Abstract
Dopamine neurons carry phasic signals for a limited number of behavioural events. The events include, in descending order, reward, physically intense stimuli, risk and punishment. Recent neurophysiological studies have provided interesting details on these functions.
Introduction and context
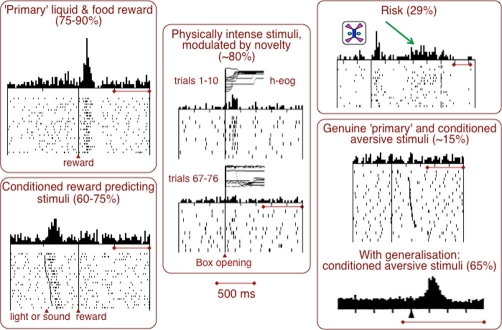
Results from lesion and psychopharmacological studies suggested a wide range of behavioural functions for midbrain dopaminergic systems. The key question is, which of these functions are actively encoded by impulse activity and dopamine release and thus give rise to a phasic dopamine signal suitable for rapid neuronal mechanisms? Promising leads come from drug addiction and electrical self-stimulation, suggesting that dopaminergic stimulation has rewarding and approach-generating effects [1,2]. The strongest dopamine signal is related to reward, as most dopamine neurons are phasically activated by reward-predicting stimuli and code bidirectional reward prediction errors in humans [3], monkeys (60-90% of dopamine neurons) [4] and rats [5]. However, dopamine neurons code more than reward (Figure 1) [6]. Substantial dopamine activations occur also with physically intense, salient stimuli (75-90% [4,7]), whereas novelty on its own fails to elicit activations [8] but enhances the response efficacy of stimuli [6]. Slightly slower activations code the predicted risk of future rewards in a fraction of dopamine neurons (29%) [9]. Only a small proportion of dopamine neurons in awake animals are activated by punishers and conditioned aversive stimuli such as air puffs, hypertonic saline or electric shock (<20% [10], 18-29% [11]), whereas depressions constitute the more frequent response. Aversive stimulation in anaesthetised animals produces varying but often low degrees of mostly slower, activating responses (50% [12], 18% [13], 17% [14], 14% [15]). Activating responses occur frequently to conditioned aversive stimuli when these are presented in random alternation with conditioned, reward-predicting stimuli of the same sensory modality (65% [10]); the activations are much less frequent when the two types of stimuli have different sensory modalities.
Figure 1. Multiple dopamine signals.
Neurophysiological activations of dopamine neurons following primary rewards and conditioned, reward-predicting stimuli, physically intense stimuli, risk, and primary and conditioned aversive stimuli. Horizontal bars indicate 500 ms in all panels. Data are from [4,9,10,26]. h-eog, horizontal electro-oculogram.
The reasons for ‘false’ aversive activations [10] might lie in generalisation with rewarding stimuli, sensitisation or pseudoconditioning, or stimulus salience. Generalisation arises from similarities between conditioned stimuli, which might explain the influence of sensory modalities on neuronal responsiveness [10]. Sensitisation or pseudoconditioning arises when a primary reinforcer sets a contextual background and provokes unspecific behavioural responses to any events within this context [16]. As dopamine neurons are very sensitive to reward, a rewarding context might induce a ‘default’ unspecific response to stimuli set in this context. Salience can be derived from physically strong stimuli or from motivationally important events like rewards, punishers or novel stimuli. Physical salience seems to drive dopamine neurons [4,7] but would not explain aversive responses to the usually employed small visual stimuli. Motivational salience might explain activations in dopamine neurons if these occur indiscriminately with both appetitive and aversive events without confounding generalisation and sensitisation-pseudoconditioning, but this remains to be shown. There are several possible explanations for the observed aversive activating responses of dopamine neurons, of which sensitisation-pseudoconditioning might be the most important one. True aversive activations do not seem to involve more than 10-20% of dopamine neurons when these confounds are ruled out, and aversive experiments that are more definitive would need to completely eliminate all contextual reward associations with the laboratory in which an awake animal is being tested.
Major recent advances
Neurophysiological reinvestigations with better identification of dopamine neurons confirmed the overall low incidence of aversive dopamine activations in anaesthetised animals [17] and located such neurons in the ventromedial tegmental area [18]. Aversive air puffs in awake monkeys produced activations in some dopamine neurons (23% [19], 11% [20]), similar to air puff to the arm (14% [10]). Interestingly, the air puff failed to induce bidirectional prediction error responses typical for reward; prediction had only modulating effects on aversive responses [20]. Conditioned, air puff-predicting stimuli activated a few dopamine neurons in one study (13% [19]) but substantially more than the air puff itself in another study (37% [20]). Since a conditioned stimulus is less aversive than the air puff it predicts, the higher number of activations to the stimulus (37%) compared with the air puff (11%) suggests an inverse relationship between aversiveness and activation, leaving the proportion of truly aversively activated dopamine neurons closer to 11% than 37%. One possible explanation for the more frequent aversive stimulus activations might lie in sensitisation or pseudoconditioning by the reward, whereas generalisation would be less with block design [20]; motivational salience would be highest for primary air puff and thus explain activations in only about 11% of neurons responding to this event. Although the stimulus activations correlated positively with air puff probability in the population, they were not assessed in individual neurons [20]. A population correlation may arise from a relatively small number of positively correlated neurons within that population.
Studies using other techniques described further functional aspects of dopamine signalling. Fast-scan voltammetry in behaving rats revealed dopamine increases with rewards that shifted to reward-predicting stimuli after conditioning [21], suggesting that impulse responses of dopamine neurons lead to corresponding dopamine release from striatal varicosities. The dopamine increase was differential for rewards (sucrose) and failed to occur with punishers (quinine) [22]. Apparently, the impulse response to punishment was insufficient for producing a measurable voltammetric dopamine change. This result contrasts with an earlier reported dopamine increase following aversive stimuli detected by in vivo dialysis [23,24]. The time courses of in vivo dialysis, which are 200 to 1800 times longer than those of fast-scan voltammetry, might allow the detection of dopamine released from the relatively few dopamine neurons activated by punishers. Finally, neuron-specific optical stimulation of dopamine neurons via genetically inserted channelrhodopsin-2 induced Pavlovian place preference in behaving rats [25], indicating an overwhelming causal influence of the rewarding rather than the aversive dopamine signal on learning and approach behaviour.
Future directions
Although the prediction error response of dopamine neurons would make a good teaching signal, the bulk of available data are correlational. Methods allowing investigators to study the causal role of dopamine in learning were initially hampered by the uncertain identity of electrically stimulated neurons [1], but these issues might be overcome by the recently emerging optogenetic methods [25]. Future optogenetic work might delineate the contributions of the different components of the prediction error response to behavioural learning and identify the particular forms of learning sensitive to dopamine signals.
Rewards, and in particular conditioned stimuli predicting such rewards, serve for choices between differently valued options. Future work may address the role of dopamine reward responses in decision making in which they might provide both a teaching signal for value updating and a value prediction signal for the different objects and actions involved in the decision process.
Future studies may investigate the role of non-reward-related dopamine signals in behavioural reactions. Although the dopamine systems appear to be more homogeneous in function compared with most other brain structures, a certain functional heterogeneity might prove advantageous for its role in behaviour. The various dopamine signals might differentially influence specific brain processes, and fine-grained studies in neuronal connectivity and receptor localisation should provide useful information.
Acknowledgements
The author's laboratory is supported by the Wellcome Trust, the Cambridge Behavioural and Clinical Neuroscience Institute (BCNI) and the Human Frontiers Science Programme.
Competing interests
The author declares that he has no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/b/2/2
References
- 1.Wise RA, Rompre P-P. Brain dopamine and reward. Ann Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 2.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 3.Zaghloul KA, Blanco JA, Weidemann CT, McGill K, Jaggi JL, Baltuch GH, Kahana MJ. Human substantia nigra neurons encode unexpected financial rewards. Science. 2009;323:1496–9. doi: 10.1126/science.1167342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–63. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- 5.Pan W-X, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005;25:6235–42. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Strecker RE, Jacobs BL. Substantia nigra dopaminergic unit activity in behaving cats: Effect of arousal on spontaneous discharge and sensory evoked activity. Brain Res. 1985;361:339–50. doi: 10.1016/0006-8993(85)91304-6. [DOI] [PubMed] [Google Scholar]
- 8.Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–8. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.4 Must ReadEvaluated by Xiao-Jing Wang 03 Jan 2002, Earl Miller 22 Jan 2002
- 9.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]; F1000 Factor 9.7 ExceptionalEvaluated by Robert Sapolsky 14 May 2003, Xiao-Jing Wang 28 May 2003, Earl Miller 02 Jun 2003
- 10.Mirenowicz J, Schultz W. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 1996;379:449–51. doi: 10.1038/379449a0. [DOI] [PubMed] [Google Scholar]
- 11.Guarraci FA, Kapp BS. An electrophysiological characterization of ventral tegmental area dopaminergic neurons during differential pavlovian fear conditioning in the awake rabbit. Behav Brain Res. 1999;99:169–79. doi: 10.1016/S0166-4328(98)00102-8. [DOI] [PubMed] [Google Scholar]
- 12.Chiodo LA, Antelman SM, Caggiula AR, Lineberry CG. Sensory stimuli alter the discharge rate of dopamine (DA) neurons: evidence for two functional types of DA cells in the substantia nigra. Brain Res. 1980;189:544–9. doi: 10.1016/0006-8993(80)90366-2. [DOI] [PubMed] [Google Scholar]
- 13.Mantz J, Thierry AM, Glowinski J. Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res. 1989;476:377–81. doi: 10.1016/0006-8993(89)91263-8. [DOI] [PubMed] [Google Scholar]
- 14.Schultz W, Romo R. Responses of nigrostriatal dopamine neurons to high intensity somatosensory stimulation in the anesthetized monkey. J Neurophysiol. 1987;57:201–17. doi: 10.1152/jn.1987.57.1.201. [DOI] [PubMed] [Google Scholar]
- 15.Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479–93. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Sheafor PJ. Pseudoconditioned jaw movements of the rabbit reflect associations conditioned to contextual background cues. J Exp Psychol Anim Behav Process. 1975;104:245–60. doi: 10.1037/0097-7403.1.3.245. [DOI] [PubMed] [Google Scholar]
- 17.Brown MTC, Henny P, Bolam JP, Magill PJ. Activity of neurochemically heterogeneous dopaminergic neurons in the substantia nigra during spontaneous and driven changes in brain state. J Neurosci. 2009;29:2915–25. doi: 10.1523/JNEUROSCI.4423-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009;106:4894–9. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Gary Aston-Jones 29 Apr 2009
- 19.Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci. 2008;28:11673–84. doi: 10.1523/JNEUROSCI.3839-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Luc Mallet 06 Jul 2009
- 20.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctively convey positive and negative motivational signals. Nature. 2009;459:837–41. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.6 Must ReadEvaluated by Kent Berridge 24 Jun 2009, James Surmeier 16 Jul 2009, Ruth Roberts 29 Jul 2009
- 21.Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–8. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.4 Must ReadEvaluated by Kent Berridge 20 Aug 2007, James Bibb 01 Oct 2007
- 22.Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–7. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.2 RecommendedEvaluated by Kent Berridge 05 Dec 2008, Norman White 16 Jan 2009
- 23.Joseph MH, Datla K, Young AMJ. The interpretation of the measurement of nucleus accumbens dopamine by in vivo dialysis: the kick, the craving or the cognition? Neurosci Biobehav Rev. 2003;27:527–41. doi: 10.1016/j.neubiorev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Young AM. Increased extracellular dopamine in nucleus accumbens in response to unconditioned and conditioned aversive stimuli: studies using 1 min microdialysis in rats. J Neurosci Meth. 2004;138:57–63. doi: 10.1016/j.jneumeth.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–4. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 9.6 ExceptionalEvaluated by Michael Frank 30 Apr 2009, John Dani 04 Sep 2009
- 26.Romo R, Schultz W. Dopamine neurons of the monkey midbrain: contingencies of responses to active touch during self-initiated arm movements. J Neurophysiol. 1990;63:592–606. doi: 10.1152/jn.1990.63.3.592. [DOI] [PubMed] [Google Scholar]