Abstract
The classification of breast cancers into multiple molecular subtypes has necessitated the need for biomarkers that can assess tumor progression and the effects of chemo-preventive agents on specific breast cancer subtypes. The goal of this study was to identify biomarkers whose expression are altered along with estrogen receptor α (ERα) in the polyoma middle-T antigen (PyMT) transgenic model of breast cancer and to investigate the chemopreventive activity of phenethyl isothiocyanate (PEITC). The diet of PyMT female mice was fortified with PEITC (8 mmol/kg) and the mammary streak and/or gross tumors and metastases in lungs were subjected to immunohistochemical analyses for ERα, FOXA1, and GATA-3. FOXA1 is associated with luminal type A cancers, while GATA-3 is a marker of luminal progenitor cell differentiation. In both control and PEITC-treated groups, there was a progressive loss of ERα and FOXA1 but persistence of GATA-3 expression indicating that the tumors retain luminal phenotype. Overall, the PyMT induced tumors exhibited the entire gamut of phenotypes from ERα+/FOXA1+/GATA-3+ tumors in the early stage to ERα±/FOXA1−/GATA-3+ in the late stage. Thus, PyMT model serve as an excellent model for studying progression of luminal subtype tumors. PEITC treated animals had multiple small tumors, indicating delay in tumor progression. Although these tumors were histologically similar to those in controls, there was a lower expression of these biomarkers in normal luminal cells indicating delay in tumor initiation. In in vitro studies, PEITC depleted AldeFluor-positive putative stem/progenitor cells, which may partly be responsible for the delay in tumor initiation.
Keywords: estrogen receptor, GATA-3, FOXA1, breast cancer
Introduction
Molecular profiling studies have led to the classification of breast cancer into five intrinsic subtypes: luminal subtype A, luminal subtype B, HER2+, basal, and normal-like (1). Although luminal type A and luminal type B breast cancers both express estrogen receptor α (ERα), luminal type A breast cancers additionally express transcription factors GATA-3 and FOXA1 (1,2). ERα, FOXA1 and GATA-3 constitute a functional transcription factor network that determines estrogen dependence and response to anti-estrogen treatment (3). On the other hand, luminal type B breast cancers express proliferation markers such as Ki67 and these cancers appear to be less dependent on estrogen and hence respond poorly to anti-estrogen treatment (4). Although biologically plausible, it is not clear whether clinically observed anti-estrogen resistance involves any progression from luminal type A to luminal type B breast cancers. Even if such a progression occurs, it is yet uncertain if it can be assessed through bio-marker profiling studies. Using MCF-7 cell model system, we have demonstrated insulin-mediated progression of luminal A to luminal B phenotype, which was accompanied by reduced expression of FOXA1 and GATA-3 but not ERα, enhanced growth factor signaling, and resistance to anti-estrogen treatment (5). Animal models provide an effective opportunity to test this concept in the biological environment and translate to human breast cancer biology.
The polyoma middle T (PyMT) transgenic mouse has proven to be an effective in vivo model that illustrates the progression of breast cancer. Mammary tumors demonstrate a distinct progression from adenoma through low grade to high-grade carcinoma and ultimately to systemic disease characterized by pulmonary metastasis (6). Polyoma middle T antigen is a membrane-attached protein and is a potent oncogene that is not expressed in human tissue. It targets signaling pathways often activated in human breast cancer including Src family kinases, ras oncogene, and PI3 kinase pathways. Its expression also results in up-regulation of the proliferation-related oncogene c-Myc, which is elevated in human breast cancer (6). Mammary hyperplasia in this mouse model can be detected at as early as four weeks. By 14 weeks many of these lesions have not only progressed to carcinoma, but also have given rise to pulmonary metastasis. The progression of these tumors from early adenoma to advanced/metastatic carcinoma has been correlated with changes in ERα and progesterone receptor (PR) status (6). The tumors retain their ERα/PR positivity through adenoma, which is similar to their expression in luminal type A tumors in humans. By the time these tumors progress to the stage of early carcinoma there is loss of PR expression subsequently followed by a decreased ERα expression in advanced/metastatic carcinoma. These expression patterns in the later stage of tumors are similar to that observed in human luminal type B tumors. If this model represents the progression of luminal type A to luminal type B tumors, then biomarkers like ERα, FOXA1 and GATA-3 could be used to study this progression. Moreover the effectiveness of chemopreventive agents in halting this progression could be studied through the use of these markers.
Phenethyl isothiocyanate (PEITC) is a compound that is derived from vegetables of the genus Brassica (7) and has demonstrable chemopreventive properties in rat esophagus, liver and bladder cancers (8). It has several proposed mechanisms of action including induction of reactive oxygen species (ROS) secondary to disruption of the glutathione antioxidant system resulting in mitochondrial damage, inactivation of redox-sensitive molecules, and massive cell death (9).
This study endeavors to investigate two specific aims. The first is to further explore the PyMT transgenic mice as an effective model of progression of invasive breast cancer by analyzing the expression of biomarkers that are characteristic of predominantly ERα-positive luminal type A tumors, specifically ERα, FOXA1, and GATA-3. Loss of FOXA1 expression has been shown to correlate with both decreased response to endocrine therapy as well as reduced survival in patients with luminal type A breast tumors (2,10). Additionally, there is evidence to suggest a correlation between loss of GATA-3 expression with poor prognosis and increased metastasis (11–14). The second aim of this study was to investigate the effectiveness of PEITC as a dietary chemopreventive agent, and to determine its effect(s) on AldeFluor expressing ‘stem’ cells and on biomarker expression.
We investigated the effectiveness of this compound by supplementing the diet of the PyMT mice with PEITC and examining its effects macroscopically as well as microscopically for mammary disease progression. Microscopic evaluation was complemented by immunohistochemical examination of serial mammary tissues for the ERα, FOXA1, and GATA-3 transcription factor network. In addition in vitro studies with a mammary tumor cell line derived from these animals were performed to investigate whether PEITC targets cancer cells with ‘stem/progenitor cell’- like properties using the AldeFluor assay.
Materials and methods
Mice and histology
All procedures involving mice were conducted in accordance with the National Institutes of Health regulations concerning the use and care of experimental animals. Approval from the Institutional Animal Care and Use Committee was obtained before initiating the study. Male PyMT mice of FVB/N strain were obtained from the National Cancer Institute mouse repository and were randomly bred with wild-type FVB/N (WT) females lacking the PyMT transgene to obtain female mice heterozygous for the PyMT transgene. All of the mice analyzed in this study were female PyMT heterozygote.
Female mice were divided into treatment and control groups after weaning at 21 days. PEITC fortified or control diet was given starting the next day after weaning. The treatment feed was made up of regular mouse feed treated with 8 mmol/kg of PEITC (Sigma, St. Louis, MO) dissolved in corn oil (Sigma), a dose that has been previously used in chemoprevention models (15). The control group received feed with the addition of plain corn oil. Based on duration of exposure to the supplemented feed prior to harvest, mice from each group were further divided into four subgroups: an indefinite or >8 weeks exposure (9 controls; 10 PEITC fed), eight weeks (8 controls; 6 PEITC fed), six weeks (7 controls; 5 PEITC fed), and four weeks (5 controls; 4 PEITC fed). All of the mice in the ‘indefinite’ group required euthanasia prior to the completion of the 16 weeks for humane reasons. These reasons included: ulceration, respiratory distress, and inhibited activity due to size of tumors such that mouse could not eat or drink.
In order to rule out any mammary tumor formation before supplementation, all mice were adequately palpated for mammary glands and/or tumors. Bilateral abdominal mammary glands were used for whole mount preparation in addition to distinctly palpable tumors. One or more tumors from the same animal were analyzed. Mammary glands were fixed in 10% formalin, and subsequently paraffin-embedded, sectioned, and stained with hematoxylin and eosin (H&E). Additionally, both lungs were also subjected to histological examination.
Cells and cell culture
Tumor sample was surgically isolated from a 14-week old PyMT female mouse, digested with collagenase/hyluranidase, and a cell line was established in vitro. Tumor cells were subsequently maintained in Dulbecco’s Modification of Eagle’s Medium (DMEM) with phenol red supplemented with 10% fetal bovine serum and penicillin/streptomycin.
Flow cytometry to measure stem-cell-like activity using AldeFluor assay
Cultured PyMT cells were treated for 24 and 48 h with PEITC concentrations at 5 nM and 10 nM. ALDH1-positivity was measured using AldeFluor assay as per instructions from the manufacturer (Stem Cell Technologies, Vancouver, Canada). The assay was performed in two parts; with and without a competitive inhibitor diethylamino-benzaldehyde (DEAB).
Immunohistochemistry (IHC)
To identify cells expressing ERα, FOXA1, and GATA-3, formalin-fixed mammary gland sections were immunostained using antibodies against ERα, FOXA1, and GATA-3 (Santa Cruz Biotechnology, Santa Cruz, CA) as described previously (2). Expression was recorded in two parts; percentage of staining that ranged from ‘0’ in case of no nuclear expression to a maximum of ‘100’ with all cells showing expression; and intensity of 0, 1+, 2+ and 3+ for none, weak, moderate and strong staining respectively. Percentage (P) and intensity (I) of nuclear expression for ERα, FOXA1 and GATA3 were multiplied to generate a numerical expression score (S = P × I) (2).
Statistical analysis
Data from animal studies as well as IHC were analyzed using Statistical Package for Social Sciences 17.0 (SPSS, Inc., Chicago, IL, USA). All continuous data were compared across the control and PEITC-supplemented groups using the Wilcoxon Signed Rank test. Survival curves were plotted using the Kaplan-Meier function. All tests were two-sided and a p=0.05 was considered as significant.
Results
PyMT transgenic mice
Mammary tissues from mice of 4 weeks age and those that demonstrated the four distinct mammary gland lesions, hyperplasia, adenoma, low-grade carcinoma and high-grade carcinoma, in PyMT as described by Lin et al (6) were analyzed for the expression of biomarkers.
Expression of only GATA-3 persists in tumors of PyMT mice
Expression of ERα, FOXA1, and GATA-3 in breast cancer is associated with a well-differentiated phenotype as well as a favorable prognosis (2). In particular, GATA-3 is required for differentiation of luminal progenitor cells (16,17). The expression pattern of ERα in the PyMT model has previously been reported (6) which states that ER-positivity decreases in tumors as they transform to the carcinomatous stage. However, the mechanisms associated with these changes have not been well studied. Also, there are no reports examining expression patterns of GATA-3 and FOXA1 in this model. To address this, we performed immunohistochemical examination of mammary tumors at different time points. When ERα and FOXA1 expression was compared across normal luminal cells, adenomas and carcinomas, there was a significant loss in their expression as lesions progressed from normal tissue to tumors (p<0.0001 for both markers) (Fig. 1). ERα expression was low in adenomas and they decreased further in carcinomas. Low FOXA1 expression was noted in adenomas but they were completely lost in carcinomas (p=0.034). Thus, it appears that FOXA1 expression parallels ERα in this model and its expression is lost. Alternatively, it is possible that FOXA1-negative tumor cells are clonally selected during progression of the tumor. GATA-3 expression was much higher than that of ERα and FOXA1 in adenomas and carcinoma stage and did not differ significantly from the normal mammary gland (p=0.157). This finding demonstrates that GATA-3 persists even in late stages of tumors in this mouse model (Fig. 1).
Figure 1.
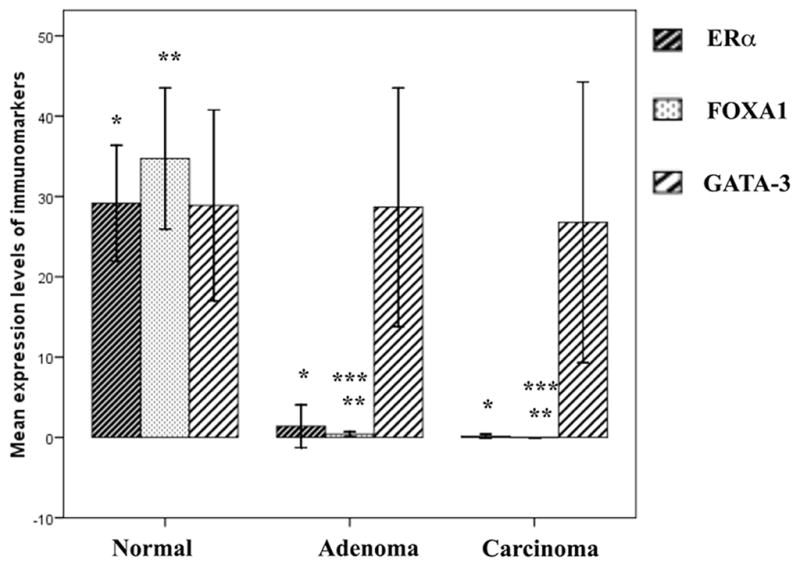
Graph (mean ± SE) demonstrating sequential loss of ERα, FOXA1 and GATA-3 in various stages of tumor progression in PyMT transgenic mouse model. Although, the expression levels of ERα and FOXA1 are negligible in late stages, we observed a persistence of GATA-3 expression even in late stages of tumors. *, **normal vs. adenoma or carcinoma for ERα and FOXA1 p<0.0001. ***FOXA1, adenoma vs. carcinoma p=0.034. Differences in GATA-3 expression were not significantly different (p=0.157).
GATA-3 expression is preserved in metastasis
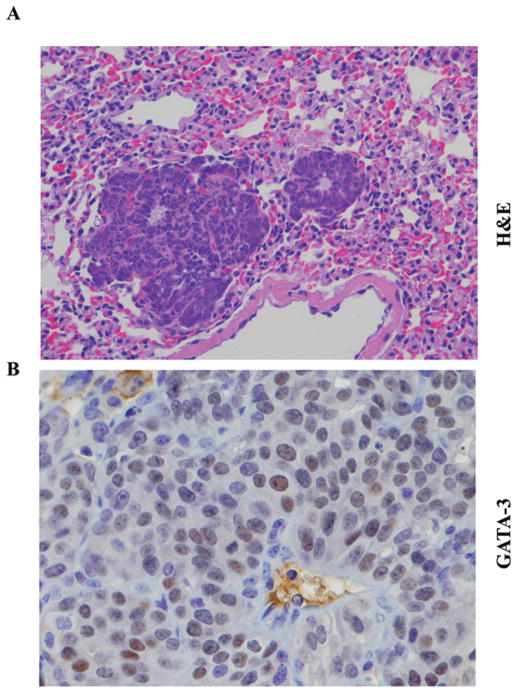
Previous studies that utilized transplantation of PyMT tumor cells into syngenic mice with or without additional manipulation of GATA-3 expression in tumor cells concluded that tumor progression involves expansion of GATA-3 negative tumor cells (12). These authors concluded that GATA-3 actively induces differentiation and represses dissemination of tumor cells. As per this model, lung metastasis should lack GATA-3 expression. To test this possibility, we performed immunohistochemistry of lung tissue for metastases. In all mice with pulmonary metastases that were analyzed, we noted persistence of GATA-3 expression in a subpopulation of metastatic cells (Fig. 2). The differences observed in the previous study could be due to variations in the methods used for inducing metastasis. Unlike GATA-3, FOXA1 expression was not observed in lung metastatic cells (Fig. 2).
Figure 2.
GATA-3 expression in lung metastatic lesions in PyMT mouse model. (A) H&E staining of lung metastasis (magnification ×20). (B) GATA-3 staining in lung metastasis (magnification ×40). Note a subpopulation of cancer cells was GATA-3-positive.
PEITC delays growth of tumors
In order to study the effect of a chemopreventive agent in this model, we supplemented one group of mice with PEITC. In both groups the mean time to appearance of tumors from weaning was about 57 days. When mice supplemented with PEITC and control diets for same duration were compared, mice from the PEITC group developed twice as many tumors as the control group. This was in part due to the small size of tumors and the fact that they did not merge to form larger tumor nodules in the PEITC group. The overall grade of tumors developed in the two supplemented groups did not vary significantly. The late tumors showed significant cytological atypia, mitotic activity and necrosis. In addition, squamous metaplasia was also noted in many tumors. The number of mice from both groups that developed lung metastases and the number of metastatic foci did not differ significantly. In addition, we did not note any difference in the survival of mice in the indefinite group by supplementation group.
PEITC delays tumor initiation
We then examined the effect of PEITC treatment on the expression pattern of above markers on normal mammary gland. Both control and PEITC-supplemented animals showed well-formed mammary glands at 4 weeks. Although the normal luminal cells expressed ERα, FOXA1 and GATA-3 in both groups, their expression was 2-to 3-fold higher in the control group (p=0.001, <0.0001 and 0.043, respectively) (Fig. 3). These differences were more significant in mice at early age. These results suggest that PEITC inhibits initiation of these tumors, possibly by reducing the proliferation of luminal cells.
Figure 3.
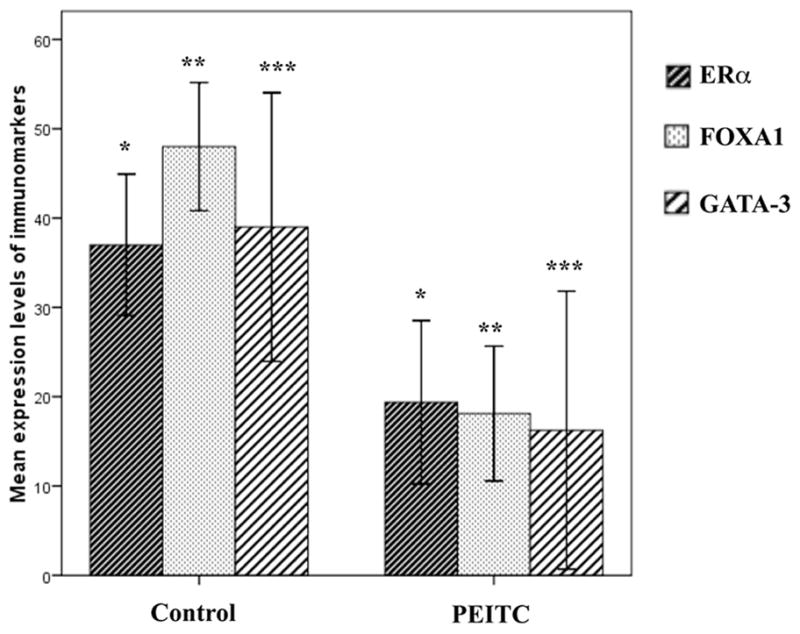
ERα, FOXA1 and GATA-3 expression in normal luminal cells in both control and PEITC supplemented mice (mean ± SE). The expression of these biomarkers in controls is 2–3 times as much as in PEITC group. Control vs. PEITC treated groups *p=0.001, **p<0.0001, and ***p=0.043.
PEITC did not have an effect on the expression pattern of ERα, FOXA1 and GATA-3 in tumors. This could be due to very low expression levels of these markers and any changes as a consequence of PEITC treatment was within the range of standard deviation. Representative FOXA1 and GATA-3 staining patterns in control and four-week PEITC-treated groups are shown in Fig. 4.
Figure 4.
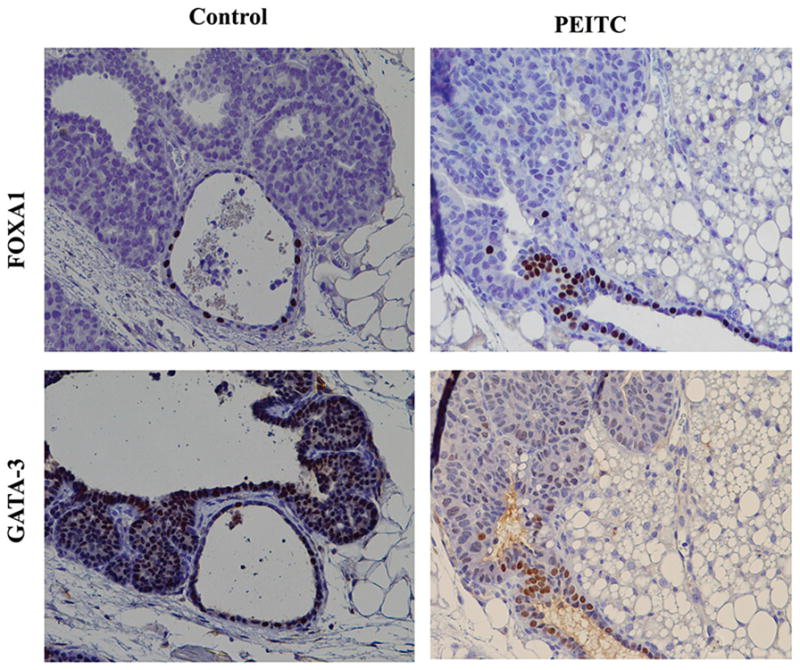
A representative FOXA1 and GATA-3 staining of mammary gland of untreated and PEITC treated animals maintained for four weeks after weaning (magnification ×20).
PEITC targets AldeFluor-positive cells
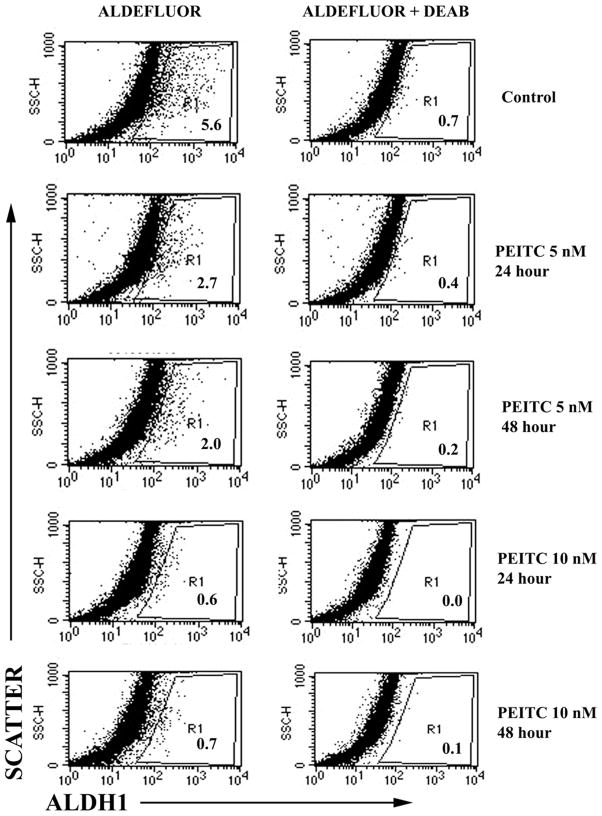
The chemo-preventive activity of PEITC involves generation of reactive oxygen species (ROS) (9). Therefore, cancer cells with effective ROS generating system are expected to be sensitive to PEITC. ‘Cancer stem cells’ have been shown to be efficient in scavenging reactive oxygen species, which enables them to be radioresistant (18). If PEITC is capable of inducing ROS in differentiated cancer cells but not in cancer stem cells, prolonged treatment with PEITC is expected to cause enrichment of cancer stem cells. To test this possibility, we examined the ‘cancer stem cell’ status of untreated and PEITC-treated PyMT mammary tumor derived cell line. Normal and cancer epithelial cells from mouse mammary gland as well as the human breast that express higher levels of aldehyde dehydrogenase 1 (ALDH-1) are considered to have stem/progenitor cell like properties (19,20). However, cancer stem/progenitor cell phenotype of PyMT-derived tumors has not been examined so far. ALDH-1 levels are usually measured using the dye AldeFluor, which provides indirect measurement of all ALDHs in cells. We examined the effect of PEITC on the AldeFluor positivity of tumor cells. As seen in Fig. 5, approximately 5% of control cells were AldeFluor-positive as against at least 2% positivity in PEITC treated cells. With increasing duration and/or concentration of exposure to PEITC, tumor cells demonstrated reduced AldeFluor-positivity. These results suggest that cancer cells with ‘stem cell’- like properties as characterized by AldeFluor assay are sensitive to PEITC.
Figure 5.
PEITC depletes AldeFluor-positive cancer cells. Mammary tumor cell line derived from PyMT mice was treated with PEITC for indicated time and examined for AldeFluor-positive cells by flow cytometry. Cells were incubated with fluorescent ALDH-1 substrate AldeFluor with or without competitive inhibitor DEAB. R1 corresponds to AldeFluor-positive cells.
Discussion
The PyMT transgenic mouse has been previously demonstrated to model human breast carcinogenesis. The main advantages of this model are that it has high incidence of lung metastasis with shorter latency periods for tumor development and not being dependent on animals getting pregnant. More importantly, it is one of the few models of ERα-positive tumors, with early stage tumors being of histological low grade and expressing both ERα and PR. The luminal A status of these tumors has been confirmed by gene expression studies (21). Consistent with this, we found the expression of FOXA1 and GATA-3 in these tumors.
Animals with mammary specific PyMT transgene show a range of histological lesions depending on their age. Similar, to the observations by Lin et al, we noted four distinct stages of tumor progression from hyperplasia to adenoma through low grade and high-grade carcinoma (6). The late stages are associated with marked cytological atypia and mitotic activity. In addition, we observed prominent necrosis and foci of squamous metaplasia in these late stages. These morphological changes in the tumor characteristics were also associated with change in the immunophenotype. Similar to Lin et al (6), late stage tumors in this study showed marked decrease in ERα. We additionally demonstrate a complete loss of FOXA1 expression with progression. These morphological and immunophenotypic changes suggest that these late stage tumors no longer represent luminal A phenotype. In this context it is worth noting that using gene expression analysis Herschkowitz et al have classified the PyMT tumors as of luminal A subtype (21). Given the findings of our study, it is imperative that these studies are repeated with tumors at later stages to confirm whether they are still of luminal A phenotype. In human breast cancers, it believed that progression of tumor grade does not occur. However, Natrajan et al recently documented 16q deletion, characteristic of low-grade cancers, in some high-grade ERα-positive tumors (22). They suggest that in at least some cases of ERα positive tumors progression do occur. A detailed analysis of the PyMT tumors, at different stages of development, might help to understand some of the changes associated with conversion of luminal A to luminal B phenotype.
GATA-3 is required for differentiation of luminal progenitor cells (16,17). Targeted deletion of this gene in the mammary gland leads to accumulation of luminal progenitor cells and concomitant block in differentiation (16). However, survival of differentiated cells also requires GATA-3 (12). GATA-3 is suggested to function as a co-regulator of ERα function. We noted that GATA-3 expression was higher than that of ERα and FOXA1 to begin with and persisted even in the late stages of tumors through metastases. In the hierarchy of luminal cell differentiation, GATA-3 appears to be upstream of FOXA1 and ERα because a substantially larger number of luminal cells express GATA-3 compared to ERα or FOXA1 both in human breast (23) and in the mouse mammary gland (Fig. 3). Therefore, differentiated luminal cells expressing GATA-3 but lacking ERα and FOXA1 must exist. The high expression of GATA-3 is in concordance to a previous study that used mouse model’s intrinsic gene set cluster analysis to demonstrate that GATA-3 gene was highly expressed in MMTV-PyMT tumors similar to that observed in human luminal tumors (21).
Dietary pro-oxidant molecules such as PEITC are believed to cause apoptosis of transformed but not normal cells (7). In vitro studies have identified multiple cellular targets of PEITC: inhibition of cap-dependent translation by regulating the level and phosphorylation of 4E-BP1, proteosomal degradation of cell division cycle 25C and cyclin-dependent kinase 1 leading to G2/M arrest, and covalent binding to tubulin, which may be essential for growth inhibition (24–26). In this study, we have noted an effect of PEITC in early stage but not late stage tumors with respect to expression of biomarkers. The novel finding in this study is that PEITC lowers the expression of biomarkers such as ERα, FOXA1 and GATA-3 in normal luminal cells as compared to those in control mice. Thus even in the early stage when the ducts show some expression of these biomarkers, PEITC inhibits the initiation of tumor formation by reducing the proliferation of luminal cells that may be prone for tumorigenesis.
Although PEITC delayed tumor initiation, it did not have an effect on overall survival or time to development of tumors. One reason may be that while PEITC reduced tumor initiation from ERα/FOXA1/GATA-3 positive luminal epithelial cells by suppressing their expression and thus the hormonal network, it may have inadvertently promoted tumor initiation/progression by ERα-negative luminal cells. It is more likely that PyMT is a very potent oncogene and chemopreventive agents can inhibit only some of its activity. However, PEITC appears to arrest the tumor progression at some stage after tumor formation, possibly only a specific subtype, although this was not evident in the immunohistochemical examination of the biomarkers that we studied. Further studies on more reliable biomarkers that help differentiate the various stages of mammary gland tumorigenesis are warranted.
In vitro study of mammary tumor cells exposed to various durations and concentrations of PEITC for AldeFluor-positivity determined that PEITC reduces cells with ‘stem cell-like’ features. This suggests that stem cells may be sensitive to PEITC, if ‘stem cells’ are defined as AldeFluor-positive. Once further studies confirm the chemopreventive role of PEITC in breast tumorigenesis, radioresistant cancers due to ‘stem cell-like’ properties could be treated with PEITC (27).
In summary, the PyMT model may be ideal to study the progression of luminal A to luminal B subtype cancer and to identify genetic/epigenetic changes associated with such a progression. PIETC at least partially inhibits tumor initiation and delays progression in this model.
Acknowledgments
We thank Poornima Bhat-Nakshatri and Susan Rice for assistance in AldeFluor assay. This study is supported by the grant R03 CA123561 from the National Cancer Institute to HN. KM is supported by T32 cancer biology training grant CA111198 from National Institutes of Health. HN is Marian J. Morrison Investigator of Breast Cancer Research.
References
- 1.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badve S, Turbin D, Thorat MA, et al. FOXA1 expression in breast cancer correlation with luminal subtype A and survival. Clin Cancer Res. 2007;13:4415–4421. doi: 10.1158/1078-0432.CCR-07-0122. [DOI] [PubMed] [Google Scholar]
- 3.Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M. Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer Res. 2007;67:6477–6483. doi: 10.1158/0008-5472.CAN-07-0746. [DOI] [PubMed] [Google Scholar]
- 4.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCune K, Bhat-Nakshatri P, Thorat MA, Nephew KP, Badve S, Nakshatri H. Prognosis of hormone-dependent breast cancers: implications of the presence of dysfunctional transcriptional networks activated by insulin via the immune transcription factor T-bet. Cancer Res. 2010;70:685–696. doi: 10.1158/0008-5472.CAN-09-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin EY, Jones JG, Li P, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu XJ, Hua X. Targeting ROS: selective killing of cancer cells by a cruciferous vegetable derived pro-oxidant compound. Cancer Biol Ther. 2007;6:646–647. doi: 10.4161/cbt.6.5.4092. [DOI] [PubMed] [Google Scholar]
- 8.Reen RK, Dombkowski AA, Kresty LA, et al. Effects of phenylethyl isothiocyanate on early molecular events in N-nitrosomethylbenzylamine-induced cytotoxicity in rat esophagus. Cancer Res. 2007;67:6484–6492. doi: 10.1158/0008-5472.CAN-06-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trachootham D, Zhou Y, Zhang H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Thorat MA, Marchio C, Morimiya A, et al. FOXA1 expression in breast cancer is associated with Luminal subtype and good prognosis. J Clin Pathol. 2007 doi: 10.1136/jcp.2007.052431. [DOI] [PubMed] [Google Scholar]
- 11.Jacquemier J, Charafe-Jauffret E, Monville F, et al. Association of GATA3, P53, Ki67 status and vascular peritumoral invasion are strongly prognostic in luminal breast cancer. Breast Cancer Res. 2009;11:R23. doi: 10.1186/bcr2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kouros-Mehr H, Bechis SK, Slorach EM, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehra R, Varambally S, Ding L, et al. Identification of GATA3 as a breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res. 2005;65:11259–11264. doi: 10.1158/0008-5472.CAN-05-2495. [DOI] [PubMed] [Google Scholar]
- 14.Dydensborg AB, Rose AA, Wilson BJ, et al. GATA3 inhibits breast cancer growth and pulmonary breast cancer metastasis. Oncogene. 2009;28:2634–2642. doi: 10.1038/onc.2009.126. [DOI] [PubMed] [Google Scholar]
- 15.Conaway CC, Wang CX, Pittman B, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 16.Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 17.Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diehn M, Cho RW, Lobo NA, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007;21:3238–3243. doi: 10.1101/gad.1616307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natrajan R, Lambros MB, Geyer FC, et al. Loss of 16q in high grade breast cancer is associated with estrogen receptor status: Evidence for progression in tumors with a luminal phenotype? Genes Chromosomes Cancer. 2009;48:351–365. doi: 10.1002/gcc.20646. [DOI] [PubMed] [Google Scholar]
- 23.Nakshatri H, Badve S. FOXA1 in breast cancer. Expert Rev Mol Med. 2009;11:E8. doi: 10.1017/S1462399409001008. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Straub J, Xiao D, et al. Phenethyl isothiocyanate, a cancer chemopreventive constituent of cruciferous vegetables, inhibits cap-dependent translation by regulating the level and phosphorylation of 4E-BP1. Cancer Res. 2007;67:3569–3573. doi: 10.1158/0008-5472.CAN-07-0392. [DOI] [PubMed] [Google Scholar]
- 25.Xiao D, Johnson CS, Trump DL, Singh SV. Proteasome-mediated degradation of cell division cycle 25C and cyclin-dependent kinase 1 in phenethyl isothiocyanate-induced G2-M-phase cell cycle arrest in PC-3 human prostate cancer cells. Mol Cancer Ther. 2004;3:567–575. [PubMed] [Google Scholar]
- 26.Mi L, Xiao Z, Hood BL, et al. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]