Abstract
Objective
The goal of this study was to investigate the relationship between plasma levels of insulin-like growth factors-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) and the risk for cervical intraepithelial neoplasia (CIN) and cervical cancer.
Methods
Plasma levels of IGF-1 and IGFBP-3 of 44 cervical cancer patients, 82 CIN patients and 40 neoplasm-free patients were investigated. Then the associations of the plasma levels of IGF-1 and IGFBP-3 with cervical neoplasm or its clinicopathologic parameters were analyzed.
Results
The mean IGF-1 concentrations were significantly different among the control, CIN, and cervical cancer groups; the levels were higher in the CIN group compared to the controls. According to the quartile category, the plasma IGF-1 level was significantly higher (p=0.0015) in the CIN group than in the controls. The IGFBP-3 level showed no association between the controls and CIN groups (p=0.842). Although the mean IGF-1/IGFBP-3 molar ratio had borderline significance (p=0.08) among the study population, the quartile comparison showed a significantly higher IGF-1/IGFBP-3 molar ratio in the CIN group compared to the control group (p=0.041).
Conclusion
Plasma levels of IGF-1 and the IGF-1/IGFBP-3 molar ratio might be useful for the development early detection of cervical lesions and used as an adjuvant diagnostic tool for cervical neoplasia after more larger scale research.
Keywords: IGF-1, IGFBP-3, IGF-1/IGFBP-3 molar ratio, Cervical neoplasia
INTRODUCTION
It is well known that cells with accelerated rates of division and proliferation are predisposed to develop into cancer cells.1,2 Cell proliferation and apoptosis are especially important for the formation of certain neoplasia. The insulin-like growth factors (IGF) are multifunctional peptides that play a pivotal role in cell regulation. The IGF family consists of IGF-1, IGF-2 and several IGF-binding proteins (IGFBP). IGF-1 is a broad-spectrum growth factor that has been shown to play an important role in regulation of cell proliferation, differentiation and apoptosis, and may thus be involved in the development of cancer;3,4 it acts in an endocrine, paracrine, and autocrine manner in many tissues, and has acute anabolic effects on protein and carbohydrate metabolism by increasing the cellular uptake of amino acids, and by stimulating glycogen and protein synthesis.5 It acts as a mitogen by increasing DNA synthesis and stimulating the expression of cyclin D1.6 IGF-1 also can stimulate the expression of Bcl proteins and the suppression of Bax, which results in blocking the initiation of the apoptotic pathway.7
The action of IGF-1 and -2 is mediated by the IGF-1 receptor (IGF-1R). The type IGF-1R is a tyrosine kinase similar to the insulin receptor; it mediates the promotion of growth by binding to both IGF-1 and IGF-2.8,9 The IGF-1R affects several growth promoting functions. It stimulates mitogenesis in many different cell types and protects the cells from apoptosis.10,11 The receptor also plays an important role in the transformation of cells induced by viral proteins and oncogene proteins.12 Previous studies have reported that overexpression of IGF-1R may be associated with certain aggressive tumors.13,14
IGF-1 interacts with its cell membrane receptor to influence various cellular activities. The bioavailability of the IGF family is regulated by IGFBPs. The IGFBPs are composed of six proteins; all IGFBPs have growth inhibitory effects.15 The inhibitory effects are caused by competitive binding of the IGFs, and prevention of their binding to the receptor. More than 90% of the plasma IGF-1 is bound to IGFBP-3, the most abundant of the six different IGFBPs identified (IGFBP 1-6). IGFBP-3 has been shown to play a major role in the regulation of the interaction between IGF and IGF-1R;16,17 it modulates free IGF and inhibits its transfer from the circulation to tissues. The inhibitory effects have been shown to be both IGF-dependent and independent.
There are several lines of evidence that support a role for the IGF family in the development of neoplasia. The results from previous studies have shown an increased risk of cancer associated with high circulating IGF-1 levels, especially in cancers of epithelial and glandular origin such as the prostate, colon, breasts, and lungs.18-21 The finding of high IGFBP-3 levels appears to be associated with a decreased risk.22-24
With regard to cervical cancer, it is a cancer of epithelial origin similar to other cancers with high plasma IGF levels. Thus, IGF may be involved in carcinogenesis of the cervix. There is some evidence that the IGF receptor is overexpressed in cervical cancers, and reports suggest that cervical cancer might be sensitive to IGF-1.25 One study reported a positive correlation between high plasma IGF levels and squamous intraepithelial lesions.26
Cervical cytology has been considered the primary screening method for early detection of cervical intraepithelial neoplasia (CIN) and cervical cancer. The measurement of the plasma concentration of IGF-1 and IGFBP-3 may be relatively easier and more convenient for patients that commonly have blood drawn at routine health examinations; this would likely be preferred to obtaining specimens for cervical cytology. IGF levels may be useful as biomarkers for the assessment of risk for CIN and/or cervical cancer. However, there is little research on the relationship between the IGF family and cervical neoplasias.
This study was performed to investigate the relationship between plasma levels of IGF-1 and IGFBP-3 and the risk for CIN and/or cervical cancer.
MATERIALS AND METHODS
1. Study population
One hundred and sixty six women that underwent surgery from November 2004 to May 2007, at the department of Gynecology at Ewha Womans University, Mokdong hospital, were included in this retrospective study. A control group consisted of 40 patients that were diagnosed with benign gynecological disease by pathology. The group with CIN consisted of 82 cases (40 cases of CIN II and 42 cases of CIN III), and the cancer group consisted of 44 cases of cervical cancer at a variety of different stages. Among all surgery cases during the study period, CIN cases and controls were selected if the patient agreed to sample collection during the study period. All participants signed an informed consent that explained the further use of blood samples and cervical tissues before donating the blood samples. The clinical and pathological data used for this study were collected by retrospective medical chart review. This study was exempted from approval of the Institutional Review Board, at the Ewha Womans University, Mokdong Hospital, because of its retrospective design.
2. Blood sample collection and analysis
From all participating women, a 10-mL blood sample was collected by venipuncture and added to a heparinized Vacutainer tube. The blood samples were centrifuged at 1,500 × g for 20 minutes. The plasma and Buffy coat were aspirated and stored separately at -70℃. Plasma levels of IGF-1 and IGFBP-3 were measured using an ELISA kit from Diagnostics Systems Laboratories Inc. (Webster, TX, USA). All samples were tested in duplicate and the mean values of the patient's plasma IGF-1 and IGFBP-3 levels were determined. If differences between the two results were more than 10%, the test was repeated. In addition, the effects of HPV infection on the levels of the IGF family were studied by dividing the patients into HPV (+) and HPV (-) groups and evaluating the levels of IGF-1 and IGFBP-3.
3. Statistical analysis
The ANOVA test was used to compare the variables between cases and controls. We used the plasma levels of IGF-1 and IGFBP-3 for the statistical analysis. The Spearman correlation coefficient was used to examine the relationship between the IGF levels and age. For evaluation of the IGF levels in cancer patients, we used the Mann-Whitney U test. We categorized their plasma levels into quartiles based on the distribution in control subjects and used the results for the analysis. The IGF-1/IGFBP-3 molar ratio that was calculated from [(IGF-1×0.13)/(IGFBP-3×0.035)] was also evaluated. The chi-square test was used to compare the variables between cases and control patients.
RESULTS
1. Characteristics of the subjects
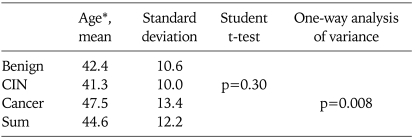
The ages of the three groups are listed in Table 1. The difference in age between the control and the CIN group was not significant (p=0.30); however, the patients were significantly older in the cancer group (p=0.008). The parity was 1.5±0.87, 1.69±1.09, and 1.97±1.42 in the controls, CIN and the cancer groups respectively; there was no significant difference among the three groups. Other factors such as smoking and the number of intimate partners were not evaluated in this study.
Table 1.
Comparison of age between the benign controls, the cervical intraepithelial neoplasia (CIN) and the cancer patients
No differences were noted between the benign controls and the CIN group, but significant difference in age was noted in the cancer group compared to the controls.
*Patient's age at diagnosis.
2. IGF-1 and IGFBP-3 levels among the study groups
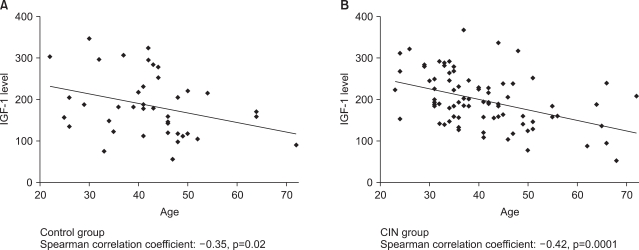
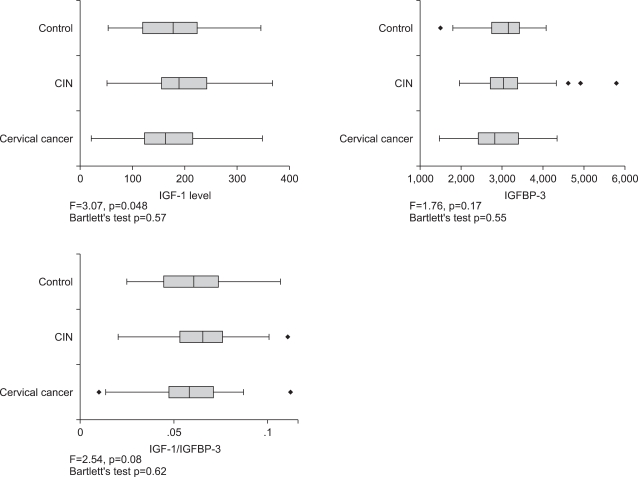
IGF-1 and IGFBP-3 levels showed an inverse correlation with age both in the control group and the CIN group (Fig. 1). The mean plasma IGF-1 levels were 184.9±74.6 ng/mL in the control group, 200±65.5 ng/mL in the CIN group, and 168.1±72.4 ng/mL in the cancer group (p=0.048). However since the mean age of the cancer group was significantly greater than the control or CIN groups, the lower plasma IGF-1 level may be affected by age factor. The levels of IGFBP-3 were 3074.9±602.0 ng/mL, 3096.9±618.4 ng/mL and 2881.5±699.9 ng/mL respectively (p=0.17). The results demonstrated a significant increase in the IGF-1 in the CIN group; however, there was no significant difference noted in the IGFBP-3 levels among the study groups (Fig. 2).
Fig. 1.
Scatter plots showing inverse correlation of serum concentrations of insulin-like growth factors-1 (IGF-1) with age, separately among the control group (A) and the cervical intraepithelial neoplasia (CIN) group (B).
Fig. 2.
Serum levels of insulin-like growth factors-1 (IGF-1), IGF-binding protein-3 (IGFBP-3), and IGF-1/IGFBP-3 molar ratio in the study population showing significantly increased levels of IGF-1 and IGF-1/IGFBP-3 molar ratio in the cervical intraepithelial neoplasia (CIN) group.
3. IGF-1/IGFBP-3 molar ratio between the controls and CIN cases
The IGF-1/IGFBP-3 molar ratio was 0.059±0.018, 0.064±0.017, and 0.057±0.019 in the controls, CIN and cancer groups (p=0.08), respectively; there was no significant association noted among the three groups (Fig. 2). However, according to the quartile category, the molar ratio was relatively high in the CIN group (p=0.041). There were 64.6% (53 cases) of the CIN group above the 3rd quartile of the controls.
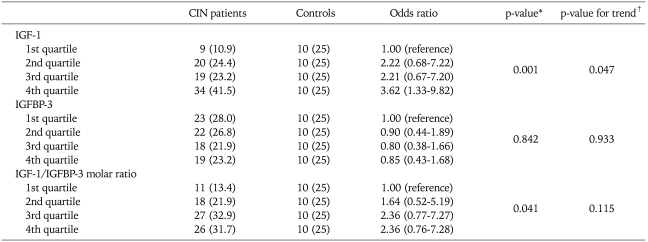
4. IGF-1 and IGFBP-3 levels among the study groups according to the quartile category
In the quartile category, the plasma IGF-1 level was significantly higher (p=0.0015) in the CIN group than the controls (Table 2). About 41.5% (34 patients) of the CIN group was in the 4th quartile group of the controls. The IGFBP-3 level showed no association between the controls and the CIN cases (p=0.842). As mentioned above, the age was significantly higher in the cancer group compared to the control and CIN groups. Therefore, the analysis based on the quartile category was performed only between the control and CIN groups.
Table 2.
Levels of IGF-1, IGFBP-3 and IGF-1/IGFBP-3 molar ratio according to the quartile category between CIN and the control group
IGF-1: insulin-like growth factors-1, IGFBP-3: IGF-binding protein-3, CIN: cervical intraepithelial neoplasia.
*chi-square test. †Weighted linear regression was performed to model the natural logarithm of odds ratios for the risk of cervical neoplasm as a function of qualitatively classified IGF-1, IGFBP-3, and IGF-1/IGFBP-3 molar ratio (1st quartile, 1; 2nd quartile, 2; 3rd quartile, 3; 4th quartile, 4) using the inverse variance calculated from confidence intervals of each category; standard error=[LN (upper limit)-LN (lower limit)]/2×1.96; inverse variance=1/(standard error×standard error).
5. IGF-1 and HPV infection
HPV testing was not performed in all patients, especially among the controls. Only 71 cases out of 82 patients in the CIN group and 34 cases out of 44 patients in the cancer group had HPV testing. We used Hybrid Capture 2® (Digene Corporation, Gaithersburg, MD, USA) assay method for HPV detection. In the CIN group, 54 patients had positive results, and their mean circulating IGF-1 level was 195.42±8.0 ng/mL. In the negative cases, the mean IGF-1 level was 220.21±18.4 ng/mL and the p-value was 0.16.
In the cancer group, 26 patients were positive for HPV infections, and their mean plasma IGF level was 167.0±67.8 ng/mL; eight cases had negative results and their mean IGF-1 level was 170.6±52.8 ng/mL. There was no significant correlation identified (p=0.92) between the HPV infection status and IGF-1 levels.
6. IGF-1 levels and cancer stages
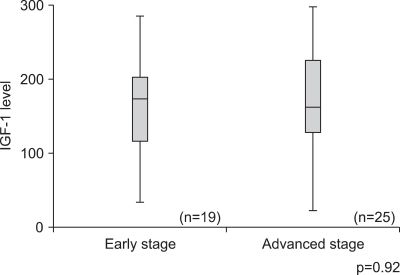
The levels of IGF-1 were evaluated according to the cervical cancer stage and no correlation was noted between IGF-1 levels and the cancer stage (p=0.92) (Fig. 3). During early stage disease (CIS-IA), the mean level of IGF-1 was 169.9±17.3 ng/mL (95% CI, 133.6 to 206.3), and for advanced stage disease (over stage IB), the mean level was 166.6±13.9 ng/mL (95% CI, 137.8 to 195.3).
Fig. 3.
Levels of insulin-like growth factors-1 (IGF-1) according to cancer stage (p-value=0.92). There was no statistical difference in serum IGF-1 between early and advanced stage cervical cancer.
DISCUSSION
The results of the current study showed that the plasma IGF-1 level and IGF-1/IGFBP-3 molar ratio were significantly associated with CIN, but had no significant association with cervical cancer. Elevated plasma IGF-1 and IGF-1/IHFBP-3 ratios were associated with the development of CIN; although it is not clear whether increased plasma levels of IGF-1 and IGF-1/IGFBP-3 were the cause or the result of CIN.
The insulin-like growth factor family was first isolated and sequenced in 1978. The "Insulin-like growth factors" were named after their primary structure that resembled proinsulin.27 These factors play an important role in human growth and maintenance of homeostasis, and recently their involvement in carcinogenesis has been recognized. IGF presumably plays an important role in carcinogenesis by regulating the growth of cancer cells and by interacting with cancer related molecules; this has been confirmed by several cell culture experiments.28 Many epidemiological studies have shown that IGF-1 levels were higher in cancer patients with sarcoma, leukemia, prostate, colorectal, breast, liver, and uterine cancers. Animal experiments have shown that over-expression of IGF-1 increases the tendency for tumor development.29
Previous studies have demonstrated some relationship between IGF-1 and cervical cancer; however, such results have been inconsistent. One study proposed that IGF-1 was associated with cervical cancer growth in vitro; however, another study found no association between high plasma IGF-1 levels and cervical cancer.30 Another study reported that low values of IGF-1 might be associated with cervical cancer.31 However, Wu et al.26 found a strong dose-dependent correlation with the IGF-1 level and squamous intraepithelial lesions of the cervix including cervical cancer.
The results of this study showed that circulating IGF-1 levels were significantly correlated with CIN. As previously reported, IGF-1 stimulates cell proliferation by influencing DNA synthesis and cyclin D production; it can suppress apoptosis by interacting with Bax and Bcl genes. In addition, IGF-1 might promote angiogenesis by effecting vascular endothelial growth factor production.32 Therefore, the circulating IGF-1 level was higher in the CIN group than in the control cases. Our study showed that IGF-1 was not associated with cervical cancer. These results can be explained by the following. First, age was an important factor associated with the IGF-1 levels. The levels of circulating IGF-1 change with age.33 They increase from birth to puberty, and decrease after puberty. The results of this study were consistent with these prior findings. Since the mean age was significantly higher in the cervical cancer group compared to controls and CIN cases, age may have affected the plasma IGF-1 levels. Second, there are likely to be many alterations not yet revealed in the microenvironment during carcinogenesis, thus these alterations may affect the circulating IGF-1 levels in patients with cervical cancer.
Our study has limitations in that plasma IGF levels may be affected by body mass index, diet, physical activity, and menstrual status, which are potential confounders of the case control study comparing plasma IGF levels. A large cohort of cases and controls should be recruited to be able to demonstrate a clear relationship between IGF variables and cervical neoplasia.
IGFBP-3 appears to have a protective effect against carcinogenesis in several cancers. One study reported that plasma levels of IGFBP-3 in patients with cervical cancer were significantly lower than in the control group; however, the levels returned to normal following cancer treatment. In addition, IGFBP-3 down-regulated growth factors in vitro in cervical cancer.34 A former study revealed that the plasma IGFBP-3 was significantly higher in groups with squamous intraepithelial lesions than in controls; however, after adjustment for IGF-1, no correlation was observed with the IGFBP-3 levels.26
In the present study, we could not find any relationship between cervical neoplasia and the plasma IGFBP-3 levels. IGFBP-3 is a binding protein, and can play a dual role. As stated above, IGFBP-3 inhibits cell growth and accelerates apoptosis in both an IGF-dependent and IGF-independent manner. However, in an environment with a high IGF-1 concentration, IGFBP-3 can enhance the effect of IGF by presenting and releasing IGF-1 for interaction with the receptor, while protecting the receptor from down-regulation by exposure to a high IGF-1 concentration.35
It is well known that HPVs are involved in the carcinogenesis of cervical cancer.36 Persistent infection with HPVs has been identified as a major risk factor for cervical cancer and CIN. However, only a small portion of persistently infected women will develop cervical cancer. Therefore, there are likely several interacting factors that influence carcinogenesis leading to cancer of the cervix. Both the viral factors, as associated with HPV infection and the humoral factors, similar to those associated with the IGF family influence cell proliferation, are resistant to apoptosis and cancer development. Immortalization of HPV-infected cervical squamous epithelial cells is a major factor in the carcinogenesis of cancer of the cervix. It may be initiated by inactivation of cell cycle regulatory p53 and retinoblastoma genes by HPV E6 and E7 genes. Thus, this may trigger the up-regulation of growth factors, such as IGF or the epidermal growth factor.37
The results of this study showed no relationship between IGF levels and HPV infections. The association between HPV infections and IGF levels has not been extensively explored in cervical neoplasia. One study found no association with the mRNA expression of IGF-1 and HPV infection status;38 however, another study revealed that cervical cells transduced with high-risk HPV E6 and E7 genes in cell cultures had an 85-fold increase in IGFBP-3 levels.39 Theoretically, high levels of IGF-1 may affect HPV infection and vice versa; however, there are no studies that have found a positive correlation between HPV infection and plasma IGF-1 and IGFBP-3 levels, including our study. Therefore, further study will be needed to determine the relationship between HPV infections and the IGF family.
According to our results, plasma levels of IGF-1 and the IGF-1/IGFBP-3 molar ratio were not associated with cervical cancer, but demonstrated significantly higher levels in the CIN group compared to the control group. Therefore, these factors may be involved in the progression from normal to precancerous lesions of the cervix. In the future, plasma levels of IGF-1 and the IGF-1/IGFBP-3 molar ratio may be useful as early detection markers, or as an adjuvant diagnostic tool or prognostic marker in patients with cervical neoplasia.
In conclusion, the results of this study show a positive correlation between IGF-1 and IGFBP-3 plasma levels and CIN. Larger studies are needed to assess the role of the IGF family in the development of cervical neoplasia.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Korea (A080884).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE. Increased cell division as a cause of human cancer. Cancer Res. 1990;50:7415–7421. [PubMed] [Google Scholar]
- 2.Kim K, Ryu SY. Major clinical research advances in gynecologic cancer 2009. J Gynecol Oncol. 2009;20:203–209. doi: 10.3802/jgo.2009.20.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sara VR, Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990;70:591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld RG, Lamson G, Pham H, Oh Y, Conover C, De Leon DD, et al. Insulinlike growth factor-binding proteins. Recent Prog Horm Res. 1990;46:99–159. doi: 10.1016/b978-0-12-571146-3.50009-2. [DOI] [PubMed] [Google Scholar]
- 5.Baxter RC, Saunders H. Radioimmunoassay of insulin-like growth factor-binding protein-6 in human serum and other body fluids. J Endocrinol. 1992;134:133–139. doi: 10.1677/joe.0.1340133. [DOI] [PubMed] [Google Scholar]
- 6.Furlanetto RW, Harwell SE, Frick KK. Insulin-like growth factor-I induces cyclin-D1 expression in MG63 human osteosarcoma cells in vitro. Mol Endocrinol. 1994;8:510–517. doi: 10.1210/mend.8.4.8052269. [DOI] [PubMed] [Google Scholar]
- 7.Minshall C, Arkins S, Straza J, Conners J, Dantzer R, Freund GG, et al. IL-4 and insulin-like growth factor-I inhibit the decline in Bcl-2 and promote the survival of IL-3-deprived myeloid progenitors. J Immunol. 1997;159:1225–1232. [PubMed] [Google Scholar]
- 8.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 9.Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 10.Esposito DL, Blakesley VA, Koval AP, Scrimgeour AG, LeRoith D. Tyrosine residues in the C-terminal domain of the insulin-like growth factor-I receptor mediate mitogenic and tumorigenic signals. Endocrinology. 1997;138:2979–2988. doi: 10.1210/endo.138.7.5281. [DOI] [PubMed] [Google Scholar]
- 11.Hongo A, D'Ambrosio C, Miura M, Morrione A, Baserga R. Mutational analysis of the mitogenic and transforming activities of the insulin-like growth factor I receptor. Oncogene. 1996;12:1231–1238. [PubMed] [Google Scholar]
- 12.LeRoith D, Baserga R, Helman L, Roberts CT., Jr Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–59. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, et al. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–1133. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 14.Xie Y, Skytting B, Nilsson G, Brodin B, Larsson O. Expression of insulin-like growth factor-1 receptor in synovial sarcoma: association with an aggressive phenotype. Cancer Res. 1999;59:3588–3591. [PubMed] [Google Scholar]
- 15.Ferry RJ, Jr, Katz LE, Grimberg A, Cohen P, Weinzimer SA. Cellular actions of insulin-like growth factor binding proteins. Horm Metab Res. 1999;31:192–202. doi: 10.1055/s-2007-978719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arany E, Afford S, Strain AJ, Winwood PJ, Arthur MJ, Hill DJ. Differential cellular synthesis of insulin-like growth factor binding protein-1 (IGFBP-1) and IGFBP-3 within human liver. J Clin Endocrinol Metab. 1994;79:1871–1876. doi: 10.1210/jcem.79.6.7527416. [DOI] [PubMed] [Google Scholar]
- 17.Ballard FJ, Knowles SE, Walton PE, Edson K, Owens PC, Mohler MA, et al. Plasma clearance and tissue distribution of labelled insulin-like growth factor-I (IGF-I), IGF-II and des (1-3) IGF-I in rats. J Endocrinol. 1991;128:197–204. doi: 10.1677/joe.0.1280197. [DOI] [PubMed] [Google Scholar]
- 18.Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36:1224–1228. doi: 10.1016/s0959-8049(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(Suppl 11):3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 21.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 22.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 23.Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello LB, Lagiou P, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 25.Steller MA, Delgado CH, Zou Z. Insulin-like growth factor II mediates epidermal growth factor-induced mitogenesis in cervical cancer cells. Proc Natl Acad Sci U S A. 1995;92:11970–11974. doi: 10.1073/pnas.92.26.11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Tortolero-Luna G, Zhao H, Phatak D, Spitz MR, Follen M. Serum levels of insulin-like growth factor I and risk of squamous intraepithelial lesions of the cervix. Clin Cancer Res. 2003;9:3356–3361. [PubMed] [Google Scholar]
- 27.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253:2769–2776. [PubMed] [Google Scholar]
- 28.Macaulay VM. Insulin-like growth factors and cancer. Br J Cancer. 1992;65:311–320. doi: 10.1038/bjc.1992.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogler CE, Yang D, Rossetti L, Donohoe J, Alt E, Chang CJ, et al. Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. J Biol Chem. 1994;269:13779–13784. [PubMed] [Google Scholar]
- 30.Steller MA, Delgado CH, Bartels CJ, Woodworth CD, Zou Z. Overexpression of the insulin-like growth factor-1 receptor and autocrine stimulation in human cervical cancer cells. Cancer Res. 1996;56:1761–1765. [PubMed] [Google Scholar]
- 31.Serrano ML, Romero A, Cendales R, Sanchez-Gomez M, Bravo MM. Serum levels of insulin-like growth factor-I and -II and insulin-like growth factor binding protein 3 in women with squamous intraepithelial lesions and cervical cancer. Biomedica. 2006;26:258–268. [PubMed] [Google Scholar]
- 32.Bustin SA, Jenkins PJ. The growth hormone-insulin-like growth factor-I axis and colorectal cancer. Trends Mol Med. 2001;7:447–454. doi: 10.1016/s1471-4914(01)02104-9. [DOI] [PubMed] [Google Scholar]
- 33.Gomez JM, Mourot B, Fostier A, Le Gac F. Growth hormone receptors in ovary and liver during gametogenesis in female rainbow trout (Oncorhynchus mykiss) J Reprod Fertil. 1999;115:275–285. doi: 10.1530/jrf.0.1150275. [DOI] [PubMed] [Google Scholar]
- 34.Mathur RS, Mathur SP. In vitro downregulation of growth factors by insulin-like growth factor binding protein-3 in cervical cancer. Gynecol Oncol. 2003;91:410–415. doi: 10.1016/s0090-8258(03)00513-4. [DOI] [PubMed] [Google Scholar]
- 35.Conover CA, Powell DR. Insulin-like growth factor (IGF)-binding protein-3 blocks IGF-I-induced receptor down-regulation and cell desensitization in cultured bovine fibroblasts. Endocrinology. 1991;129:710–716. doi: 10.1210/endo-129-2-710. [DOI] [PubMed] [Google Scholar]
- 36.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 37.Dassonville O, Formento JL, Francoual M, Ramaioli A, Santini J, Schneider M, et al. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J Clin Oncol. 1993;11:1873–1878. doi: 10.1200/JCO.1993.11.10.1873. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura K, Hongo A, Kodama J, Miyagi Y, Yoshinouchi M, Kudo T. Down-regulation of the insulin-like growth factor I receptor by antisense RNA can reverse the transformed phenotype of human cervical cancer cell lines. Cancer Res. 2000;60:760–765. [PubMed] [Google Scholar]
- 39.Berger AJ, Baege A, Guillemette T, Deeds J, Meyer R, Disbrow G, et al. Insulin-like growth factor-binding protein 3 expression increases during immortalization of cervical keratinocytes by human papillomavirus type 16 E6 and E7 proteins. Am J Pathol. 2002;161:603–610. doi: 10.1016/S0002-9440(10)64215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]