Abstract
BRCA and poly-ADP ribose polymerase (PARP) regulate pathways of DNA repair. Due to the accumulation of mutations introduced by error-prone DNA repair, breast and ovarian cancers develop in the setting of BRCA deficiency. A series of recent clinical trials has tested the use of PARP inhibition as a therapeutic strategy to target BRCA-deficient tumors.
Introduction and context
DNA sustains damage through exposure to chemicals, UV light, ionizing radiation, chemotherapy, or products of cellular metabolism [1]. Damaged DNA triggers cell cycle arrest and transcriptional activation. Single-strand breaks are faithfully repaired through base excision, nucleotide excision, or mismatch repair. Double-strand breaks are repaired by homologous recombination (HR), a process that restores the original genetic sequence, or by non-homologous end joining or single-strand annealing, processes that lack fidelity to the germline DNA sequence. Use of DNA repair pathways that are prone to error generates a new erroneous sequence. Malignancy arises when DNA is repaired with errors causing mutations that activate oncogenes or knock out tumor suppressor genes. If DNA is damaged to such an extent that it cannot be repaired by any mechanism, then cells cannot transcribe genes or replicate chromosomes, and will die.
The BRCA1 gene was identified in 1990 as a cause of hereditary breast cancer and was sequenced in 1994 and has since been shown to function in the complex HR pathway of DNA repair [2]. Women carrying a heterozygous deleterious mutation in the BRCA1 gene carry a 57% cumulative risk of developing breast cancer by the age of 70 years and a 40% risk of developing ovarian cancer [3]. In the case of a deleterious BRCA2 mutation, women have a 49% risk of developing breast cancer and an 18% risk of developing ovarian cancer by age 70. Tumors that arise in BRCA mutation carriers have lost the wild-type allele of the BRCA gene and express only the mutated/truncated allele. Therefore, the tumor cells are unable to repair DNA through BRCA-dependent mechanisms.
BRCA1 and BRCA2 regulate repair of damaged DNA through HR [2]. Active BRCA1 promotes cell cycle arrest in conjunction with p53 and associates with DNA double-strand breaks marked by RAD51 foci. ATM (ataxia telangiectasia mutated) and BRCA2 accumulate with BRCA1 and RAD51 at the sites of double-strand DNA damage. RAD51 promotes association with the sister chromatid, and DNA polymerase proceeds using the sister chromatid as the template for repair.
In cells lacking functional BRCA1 or BRCA2, HR is deficient. DNA repair proceeds through more error-prone repair pathways. Repair triggered in this manner typically removes the region of damaged DNA and joins the truncated DNA fragment with intact DNA. The cell cycle then resumes, propagating the deletion-mutated sequence. Chromosome instability in the BRCA-mutated HR-deficient cells thus perpetuates cellular proliferation in the setting of potential oncogene activation. It is unclear why breast or ovarian epithelial cells are more susceptible to the oncogenic outcome of BRCA deficiency.
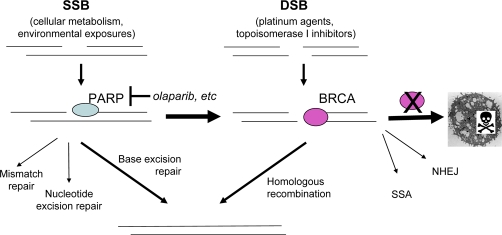
HR-deficient cells are exquisitely sensitive to inhibition of PARP (poly-ADP ribose polymerase 1 and 2) [4]. PARP is activated by DNA damage and subsequently modifies histones and DNA-associated proteins by ADP ribosylation. Areas of single-strand DNA damage are thus marked and signal the assembly of DNA repair complexes, primarily in the base excision repair pathway. With continuous PARP inhibition, single-strand breaks are converted to double-strand breaks at replication forks, which predominantly depend on HR. The accumulation of double-strand breaks overwhelms the DNA repair mechanisms in HR-deficient cells. Irreparable DNA damage triggers cell death (Figure 1). Therefore, inhibiting PARP has been undertaken as a strategy to selectively kill cells with dysfunctional BRCA proteins.
Figure 1. Mechanism of sensitivity to PARP inhibition in BRCA-deficient cells.
Cells acquire DNA damage through environmental exposures or chemotherapy agents. Repair of single-strand breaks relies on PARP; repair of double-strand breaks depends on BRCA. With PARP inhibition, single-strand breaks progress to double-strand breaks. In the absence of functional BRCA, the accumulation of double-strand breaks overwhelms DNA repair mechanisms. Irreparable DNA damage triggers cell death. DSB, double-strand break; NHEJ, non-homologous end joining; PARP, poly-ADP ribose polymerase; SSA, single-strand annealing; SSB, single-strand break.
Major recent advances
PARP inhibitors in clinical development
Small-molecule inhibitors of PARP activity began development as sensitizers to DNA-damaging chemotherapy or ionizing radiation. Due to the intrinsic synthetic lethality of the underlying defect in BRCA-dependent DNA repair, BRCA-deficient cancer cells are 1000-fold more sensitive to single-agent PARP inhibition. The concept of synthetic lethality - in which functional inhibition of two proteins leads to cell death but blockade of either alone does not - is well illustrated by the use of PARP inhibitors in BRCA-deficient cancers. If either PARP or BRCA function remains intact, a cell will continue to survive. Therefore, inhibiting PARP should not affect the non-cancerous cells that contain one functional copy of BRCA. Loss of both functions, however, is incompatible with life [5,6]. Preclinical studies supported this concept in vitro and in mouse models of BRCA-deficient tumors, thus prompting the clinical development of PARP inhibitors for these genetic cohorts of patients [4].
Six highly potent and specific PARP inhibitors are currently in clinical development in oncology [7]. BSI 201 (BiPar Sciences Inc., South San Francisco, CA, USA) has entered a phase III trial for triple-negative breast cancer in combination with gemcitabine and carboplatin (G/C). Three agents - olaparib (AZD 2281; AstraZeneca, London, UK), ABT888 (Abbott Laboratories, Abbott Park, IL, USA), and AG014966 (Pfizer Inc., New York, NY, USA) - are in phase II clinical trials as single agents or in combination with chemotherapy. Two PARP inhibitors are in phase I trials: MK4827 (Merck, Darmstadt, Germany) and CEP9722 (Cephalon, Inc., Frazer, PA, USA). Two additional agents entered clinical development but have not been pursued: GPI 21016 (Sanofi-Aventis, Paris, France) and INO-1001 (Genentech, Inc., South San Francisco, CA, USA).
PARP inhibitors in BRCA-deficient cancer
A recent phase I clinical trial identified the maximum tolerated dose of olaparib in patients with solid tumors and evaluated activity in an expansion cohort of 20 patients with BRCA-deficient tumors [8]. Of the 19 patients evaluable, 12 (63%) showed evidence of clinical benefit as defined by objective response (radiographic or tumor marker) or stabilization of disease for 4 months or longer. These results prompted phase II trials to extend the evaluation of efficacy specifically in BRCA-deficient ovarian cancer or breast cancer.
Early results of these phase II trials were presented at the American Society of Clinical Oncology (ASCO) 2009 Annual Meeting. The phase II trial of the PARP inhibitor olaparib in BRCA-deficient advanced breast cancer accrued two sequential cohorts of patients [9]. The first 27 patients received olaparib at 400 mg twice daily, and the second 27 patients received olaparib 100 mg twice daily since the pharmacodynamic results from the phase I trial showed maximal PARP inhibition in peripheral blood cells at the 100 mg dose. The therapy was well tolerated, and investigators reported a 41% overall response rate in the patients of the first cohort, with a median progression-free survival of 5.7 months. The second clinical trial tested the same two sequential dose cohorts in women with BRCA-deficient advanced ovarian cancer [10]. At the time of the preliminary report, 33 patients were treated at the 400 mg dose, with response in 57.6% of patients and a median progression-free survival of 5.8 months. Of 24 other patients treated at the 100 mg dose, 16.7% had achieved a response, with a progression-free survival of 1.9 months. Although not statistically powered to evaluate differences between the two dose cohorts, these preliminary results suggest that the pharmacodynamic saturation of PARP inhibition, as tested in peripheral blood cells of the patients in the phase I trial, may not have adequately reflected the level of activity of this compound in the tumors.
Triple-negative breast cancer - lacking expression of estrogen receptor (ER), progesterone receptor, and HER2 - is an aggressive breast cancer subtype that shares molecular and pathologic features with BRCA1-related breast cancers. The majority of BRCA1-deficient breast cancers occur as the triple-negative phenotype. Recent molecular evidence suggests a necessity for functional BRCA1 protein in inducing expression of ER in breast cell progenitors [11,12]. Sporadic triple-negative breast cancer occurring in non-mutation carriers may similarly result from epigenetic silencing of BRCA1 and ER by aberrant methylation of these genes’ promoters [13]. A randomized phase II trial, also presented at the ASCO 2009 Annual Meeting, evaluated the efficacy of G/C with or without the PARP inhibitor BSI-201 [14]. Patients were randomly assigned to G/C alone or G/C + BSI-201. Endpoints were clinical benefit rate (CBR) (that is, complete response + partial response + stable disease of at least 6 months), progression-free survival, and overall survival. Early results of this trial showed that BSI-201 improved CBR from 21% to 62% (P = 0.0002), increased progression-free survival from 3.3 to 6.9 months (P <0.0001), and extended overall survival from 5.7 to 9.2 months (P = 0.0005) in these patients.
Future directions
In summary, these trials have provided proof of principle in achieving synthetic lethality of PARP inhibition in the setting of BRCA deficiency in human cancer. BRCA-deficient cancers typically show heightened sensitivity to DNA-damaging chemotherapeutic agents that cause double-strand breaks in DNA typically repaired by HR [2]. Ongoing clinical trials at the National Cancer Institute and elsewhere are testing the safety and efficacy of using PARP inhibitors in combination with chemotherapeutic agents that induce double-strand breaks, such as carboplatin, topotecan, cyclophosphamide, or temozolomide, in patients carrying BRCA1 or BRCA2 germline mutations. It has been postulated that the combination of a PARP inhibitor with a DNA-damaging agent may lead to excessive myelosupression. Our general impression thus far, however, is that tolerance of the combined regimen is more closely related to prior platinum therapy rather than the existence of the germline mutation. Ongoing studies will address this question in parallel cohorts. Results of these trials are eagerly awaited in hopes of improving response rate and duration and eliminating these hereditary cancers.
Acknowledgments
The authors would like to acknowledge the support of the National Cancer Institute (NCI) Intramural Research Program.
Abbreviations
- ASCO
American Society of Clinical Oncology
- CBR
clinical benefit rate
- ER
estrogen receptor
- G/C
gemcitabine and carboplatin
- HR
homologous recombination
- PARP
poly-ADP ribose polymerase
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/b/2/10
References
- 1.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–85. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 2.Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. 2002;8:571–6. doi: 10.1016/S1471-4914(02)02434-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lord CJ, Ashworth A. Targeted therapy for cancer using PARP inhibitors. Curr Opin Pharmacol. 2008;8:363–9. doi: 10.1016/j.coph.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Iglehart JD, Silver DP. Synthetic lethality--a new direction in cancer-drug development. N Engl J Med. 2009;361:189–91. doi: 10.1056/NEJMe0903044. [DOI] [PubMed] [Google Scholar]
- 6.Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–98. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 7.ClincialTrials.gov homepage. [ www.clinicaltrials.gov]
- 8.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]; f1000 Factor 4.8 Must ReadEvaluated by Christos Sotiriou 23 Jul 2009, Jeffrey Evans 26 Aug 2009
- 9.Tutt A, Robson M, Garber JE, Domchek S, Audeh MW, Weitzel JN, Friedlander M, Carmichael J. Phase II trial of the oral PARP inhibitor olaparib in BRCA-deficient advanced breast cancer. J Clin Oncol. 2009;27:18s. [Google Scholar]
- 10.Audeh MW, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Carmichael J, Tutt A. Phase II trial of the oral PARP inhibitor olaparib (AZD2281) in BRCA-deficient advanced ovarian cancer. J Clin Oncol. 2009;27:15s. [Google Scholar]
- 11.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, Feleppa F, Huschtscha LI, Thorne HJ, kConFab. Fox SB, Yan M, French JD, Brown MA, Smyth GK, Visvader JE, Lindeman GJ. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–13. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]; f1000 Factor 6.0 Must ReadEvaluated by Ruth Keri 09 Oct 2009
- 12.Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci U S A. 2008;105:1680–5. doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000 Factor 6.0 Must ReadEvaluated by Ruth Keri 17 Apr 2008
- 13.Wei M, Xu J, Dignam J, Nanda R, Sveen L, Fackenthal J, Grushko TA, Olopade OI. Estrogen receptor alpha, BRCA1, and FANCF promoter methylation occur in distinct subsets of sporadic breast cancers. Breast Cancer Res Treat. 2008;111:113–20. doi: 10.1007/s10549-007-9766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Shaughnessy J, Osborne C, Pippen J, Yoffe M, Patt D, Monaghan G, Rocha C, Ossovskaya V, Sherman B, Bradley C. Efficacy of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in combination with gemcitabine/carboplatin (G/C) in patients with metastatic triple-negative breast cancer (TNBC): results of a randomized phase II trial. J Clin Oncol. 2009;27:18s. [Google Scholar]