Abstract
Objective
Glomerular capillary hemorrhage (GCH) has been reported and confirmed as a consequence of contrast-enhanced diagnostic ultrasound (CEDUS) of rat kidney. This study assessed renal tissue injury in the larger porcine model.
Methods
The right kidneys of anesthetized pigs were imaged in 8 groups of 4 pigs. A Vingmed System Five (General Electric Co. Cincinnati OH) was used at 1.5 MHz in B-mode to intermittently scan the kidney at 4 s intervals. A Sequoia 512 (Acuson, Mountain View CA) was used in the 1.5 MHz Cadence CPS mode with intermittent agent-clearance bursts at 4 s intervals. Kidneys were scanned transabdominally, or after laparotomy through a saline standoff. The Sequoia 512 probe was placed in contact with the kidney for one group. Definity (Lantheus Medical Imaging, N. Billerica, MA) was infused at 4 μl/kg/min (diluted 33:1 in saline) for 4 min during scanning.
Results
Blood-filled urinary tubules were evident on the kidney surface for all groups, except for the group with the probe in contact with the kidney. GCH was found by histology in 31.7 % ± 9.8 % of glomeruli in the center of the scan plane for 1.7 MPa transabdominal scanning and 1.5 % ± 2.9 % of glomeruli in sham samples (P<0.05). In addition, hematuria was detected after scanning, and tubular obstruction occurred in some nephrons.
Conclusion
Renal tissue damage was induced by CEDUS in the porcine model. This result, together with previous studies in rats, support an hypothesis that GCH would occur in humans from similar CEDUS.
Keywords: diagnostic ultrasound adverse effects, ultrasound contrast agent, glomerular hemorrhage, Bowman’s space clot, renal intra-tubular obstruction
INTRODUCTION
Contrast enhanced diagnostic ultrasound (CEDUS) has shown great promise for augmenting the information available from ultrasound examinations. Ultrasound contrast agents are injectable suspensions of gas bodies (stabilized microbubbles). The gas bodies are scientifically engineered to persist in the circulation and provide a strong echo-return for image enhancement [1]. Under some conditions, the gas bodies can also serve as near-optimal nuclei for ultrasonic cavitation, which can lead to localized tissue damage [2]. CEDUS methods which have been associated with a risk of bioeffects involve intermittent imaging at relatively high diagnostic pulse amplitudes. These modes utilize gas body destabilization and destruction for imaging of perfusion either by “flash” images at different intervals, or by agent-destructive exposure followed by nondestructive imaging of the re-fill of the imaged tissue with blood containing gas bodies. The agent-destructive pulses can induce ultrasonic cavitation-behavior for some gas bodies within capillaries leading to capillary rupture and tissue injury, which has been observed in heart, kidney and other tissues [2].
Capillary rupture in kidney glomeruli is particularly important because of the unique structure of the kidney [3, 4]. Due to the high kidney blood flow, the concentration of gas bodies is higher in kidney, especially in the glomerular tuft, than in most other tissues. Glomerular capillary hemorrhage (GCH) flows into Bowman’s space and urinary tubules, which amplifies the impact of the capillary rupture by effecting the entire nephron functional unit. The relatively high hydrostatic pressure of glomerular capillaries can result in sufficient hemorrhage to precipitate fibrin formation in Bowman’s space, intratubular obstruction, and acute tubular necrosis [5, 6]. Because of the similarity of kidney structure in different mammalian species, the observation of GCH in rats also implies that similar effects would occur in other species, including humans, under similar conditions of CEDUS [5].
Our previous studies using rats involved B-mode intermittent CEDUS with a phased array probe [4]. In order to simulate human scanning, the rats were scanned in a water bath with the ultrasound focal zone placed in the kidney. GCH was demonstrated for a range of examination scan conditions. Recently, Jimenez et al. have reported that CEDUS in pig kidney did not cause renal tissue damage [7]. For that study, the kidney was scanned with a curved linear array probe using a contrast-specific mode and intermittent scan bursts for contrast-clearance. The kidneys were accessed by laparotomy and the probe was placed in contact with the kidney surface. The rationale for contact scanning of the kidney was not clear, particularly since the method does not allow clear imaging of the proximal cortex for normal settings of the focal zone depth. No visual or histological evidence of GCH in the proximal cortex or other renal tissue damage was found in this porcine model of CEDUS [7]. These contradictory results for rats and pigs are puzzling and worthy of further investigation. This present study was undertaken to assess possible renal tissue damage in pigs using methods which replicated, as much as possible, the methods which would be used in humans. In addition, methods similar to those of Jimenez et al. [7] were tested in order to understand the origin of the contradictory results.
METHODS
Animal preparation
This investigation was conducted with the approval of the University Committee on the Use and Care of Animals, University of Michigan. A total of 48 female pigs (crossbred Yorkshire and Landrace breeds), which weighed 27.4 ± 3.0 kg, were obtained from Michigan State Swine Research (East Lansing, MI). Pigs were fasted for 12 hr before beginning testing. The induction anesthetic was 6 mg/kg Telazol plus 2.2 mg/kg Xylazine injected IM. The pigs were maintained at the desired level of anesthesia by 1-3 % Isoflurane in oxygen which was delivered via a breathing bag and snout cone. Atropine (0.05 mg/kg IM) was given to control respiratory secretions. For intravenous injections and sterile saline infusion, 22 gauge cannulae were inserted into a vein on both ears. Infusion of 0.9 % sodium chloride from 1 L bags via a primary IV line was continuous throughout all procedures. The pigs were placed on their left side and vital signs were monitored with a veterinary monitor (Vet/Ox Plus 4800, Sims BCI Inc. Waukesha WI). Body temperature maintenance was aided by use of warming pads and insulating drapes. Average values and standard deviations at the time of scanning were: SpO2 96 ± 2, heart rate 113 ± 23 min−1, respiration rate 37.5 ± 9.6 min−1 and temperature 36.8 ± 0.9 C. For transabdominal scanning, the skin over the right kidney was cleaned and depilated. For direct access to the right kidney, a laparotomy was performed to expose the kidney surface without breaching the peritoneum. This allowed scanning of the kidney with the normal tissues in place around it, other than the abdominal wall. Retractors were used to produce an opening of about 8 cm by 10 cm, but, typically, several cm of the kidney remained hidden under the lowest ribs. This method differed from that of Jimenez et al. [7], in which the intestines were moved, and the abdominal cavity was filled with saline for complete access to the kidneys.
The ultrasound contrast agent for this study was Definity® (perflutren lipid microsphere injectable suspension, Lantheus Medical Imaging, Inc., N. Billerica, MA USA). A fresh vial was prepared each day according to the manufacturer’s instructions. 0.6 ml of Definity was diluted in 20 ml of sterile saline in a syringe, which was connected to an ear vein cannula with an extension tube. The diluted agent was infused at 0.14 ml/kg/min with a syringe pump (Aladdin 1000, World Precision Instruments, Sarasota FL USA), which delivered 16 μl/kg of Definity in 4 min at a 4 μl/kg/min rate (approximating the infusion method and maximum rate specified in the package insert). The use of Definity allows for comparison of results to previous study results in rats. Jimenez et al. used Sonovue (Bracco SpA, Milan IT) [7], which at this time has not been approved for clinical use in the USA. Unfortunately, Bracco representatives declined to provide Sonovue for this investigation, which would have allowed a more direct comparison to the Jimenez et al. [7] study.
During initial tests, some pigs suffered anaphylactoid-like reactions to the contrast agent, which included erythema, bradycardia and reduced respiration, after 1-2 min of agent infusion. Such reactions are known to occur from ultrasound contrast agents, particularly in pigs, including Definity [8], Albunex [9] and Sonovue [10]. Both Definity and Sonovue have associated warnings concerning possible human reactions. Pigs were given IV injections of 1 mg/kg diphenhydramine and 2 mg of dexamethasone about 1 hr before CEDUS as a preventative measure. This type of preventative strategy has also been used for other radiological contrast media reactions [11, 12].
Urinalysis was conducted to determine if hematuria, which was detected in rats [5], also occurred in swine. For rats, the animals were placed in urine collection cages and the urine sample was collected after 4 h. This method could not be used for the swine. The cannulation of the urethra was very difficult in these animals, and could not be used for urine samples. In one group, urine samples were collected before and 2 hr after exposure by transabdominal puncture of the bladder using a syringe and 21 Ga needle. This method was poor because it involved dilution of any post-exposure blood in a variably-filled bladder. For another group of pigs with transabdominal exposure, the right ureter was accessed by laparotomy and a 3.6 mm diameter cannula inserted for urine sampling at 10 min intervals before, during and 3 times after exposure. This method was also difficult and some blood was present in initial samples due to the cannulation procedure. Pre-exposure sampling of urine was done to assure a negative hematuria reading before exposure. The hematuria was read from Hemastix (Bayer Corp., Elkhart IN) and a hematuria score was calculated as described previously [5].
Diagnostic Ultrasound
Two diagnostic ultrasound machines were used for exposure-scanning. The first machine was a Vingmed System Five (General Electric Co., Cincinnati OH) with FPA2.5 phased array probe. This probe was protected with a probe cover (Cone Instruments, Solon OH) and clamped in an adjustable support stand and aimed downward at the kidney. A bag, which was made from a 14 cm wide polyurethane ultrasound probe cover, was secured over the end of the probe and filled with saline to allow variation in the distance between the probe and the kidney cortex. The acoustic coupling of the bag to the kidney was by saline, or to the skin was by ultrasound coupling gel and saline. The distance from probe to kidney was adjusted to place the proximal cortex in the focal zone 3.5-4.5 cm from the transducer face where the −6 dB scan plane thickness (beam width) was 4.3 mm. The machine was set to 1.5 MHz, 5 cm focus, 10 cm depth and a 4 s interval between dual scans (50 ms apart). Dual scans were employed to show contrast destruction by the loss of enhanced contrast in the second image. This gave 60 scans during 4 min of agent infusion, which was intended to parallel the use of 60 scans during 1 min of agent infusion in the previous studies with rats [4]. The peak rarefactional pressure amplitude (PRPA) of the 1.5 μs pulses (about 2 cycles) was measured in a water bath with a calibrated hydrophone (model 805, Sonora Medical Systems Inc., Longmont CO USA). At the maximum (0 dB) setting, the PRPA was 2.3 MPa. Each scan had a pulse amplitude sequence previously found to be 4.6 ms in duration [13]. For transabdominal scanning, the PRPA at the kidney was estimated by inserting samples of the abdominal wall between the probe and hydrophone. Four 10 cm by15 cm samples of abdominal wall over the kidney were taken from a group with transabdominal exposure and measured at four different positions to obtain a nominal (average of the four measurements) reading for each sample. The mean PRPA was 1.66 ± 0.14 MPa for the mean thickness of 1.9 ± 0.2 cm. Comparing this result to the water PRPA value gives an attenuation of −2.8 dB (a factor of 0.73) or about 1.0 dB/cm/MHz for the skin plus muscle tissues. The on-screen Mechanical Index (MI) was 1.7 for the 0 dB setting. The measured MI equivalent (given by the PRPA divided by the square-root of the frequency) was MIe = 1.9 for the water path and MIe = 1.4 for the transabdominal path.
The second diagnostic ultrasound machine was a Sequoia 512 (Acuson, Mountain View CA), which was the same model machine used by Jimenez et al. [7]. The 4C1-S probe was used in 1.5 MHz Cadence CPS mode, which is a contrast specific mode, with 10 cm image depth and foci at 4 and 6 cm. This probe also was protected with a probe cover and was clamped for kidney scanning through a saline-filled bag as described above. The repeated burst mode was used for clearance of the contrast agent from the image with 5 frame bursts (the default setting), which totaled 110 ms in duration, each 4 s. Low power imaging with a 1 s interval and a PRPA of 0.1 MPa occurred between the clearance bursts. This was below the exposure levels required for gas body destabilization or GCH, so that the low power imaging can be neglected with regard to the GCH bioeffect. The exposure timing was therefore essentially the same as the Vingmed System Five exposure (i. e. intermittent high-power agent destructive bursts at 4 s intervals). The maximum PRPA was measured in the water bath to be 1.9 MPa for the 3.06 μs pulses (about 5 cycles) in the clearance burst. The actual duration of 5 cycles was 2.77 μs, which represents an ultrasound frequency of 1.8 MHz. For this setting, the burst MI was listed at 1.9 on screen but the measured equivalent MI was MIe = 1.4 even for the water path. The reason for this discrepancy between the output expected according to the on screen MI and the measured values was uncertain. The value of MI=1.9 is the USFDA guideline upper limit for diagnostic ultrasound. However, the measured values were consistent with the information given in the Transducer Specification Manual for this probe, which listed the maximum MI as 1.2. For one group, the saline-filled bag was omitted and the probe was placed in contact with the kidney, which was the method used by Jimenez et al. [7]. This method necessitated some pressure on the kidney by the curved array to increase the width of the contact region in the image, but the ends of the probe typically were not in contact. The largest rarefactional pressure amplitude was 0.96 MPa at 0.5 cm and 1.05 MPa at 1.5 cm, which included the proximal cortex (i. e. the focal zone and PRPA were deeper into the kidney in the medulla or distal portions of the cortex).
In addition, the wash-in and wash-out of contrast enhancement was observed in the left kidney for one group of pigs using the flash echo imaging with a Powervision 8000 ultrasound machine (model SSA-390A, Toshiba Corporation, Tochigi-Ken, JPN) with curved linear array (PVN-375AT). This procedure was used previously for assessing the loss of circulating contrast agent gas bodies in rats due to natural loss mechanisms and to contrast agent destruction by exposure of the right kidney [14]. For the present study, this method was used to assess the gas body concentration in the larger animal for comparison to the results in rats. The image depth was 8 cm with a 6 cm focus for the harmonic imaging mode (2.3 MHz transmit and 4.6 MHz receive). A region of interest was selected in the proximal renal cortex and time intensity-curves for 4 min during infusion and 4 min after infusion were obtained twice, once without and once with exposure-scanning of the right kidney. The curve data were collected at 10 s intervals. The time-intensity curves were evaluated by nonlinear curve fitting to obtain the plateau level and the decay (wash-out) time constant, as described previously [14].
Measured Endpoints
At the conclusion of testing, the animals were euthanized while under anesthesia, and the right kidney was removed. The kidney surface was photographed after removal of the renal capsule. Exposed kidney samples were taken for histology from the scan plane identified by blood filled urinary tubules visible on the surface, and an adjacent sham sample was taken outside the scanned region of each kidney. All scans were approximately parallel to the sagittal plane to provide a longitudinal view of the kidney, which was the orientation used by Jimenez et al. [7]. For open-abdomen exposure, the observable band of blood filled tubules was 6-7 cm long and 4 exposed samples were collected. For transabdominal exposure, the ribs restricted the view of the kidney to a greater extent, so that the observable band was 4.5-6 cm long and 3 or 4 exposed samples were collected. For scanning with the probe in contact with the kidney, the scan plane was marked after euthanasia by making small cuts on either side of the probe. No blood filled tubules were seen on the proximal side of the kidney for contact scanning, but some were seen on the distal side: two samples of cortex were collected from the proximal side and two from the distal side for this group. Kidney samples were immersion fixed in neutral buffered formalin and trimmed for placement in histological cassettes. Histological processing was performed at the Research Histology and Immunoperoxidase Laboratory of the University of Michigan Comprehensive Cancer Center Tissue Core. Glomerular capillary hemorrhage (GCH) was evaluated by histology with hematoxylin and eosin staining as described previously [4]. Glomeruli with and without evidence of hemorrhage were counted and the counts used to calculate the percentage of GCH. Results for the 2, 3 or 4 exposed samples were summed to represent the exposed region.
In rats, intratubular obstruction was shown to occur from CEDUS by measurements of the width of Bowman’s space and detection of fibrin in the urinary space [6]. Possible intratubular obstruction in the swine kidney was assessed, using the histological methods described previously [6]. In addition, antibody staining for fibrin was used to identify clots in glomeruli for the two groups with delayed sacrifice (30 min or 4 hr) for urine collection. Fibrin staining was performed on serial sections adjacent to the H&E stained slides used for GCH scoring. Rabbit anti-human antibody to fibrinogen (Dako North America, Capinteria CA, USA) was used to tag the clots followed by peroxidase labeled anti-rabbit antibody and DAB staining. Negative controls were run on separate slides using normal rabbit immunoglobulin fraction. The slides stained for fibrin were scored for the percentage of glomeruli with clearly identifiable clots, which stained dark brown.
Experimental plan and statistics
A total of 48 pigs were obtained for this study. Seven of these were used in preliminary testing to develop the best methods for the research. The preventative treatment noted above appeared to help prevent mild reactions, such as erythema, but 4 pigs suffered cessation of respiration or of the heartbeat during agent infusion and were lost from the study. These severe reactions evince the failure of the preventative pretreatment (see Methods) to eliminate the anaphylactoid reactions. In addition, results from 5 pigs were excluded due to technical problems with performing the experiment (e.g. movement of the animal). The final study results included 8 separate groups of 4 pigs each, which are listed in Table 1. Numerical results are presented as the mean plus/minus the standard deviation, or plotted as the mean with standard error bars. For statistical analysis, Mann-Whitney rank sum tests were used to compare means of the measured parameters, with statistical significance assumed at P=0.05. For multiple comparisons, a one-way ANOVA was performed with pair wise comparisons made using the Holm-Sidak method.
Table 1.
A listing of the 8 test groups, for which a standoff (so) was normally used to place the focal zone in the proximal renal cortex.
| Diagnostic | Probe | PRPA | Sacrifice | Test | Exp GCH | Shm GCH | ||
|---|---|---|---|---|---|---|---|---|
| Group | Ultrasound | Placement | MPa | Delay | Endpoints | % | % | P < |
| 1 | Vingmed | open, so | 1.3 | < 5 min | GCH | 0.3 ± 0.4 | 0 ± 0 | ns |
| 2 | Vingmed | open, so | 1.7 | < 5 min | GCH | 26.5 ± 10.5 | 0 ± 0 | 0.005 |
| 3 | Vingmed | open, so | 2.3 | < 5 min | GCH, B, T | 59.2 ± 2.2 | 1.1 ± 2.2 | 0.001 |
| 4 | Vingmed | open, so | 2.3 | 2 hr | GCH, F, H | 26.6 ± 10.2 | 0 ± 0 | 0.05 |
| 5 | Vingmed | closed, so | 1.7 | < 5 min | GCH | 29.6 ± 11.5 | 1.5 ± 2.9 | 0.05 |
|
| ||||||||
| 6 | Sequoia | open, so | 1.9 | < 5 min | GCH | 42.7 ± 2.8 | 0 ± 0 | 0.05 |
| 7 | Sequoia | cont, prox | 0.96-1.05 | < 5 min | GCH | 0.06 ± 0.11 | 0 ± 0 | ns |
| cont, dist | nd | 6.6 ± 4.9 | 0.05 | |||||
| 8 | Sequoia | closed, so | 1.3 | 30 min | GCH, F, H | 7.9 ± 5.6 | 0 ± 0 | 0.05 |
For the Sequoia exposure with probe contact, the rarefactional pressure amplitude in the proximal cortex is given (rather than the PRPA), and for the closed abdomen exposure the PRPA was derated by the 1.0 dB/cm/MHz attenuation measured for the intervening abdominal wall. Abbreviations are: open abdomen (open), closed abdomen (closed), standoff (so), contact (cont), proximal (prox), distal (dist), exposed (Exp), sham (Shm) peak rarefactional pressure amplitude (PRPA), statistical probability (P), not determined (nd) and not significant (ns). The endpoints are glomerular capillary hemorrhage (GCH), Bowman’s space measurement (B), time-intensity analysis (T), immunohistological fibrin staining (F) and testing for hematuria in urine samples (H).
RESULTS
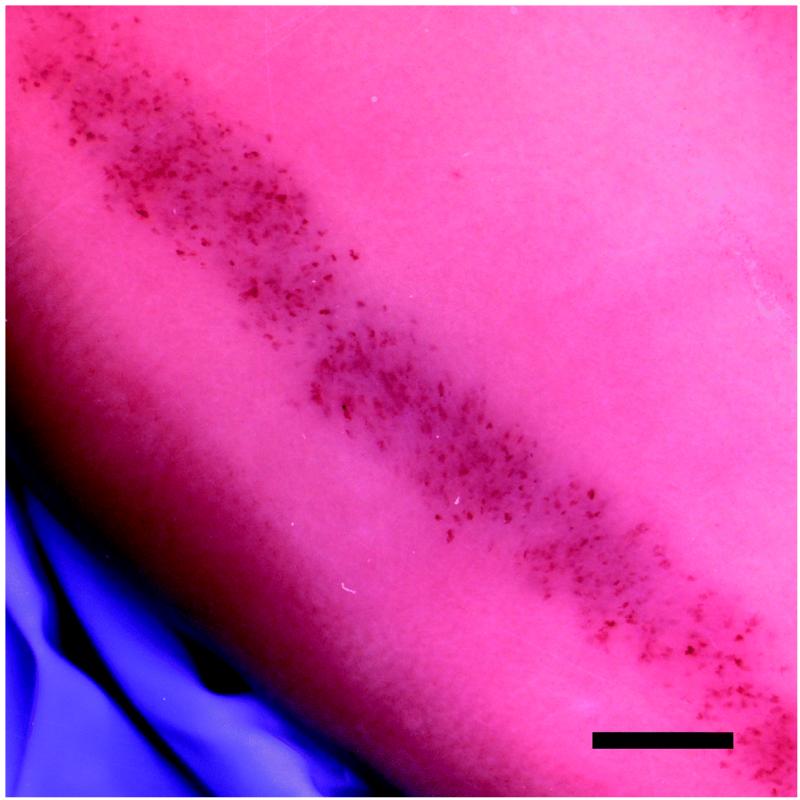
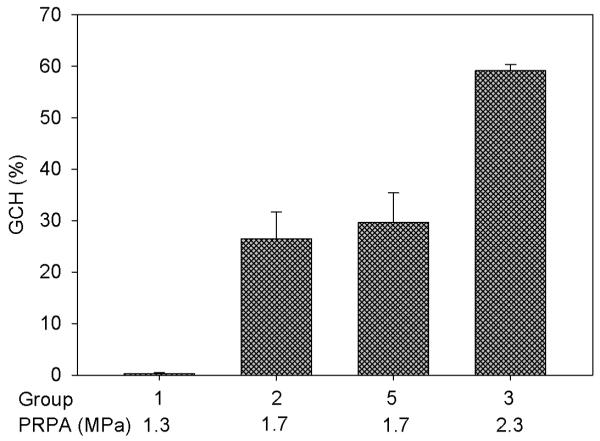
A band of blood-filled tubules due to GCH was typically seen on the surface of the kidneys. Figure 1 shows the hemorrhage marks in the scan plane and a cross section of the cortex for one of the kidneys of Group 5. The percentage of GCH (glomeruli containing red cells within Bowman’s capsule) at the approximate center of the scan plane for each group is listed in Table 1, together with the GCH in the adjacent sham sample and P values comparing each set of sham and exposed data. Some sham samples had evident GCH in histology (presumably these samples were not completely outside the scanned volumes) but the sham GCH results were not significantly different from zero. The results for groups 1, 2 and 3 with open abdomen exposure of the kidney through a saline stand off form a small exposure-response test as shown in Fig. 2. The closed-abdomen group 5 was added to this plot because the attenuation through the abdominal wall approximated the reduction (− 3 dB) from maximum output for group 2, and the GCH results are essentially the same for these two groups. The lowest PRPA exposure (group 1) had non-significant GCH in histology, but did show blood filled tubule markings on the surface of each exposed kidney with 8, 5, 8, and 8 marks in the four kidneys. The blood filled tubule markings are a more sensitive test for low effects, as noted previously [15]. The mean of 7.3 ± 1.5 marks was significantly greater (P<0.05) than the zero effect outside the scan plane.
Figure 1.
The band of marks seen on the kidney surface, resulting from hemorrhage into urinary tubules, on a group 5 kidney with closed abdomen image-exposure (a), and a cross-section cut through the band to show the penetration of tissue injury through the cortex (b). Scale bars are 5 mm (a) and 2 mm (b).
Figure 2.
The mean percentage with standard error bars of glomerular capillary hemorrhage (GCH) induced by the Vingmed System Five machine in the center of the scan plane for the groups 1, 2, 3 and 5 listed in Table 1 with the specified peak rarefactional pressure amplitude (PRPA) for the scans.
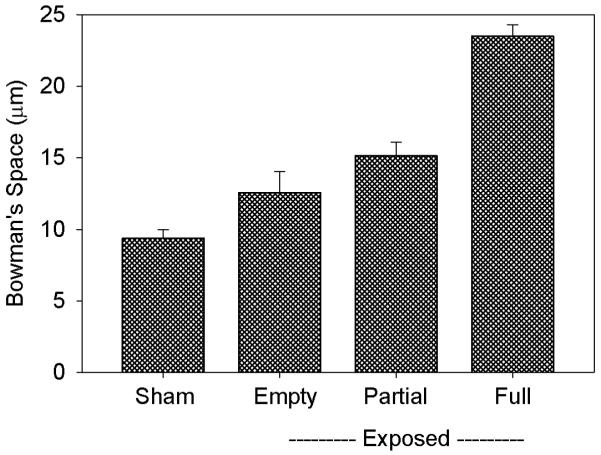
The width of Bowman’s space was determined for Group 3 for the sham and exposed samples. For the exposed samples, glomeruli were categorized as having Bowman’s space empty (40.9 ± 2.4 %), partially full (44.4 ± 3.7 %) and essentially full (14.7 ± 3.4 %) of erythrocytes. The results are shown in Fig. 3. Pair wise comparisons indicated that the mean widths for partial and full categories of the exposed samples were significantly larger than for sham samples (P<0.01), and that the mean widths for the sham and empty scores were not significantly different. The substantial enlargement of Bowman’s space was indicative of intratubular obstruction.
Figure 3.
Measurements of the width of Bowman’s urinary space (between the glomerular tuft and capsule) for samples from group 3 pigs. Exposed Bowman’s spaces were measured for erythrocyte content categorized as empty, partially full and essentially full of hemorrhage. The enlargement of Bowman’s space is indicative of intratubular obstruction by the hemorrhage into the urinary space and tubules.
Time intensity curves were generated from the left kidney of each pig in Group 3, one without exposure of the right kidney and one with exposure. The plateau values of 22.1 ± 18.7 relative units without and 23.2 ± 18.3 relative units with exposure of the right kidney were not significantly different. This indicated that the destruction of the contrast agent in the right kidney during scanning (i. e. exposure) did not substantially reduce the circulating dose of contrast agent. The intensity decay constants after the end of infusion were 26.0 ± 6.0 s and 28.4 ± 11.3 s (not significantly different) with and without exposure of the right kidney, respectively.
Group 4 was scanned in the same way as Group 3 but sacrifice was delayed for two hours to allow for urine collection and fibrin formation in Bowman’s space. This group had a GCH score of 26.6 ± 10.2 %, which indicated that many glomeruli had not cleared the hemorrhage by 2 hr. Slides stained for fibrin showed that 34.1 ± 21.7 % of glomeruli had evidence of fibrin formation, which was indicative of activation of the coagulation system in Bowman’s space. A few glomeruli (5.3 ± 6.8 %) had fibrin nearly filling Bowman’s space and often extending into the tubule, as shown in Fig. 4. Urine collections from the full bladder for this group showed test strip results which were negative for hematuria before scanning and positive for hematuria after scanning (P<0.05).
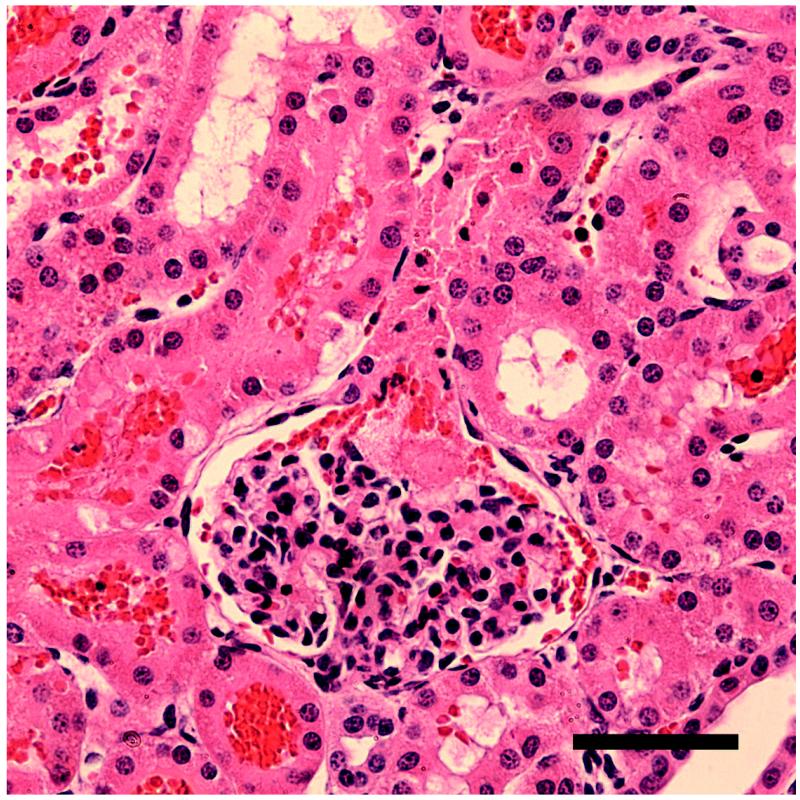
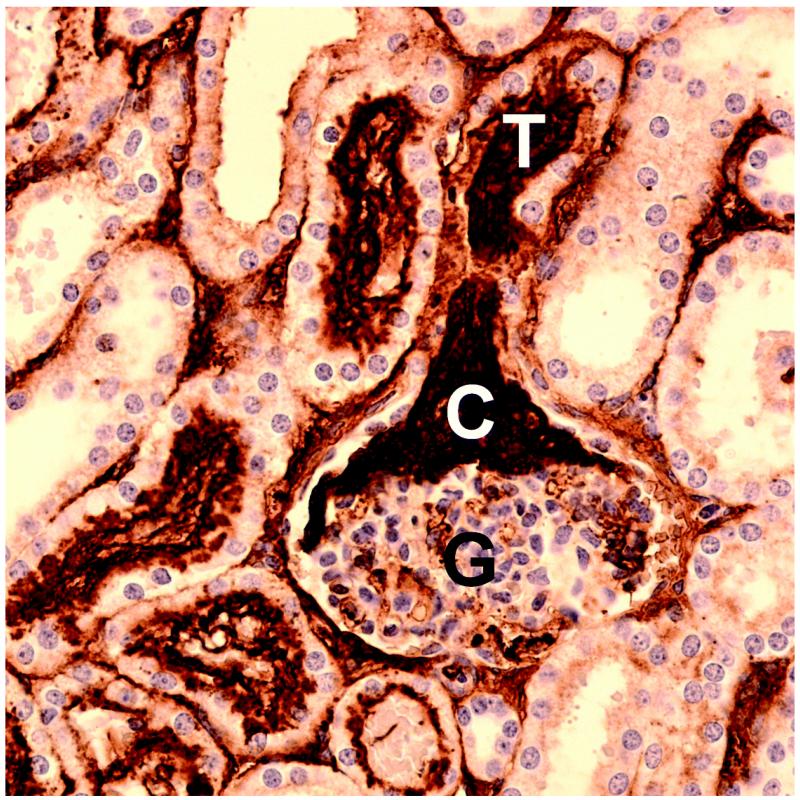
Figure 4.
Serial sections of a glomeruli found in a kidney from group 4 with normal hematoxylin (blue) and eosin staining (a) and with hematoxylin and immunohistological staining for fibrinogen/fibrin, which stains clots dark brown (b). The clot (C) in (b) extends from the glomerular capsule (G) into the neck of the first convoluted tubule (T). Scale bars: 50 μm.
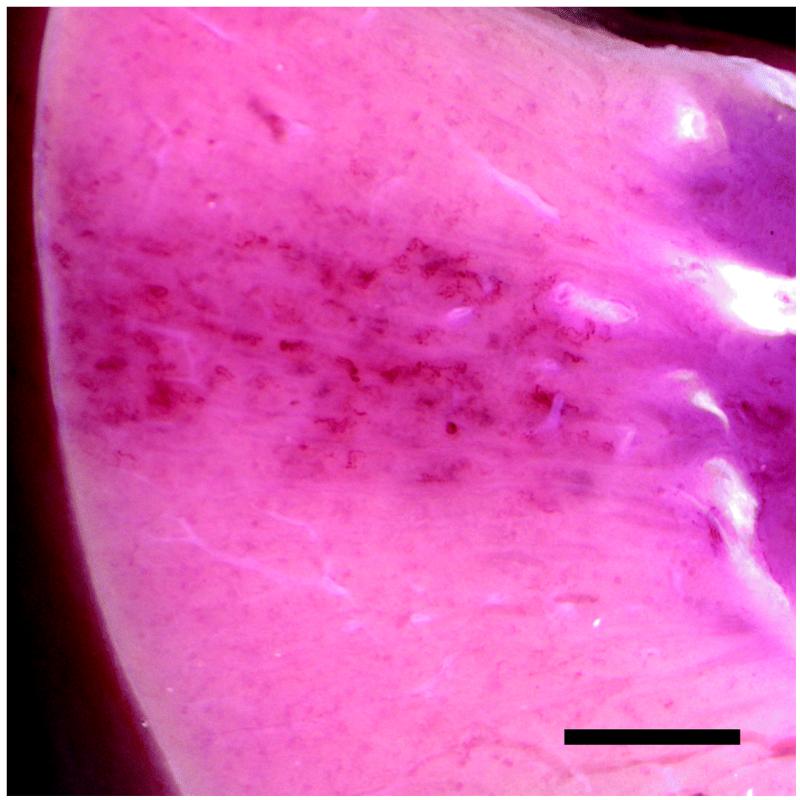
Three groups of pigs were scanned with the Sequoia 512 as listed in Table 1. Group 6 with open abdomen exposure and the saline standoff gave 42.7 ± 2.8 % GCH, which was commensurate with the results for the Vingmed machine. The hemorrhage marks for one of the Group 6 kidneys is shown in Fig. 5. Group 7 with open abdomen and scanning with the probe in contact with the kidney (i. e. without a standoff) had one GCH detected in four pigs, which was not significant. The different ultrasound images obtained by the contact scanning and closed abdomen scanning are shown in Fig. 6. For the contact scanning, the machine had difficulty forming an image near the probe (the focal points remained at 4 and 6 cm, as for the other groups), and there was no clear indication of enhanced contrast within the proximal cortex. However, there were indications of enhanced contrast in the distal cortex, and samples taken from the distal side of the kidneys had 6.6 ± 4.9 % GCH (P<0.05). Group 8 with transabdominal scanning had 7.9 ± 5.6 % GCH (P<0.05 relative to zero in sham samples) in the proximal cortex. Fibrin staining was done on adjacent serial sections and 4.5 ± 2.7 % of glomeruli had evidence of clotting in Bowman’s space (P<0.05). Urine collections for this group were positive for blood for the samples collected 10 and 20 min after exposure (P<0.05, relative to negative pre- and during-exposure samples) but were inconsistent (not statistically significant) for the 30 min collection.
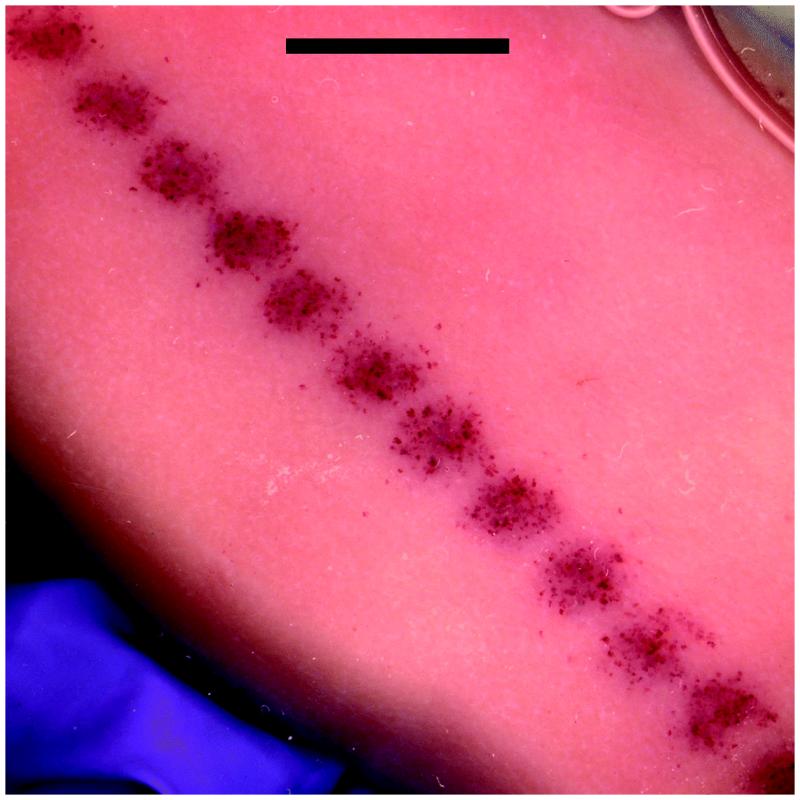
Figure 5.
A band of marks from hemorrhage into tubules on a group 6 kidney with open-abdomen scanning with the Sequoia 512. Note the regular pattern of maxima in the marks with a spacing of about 4 mm. Scale bar: 10 mm.
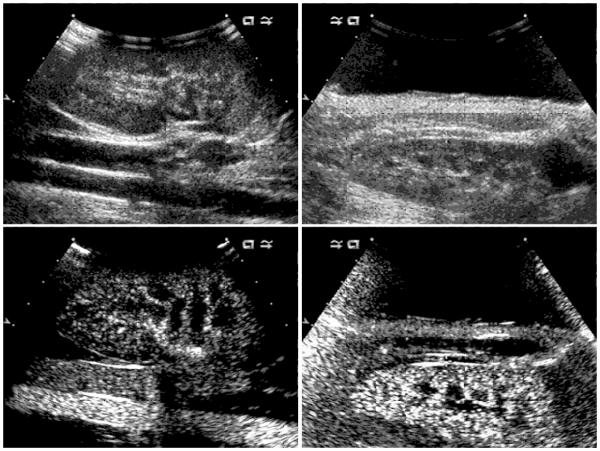
Figure 6.
Images obtained with the Sequoia 512 for the probe in contact with the kidney (left) and for the probe with saline standoff and closed abdominal wall (right). The images were obtained with the tissue harmonic mode (top row), and with the contrast mode (bottom row) which shows contrast agent gas bodies in the renal circulation. The contrast mode utilized balance setting 1 (contrast agent only) in (left lower), and setting 0 (contrast plus B mode background) in (right lower).
DISCUSSION
Contrast enhanced diagnostic ultrasound (CEDUS) was investigated in swine with regard to production of renal tissue damage. Glomerular capillary hemorrhage (Table 1, Figs. 1, 2, 4 and 5) was produced with open or closed abdomen exposure and was similar to the effect previously found in rat kidneys. In rats, CEDUS involved 10 μl/kg/min infusion for 1 min and 1 s intermittent image-exposure using the Vingmed System Five. GCH was found to be 0 % at 0.8 MPa, 5.6 ± 1.4 at 1.2 MPa, 37.0 ± 11.1 % at 1.8 MPa [4] and 49.2 ± 12.2 % at 2.3 MPa [13]. This the Vingmed System Five was very low at 1.3 MPa (Fig. 2), but GCH was evinced by blood filled was quite similar to results in swine (Fig. 2). The effect induced by intermittent scanning with tubules on the surface. However, the effect produced by agent-destructive bursts during contrast mode imaging with the Sequoia 512 was substantial at 1.3 MPa (Table 1, closed abdomen), possibly due to the longer pulses and burst duration. The threshold for GCH was found to be 0.73 MPa at 1.5 MHz in rats using more sensitive testing methods [4].
The relative species sensitivity depends on several factors, all of which may not be known. For example, the anaphylactoid reaction induced in pigs could reduce the circulating dose of contrast agent gas bodies and the resulting bioeffects compared to rats. The time intensity curves obtained in the left kidney had a sham decay constant of about 26 s for pigs, which was much faster than the sham decay constant of about 74 s in rats [14]. The natural gas-body removal by the mononuclear phagocyte system may work faster in pigs than in rats. The time intensity curves did not, however, reveal an important loss of gas bodies through agent destruction in the exposed right kidney, which was seen in rats.
Hematuria was detected in urine samples from the swine, but were not comparable to the previous results in rats [5], due to the very different urine collection methods. In addition, the occurrence of Bowman’s space enlargement when full of blood (indicative of intratubular obstruction) and formation of clots in Bowman’s space, which had been demonstrated in rats under different conditions [6], were also observed in the swine. The intratubular obstruction would be expected to cause acute tubular necrosis in effected nephrons, which had been previously seen in rats [5].
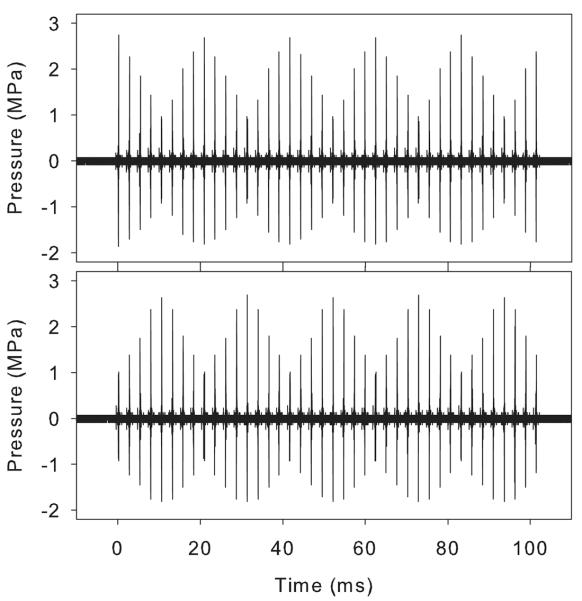
An interesting feature of the exposure with the Sequoia 512 was that the band of blood filled tubule marks produced on the kidney was variable (Fig. 5) with maxima in density of the marks having a regular spacing of about 4 mm. Hydrophone examination of the pulse sequences produced by the clearance burst revealed that the two patterns of pulse amplitudes shown in Fig. 7 could be seen at regular intervals. The lateral spacing between the occurrence of either extreme pattern was 4.2 mm at 4 cm depth, and varied with depth. Previously, a large difference in the GCH effect for pulse pressure-amplitude sequences which start high and decrease (ramp down) and sequences which start low and increase (ramp up) was found: the ramp-down sequence had much more effect [13]. The low effect for the ramp-up sequence was attributed to the destruction of the optimum gas bodies before the highest PRPA pulses arrived. The ramp-down and up sequences occur at regular intervals for the initial pulses in the agent-destruction bursts shown in Fig. 7. This observation explains the regular spacing of maxima in the hemorrhage-filled tubule marks shown in Fig. 5, as the positions of the ramp down sequence.
Figure 7.
Ultrasonic pressure plots for the clearance burst of the Sequoia 512 in contrast mode at two lateral positions about 2 mm apart in the focal zone at the same 4 cm image depth. The pulse pressure amplitudes vary in a regular way with five peaks. However, note that the sequence in the upper panel starts (on the left) with a maximal pulse followed by decreasing pulse heights, while the sequence in the lower panel starts with a minimal pulse followed by increasing pulse heights. Sequences displayed a transition between the two extreme cases as the hydrophone was moved transversely along the scan plane.
Jimenez et al. found no renal tissue damage in swine kidney after CEDUS imaging with a Sequoia 512 ultrasound machine with the probe in contact with the kidney [7]. The depth of the maximum pulse pressure-squared integral (focal zone) was listed as 87 to 38 mm [7]. The reason for the lack of tissue injury appears to be the placement of the probe in contact with the kidney, which placed the focal zone outside the proximal cortex. This configuration did not produce a clear image of the proximal cortex (Fig. 7). This same negative result in the proximal cortex was obtained in the present study for scanning with the probe in contact with the kidney (Table 1). However, GCH was observed on the distal side of the kidney in this study, apparently because some portion of the distal cortex was within the focal zone (Fig. 6). Another reason for the lack of tissue injury may have been the discrepancy in measured PRPA for the Sequoia 512, which was much lower than expected for the displayed MI value of 1.9 (see Methods). The differences between Definity and Sonovue do not seem important, because both agents are suspensions of gas bodies, which are strongly activated by the diagnostic ultrasound exposure in order to produce enhanced image contrast. Unfortunately, there have been very few published bioeffects studies using Sonovue, so that no other research data appear to be available for comparison. A study employing the methods used in the present study but with Sonovue contrast agent would be a valuable addition to the literature on this subject.
In conclusion, renal tissue damage was produce in pigs by CEDUS. These animals were difficult subjects, due to the anaphylactoid reactions to the agent and other factors. This reaction may have reduced substantially the circulating contrast agent dose, based on the wash out time constant (see Results). Nevertheless, the bioeffects were substantially the same as the bioeffects seen previously in rats [4-6]. Glomerular capillary hemorrhage was induced and led to hematuria and intratubular obstruction in some nephrons. There was no reason to believe that similar bioeffects would not be induced in human kidneys under similar imaging-exposure conditions. The medical significance of these bioeffects is uncertain, but they are certainly undesirable. Tests for clinically observable effects, such as hematuria, should be included in clinical trials of renal CEDUS. Furthermore, the inclusion of information on ultrasound-induced bioeffects and their mitigation in contrast-agent package inserts would be of value to help the sonographer ensure the optimal risk-benefit profile for the patient.
ACKNOWLEDGMENT
We thank Dr. Jonathan Rubin for the loan of the Sequoia 512 ultrasound system during out of service periods. This work was supported by PHS grant EB00338 awarded by the National Institutes of Health, DHHS.
REFERENCES
- 1.Averkiou M, Powers J, Skyba D, Bruce M, Jensen S. Ultrasound contrast imaging research. Ultrasound Q. 2003;19:27–37. doi: 10.1097/00013644-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Miller DL, Averkiou MA, Brayman AA, et al. Bioeffects considerations for diagnostic ultrasound contrast agents. J Ultrasound Med. 2008;27:611–632. doi: 10.7863/jum.2008.27.4.611. [DOI] [PubMed] [Google Scholar]
- 3.Wible JH, Jr, Galen KP, Wojdyla JK, Hughes MS, Klibanov AL, Brandenburger GH. Microbubbles induce renal hemorrhage when exposed to diagnostic ultrasound in anesthetized rats. Ultrasound Med Biol. 2002;28:1535–1546. doi: 10.1016/s0301-5629(02)00651-8. [DOI] [PubMed] [Google Scholar]
- 4.Miller DL, Dou C, Wiggins RC, Wharram BL, Goyal M, Williams AR. An in vivo rat model simulating imaging of human kidney by diagnostic ultrasound with gas-body contrast agent. Ultrasound Med Biol. 2007;33:129–135. doi: 10.1016/j.ultrasmedbio.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Williams AR, Wiggins RC, Wharram BL, et al. Nephron injury induced by diagnostic ultrasound imaging at high mechanical index with gas body contrast agent. Ultrasound Med Biol. 2007;33:1336–1344. doi: 10.1016/j.ultrasmedbio.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller DL, Dou C, Wiggins RC. Glomerular capillary hemorrhage induced in rats by diagnostic ultrasound with gas-body contrast agent produces intratubular obstruction. Ultrasound Med Biol. 2009;35:869–877. doi: 10.1016/j.ultrasmedbio.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiménez C, de Gracia R, Aguilera A, et al. In situ kidney insonation with microbubble contrast agents does not cause renal tissue damage in a porcine model. J Ultrasound Med. 2008;27:1607–1615. doi: 10.7863/jum.2008.27.11.1607. [DOI] [PubMed] [Google Scholar]
- 8.Grauer SE, Pantely GA, Xu J, et al. Myocardial imaging with a new transpulmonary lipid-fluorocarbon echo contrast agent: experimental studies in pigs. Am Heart J. 1996;132:938–945. doi: 10.1016/s0002-8703(96)90002-2. [DOI] [PubMed] [Google Scholar]
- 9.Ostensen J, Hede R, Myreng Y, Ege T, Holtz E. Intravenous injection of Albunex microspheres causes thromboxane mediated pulmonary hypertension in pigs, but not in monkeys or rabbits. Acta Physiol Scand. 1992;144:307–315. doi: 10.1111/j.1748-1716.1992.tb09299.x. [DOI] [PubMed] [Google Scholar]
- 10.Bramos D, Tsirikos N, Kottis G, et al. The acute effect of an echo-contrast agent on right ventricular dimensions and contractility in pigs. J Cardiovasc Pharmacol. 2008;51:86–91. doi: 10.1097/FJC.0b013e31815c660c. [DOI] [PubMed] [Google Scholar]
- 11.Bush WH, Swanson DP. Acute reactions to intravascular contrast media: types, risk factors, recognition, and specific treatment. AJR Am J Roentgenol. 1991;157:1153–1161. doi: 10.2214/ajr.157.6.1950858. [DOI] [PubMed] [Google Scholar]
- 12.Goss JE, Chambers CE, Heupler FA., Jr. Systemic anaphylactoid reactions to iodinated contrast media during cardiac catheterization procedures: guidelines for prevention, diagnosis, and treatment. Laboratory Performance Standards Committee of the Society for Cardiac Angiography and Interventions. Cathet Cardiovasc Diagn. 1995;34:99–104. doi: 10.1002/ccd.1810340403. [DOI] [PubMed] [Google Scholar]
- 13.Miller DL, Dou C, Wiggins RC. Simulation of diagnostic ultrasound image pulse sequences in cavitation bioeffects research. J Acoust Soc Am. 2007;122:2002–2008. doi: 10.1121/1.2773991. [DOI] [PubMed] [Google Scholar]
- 14.Miller DL, Dou C, Wiggins RC. In vivo gas body efficacy for glomerular capillary hemorrhage induced by diagnostic ultrasound in rats. IEEE Trans Biomed Eng. 2010;57:167–174. doi: 10.1109/TBME.2009.2030960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller DL, Dou C, Wiggins RC. Doppler mode pulse sequences mitigate glomerular capillary hemorrhage in contrast-aided diagnostic ultrasound of rat kidney. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:1802–1810. doi: 10.1109/tuffc.2007.464. [DOI] [PubMed] [Google Scholar]