Abstract
Recent studies have begun to illuminate the mechanism of T-cell export from the thymus, with the identification of a required lysophospholipid receptor, two upstream transcription factors, and several downstream regulators of cytoskeleton dynamics. This work has generated immediate translational impact, aiding the design of immunosuppressant drugs and the identification of a novel form of human immunodeficiency.
Introduction and context
Human immunocompetence and survival depend on the egress of newly produced T cells from the thymus. Approximately 1% of the thymocyte population emigrates from the thymus each day to populate the peripheral T-cell compartment [1]. Following the arrival of early thymic progenitors, the thymus supports all steps of T-cell development, from the differentiation of progenitors into T-cell receptor (TCR)-expressing CD4 and CD8 double-positive (DP) cells in the cortex to the maturation of DP into CD4 or CD8 single-positive (SP) cells that localize to medullary regions. SP thymocytes initially possess an immature phenotype but over time acquire markers associated with maturation (Figure 1). Egress is thought to occur via blood vessels and lymphatics, but the route that predominates remains unknown [2,3]. An initial insight into the mechanism of thymic egress came with the finding that transgenic expression in thymocytes of pertussis toxin, an inhibitor of Gαi signaling, strongly inhibited egress [4]. A role for Gαi2 in thymocyte emigration was later suggested [5] though may instead reflect an accelerated transition of Gαi2-deficient cells from the DP to SP stage of development [6]. Important further advances regarding the mechanism of thymic egress emerged from a combination of pharmacological and genetic studies that established an essential role for sphingosine-1-phosphate (S1P) and one of its G-protein-coupled receptors.
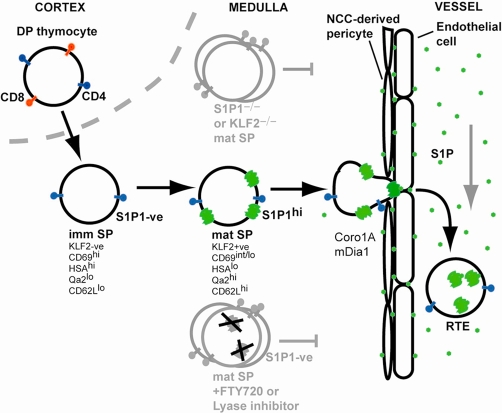
Figure 1. Thymocyte maturation and egress via a blood vessel.
A thymocyte is depicted undergoing maturation from the cortical CD4 and CD8 double-positive (DP) stage to the medullary CD4 single-positive (SP) stage. The SP cell is initially in an immature state, lacking Krüppel-like factor-2 (KLF2) and sphingosine-1-phosphate receptor-1 (S1P1) and having a characteristic surface phenotype. After a period of a few days, if the cell survives negative selection, it upregulates KLF2 and then S1P1 (green 7-transmembrane structure) and undergoes other changes in surface marker expression. Acquisition of S1P1 allows the cell to respond to S1P (green dot) that is present at approximately 1,000 nM in the vessel lumen (supplied by red blood cells) and locally supplied by radiation-resistant cells, perhaps endothelial cells or pericytes. Thymic pericytes are unusual in being neural crest cell (NCC)-derived. The final egress step triggered by S1P, likely the reverse transmigration step, requires coronin-1A- and mDia1-mediated reshaping of the actin cytoskeleton and thus the cell. Cells that have just exited the thymus are referred to as recent thymic emigrants (RTEs). T cells that are genetically deficient in S1P1 or KLF2 can reach the mature SP stage but then fail to egress and accumulate in the medulla. Treatment with FTY720 or with S1P lyase inhibitors that promote increases in intrathymic S1P causes S1P1 down-modulation from the cell surface and likely degradation (black crosses) and interrupts egress, again causing accumulation of mature SP cells in the medulla. Cells lacking coronin-1A or mDia1 are also egress-defective. imm, immature, mat, mature.
Major recent advances
The compound FTY720, identified during a screen for immunosuppressants, blocks thymic egress [7]. The active phosphoryl metabolite of FTY720 acts as an agonist for four of five S1P receptors [8], and of these, S1P1 is strongly upregulated in maturing SP thymocytes [9]. Remarkably, genetic deletion of S1P1 in thymocytes phenocopied the pertussis transgenic mouse, indicating that S1P1 is the primary Gi-coupled receptor mediating egress [9,10]. Deficiency in the two kinases that generate S1P led to a similar thymic egress defect [3]. A second small molecule, 2-acetyl-4-tetrahydroxybutyl-imidazole (THI), known to reduce thymic egress when fed to mice [11], was found to inhibit S1P lysase, causing 1,000-fold increases in thymic S1P and down-modulation of thymocyte S1P1 [12]. Similar findings were made when S1P lyase was knocked down using a short hairpin RNA (shRNA) [12] and through analysis of mice lacking the S1P lyase gene [13]. The latter mice showed severe growth abnormalities and poor viability, perhaps reflecting roles of the lyase in sphingolipid metabolism, and thymic function was strongly affected. Introduction of a low-expressing S1P lyase transgene rescued non-lymphoid pathologies while failing to fully restore thymic egress [13]. These studies highlight the importance of appropriately compartmentalized S1P distribution to support normal thymic egress (Figure 1).
A study aimed at characterizing the altered T-cell compartment in mice lacking Krüppel-like factor-2 (KLF2), a zinc-finger transcription factor, revealed a block in thymic egress associated with reduced S1P1 mRNA in mature thymocytes [14]. KLF2 co-immunoprecipitated with the proximal S1P1 promoter and could transactivate the promoter. However, sufficient S1P1 may remain in KLF2-deficient cells to permit low amounts of thymic egress [15] and further work will be needed to fully define the factors controlling S1P1 expression. Insight into factors that act upstream of KLF2 has come from work on the Foxo family of transcription factors. Foxo1 promotes expression of KLF2 and, likely in turn, S1P1 [16]. Deficiency in Foxo1 led to a slight accumulation of mature SP thymocytes, particularly of the CD8 lineage [17], though the block was less severe than for KLF2 deficiency. Other Foxos appear likely to help drive KLF2 expression. The inhibition of Foxo transcription factors by phosphoinositide 3-kinase (PI3K) signaling [16] might explain the thymic egress block seen in transgenic mice overexpressing PI3K in thymocytes [18].
The factors determining when expressions of Foxo, KLF2, and thus S1P1 are turned on remain undefined, but recent work suggests the involvement of a timer mechanism. In transgenic mice expressing green fluorescent protein (GFP) from the Rag2 locus, the amounts of GFP in the cell can be used as a molecular timer to determine how much a thymocyte has aged since its Rag2-expressing DP stage of development [19,20]. By means of this approach, a medullary dwell time for SP thymocytes of 4 to 5 days was calculated, with egress occurring in a ‘conveyor belt’ fashion with cells that enter the medulla first exiting the thymus first [20].
To exit the thymus, T cells must reverse-transmigrate across an endothelial barrier. Several recent observations suggest that molecules required for reorganization of the thymocyte actin cytoskeleton are central to this process. One study followed up on the description of a spontaneous mutant mouse line with a thymic egress defect and peripheral T-cell deficiency [21] to identify a role for the Arp2/3 regulatory protein coronin-1A in thymic egress [22]. Notably, although DP cell migration was also affected by the mutation when tested in vitro, the in vivo thymic defect revealed itself only as an accumulation of mature SP cells, suggesting an especially stringent need for appropriate control of actin branching during the transmigration step. Mice deficient in the formin protein, mDia1, also suffered reduced thymic egress [23]. Since formins support polymerization of unbranched actin, this work further highlights the necessity of fine actin cytoskeleton control to coordinate the cell shape changes required for egress.
In addition to molecules involved in cytoskeletal reorganization, cell adhesion molecules may play a role in thymocyte egress. Recent data suggest that P-selectin glycoprotein ligand-1 (PSGL-1) may be involved [24]. P-selectin marks a subset of thymic endothelial cells, and thymocytes express PSGL-1 [24]. However, the accumulation of thymocytes at the immature SP stage in PSGL-1-deficient mice [24] is unusual for a thymic egress defect (Figure 1). Further study of how PSGL-1 influences SP thymocyte behavior is needed before it can be concluded to function at the egress step.
In addition to endothelial cells, the vascular barrier involves a layer of pericytes and two layers of basement membrane, the latter forming the so-called perivascular space (PVS) [2]. The significance of the PVS in egress remains unclear, though a reconstitution study has correlated the appearance of SP thymocytes in the PVS with the time when cells begin to egress from the thymus [25]. Two recent studies made the striking observation that the pericytes surrounding thymic blood vessels are neural crest cell-derived [26,27]. The significance of this specialized origin for thymic pericytes is unknown but may relate to the barrier properties of thymic vessels [28] or to their unique requirement to support thymocyte egress.
Future directions
Our current understanding of T-cell egress has profited enormously from the discovery of the egress inhibitory drug, FTY720, and the potential of this molecule for treatment of human autoimmune disorders is under ongoing investigation [29]. The immunosuppressive potential of S1P lyase inhibition is also under exploration with a small molecule inhibitor, LX2931, entering clinical trial [30]. An important question in this area will be to define the impact of prolonged inhibition of thymic egress on thymic function.
The importance of normal thymic egress in humans has also been highlighted by the identification of a T-B+ natural killer (NK)+ severe combined immune deficiency (SCID) patient who lacked functional alleles of the coronin-1A gene [22]. Unusual for T-B+NK+SCID but consistent with a thymic egress defect, the patient possessed normal thymic mass [31]. Given that the coronin-1A gene resides in a region of the human genome subject to copy number variation, it seems likely that further cases of SCID resulting from deficiency in this gene will be identified.
Work over the last several years has led to an increasingly detailed model for the essential final step in thymocyte maturation, acquisition of egress competence, but many questions remain. Chief among these are identifying the precise location and cellular dynamics of thymocyte egress, understanding how KLF2 induction is triggered over time, and establishing whether alterations in thymic egress occur as part of the change in thymic function that takes place in certain infectious and autoimmune diseases and during aging. Given the translational advances that have already been made, we can be sure that further research on thymic egress will continue to impact human medicine.
Acknowledgments
We apologize to colleagues whose work we were unable to cite owing to space limitations. MAZ is in the Biomedical Sciences Graduate Program and Medical Scientist Training Program. JGC is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- DP
double-positive
- GFP
green fluorescent protein
- KLF2
Krüppel-like factor-2
- NK
natural killer
- PI3K
phosphoinositide 3-kinase
- PSGL-1
P-selectin glycoprotein ligand-1
- PVS
perivascular space
- S1P
sphingosine-1-phosphate
- S1P1
sphingosine-1-phosphate receptor-1
- SCID
severe combined immune deficiency
- shRNA
short hairpin RNA
- SP
single-positive
- TCR
T-cell receptor
- THI
2-acetyl-4-tetrahydroxybutyl-imidazole
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://F1000.com/Reports/Biology/content/1/60
References
- 1.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–8. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 2.Kato S. Thymic microvascular system. Microsc Res Tech. 1997;38:287–99. doi: 10.1002/(SICI)1097-0029(19970801)38:3<287::AID-JEMT9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–8. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.4 Must ReadEvaluated by Andy Chan 30 Apr 2007, Dan Conrad 14 May 2007
- 4.Chaffin KE, Perlmutter RM. A pertussis toxin sensitive process controls thymocyte emigration. Eur J Immunol. 1991;21:2565–73. doi: 10.1002/eji.1830211038. [DOI] [PubMed] [Google Scholar]
- 5.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–50. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Finegold MJ, Jin Y, Wu MX. Accelerated transition from the double-positive to single-positive thymocytes in G alpha i2-deficient mice. Int Immunol. 2005;17:233–43. doi: 10.1093/intimm/dxh204. [DOI] [PubMed] [Google Scholar]
- 7.Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–19. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Rosen H, Sanna MG, Cahalan SM, Gonzalez-Cabrera PJ. Tipping the gatekeeper: S1P regulation of endothelial barrier function. Trends Immunol. 2007;28:102–7. doi: 10.1016/j.it.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]; F1000 Factor 8.4 ExceptionalEvaluated by Michael Dustin 03 Feb 2004, Steve Ward 20 Feb 2004, Reina Mebius 16 Mar 2004, Klaus Ley 07 Apr 2004, Pamela Schwartzberg 07 Jul 2004
- 10.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine-1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Pamela Schwartzberg 09 Jul 2004
- 11.Gobin SJ, Paine AJ. Effects of 2-acetyl-4-tetrahydroxybutyl imidazole (THI) on the thymus of rats. Thymus. 1992;20:17–30. [PubMed] [Google Scholar]
- 12.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–9. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Steve Ward 14 Sep 2005
- 13.Vogel P, Donoviel MS, Read R, Hansen GM, Hazlewood J, Anderson SJ, Sun W, Swaffield J, Oravecz T. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS ONE. 2009;4:e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 15.Sebzda E, Zou Z, Lee JS, Wang T, Kahn ML. Transcription factor KLF2 regulates the migration of naive T cells by restricting chemokine receptor expression patterns. Nat Immunol. 2008;9:292–300. doi: 10.1038/ni1565. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Steve Ward 14 Apr 2008
- 16.Fabre S, Carrette F, Chen J, Lang V, Semichon M, Denoyelle C, Lazar V, Cagnard N, Dubart-Kupperschmitt A, Mangeney M, Fruman DA, Bismuth G. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–9. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 17.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–84. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Toshinori Nakayama 17 Apr 2009
- 18.Barbee SD, Alberola-Ila J. Phosphatidylinositol 3-kinase regulates thymic exit. J Immunol. 2005;174:1230–8. doi: 10.4049/jimmunol.174.3.1230. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Steve Ward 02 Feb 2005
- 19.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–25. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 20.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–84. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yagi H, Matsumoto M, Nakamura M, Makino S, Suzuki R, Harada M, Itoh T. Defect of thymocyte emigration in a T cell deficiency strain (CTS) of the mouse. J Immunol. 1996;157:3412–9. [PubMed] [Google Scholar]
- 22.Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, An J, Xu Y, Jenne CN, Foger N, Sorensen RU, Goodnow CC, Bear JE, Puck JM, Cyster JG. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol. 2008;9:1307–15. doi: 10.1038/ni.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Francisco A Bonilla 15 Dec 2008
- 23.Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, Miki T, Minato N, Narumiya S. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204:2031–8. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Giulio Gabbiani 15 Aug 2007
- 24.Gossens K, Naus S, Corbel SY, Lin S, Rossi FM, Kast J, Ziltener HJ. Thymicprogenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J Exp Med. 2009;206:761–78. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Avinash Bhandoola 19 May 2009
- 25.Mori K, Itoi M, Tsukamoto N, Kubo H, Amagai T. The perivascular space as a path of hematopoietic progenitor cells and mature T cells between the blood circulation and the thymic parenchyma. Int Immunol. 2007;19:745–53. doi: 10.1093/intimm/dxm041. [DOI] [PubMed] [Google Scholar]
- 26.Muller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson WD, Wegner M, Rodewald HR. Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol. 2008;180:5344–51. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- 27.Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, Kioussis D, Coles M. Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol. 2008;180:3183–9. doi: 10.4049/jimmunol.180.5.3183. [DOI] [PubMed] [Google Scholar]
- 28.Raviola E, Karnovsky MJ. Evidence for a blood-thymus barrier using electron-opaque tracers. J Exp Med. 1972;136:466–98. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Lexicon Pharmaceuticals, Inc. homepage. [ http://www.lexicon-genetics.com]
- 31.Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU, Puck JM. Severe combined immunodeficiency (SCID) and attention deficit hyperactivity disorder (ADHD) associated with a coronin-1A mutation and a chromosome 16p11.2 deletion. Clin Immunol. 2008;131:24–30. doi: 10.1016/j.clim.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Antony Basten 13 Jan 2009