Abstract
PURPOSE
To acquaint wound care practitioners with new information related to debridement of chronic wounds.
TARGET AUDIENCE
This continuing education activity is intended for physicians and nurses with an interest in wound care.
OBJECTIVES
After reading this article and taking this test, the reader should be able to:
Explain the role of keratinocytes in wound healing.
Discuss new research findings on the physiological differences between healing and nonhealing wounds.
Identify implications of the new research for debridement of chronic wounds.
As we stand at the beginning of the 21st century, wounds continue to have an enormous burden worldwide. In the United States, more than $25 billion is spent annually on wound care.1 The true cost, however, is the mortality and morbidity associated with chronic wounds, primarily in older adults, those with disabilities, and those with pressure, venous, and diabetic foot ulcers.2 With more than 28 different specialties involved in wound care and the broad spectrum of treatment and therapies available, the challenge remains for clinicians to use evidence-based protocols3–8 to achieve 100% healing and avoid the majority of morbidity and mortality associated with chronic wounds. Given the enormous amount of evidence and treatment options available, what guides can clinicians use to best help their patients?
A ROAD MAP FOR WOUND HEALING
The goal is for clinicians to obtain a picture similar to a product “bar code scan” of the status of the chronic wound they are trying to heal. New research into the molecular biology of chronic wounds reveals specific patterns in the edges of nonhealing wounds that differ from the patterns of a healing wound. These patterns can provide the clinician with a “road map” to guide selection of treatments in addition to evaluating their effectiveness.9
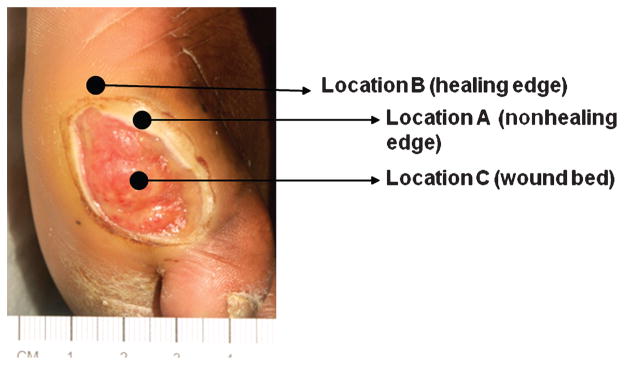
Clinicians are defining wound topography, particularly that of the wound edge. For example, when looking at a chronic wound, the wound edge (location A) and wound bed (location C) are readily identifiable (Figure 1). Research has discovered that the wound really extends beyond this traditional boundary to include the area marked as location B.9,10 Much has been written about the preparation of the wound bed, ie, at location C.11,12 Wound closure (100% epithelialization) and restoration of the skin barrier are essential for wound healing. The focus of research about wound topography has been to understand the biological properties of the cells at the wound boundary, which has 2 edges that are biologically distinct: a healing edge, indicated by location B in Figure 1, and a nonhealing edge, indicated by location A.
Figure 1. KERATINOCYTE TOPOGRAPHY IN A WOUND.
A typical diabetic ulcer is shown. Arrows indicate specific wound locations: Wound bed = location C; nonhealing edge = location A; healing edge = location B.
KERATINOCYTES AS RESPONDERS IN WOUND HEALING
Keratinocytes are responsible for the formation and maintenance of the skin’s barrier. Disruption of the epidermal integrity activates a homeostatic response collectively called wound healing. This response is tailored to rapidly restore a functional epidermal lining over the wound site. Therefore, the entire wound healing process is designed to restore the barrier, which is produced by keratinocytes.13,14
Why are keratinocytes important for the wound healing process? The critical answer is that they initiate and maintain the entire process. Keratinocytes are the primary cells that respond in the wound by releasing prestored interleukin 1 (IL-1). Released IL-1 has a dual function. It represents “a call to arms” as it communicates to other cells in the wound site that the barrier has been broken and that pathogens may enter. Interleukin-1 also promotes activation of keratinocytes. This phenotypic change stimulates keratinocyte migration and proliferation, changing their phenotype from differentiating to activated. When activated, keratinocytes produce, secrete, and respond to multiple growth factors and cytokines, such as tumor necrosis factor α or epidermal growth factor/tumor necrosis factor α. This “cellular cross-talk” is an important characteristic of the wound healing process.13–15
Keratinocytes have the ability to fight infection by making antimicrobial peptides (AMPs). Antimicrobial peptides, such as cathelicidins and defensins, act as both natural antibiotics and as signaling molecules that activate the host cell process involved in immune defense and tissue repair.16
THE NONHEALING EDGE
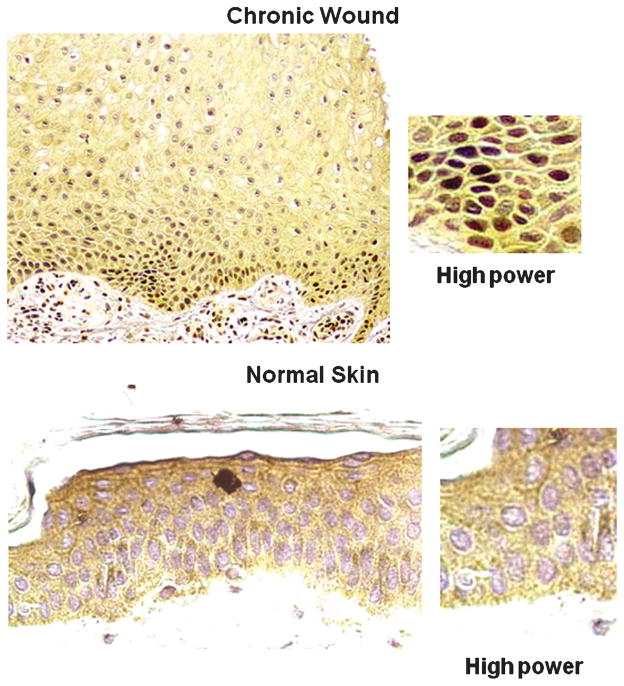
At first blush, it would appear that the edge of a chronic wound is made up of a uniform cell population. Studying tissues taken from chronic wounds in humans, the researchers discovered that skin cells get stuck in the middle of the normal healing process and cannot migrate to the wound site (Figure 2, left panel is chronic wound, right panel is normal skin). In skin with chronic wounds (location A in Figure 1), the cells multiply at a higher rate than usual (hyperproliferative), yet they are unable to migrate into the wound to close it as would be expected from normally activated keratinocytes.9,10 Instead, they form thickened layers around the edge, much like a callus or a corn. As keratinocytes move upward, they normally lose their nuclei and form sturdy layers of cross-linked proteins, creating a protective layer over the wound. But in chronic wounds, the skin cells are unable to progress to this stage of differentiation, and they remain nucleated. Thus, the keratinocytes of the chronic wound seem to be trapped in the middle of these 2 normal processes and cannot seem to complete either of them.
Figure 2. HISTOLOGY OF WOUND EDGE.
Histopathology of nonhealing edge (left) reveals thick cornified layer (stratum corneum) and thick, hyperproliferative epidermis. Section of normal skin (right) is shown for comparison.
This lack of keratinocyte migration and epithelialization, sometimes in the presence of adequate granulation tissue and the stalling of the healing process, is caused by the over-abundance of a molecule called c-myc (Figure 3, darkened cells in chronic wound), a product of the ubiquitous myc gene, which has been implicated in many human cancers. This molecule is known to suppress cell migration and to cause the skin to thicken, obstructing reparative cells from reaching the edge of the wound. The cause of c-myc overproduction was then traced one step up the molecular pathway to β-catenin, a critical regulator of cell behavior. β-catenin activates the production of c-myc as well as other pathways that affect the migration, growth, and regulation of skin cells. This is the first study to investigate the roles of c-myc and β-catenin in impairment of wound healing in humans.10
Figure 3. MOLECULAR MARKERS OF CHRONIC WOUNDS.
C-myc is a pathogenic marker of chronic wounds.10 Immunohistochemistry is showing positive staining with c-myc–specific antibody of the nuclei of the biopsy from a patient with chronic wound (top), whereas there is no c-myc staining in biopsy of normal skin (bottom).
In the healing edge (location B in Figure 1), however, keratinocytes are normalized and have restored capacity for migration and proliferation. These cells can respond to molecular wound healing signals. These exciting, new findings indicate that a potentially healing edge that can be mobilized to heal is behind every stalled nonhealing edge.
THE WOUND “BAR CODE”
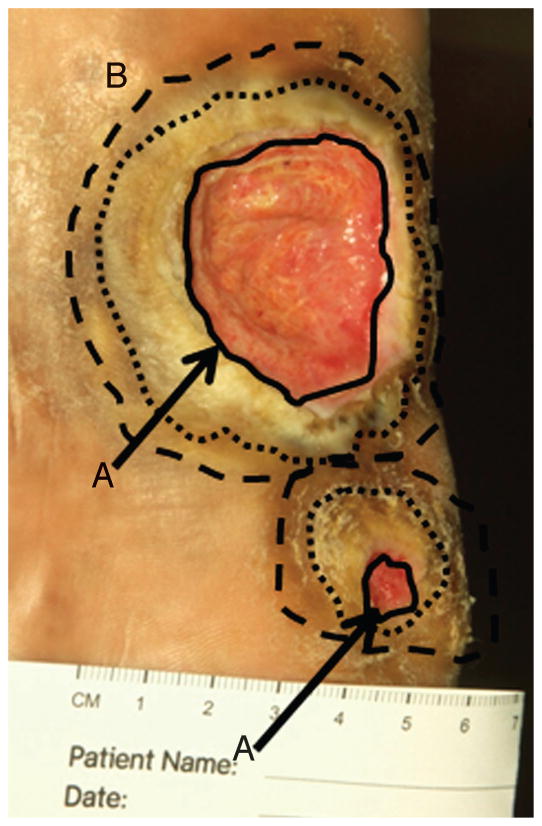
When looking at the edge of a chronic wound (Figure 4, solid black circle), most clinicians would readily identify a “stalled” rounded edge, ie, a callus that indicates debridement to facilitate wound healing.11,17 Research, however, suggests that debriding only to this traditional boundary may be inadequate, still leaving impaired cells remaining. What would be helpful is a way to clearly and easily identify the nonhealing edge of location A from the healing edge of location B (Figure 4, possible location B indicated by broken lines). What if clinicians had a road map of these 2 wound edges? Research shows that the true healing edge of the wound, location B, lies further than traditionally thought.
Figure 4. BIOLOGICALLY BASED MARGINS OF DEBRIDEMENT.
Digital photograph of a neuropathic plantar foot ulcer in a patient with diabetes. The solid black lines indicate nonhealing edge (location A, indicated by black arrows). Two outer circles (broken lines) indicate 2 possible margins of debridement, the presumed location B or healing-edge of the wound. At this edge, keratinocytes have the ability to migrate and participate in the wound healing process.
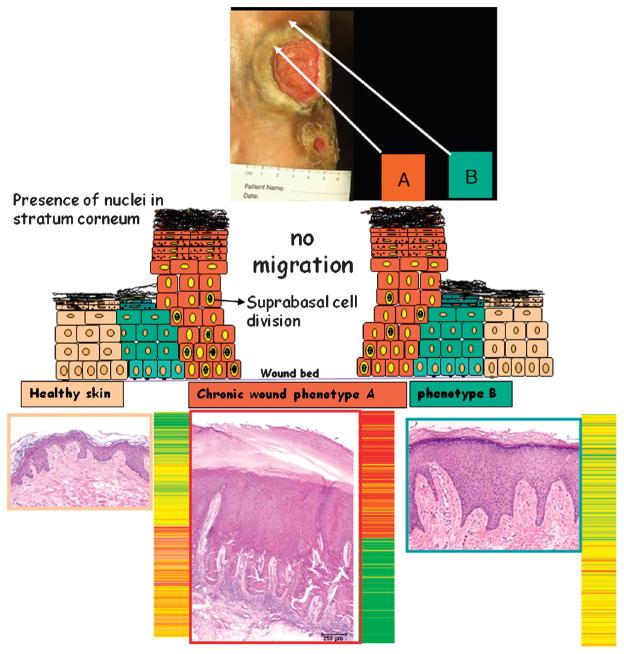
A new pilot investigation identified the markers that can differentiate the nonhealing edge from the healing edge.9 Venous ulcers using biopsies from nonhealing edge (location A) and healing edge (location B) were studied to characterize them on the molecular level. For each of them, histology (Figure 5) and isolation of RNA from the samples were performed. The level of messenger RNA (mRNA) corresponds to the level of the gene activity in each sample. The mRNA levels from 2 wound locations were quantified using microarrays. Graphic presentation of the gene activity (expression pattern) results in the color pattern corresponding to a specific wound location (Figure 6). Using this method, one can translate gene activity into a graphic picture similar to the product bar code that everyone sees in everyday products (eg, the boarding pass on an airplane ticket). Therefore, each location within a wound has a specific “wound bar code.” The bar code for a sample taken at location B is different from the bar code at location A in the wound regardless of the type of wound.
Figure 5. WOUND BAR CODE.
Histology and gene expression profiles obtained from biopsies from a patient with venous ulcer are shown for 3 locations: nonhealing edge (location A), healing edge (location B), and normal skin. Specific gene expression pattern is detected from each of the locations, indicating that it can be used to determine the subpopulation of cells within the wound. Colors indicate the degree of regulation of specific genes.
Figure 6. WOUND BAR CODE LOCATION.
Debridement aims at removal of cells from location A, thus exposing cells from location B exposed to wound healing stimuli.
IMPLICATIONS FOR CLINICAL PRACTICE
These studies can assist clinicians as they readily identify the stalled rounded wound edge versus the healing edge of cells capable of advancing. Clearly, the nonmigrating thickened wound edge at location A needs to be debrided to facilitate wound bed preparation and wound healing (Figure 6). The goal of surgical/sharp debridement is to remove the abnormal keratinocytes at location A while sparing the keratinocytes at location B, which are still capable of movement and stimulation, thus triggering the cells to commit to healing (Figure 4).
The question is: What area should be debrided? As debridement progresses and the nonhealing edge is removed, the part of the wound edge is reached that contains cells capable of migration and participating in wound healing. The clinician’s challenge is how to identify this region and determine the proper debridement margin?
The ideal road map for debridement would be an easily seen visible line of demarcation between the migrating and nonmigrating cells. Research has shown that the color patterns of gene expression of the wound edges at locations A and B are very different from each other. This means that during the debridement procedure, one can identify the specific bar code of the location before and after debridement, thus determining if the extent of debridement was sufficient. In practical terms, the clinicians may have a tool (a road map) to guide the debridement. In removing a cancer, the surgeon resects until “negative margins” are achieved as defined by pathology. Similarly, wound surgeons of the future may debride using the wound bar code until a “margin of response” is reached18: the margin at which the cells left behind after debridement can respond to the body’s endogenous wound healing signals and migrate as programmed. This margin is clinically identified by soft tissue. Although debridement of surrounding soft tissue may cause the ulcer to appear larger, these cells are typically impaired. A clear margin or border of debridement would also maximize the efficacy of advanced topical wound healing treatments by minimizing the number of cells unable to respond to the therapy.
In summary, by generating the gene expression profiles of the specific wound region by creating a simple color pattern, gene tree (analogous to how a blue dye is positive for pregnancy test), one can quickly identify from which region of the wound the biopsy originates and how well the wound was debrided.
CONCLUSIONS
Even with current treatment protocols, this new knowledge can be used to maximize the effect of current technology. At present, generating a wound bar code is an expensive and time-consuming procedure. It takes a minimum of 3 days to “develop” the bar code picture. Development of this technology will shorten the time and decrease the cost of wound management. So, just as an airline boarding pass is quickly scanned, the chronic stalled wound edge can be scanned in the operating room, speeding its journey to healing.
Acknowledgments
The authors thank the members of the Wound Healing Program at New York University, as well as the Tissue Repair Laboratory and Tissue Engineering, Regeneration and Repair Program of the Hospital for Special Surgery of the Weill Cornell Medical College, New York, NY. The authors’ research is supported by the National Institutes of Health grants DK59424 (H.B.) and NR08029 (M.T.-C.). The authors also acknowledge the use of the Musculoskeletal Repair and Regeneration Core Center of the Hospital for Special Surgery (AR046121).
Footnotes
Dr Tomic-Canic has disclosed that she has no significant relationships with or financial interest regarding this educational activity. Dr Ayello has disclosed that she is/was a consultant/advisor for Smith & Nephew, KCI, Molnlycke, Hill-Rom, and Sage; is a consultant/advisor for Covidien and Healthpoint; was a consultant/advisor for Coloplast and IVIVI; is/was a member of the speaker’s bureau for Smith & Nephew, KCI, Hill-Rom, Ross, and Organogenesis; is a member of the speaker’s bureau for 3M and Molnlycke; others: Lippincott Williams & Wilkins, New Jersey Hospital Association (with funding from 3M, Sage, and Healthpoint), World Union of Wound Healing Societies, American Professional Wound Care Association, Hollister, and Healthpoint. Drs Stojadinovic, Golinko, and Brem have disclosed that they have no significant relationships with or financial interest regarding this educational activity. All staff in a position to control the content of this CME activity have disclosed that they have no financial relationship with, or financial interests in, any commercial companies pertaining to this educational activity.
Lippincott CME Institute, Inc, has identified and resolved all faculty and staff conflicts of interest regarding this educational activity.
Contributor Information
Marjana Tomic-Canic, Tissue Repair Laboratory, Tissue Engineering Regeneration and Repair Program, Associate Scientist, Hospital for Special Surgery, Associate Professor of Dermatology, Weill Cornell Medical College, New York, NY.
Elizabeth A. Ayello, Ayello, Harris & Associates, Inc, Faculty, Excelsior College, School of Nursing, Senior Advisor, John A. Hartford Institute for Geriatric Nursing, New York, NY, Clinical Associate Editor, Advances in Skin and Wound Care, Ambler, PA, Co-Secretary, World Union of Wound Healing Societies.
Olivera Stojadinovic, Tissue Repair Laboratory, Hospital for Special Surgery of the Weill Cornell Medical College, New York, NY.
Michael S. Golinko, Department of Surgery, New York University School of Medicine, New York, NY.
Harold Brem, Division of Wound Healing & Regenerative Medicine, New York University Langone Medical Center, New York, NY, Associate Professor of Pathology, Department of Surgery, New York University School of Medicine New York, NY.
References
- 1.Center for Medicare & Medicaid Services. Expert Advisory Panel on the Usual Care of Chronic Wounds [cited 10/28/2006] [Last accessed August 1, 2008]; Available at: http://www.cms.hhs.gov/mcd/viewmcac.asp?where=index&mid=28.
- 2.Brem H, Jacobs T, Vileikyte L, et al. Wound-healing protocols for diabetic foot and pressure ulcers. Surg Technol Int. 2003;11:85–92. [PubMed] [Google Scholar]
- 3.Whitney J, Phillips L, Aslam R, et al. Guidelines for the treatment of pressure ulcers. Wound Repair Regen. 2006;14:663–79. doi: 10.1111/j.1524-475X.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- 4.Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity ulcers. Plast Reconstr Surg. 2006;117(7 Suppl):143S–149S. doi: 10.1097/01.prs.0000222526.21512.4c. discussion 150S–151S. [DOI] [PubMed] [Google Scholar]
- 5.Robson MC, Cooper DM, Aslam R, et al. Guidelines for the treatment of venous ulcers. Wound Repair Regen. 2006;14:649–62. doi: 10.1111/j.1524-475X.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 6.Brem H, Lyder C. Protocol for the successful treatment of pressure ulcers. Am J Surg. 2004;188(1A Suppl):9–17. doi: 10.1016/S0002-9610(03)00285-X. [DOI] [PubMed] [Google Scholar]
- 7.Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence-based protocol for diabetic foot ulcers. Plast Reconstr Surg. 2006;117(7 Suppl):193S–209S. doi: 10.1097/01.prs.0000225459.93750.29. discussion 210S–211S. [DOI] [PubMed] [Google Scholar]
- 8.Brem H, Kirsner RS, Falanga V. Protocol for the successful treatment of venous ulcers. Am J Surg. 2004;188(1A Suppl):1–8. doi: 10.1016/S0002-9610(03)00284-8. [DOI] [PubMed] [Google Scholar]
- 9.Brem H, Stojadinovic O, Diegelmann RF, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med. 2007;13(1–2):30–9. doi: 10.2119/2006-00054.Brem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stojadinovic O, Brem H, Vouthounis C, et al. Molecular pathogenesis of chronic wounds: the role of the beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167(1):59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11 (Suppl 1):S1–S28. doi: 10.1046/j.1524-475x.11.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 12.Falanga V, Saap LJ, Ozonoff A. Wound bed score and its correlation with healing of chronic wounds. Dermatol Ther. 2006;19(6):383–90. doi: 10.1111/j.1529-8019.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 13.Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2005;97(3):173–83. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomic-Canic M, Komine M, Freedberg IM, Blumenberg M. Epidermal signal transduction and transcription factor activation in activated keratinocytes. J Dermatol Sci. 1998;17(3):167–81. doi: 10.1016/s0923-1811(98)00016-4. [DOI] [PubMed] [Google Scholar]
- 15.Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. Keratins and the keratinocyte activation cycle. J Invest Dermatol. 2001;116(5):633–40. doi: 10.1046/j.1523-1747.2001.01327.x. [DOI] [PubMed] [Google Scholar]
- 16.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- 17.Steed DL, Donohoe D, Webster MW, Lindsley L. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. Diabetic Ulcer Study Group. J Am Coll Surg. 1996;183(1):61–4. [PubMed] [Google Scholar]
- 18.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–22. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]