proteins. The 21 amino acid insert contains a tyrosine (Y32) residue (see Figure 1) that could potentially be phosphorylated. As identified from Prosite database analysis, the amino acid sequence surrounding Y32 has some structural homology with the Y43 site on enolase, which is a protein readily phosphorylated by tyrosine kinases (31). We explored this possibility and found no evidence for phosphorylation of Y32 nor did a Y32F mutant of Lyn A show altered Ca2+ responses or degranulation (data not shown). In contrast, the marked difference of Lyn A and B isoforms in interacting with phosphoproteins suggests that the 21 amino acid insert functions to control Lyn A interactions presumably by creating a conformation that limits access to its protein interaction domains (SH2 and/or SH3). How this may occur is undetermined, however, given that GST fusion proteins of Lyn A and Lyn B (which are not tyrosine phosphorylated) maintained their differing ability to bind phosphoproteins, one can conclude that this behavior is not likely to be regulated by tyrosine phosphorylation of the 21 amino acid insert but most likely results from differences in protein folding/conformation.
Unlike Lyn A, Lyn B poorly induced FcεRI-mediated Ca2+ mobilization and mast cell degranulation. The underlying mechanism for the effect on Ca2+ mobilization and mast cell degranulation was the inability of the Lyn B isoform to effectively promote the phosphorylation of PLCγ and the production of IP3, crucial steps for these responses (9). Phosphorylated LAT1 was co-immunoprecipitated with PLCγ from Lyn A-expressing cells in greater amounts than from cells expressing Lyn B. This suggests that Lyn B fails to promote and/or impairs this interaction. This finding is consistent with the previous observation that loss of LAT1, or mutation of the PLCγ binding site on LAT1, causes loss of PLCγ phosphorylation, impaired Ca2+ responses, and defective mast cell degranulation (32, 33).
The ability of Lyn B, and not Lyn A, to efficiently associate with SHIP-1 may also be the key factor in the impaired PLCγ activity and reduced Ca2+ response. It has been shown that SHIP-1 co-immunoprecipitates with Lyn kinase from monocytes (34) and from mast cells (35). Moreover, SHIP-1 association with FcγRIIB is a key step for the inhibitory properties of this receptor on FcεRI-mediated Ca2+ responses, following co-ligation of these receptors (36). Recently, the negative regulatory role for SHIP-1 has been extended to its association with LAT1 in both mast cells as well as in T cells. In T cells, LAT1 organizes an inhibitory complex that includes the adapter Dok-2 and SHIP-1, which serves to dampen the extent of early activation upon TCR engagement (37). In mast cells, LAT1 can also exert an inhibitory role under certain circumstances. Analysis of LAT1 mutants showed the recruitment of SHIP-1 into an FcεRI-induced signaling complex (38). SHIP-1 was found to be recruited to LAT1 in a cooperative manner, in which binding of Grb-2 to tyrosine Y195 potentiates the binding of SHIP-1 to tyrosine Y235. However, our findings suggest that LAT1 is not required for the association of Lyn B with SHIP-1. Deficiency in LAT1 had minimal effect on this interaction, suggesting that the interaction of SHIP-1 with Lyn B is not mediated through this adapter protein. Instead, Lyn B may bring SHIP-1 to LAT1 where it might exert its control by destabilizing PLCγ interaction with the plasma membrane (and thus with LAT1) possibly by reducing the levels of PIP3, a lipid required for PLCγ translocation to the plasma membrane. It should be noted, that other modes of inhibition by SHIP-1 have been described. Dok-1, an adapter that binds SHIP-1, can associate with and negatively regulate FcεRI signaling without the involvement of inhibitory receptors (39, 40). In mast cells, Dok-1 was found to associate with the FcεRI β-chain (40) and its phosphorylation was demonstrated to be Lyn-dependent (41). Thus, it is possible that Lyn B interactions with SHIP-1 might be mediated through the adapter Dok-1. Nonetheless, our analysis of Lyn B immunoprecipitates for the presence of Dok-1 did not reveal detectable levels of this adapter protein (data not shown). Yet, mutation of the non-canonical tyrosine residue that lies in the middle of the FcεRI β-chain caused increased cytokine production in mast cells and led to a modest loss of SHIP-1 phosphorylation, suggesting that this tyrosine residue might interact with this negative regulator (42).
Here we also investigated whether the previously described negative regulatory role of Lyn kinase on cytokine production (11, 13) could be ascribed to Lyn A or Lyn B. While Lyn A consistently showed a moderate dampening of cytokine responses, when compared to WT cells, this effect was incomplete. That Lyn A showed a greater dampening effect than Lyn B was unexpected, given that the association of SHIP-1 is more prominent with Lyn B and thus one might expect that Lyn B would have a greater dampening effect. Our findings show that expression of both Lyn isoforms is required to restore the control on cytokine production. It is unlikely that the suppression of cytokine responses is related to dampening of Ca2+ mobilization, since expression of both Lyn A and Lyn B increased Ca2+ fluxes, following FcεRI stimulation. A plausible explanation could derive from our previous finding that mutation of the FcεRI β-chain ITAM tyrosines, which causes the loss of Lyn association with this receptor, induced increased IκBα degradation and enhanced nuclear NFκB activity following FcεRI stimulation (42). It is well established that in mast cells, TNF and IL-6 production are highly dependent on NFκB activity (43, 44). Thus, both Lyn A and Lyn B may be required to prevent the excessive degradation of IκBα and thus dampen nuclear NFκB activity. However a test of this hypothesis showed the inverse, as FcεRI stimulated cells expressing the individual Lyn A or Lyn B isoforms were found to have higher amounts of IκBα protein remaining when compared to cells expressing both isoforms, which had a similar content of IκBα protein to stimulated WT cells (data not shown). Thus, it is unlikely that increased NFκB activity could account for increased cytokine response of Lyn A or Lyn B -expressing cells, and how both Lyn kinase isoforms complement to dampen cytokine production remains to be determined.
We now appreciate that the 21 amino acid NH2-terminal insert found in Lyn A, and not in Lyn B, determines the efficacy of each isoform in activating PLCγ and generating IP3, and thus in promoting Ca2+ fluxes. Additionally, key differences were seen in the ability of each isoform to associate with phosphoproteins like LAT and SHIP-1, suggesting that the 21 amino acid insert governs protein interactions. Most importantly, our findings demonstrate that the Lyn A and Lyn B isoforms have distinct roles with regards to mast cell signal transduction. Nonetheless, the two isoforms complement in inducing both positive and negative regulation of mast cell effector functions. These findings provide an explanation for the presence of both Lyn A and Lyn B isoforms in mast cells, and likely for other cell types as well
Acknowledgements
We acknowledge the invaluable support of the Laboratory Animal Care and Use Section, the Light Imaging Section and the Flow Cytometry Section, Office of Science and Technology, NIAMS.
Footnotes
This research was supported by the intramural program of the National Institute of Arthritis and Musculoskeletal and Skin Disease of the National Institutes of Health.
References
- 1.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 2.Pribluda VS, Pribluda C, Metzger H. Transphosphorylation as the mechanism by which the high-affinity receptor for IgE is phosphorylated upon aggregation. Proc Natl Acad Sci USA. 1994;91:11246–11250. doi: 10.1073/pnas.91.23.11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holowka D, Baird B. FcεRI as a paradigm for a lipid raft-dependent receptor in hematopoietic cells. Semin Immunol. 2001;13:99–105. doi: 10.1006/smim.2000.0301. [DOI] [PubMed] [Google Scholar]
- 4.Paolini R, Jouvin MH, Kinet JP. Phosphorylation and dephosphorylation of the high-affinity receptor for immunoglobulin E immediately after receptor engagement and disengagement. Nature. 1991;353:855–858. doi: 10.1038/353855a0. [DOI] [PubMed] [Google Scholar]
- 5.Benhamou M, Ryba NJP, Kihara H, Nishikata H, Siraganian RP. Protein-tyrosine kinase p72syk in high affinity receptor signaling. Identification as a component of pp72 and associaition with the receptor γ chain after receptor aggregation. J Biol Chem. 1993;268:23318–23324. [PubMed] [Google Scholar]
- 6.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 7.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nechushtan H, Leitges M, Cohen C, Kay G, Razin E. Inhibition of degranulation and interleukin-6 production in mast cells derived from mice deficient in protein kinase Cβ. Blood. 2000;95:1752–1757. [PubMed] [Google Scholar]
- 9.Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami Y, Kitaura J, Satterthwaite AB, Kato RM, Asai K, Hartman SE, Maeda-Yamamoto M, Lowell CA, Rawlings DJ, Witte ON, Kawakami T. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- 11.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Hansen V, Smith AJ, Surviladze Z, Chigaev A, Mazel T, Kalesnikoff J, Lowell CA, Krystal G, Sklar LA, Wilson BS, Oliver JM. Dysregulated FcεRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J Immunol. 2004;173:100–112. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita Y, Charles N, Furumoto Y, Odom S, Yamashita T, Gilfillan AM, Constant S, Bower MA, Ryan JJ, Rivera J. Cutting edge: genetic variation influences FcεRI-induced mast cell activation and allergic responses. J Immunol. 2007;179:740–743. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]
- 14.Rivera J, Tessarollo L. Genetic background and the dilemma of translating mouse studies to humans. Immunity. 2008;28:1–4. doi: 10.1016/j.immuni.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Sidorenko SP, Law CL, Chandran KA, Clark EA. Human spleen tyrosine kinase p72Syk associates with the Src-family kinase p53/56Lyn and a 120-kDa phosphoprotein. Proc Natl Acad Sci U S A. 1995;92:359–363. doi: 10.1073/pnas.92.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi TL, Bolen JB, Ihle JN. Hematopoietic cells express two forms of lyn kinase differing by 21 amino acids in the amino terminus. Mol Cell Biol. 1991;11:2391–2398. doi: 10.1128/mcb.11.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita T, Mao SY, Metzger H. Aggregation of the high-affinity IgE receptor and enhanced activity of p53/56lyn protein-tyrosine kinase. Proc Natl Acad Sci U S A. 1994;91:11251–11255. doi: 10.1073/pnas.91.23.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Field KA, Holowka D, Baird B. FcεRI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc Natl Acad Sci USA. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovarova M, Wassif CA, Odom S, Liao K, Porter FD, Rivera J. Cholesterol deficiency in a mouse model of Smith-Lemli-Opitz syndrome reveals increased mast cell responsiveness. J Exp Med. 2006;203:1161–1171. doi: 10.1084/jem.20051701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, Klinman NR, Katz DH. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- 21.Rivera J, Kinet J-P, Kim J, Pucillo C, Metzger H. Studies with a monoclonal antibody to the β subunit of the receptor with high affinity for immunoglobulin E. Mol Immunol. 1988;25:647–661. doi: 10.1016/0161-5890(88)90100-9. [DOI] [PubMed] [Google Scholar]
- 22.Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting Edge: Lentiviral shRNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- 23.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, Rivera J. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita T, Suzuki R, Backlund PS, Yamashita Y, Yergey AL, Rivera J. Differential dephosphorylation of the FcRγ immunoreceptor tyrosine-based activation motif tyrosines with dissimilar potential for activating Syk. J Biol Chem. 2008;283:28584–28594. doi: 10.1074/jbc.M802679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blank U, Rivera J. Assays for regulated exocytosis of mast cell granules. Chapter 15. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb1511s32. Unit 15 11. [DOI] [PubMed] [Google Scholar]
- 26.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber M, Helgason CD, Damen JE, Liu L, Humphries RK, Krystal G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci U S A. 1998;95:11330–11335. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song M, Kim MJ, Ha S, Park JB, Ryu SH, Suh PG. Inositol 5'-phosphatase, SHIP1 interacts with phospholipase C-γ1 and modulates EGF-induced PLC activity. Exp Mol Med. 2005;37:161–168. doi: 10.1038/emm.2005.22. [DOI] [PubMed] [Google Scholar]
- 29.Rawlings DJ, Scharenberg AM, Park H, Wahl MI, Lin S, Kato RM, Fluckiger AC, Witte OW, Kinet J-P. Activation of BTK by a phosphorylation mechanism initiated by Src family kinases. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 30.Hata D, Kawakami Y, Inagaki N, Lantz CS, Kitamura T, Khan WN, Maeda-Yamamoto M, Miura T, Han W, Hartman SE, Yao L, Nagai H, Goldfeld AE, Alt FW, Galli SJ, Witte ON, Kawakami T. Involvement of Bruton's tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper JA, Esch FS, Taylor SS, Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984;259:7835–7841. [PubMed] [Google Scholar]
- 32.Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, Rivera J, Samelson LE. LAT is essential for FcεRI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 33.Saitoh S, Odom S, Gomez G, Sommers CL, Young HA, Rivera J, Samelson LE. The four distal tyrosines are required for LAT-dependent signaling in FcεRI-mediated mast cell activation. J Exp Med. 2003;198:831–843. doi: 10.1084/jem.20030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB. The inositol 5"-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity. J Biol Chem. 2003;278:38628–38636. doi: 10.1074/jbc.M305021200. [DOI] [PubMed] [Google Scholar]
- 35.Grochowy G, Hermiston ML, Kuhny M, Weiss A, Huber M. Requirement for CD45 in fine-tuning mast cell responses mediated by different ligand-receptor systems. Cell Signal. 2009;21:1277–1286. doi: 10.1016/j.cellsig.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Isnardi I, Lesourne R, Bruhns P, Fridman WH, Cambier JC, Daeron M. Two distinct tyrosine-based motifs enable the inhibitory receptor FcγRIIB to cooperatively recruit the inositol phosphatases SHIP1/2 and the adapters Grb2/Grap. J Biol Chem. 2004;279:51931–51938. doi: 10.1074/jbc.M410261200. [DOI] [PubMed] [Google Scholar]
- 37.Dong S, Corre B, Foulon E, Dufour E, Veillette A, Acuto O, Michel F. T cell receptor for antigen induces linker for activation of T cell-dependent activation of a negative signaling complex involving Dok-2, SHIP-1, and Grb-2. J Exp Med. 2006;203:2509–2518. doi: 10.1084/jem.20060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roget K, Malissen M, Malbec O, Malissen B, Daeron M. Non-T cell activation linker promotes mast cell survival by dampening the recruitment of SHIP1 by linker for activation of T cells. J Immunol. 2008;180:3689–3698. doi: 10.4049/jimmunol.180.6.3689. [DOI] [PubMed] [Google Scholar]
- 39.Ott VL, Tamir I, Niki M, Pandolfi PP, Cambier JC. Downstream of kinase, p62(dok), is a mediator of FcγIIB inhibition of FcεRI signaling. J Immunol. 2002;168:4430–4439. doi: 10.4049/jimmunol.168.9.4430. [DOI] [PubMed] [Google Scholar]
- 40.Abramson J, Rozenblum G, Pecht I. Dok protein family members are involved in signaling mediated by the type 1 Fcε receptor. Eur J Immunol. 2003;33:85–91. doi: 10.1002/immu.200390011. [DOI] [PubMed] [Google Scholar]
- 41.Liang X, Wisniewski D, Strife A, Shivakrupa, Clarkson B, Resh MD. Phosphatidylinositol 3-kinase and Src family kinases are required for phosphorylation and membrane recruitment of Dok-1 in c-Kit signaling. J Biol Chem. 2002;277:13732–13738. doi: 10.1074/jbc.M200277200. [DOI] [PubMed] [Google Scholar]
- 42.Furumoto Y, Nunomura S, Terada T, Rivera J, Ra C. The FcεRIβ immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IκB kinase phosphorylation and mast cell cytokine production. J Biol Chem. 2004;279:49177–49187. doi: 10.1074/jbc.M404730200. [DOI] [PubMed] [Google Scholar]
- 43.Pelletier C, Varin-Blank N, Rivera J, Iannascoli B, Marchand F, David B, Weyer A, Blank U. FcεRI-mediated induction of TNF-α gene expression in the RBL-2H3 mast cell line: regulation by a novel NF-κB-like nuclear binding complex. J.Immunol. 1998;161:4768–4776. [PubMed] [Google Scholar]
- 44.Marquardt DL, Walker LL. Dependence of mast cell IgE-mediated cytokine production on nuclear factor-κB activity. J Allergy Clin Immunol. 2000;105:500–505. doi: 10.1067/mai.2000.104942. [DOI] [PubMed] [Google Scholar]