Abstract
Multiple endocrine neoplasia syndrome type 1 (MEN1) syndrome has benefited from the identification of the gene whose mutations account for the genetic susceptibility to develop endocrine tumors. Asymptomatic MEN1 mutant carriers need to be clearly recognized because the gene-related mutations confer a high risk of multiple primary cancers, occur at younger ages, and affect multiple family members who inherit the cancer-predisposing genetic mutation.
Introduction and context
Multiple endocrine neoplasia syndrome type 1 (MEN1) syndrome is characterized by the occurrence of varying combinations of more than 20 endocrine and non-endocrine tumors. Endocrine tumors are represented mainly by the ‘classic’ P-triad originally described by Wermer: parathyroid, pituitary, and pancreatic tumors [1-4]. Tables 1 and 2 describe the endocrine and non-endocrine tumors associated with MEN1.
Table 1. Multiple endocrine neoplasia syndrome type 1 (MEN1)-related endocrine tumors and their prevalence (40 years).
| Tumor type | Tumor subtype | Prevalence in MEN1 syndrome |
|---|---|---|
| Parathyroida | Not applicable | 100% by age 50 years |
| Anterior pituitary (~10-60% of cases have anterior pituitary tumors) | ||
| Prolactinoma (PRL-oma) | Most common anterior pituitary tumor | |
| Growth hormone-secreting | 5% | |
| Growth hormone/Prolactin-secreting | 5% | |
| TSH-secreting | Rare | |
| ACTH-secreting | 2% | |
| Well-differentiated endocrine tumors | ||
| Gastrinomab | 40% | |
| Insulinoma | 10% | |
| Glucagonoma | 2% | |
| VIPoma | 2% | |
| Carcinoid | ||
| Bronchial | 10% | |
| Thymicc | ||
| Adrenocortical (~20-40% of cases have adrenocortical tumors) | ||
| Cortisol-secreting | Rare | |
| Aldosterone-secreting | Rare | |
| Pheochromocytoma | <1% |
aParathyroid tumors represent the main MEN1-associated endocrinopathy whose onset, in 90% of individuals, is between the ages of 20 and 25 years with hypercalcemia evident by the age of 50 years. bThe MEN1 gastrinomas, located mainly at the duodenal level, are frequently multiple and usually malignant, and half of them have metastasized before diagnosis. cThymic carcinoids of MEN1 syndrome tend to be aggressive and are highly lethal, particularly in male smokers [16]. Adrenocortical tumors are rarely associated with primary hypercortisolism or hyperaldosteronism. Among the non-endocrine tumors, facial angiofibromas, collagenomas, lipomas, meningiomas, ependymomas, and leiomyomas have been described in MEN1 subjects [16]. ACTH, adrenocorticotropic hormone; PRL-oma, prolactin-secreting adenoma; TSH, thyroid-stimulating hormone; VIPoma, vasoactive intestinal peptide-producing tumor.
Table 2. Multiple endocrine neoplasia syndrome type 1 (MEN1)-related non-endocrine tumors and their prevalence (40 years).
| Tumor type | Tumor subtype | Prevalence in MEN1 syndrome |
|---|---|---|
| Cutaneous tumors | Lipomas | 30% |
| Facial angiofibromas | 85% | |
| Collagenomas | 70% | |
| Central nervous system | Meningiomas | 5% |
| Ependymomas | 1% | |
| Other | Leyomiomas | 10% |
Familial and simplex MEN1 forms
The familial form of MEN1 syndrome occurs with a significantly higher frequency (90% of cases) than the simplex form, where only one individual is affected within a family with no history of the disease (10% of cases). Familial MEN1 is defined in an individual who has at least one first-degree relative with one or more main endocrine tumors or involvement of only one organ and a MEN1 disease-causing germline mutation. MEN1 syndrome is inherited in an autosomal dominant manner, and each child of an affected individual has a 50% chance of inheriting the mutation [5].
Clinical definition of MEN1
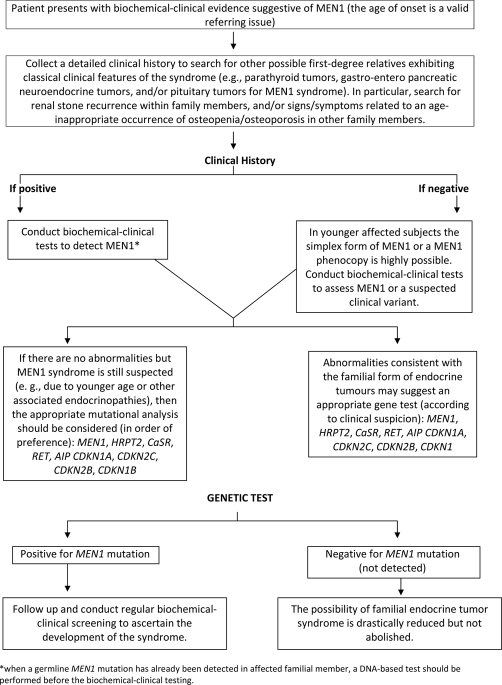
MEN1 syndrome can be defined by the presence of two ‘classic’ endocrine tumors (parathyroid, pituitary, or tumors of the gastro-entero-pancreatic tract) in an affected subject. In Figure 1, an algorithmic summary of the possible diagnostic scenario is presented.
Figure 1. Algorithmic summary of the diagnostic protocols.
MEN1, multiple endocrine neoplasia syndrome type 1.
Chromosomal location of the MEN1 gene and related tumorigenesis
The MEN1 gene was originally located on chromosome 11q13 [6-8]. The related tumorigenesis was according to Knudson’s ‘two hits’ hypothesis [9], strongly suggesting a gene inactivation.
MEN1 gene and its mutations
The MEN1 gene spans 9 kb and consists of 10 exons with a 1830-bp/1845-bp coding region encoding a novel 610/615-amino acid protein (two isoforms [10]), referred to as menin [11-13]. More than 1000 different germline MEN1 mutations, without evidence of hot-spot regions, have been described [14-18], mainly predicting absent or truncated menin. Approximately 1-3% of MEN1 germline mutations consist of large deletions detectable by Southern blot analysis or other gene dosage procedures (i.e., based on polymerase chain reaction) [15-18]. Polymorphic variants have also been described [18]. Neither the finding of a tumor suppressor mechanism nor the identification of binding partners has established the ultimate pathways of menin action in normal tissues or in tumors [16].
When and how to perform the genetic screening
A DNA test of the MEN1 gene detects mutations in 80-90% of probands with familial MEN1 and in 65% of individuals with simplex MEN1 [18] (Table 3).
Table 3. General features of multiple endocrine neoplasia syndrome type 1 (MEN1) predictive testing.
| •Diagnostic testing is appropriate in symptomatic individuals of any age. |
| •Confirming a diagnosis may alter medical management for the individual. |
| •It is medically indicated since early diagnosis allows interventions that reduce morbidity or mortality. |
| •Even in the absence of medical indications, predictive testing can influence life planning decisions. |
| •Molecular genetic testing of an affected family member may be required to determine the disease-causing mutation(s) present in the family. |
| •Genetic testing should be offered to at-risk members of a family in which a germline MEN1 mutation has been identified in an affected relative. Identifying carriers allows reproductive choices. |
| •A DNA test in MEN1 may be offered to children within the first decade because tumors such as insulinoma and pituitary adenomas have developed in some children by the age of 5 years. |
| •Genetic counseling and education should accompany carrier testing because of the potential for personal and social concerns. |
| •Many laboratories will not proceed with predictive testing without proof of informed consent and genetic counseling. |
| •Identification of the specific gene mutation in an affected relative or establishment of linkage within the family should precede predictive testing. |
| •Because predictive testing can have psychological ramifications, careful patient assessment, counseling, and follow-up are important. |
| •Predictive testing of asymptomatic children at risk for an adult-onset or later-onset disorder is strongly discouraged when no medical intervention is available (American Society of Human Genetics/American College of Medical Genetics Policy Statement - 1995) [38]. |
| •If molecular genetic testing is not possible or is not informative, individuals at 50% risk (first-degree relatives of an individual with MEN1 syndrome) should undergo routine biochemical-clinical evaluation. |
| •Currently, a DNA test identifying an individual as a MEN1 mutant gene carrier does not usually lead to immediate medical or surgical treatment, but it suggests precocious and frequent clinical screening. |
Genetic counseling in MEN1
Genetic counseling has a central role in the management of MEN1 patients and their closely related family members. MEN1 genetically predisposed subjects may benefit greatly from early identification by DNA analysis, especially at a presymptomatic stage [15,17]. Once a pathogenic MEN1 mutation has been identified in a proband, referral to a clinical geneticist is advised. Since it is recommended that adequate genetic counseling be given prior to DNA testing, presymptomatic testing in families with an identified MEN1 mutation should be performed within the context of genetic counseling [19] (Tables 3 and 4).
Table 4. Considerations when a multiple endocrine neoplasia syndrome type 1 (MEN1) genetic test has to be ordered.
| The choosing of an adequate laboratory |
| Pretest counseling and appropriate informed consent |
| Sample logistics and supporting documentation |
| Test result interpretation and follow-up program |
Individuals at risk
Subjects in whom the germline mutation has not been identified are at risk if they have inherited the MEN1 mutation from one affected parent or if they are the relatives of subjects clinically defined as suffering from MEN1.
Testing of relatives at risk
Genetic testing should be offered to at-risk members of a family in which a germline MEN1 mutation has been identified in an affected relative [18]. If molecular genetic testing is not possible or is not informative, individuals with a 50% risk (first-degree relatives of an individual with MEN1 syndrome) should undergo routine evaluation (Table 4). A DNA test for MEN1 may be offered to children within their first decade because tumors such as insulinoma and pituitary adenomas have developed in some children by the age of 5 years [2,20,21] (Table 4). Unfortunately, the great diversity and the lack of both mutational hot-spots and genotype-phenotype correlation make mutational screening time-consuming, arduous, and expensive [14]. Currently, a DNA test identifying an individual as a mutant gene carrier does not usually lead to immediate medical or surgical treatment, but it does suggest that precocious and frequent clinical screening should be carried out. Since we are still unable to predict tumor penetrance and malignancy individually, lifelong follow-up of MEN1 carriers is strongly recommended to prevent tumor morbidity.
Risk to family members
Approximately 90% of MEN1 individuals have an affected parent. However, the family history may appear negative because of (a) failure to recognize the disorder in family members, (b) early death of the parent before the onset of symptoms, or (c) late onset of the disease in the affected parent [17]. The risk to the siblings of the proband depends on the genetic status of the proband’s parents. If a parent of the proband is affected or has a disease-causing mutation, the risk to the siblings is 50%. If the disease-causing mutation found in the proband cannot be detected in the DNA of either parent, two possible explanations exist: (a) germline mosaicism in a parent or (b) a de novo mutation in the proband [17]. Each child of an individual with MEN1 has a 50% chance of inheriting the mutation. The risk to other family members depends on the status of the proband’s parents. If a parent is found to be affected or to have a disease-causing mutation or both, his or her family members are at risk [17].
Families with an apparent de novo mutation
When neither parent of a proband with MEN1 has the disease-causing mutation or clinical evidence of the disorder, it is likely that the proband has a de novo mutation (approximately 10%). However, explanations such as alternate paternity or maternity (i.e., with assisted reproduction), undisclosed adoption, or secretiveness within the family could also be considered [17].
Testing of at-risk asymptomatic individuals
When a disease-causing germline mutation has been identified in an affected family member, the genetic testing of at-risk asymptomatic individuals is appropriate for surveillance. When a known disease-causing mutation is not identified, linkage or haplotype analysis can be considered in families with more than one affected family member from different generations. Early detection of at-risk individuals affects medical management, and testing of asymptomatic individuals during childhood is beneficial [17].
Prenatal testing
Prenatal testing for MEN1 syndrome is not commonly requested, partly due to the lack of a universal consensus on performing such a diagnosis in MEN1. The disease-causing allele of an affected family member must be identified or linkage established in the family before prenatal testing can be performed [17].
Detection of MEN1 gene mutations
The advantages of DNA analysis are that (a) it requires a single blood sample and (b) it does not need to be repeated since the analysis is independent of the age of the individual and provides an objective result. Approximately 45% of germline mutations detected by sequence analysis are small deletions, and approximately 15% are small insertions [5]. The likelihood of detecting a MEN1 mutation is higher in individuals with more main P-triad tumors, especially from families with hyperparathyroidism and pancreatic islet tumors [22-24]. MEN1 genetic screening should also be offered to patients with primary hyperparathyroidism or gastrinomas after thorough investigation into the family history [25]. Simplex MEN1 cases are less likely to test positive than familial cases, in part because some of these simplex cases may be caused by somatic mosaicism [24]. Individuals who have a single MEN1-related tumor and no family history of MEN1 syndrome rarely have germline MEN1 mutations [22].
Recent advances
Intronic MEN1 mutations, such as SpaGVs (splicing-affecting genomic variants), have been recently reported. They are likely to be of significance in the 10% of MEN1 patients who do not have coding region mutations [26,27]. A new intron 3 mutation associated with PRL-oma (prolactin-secreting adenoma), decreased familial penetrance, and variable effects on MEN1 mRNA and menin have recently been described [28]. The MLPA (multiplex ligation-dependent probe amplification) assay may detect large deletions (4%) as germline mutation in MEN1 [29]. Through polymorphism analyses, gene dose assays, and nucleotide sequencing, a large germline deletion (approximately 29-kb pairs), spanning the whole MEN1 gene, has been identified in one patient with a positive family history for MEN1 whose germline MEN1 mutation was undetectable by conventional sequencing analysis [30]. Moreover, genetically diagnosed patients already harbor manifestations at the time of diagnosis, confirming that screening for a MEN1 mutation should be done at an early age [31].
It is very important to consider that germline mutations in other genes may cause a MEN1-like disorder in MEN1 mutation-negative families, namely the AIP gene [32] and the four cyclin-dependent kinase inhibitor genes CDKN1A/p15, CDKN2C/p18, CDKN2B/p21, and CDKN1B/p27 [33-35]. Interestingly, several of the proteins encoded by these genes play a role within the same molecular pathway as the menin protein. Although germline mutations in these genes appear to be rare (probably explaining only a small fraction of the MEN1 mutation-negative families), it may still be important to consider analysis of these genes in such families.
Implications for clinical practice
MEN1 mutant gene carriers must be followed by periodic clinical tumor surveillance as well as surveillance of recurrence after treatment or progression of the disease. The knowledge about carrier status enables early diagnosis and intervention [2,17]. A prospective clinical study on MEN1 mutant gene carriers revealed that biochemical evidence of neoplasia could be identified an average of 10 years before the clinical evidence of the disease, allowing early surgery. Thus, genetically positive individuals should undergo a focused surveillance for early identification of potentially malignant neuroendocrine tumors accounting for morbidity and/or mortality related to MEN1 [18].
Importantly, a very recent study having as the primary endpoint the evaluation of the occurrence of non-functioning pancreatic tumors (PETs) in asymptomatic MEN1 children carriers revealed the presence of non-functioning PETs, providing the opportunity to perform clinical surveillance to unravel their growth [36]. Thus, according to Triponez et al. [37], the possibility of precociously identifying asymptomatic MEN1 children carriers, as well as young adults with MEN1, may be helpful for the early identification of non-functioning PETs that otherwise may not be biochemically identified.
Gene testing decreased the morbidity and mortality associated with MEN1
A multicenter study of more than 250 MEN1 gene carriers revealed that, as a result of differential tumor detection, MEN1 carriers born during the second half of the 20th century tend to have their tumors diagnosed earlier than carriers of the same age born in the first half [31], a known general phenomen (anticipation phenomenon) observed in several other inherited tumors.
Conclusions
The identification of many molecular partners interacting with menin has increased our knowledge of its pathophysiology. However, more studies are necessary to clarify the MEN1-dependent tumorigenesis and the role that menin has in the development of endocrine and non-endocrine tumors. In the near future, there are prospects for novel treatments based on DNA, RNA, or even other small molecules. A better understanding of the intricate molecular pathway networks related to menin will be helpful for designing novel therapeutic strategies.
Abbreviations
- MEN1
multiple endocrine neoplasia syndrome type 1
- PET
pancreatic tumor
Competing interests
The author declares that he has no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/m/2/14
References
- 1.Wermer P. Genetic aspects of adenomatosis of endocrine glands. Am J Med. 1954;16:363. doi: 10.1016/0002-9343(54)90353-8. [DOI] [PubMed] [Google Scholar]
- 2.Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA, Jr, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658–71. doi: 10.1210/jc.86.12.5658. [DOI] [PubMed] [Google Scholar]
- 3.Anlauf M, Perren A, Meyer CL, Schmid S, Saremaslani P, Kruse ML, Weihe E, Komminoth P, Heitz PU, Klöppel G. Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology. 2005;128:1187–98. doi: 10.1053/j.gastro.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 4.Fendrich V, Langer P, Waldmann J, Bartsch DK, Rothmund M. Management of sporadic and multiple endocrine neoplasia type 1 gastrinomas. Br J Surg. 2007;94:1331–41. doi: 10.1002/bjs.5987. [DOI] [PubMed] [Google Scholar]
- 5.Brandi ML, Bordi C, Tonelli F, Falchetti A, Marx SJ. Multiple endocrine neoplasia type 1. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 3. Vol. 2. San Diego, CA, USA: Academic Press; 2008. pp. 1345–74. [DOI] [Google Scholar]
- 6.Larsson C, Skogseid B, Oberg K, Nakamura Y, Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988;332:85–7. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- 7.Friedman E, Sakaguchi K, Bale AE, Falchetti A, Streeten E, Zimering M, Weinstein L, Mc Bride WO, Nakamura Y, Brandi ML, Norton JA, Aurbach GD, Spiegel AM, Marx SJ. Clonality of parathyroid tumors in familial multiple endocrine neoplasia type 1. N Engl J Med. 1989;321:213–8. doi: 10.1056/NEJM198907273210402. [DOI] [PubMed] [Google Scholar]
- 8.Emmert-Buck MR, Lubensky IA, Dong Q, Manickam P, Guru SC, Kester MB, Olufemi SE, Agarwal S, Burns AL, Spiegel AM, Collins FS, Marx SJ, Zhuang Z, Liotta LA, Chandrasekharappa SC, Debelenko LV. Localization of the multiple endocrine neoplasia type I (MEN1) gene based on tumor loss of heterozygosity analysis. Cancer Res. 1997;57:1855–8. [PubMed] [Google Scholar]
- 9.Knudson AG. Antioncogenes and human cancer. Proc Natl Acad Sci U S A. 1993;90:10914–21. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menin - protein results National Center for Biotechnology Information. [ http://www.ncbi.nlm.nih.gov/protein?term=menin]
- 11.Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–7. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 12.Lemmens I, Van de Ven WJ, Kas K, Zhang CX, Giraud S, Wautot V, Buisson N, De Witte K, Salandre J, Lenoir G, Pugeat M, Calender A, Parente F, Quincey D, Gaudray P, De Wit MJ, Lips CJ, Höppener JW, Khodaei S, Grant AL, Weber G, Kytölä S, Teh BT, Farnebo F, Thakker RV. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum Mol Genet. 1997;6:1177–83. doi: 10.1093/hmg/6.7.1177. [DOI] [PubMed] [Google Scholar]
- 13.Gao SB, Feng ZJ, Xu B, Wu Y, Yin P, Yang Y, Hua X, Jin GH. Suppression of lung adenocarcinoma through menin and polycomb gene-mediated repression of growth factor pleiotrophin. Oncogene. 2009;28:4095–104. doi: 10.1038/onc.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verges B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, Cougard P, Chambe B, Montvernay C, Calender A. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87:457–65. doi: 10.1210/jc.87.2.457. [DOI] [PubMed] [Google Scholar]
- 15.Falchetti A, Marini F, Luzi E, Giusti F, Cavalli L, Cavalli T, Brandi ML. Multiple endocrine neoplasia type 1 (MEN1): not only inherited endocrine tumors. Genet Med. 2009;11:825–35. doi: 10.1097/GIM.0b013e3181be5c97. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal SK, Lee Burns A, Sukhodolets KE, Kennedy PA, Obungu VH, Hickman AB, Mullendore ME, Whitten I, Skarulis MC, Simonds WF, Mateo C, Crabtree JS, Scacheri PC, Ji Y, Novotny EA, Garrett-Beal L, Ward JM, Libutti SK, Richard Alexander H, Cerrato A, Parisi MJ, Santa Anna-A S, Oliver B, Chandrasekharappa SC, Collins FS, Spiegel AM, Marx SJ. Molecular pathology of the MEN1 gene. Ann N Y Acad Sci. 2004;1014:189–98. doi: 10.1196/annals.1294.020. [DOI] [PubMed] [Google Scholar]
- 17.Falchetti A, Marini F, Brandi ML. Multiple endocrine neoplasia type 1. GeneReviews section of the GeneTests website of the University of Washington, Seattle, WA, USA; 2005. [ http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=men1#men1] [Google Scholar]
- 18.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]; f1000 Factor 6.0 Must ReadEvaluated by Gilbert Cote 20 Nov 2007
- 19.Lips CJ, Hoppener JW, Van Nesselrooij BP, Van der Luijt RB. Counselling in multiple endocrine neoplasia syndromes: from individual experience to general guidelines. J Internal Med. 2005;257:69–77. doi: 10.1111/j.1365-2796.2004.01429.x. [DOI] [PubMed] [Google Scholar]
- 20.Lairmore TC, Piersall LD, DeBenedetti MK, Dilley WG, Mutch MG, Whelan AJ, Zehnbauer B. Clinical genetic testing and early surgical intervention in patients with multiple endocrine neoplasia type 1 (MEN 1) Ann Surg. 2004;239:637–45. doi: 10.1097/01.sla.0000124383.98416.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stratakis CA, Schussheim DH, Freedman SM, Keil MF, Pack SD, Agarwal SK, Skarulis MC, Weil RJ, Lubensky IA, Zhuang Z, Oldfield EH, Marx SJ. Pituitary macroadenoma in a 5-year-old: an early expression of multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2000;85:4776–80. doi: 10.1210/jc.85.12.4776. [DOI] [PubMed] [Google Scholar]
- 22.Ellard S, Hattersley AT, Brewer CM, Vaidya B. Detection of an MEN1 gene mutation depends on clinical features and supports current referral criteria for diagnostic molecular genetic testing. Clin Endocrinol (Oxf) 2005;62:169–75. doi: 10.1111/j.1365-2265.2005.02190.x. [DOI] [PubMed] [Google Scholar]
- 23.Klein RD, Salih S, Bessoni J, Bale AE. Clinical testing for multiple endocrine neoplasia type 1 in a DNA diagnostic laboratory. Genet Med. 2005;7:131–8. doi: 10.1097/01.GIM.0000153663.62300.F8. [DOI] [PubMed] [Google Scholar]
- 24.Odou MF, Cardot-Bauters C, Vantyghem MC, Carnaille B, Leteurtre E, Pigny P, Verier-Mine O, Desailloud R, Porchet N. Contribution of genetic analysis in screening for MEN1 among patients with sporadic disease and one or more typical manifestation. Ann Endocrinol (Paris) 2006;67:581–7. doi: 10.1016/S0003-4266(06)73010-4. [DOI] [PubMed] [Google Scholar]
- 25.Jäger AC, Friis-Hansen L, Hansen TV, Eskildsen PC, Sølling K, Knigge U, Hansen CP, Andersen PH, Brixen K, Feldt-Rasmussen U, Kroustrup JP, Mollerup CL, Rehfeld JF, Blichert-Toft M, Nielsen FC. Characteristics of the Danish families with multiple endocrine neoplasia type 1. Mol Cell Endocrinol. 2006;249:123–32. doi: 10.1016/j.mce.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Raghavan R, Shah S, Kondkar AA, Dherai AJ, Desai D, Chauhan P, Lala M, Ashavaid TF. MEN1 935-1G>C splicing mutation in an Indian patient with multiple endocrine neoplasia type 1. Mol Diagn Ther. 2007;11:129–31. doi: 10.1007/BF03256233. [DOI] [PubMed] [Google Scholar]
- 27.Lemos MC, Harding B, Shalet SM, Thakker RV. A novel MEN1 intronic mutation associated with multiple endocrine neoplasia type 1. Clin Endocrinol (Oxf) 2007;66:709–13. doi: 10.1111/j.1365-2265.2007.02806.x. [DOI] [PubMed] [Google Scholar]
- 28.Drori-Herishanu L, Horvath A, Nesterova M, Patronas Y, Lodish M, Bimpaki E, Patronas N, Agarwal S, Salvatori R, Martari M, Mericq V, Stratakis CA. An intronic mutation is associated with prolactinoma in a young boy, decreased penetrance in his large family, and variable effects on MEN1 mRNA and protein. Horm Metab Res. 2009;41:630–4. doi: 10.1055/s-0029-1216358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tham E, Grandell U, Lindgren E, Toss G, Skogseid B, Nordenskjöld M. Clinical testing for mutations in the MEN1 gene in Sweden: a report on 200 unrelated cases. J Clin Endocrinol Metab. 2008;92:3389–95. doi: 10.1210/jc.2007-0476. [DOI] [PubMed] [Google Scholar]
- 30.Fukuuchi A, Nagamura Y, Yaguchi H, Ohkura N, Obara T, Tsukada T. A whole MEN1 gene deletion flanked by Alu repeats in a family with multiple endocrine neoplasia type 1. Jpn J Clin Oncol. 2006;36:739–44. doi: 10.1093/jjco/hyl089. [DOI] [PubMed] [Google Scholar]
- 31.Machens A, Schaaf L, Karges W, Frank-Raue K, Bartsch DK, Rothmund M, Schneyer U, Goretzki P, Raue F, Dralle H. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin Endocrinol (Oxf) 2007;67:613–22. doi: 10.1111/j.1365-2265.2007.02934.x. [DOI] [PubMed] [Google Scholar]; f1000 Factor 3.2 RecommendedEvaluated by Gilbert Cote 24 Aug 2007, Alberto Falchetti 17 Sep 2007
- 32.Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gündogdu S, De Menis E, Mäkinen MJ, Launonen V, Karhu A, Aaltonen LA. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312:1228–30. doi: 10.1126/science.1126100. [DOI] [PubMed] [Google Scholar]; f1000 Factor 6.4 Must ReadEvaluated by Paolo Beck-Peccoz 21 Jun 2006, Anne-Paule Gimenez-Roqueplo 20 Oct 2006
- 33.Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Höfler H, Fend F, Graw J, Atkinson MJ. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A. 2006;103:15558–63. doi: 10.1073/pnas.0603877103. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000 Factor 3.0 RecommendedEvaluated by Patrick Gaudray 01 Nov 2006
- 34.Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, Vierimaa O, Mäkinen MJ, Tuppurainen K, Paschke R, Gimm O, Koch CA, Gündogdu S, Lucassen A, Tischkowitz M, Izatt L, Aylwin S, Bano G, Hodgson S, De Menis E, Launonen V, Vahteristo P, Aaltonen LA. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab. 2007;92:3321–5. doi: 10.1210/jc.2006-2843. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94:1826–34. doi: 10.1210/jc.2008-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000 Factor 6.0 Must ReadEvaluated by Branca Cavaco 06 Feb 2009
- 36.Newey PJ, Jeyabalan J, Walls GV, Christie PT, Gleeson FV, Gould S, Johnson PR, Phillips RR, Ryan FJ, Shine B, Bowl MR, Thakker RV. Asymptomatic children with multiple endocrine neoplasia type 1 mutations may harbor nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2009;94:3640–6. doi: 10.1210/jc.2009-0564. [DOI] [PubMed] [Google Scholar]; Changes Clinical Practicef1000 Factor 4.8 Must ReadEvaluated by Patrick Gaudray 29 Jul 2009, Alberto Falchetti 27 Aug 2009
- 37.Triponez F, Goudet P, Dosseh D, Cougard P, Bauters C, Murat A, Cadiot G, Niccoli-Sire P, Calender A, Proye CA, French Endocrine Tumor Study Group Is surgery beneficial for MEN1 patients with small (< or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg. 2006;30:654–62. doi: 10.1007/s00268-005-0354-9. [DOI] [PubMed] [Google Scholar]
- 38.The American Society of Human Genetics Board of Directors and The American College of Medical Genetics Board of Directors Points to Consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. J Hum Genet. 1995;57:1233–41. [PMC free article] [PubMed] [Google Scholar]