Abstract
Because there are few reports using gene delivery in clinically-approved synthetic matrices, we examined the feasibility of using a noninvasive imaging system to study the kinetics of luciferase gene expression when delivered in an adenoviral vector. Using a mouse model of full thickness injury, we quantified the kinetics of gene expression, determined the optimal dose of particle delivery, and established the temporal importance of drug delivery in obtaining optimal gene expression. Specifically, we found that the ideal time to deliver adenovirus to a graft is during the early phase of graft wound closure (days 0–3 post-operatively) for a peak of gene expression to occur 7 days after delivery. Under these conditions, there is a saturating dose of 6 × 108 adenoviral particles per graft. In light of these findings, we examined whether the efficacy of delivery could be increased by modulating the composition of the grafts. When a collagen gene-activated matrix (GAM) containing basic fibroblast growth factor (FGF2) was compared to matrix alone, a significant increase in gene expression is observed when identical amounts of vector are delivered (p < 0.05). Taken together, these results show how a noninvasive and quantitative assessment of gene expression can be used to optimize gene delivery and that the composition of matrices can dramatically influence gene expression in the wound bed.
Keywords: Wound healing, Adenovirus, Gene therapy, Bioluminescence imaging, Growth factors, Scaffolds
1. Introduction
Cutaneous wounds are a significant clinical problem in acute and chronic injury and, because they are often associated with unsatisfactory outcomes for patients, there have been numerous efforts to develop optimized biomaterials to promote tissue repair. While advances in wound care technologies have increased the range of treatment options, there remains a significant clinical need to accelerate healing, limit infection, and reduce scarring. Partial or full thickness excisions followed by grafting are among the most widely used treatments for cutaneous wounds, including burns, and chronic wounds. To date however, the deployment of gene-based medicines in conjunction with these grafts has not been fully exploited in the post-genomic era. Accordingly, while the benefit of specific cellular and acellular graft matrices is clinically accepted [1–3], the delivery of genes to the wound bed has largely focused on strategies using incisional and punch wounds [4–9] that are distinct from accepted clinical protocols for severe burn injury and wound healing. With the widespread use of biosynthetic grafts, advances in noninvasive imaging, and a better understanding of the cell biology of synthetic graft models [2,10], we are now able to better examine gene delivery to the wound bed through quantitative and noninvasive techniques.
Recently, several strategies have been tested to restrict foreign genes to the wound bed [4–7,9,11,12]. One approach, for example, is to embed gene delivery vectors in a porous degradable matrix prior to implantation [9,11,13]. In this approach, matrices stabilize vectors for the purpose of gene delivery and likely serve the dual function of also promoting the recruitment and infiltration of host cells adjacent to the wound to stimulate the internalization and expression of the gene of interest [3,14,15]. We have recently characterized the kinetics of vascular remodeling in a synthetic graft model and identified distinct phases of vascular leak that precedes re-epithelialization (early phase) vs. neovascularization and re-epithelialization (late phase) that likely influences gene transduction in vivo [16]. Using a full thickness cutaneous wound grafted with a synthetic cross-linked collagen–glycosaminoglycan matrix (Integra™) in mice, full wound coverage and healing occurs within 7–10 days, followed by contraction and vascular consolidation (i.e. reduced vascular permeability and stabilized angiogenesis) of the wound bed by 21 days [16]. We propose that these biosynthetic grafts provide a protected wound microenvironment suitable for the delivery of genes and growth factors. While the topical treatment of wounds with various gene products has been shown to lead to accelerated wound closure in many animal models [9,17–19], the clinical outcomes following these therapies has been generally poor.
Evaluation of the host response to various treatments and biomaterials has traditionally involved excision of the area of interest and ultimately, the loss of the animal. Noninvasive techniques, such as bioluminescence, which uses a cooled CCD camera to quantify photons of light emitted from specific molecules, allows investigators to evaluate the response to a treatment over time in each animal. Not only can this be more cost-effective by limiting the materials and time needed for tissue analysis, but it can also be tailored to test specific therapeutic strategies. The gene for luciferase, the enzyme which converts D-luciferin to oxyluciferin thereby emitting photons of light [20], can be incorporated into various vectors for delivery to target tissues [21,22]. Using firefly luciferase as an in vivo reporter that we can measure noninvasively, we were able to assess the localization and quantify the expression levels dynamically over an extended time (up to 40 days post-operative). These studies establish the preclinical parameters for the design and delivery of adenovirus vectors, a gene delivery system with a generally well-tolerated profile in animal models and humans [23–25].
The first goal of this study was to define the parameters (i.e. kinetics of expression, dosing, and timing) of gene delivery using aqueous Ad-luc as a sensitive, quantitative, and noninvasive reporter in the grafted wound bed. The second goal was to assess the potential for growth factor inclusion (i.e. FGF2) within lyophilized type I collagen matrices to mediate a more efficient gene expression with a stabilized gene-activated matrix (GAM). Previous studies with GAMs have shown the capacity to mediate the delivery of growth factors in several models of healing [26–29], but they have not been previously tested in a grafted wound model.
2. Materials and methods
2.1. Reagents
Replication-deficient adenovirus (Ad5) expressing either firefly luciferase (Ad-luc) was amplified in 293 cells and purified to obtain titers of at least 2 × 1010 particles per mL that were subject to serial dilution for the various assays. FGF2 (R & D Systems, Minneapolis, MN), and type I collagen (PureCol, 3.1 mg/mL) were obtained from INAMED (Fremont, CA). Silicone-backed Integra™ was used in all graft studies (Integra Life Sciences, Plainsboro, NJ).
2.2. GAM preparation
To prepare a 1 mL GAM (i.e. the volume used to prepare a lyophilized GAM for one mouse), 0.1 mL of 10× PBS was added to 0.8 mL bovine type I collagen and mixed. pH was adjusted to 7.4 with 0.1 N NaOH and 0.05 mL of Ad-luc (2 × 1011 particles/mL) added with or without FGF2 to promote invasion of host cells into the GAM (4 μg/mL final concentration). Following flash freezing in liquid nitrogen, samples were lyophilized and administered to the animal by insertion under the graft.
2.3. Mouse graft model
All procedures were approved by the UCSD Institutional Animal Care and Use Committee. Male C57BL/6 mice, ranging in age from eight to sixteen weeks old, were used for the experiments. Mice were anesthetized with isoflurane, the dorsum of the animal was removed of hair and the surgical site prepared using aseptic technique. A full thickness circular wound measuring 1.5 cm in diameter was marked to the right of midline using a standard template and the skin, subcutaneous tissue, and fascia was excised. Grafts of 1.5 cm in diameter were secured with approximately seven silk sutures. Animals received 1.4 mL normal saline resuscitation and 0.05 mg/kg buprenorphine subcutaneously post-procedure. Animals were recovered and returned to the vivarium with twelve hours of light/dark cycles and free access to food and water. At the completion of the study, animals were euthanized and tissue was collected for analysis. Aqueous Ad-luc was injected (50 μl of 2 × 1011 particles/mL diluted to a total volume of 150 μl sterile PBS) with a 0.5 mL low void volume syringe three days after grafting surgery (Figs. 1 and 2), or at various days following surgery (Fig. 3). Alternatively, lyophilized GAM containing Ad-luc was placed under the graft immediately following the grafting as the last step before closing the wound site (Fig. 4).
Fig. 1.
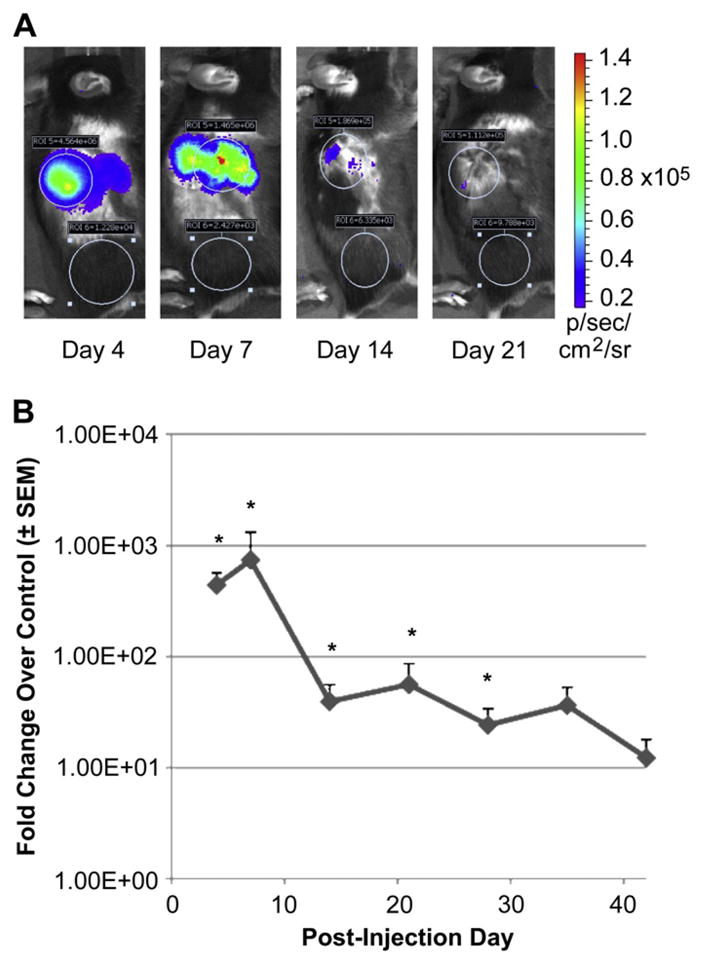
Kinetics of adenovirus-mediated gene delivery in grafts of a full thickness wound. (A) Noninvasive whole animal image of adenoviral-mediated delivery of firefly luciferase (Ad-luc) following injection under an Integra™ graft placed in a full thickness excisional wound using a cooled CCD imaging system. (B) Quantification of luciferase expression kinetics following injection of Ad-luc. Each time point is the average of noninvasive imaging in anesthetized animals with matched acquisition settings as described in Materials and methods (n ≥ 4 per time point, *p < 0.05 compared to background luminescence).
Fig. 2.
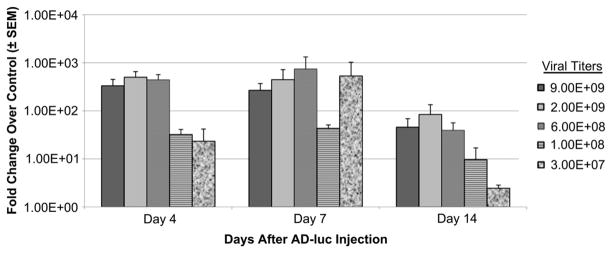
Optimization of Ad-luc dosing in grafts of a full thickness wound. Serial dilutions of Ad-luc particles were assessed for gene transduction under a graft in a full thickness wound. Ad-luc injections were performed three days after the wound and graft surgery, and imaged after an additional incubation of 4, 7 or 14 days.
Fig. 3.
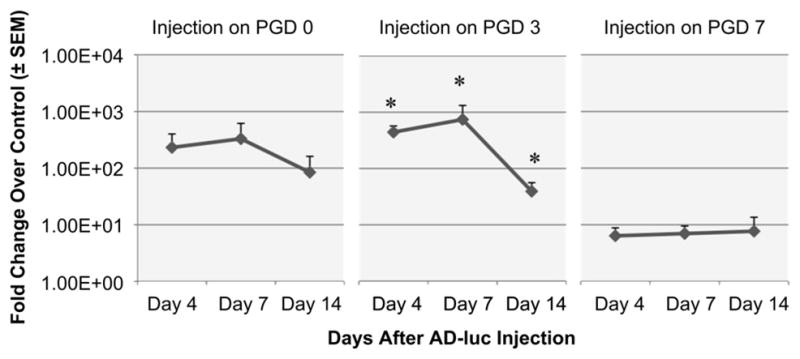
Optimization of timing of adenovirus injection following grafting. Ad-luc was injected at a fixed dose (1 × 1010 particles/graft) under the graft immediately following the surgery (PGD 0), 3 days post-grafting (PGD 3), or 7 days post-grafting (PGD 7). Bioluminescence was monitored in each surgical group for an additional 4, 7 or 14 days (n ≥ 4 per time point, *p < 0.05 compared to background luminescence).
Fig. 4.
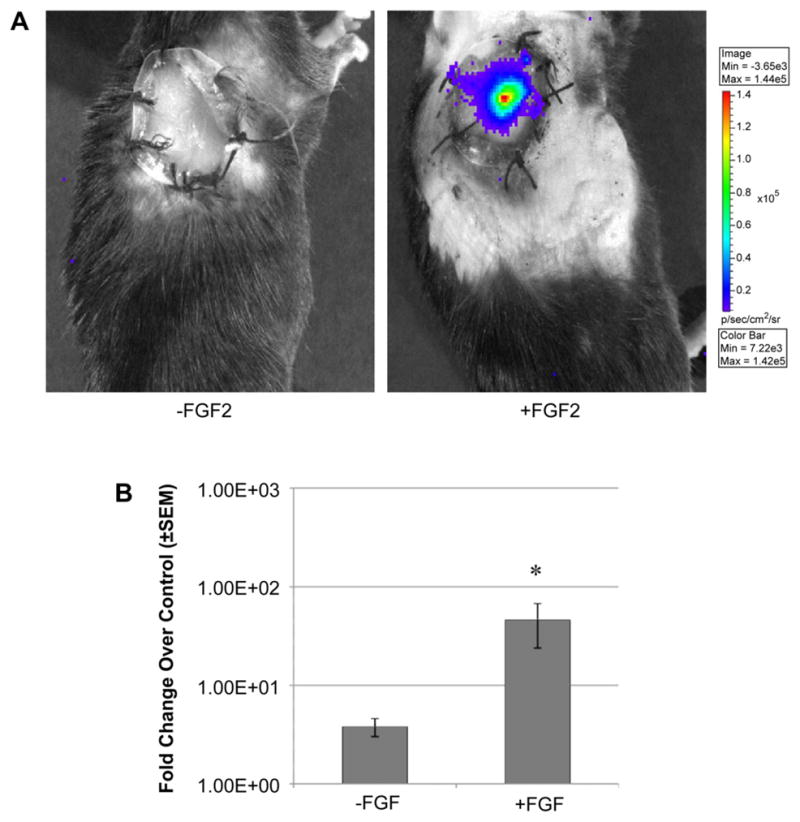
Matrix-stabilized adenovirus-mediated delivery of reporter gene in grafting. Ad-luc in lyophilized type I collagen GAMs with and without FGF2 were placed under the full thickness wound graft. (A) Representative images were collected after a 7 day incubation (B) and quantified (n ≥ 4 per time point, *p < 0.05 compared to background luminescence).
2.4. Noninvasive in vivo imaging of wound healing
Mice were anesthetized with isoflurane and subjected to intraperitoneal injection with the substrate D-luciferin (1.5 mg in a volume of 150 μl in saline) (Caliper Life Sciences, Hopkinton, MA). Following a five-minute incubation to obtain steady state kinetics of substrate distribution, mice were imaged on a Lumina CCD Imaging System (Caliper Life Sciences, Hopkinton, MA) according to manufacturer recommendations. Exposure-matched images were acquired and analyzed with Living Image software (Version 3.0) (Caliper Life Sciences, Hopkinton, MA). All animal images shown in this study were exposure-matched (i.e. matching color bar upper and lower limits in each panel). Regions of interest with bioluminescence in the wound area vs. a matched size in the flank were used as controls for image acquisition. Each image was quantified in units of photons/s/cm2/steradian to obtain a fold change of wound area signal over control area signal (i.e. Fold Change Over Control), as plotted in the figures.
2.5. Statistical analysis
The fold change of bioluminescence in the wound vs. control was analyzed in at least four animals in each group at each time point to obtain statistical significance. The Mann–Whitney U test was used to analyze the data and data with a p-value of less than 0.05 was deemed significant.
3. Results
3.1. Quantification of adenovirus-mediated gene expression
To quantify the kinetics of adenoviral-mediated gene delivery in a full thickness wound graft, we examined the persistence of firefly luciferase gene expression over an extended time. Mice (C57Bl/6) were subjected to full thickness wounds and grafted on Day 0 with Integra™ matrices as previously described [16]. On the third day post-surgery, animals were injected with aqueous Ad-luc (5 × 108 particles/graft) under the graft and the effects of the biomaterial on gene expression were evaluated. Following incubation for various time points (3, 7 and 10 days post-injection), mice were anesthetized and imaged with a deep-cooled CCD camera imaging system as described in Materials and methods. Representative exposure-matched images of the same mouse demonstrate the capacity to serially image the same subject, quantify gene expression, and localize expression to the grafting site (Fig. 1A). Quantification of the response over an extended time of up to 42 days post-injection revealed a significant increase in gene expression that, when expressed as fold increase in the local wound bed vs. control flank tissue, showed a maximum increase of >700 fold at 7 days (Fig. 1B, p < 0.05). This gene expression decreased to a steady and lower level of 10-fold for up to 42 days. These findings suggest that a high initial gene expression (i.e. within 7 days of injury) is compatible with a likely optimal therapeutic window and correlates with the kinetics of graft re-vascularization and subsequent wound closure previously shown in this graft model [16].
3.2. Determination of the optimal dose of adenovirus vector
We next determined the optimal dose of adenovirus in the full thickness wound graft model. Serial dilutions of Ad-luc were injected under the graft on the third day after wounding and imaged over 4–14 days post-injection. Gene expression was quantified as described in Materials and methods. By 4 days post-injection, the dose of 6 × 108 particles led to a similar transduction level as doses up to 9 × 109, suggesting saturation of this model at 6 × 108 particles per graft. This response was generally maintained when imaged and quantified at days 7 and 14 after injection (Fig. 2). Lower concentrations of Ad-luc (i.e. 3 × 107 to 1 × 108 particles/graft) lead to decreased transduction levels throughout the 14-day time course.
3.3. Establishment of the optimal time of adenovirus injection after wounding
We have previously shown that extensive vascular remodeling occurs in the first 7 days following graft placement on a full thickness excisional wound [16]. Therefore, we examined whether administration of adenoviral particles at different days following the surgical grafting would influence gene delivery. While studies in Figs. 1 and 2 evaluated the effects of injecting virus under the graft at day 3 post-grafting, we examined the response if mice were injected either immediately after grafting at day 0 (i.e. Post-Grafting Day (PGD) 0), day 3 (PGD 3), or day 7 post-graft (PGD 7, Fig. 3), Images were taken at 4, 7, 14 days after the injection of adenovirus. Significant increases in adenoviral-mediated gene delivery of luciferase over control flank regions were observed in the PGD 3 group (Fig. 3, p < 0.05), similar to levels observed when the virus was injected immediately after grafting (PGD 0). In contrast, there was a substantial decrease in gene transduction when the injection was performed 7 days after placement of the graft (i.e. PGD 7). These data suggest that the early time points (i.e. PGD 0 or 3), which are associated with tissue remodeling rather then consolidation, are most amenable to adenoviral-mediated gene transduction.
3.4. Evaluation of the effects of matrix-stabilized adenovirus with fibroblast growth factor-2
If the results above point towards the importance of the time of delivery to observe gene expression, they also infer the importance of administering virus during the proliferative phase of tissue remodeling. This in and of itself suggests that the biomaterials used to construct the grafts could be modified to enhance gene delivery. Indeed, whereas aqueous gene delivery is the most common approach for administering viral vectors in vivo, lyophilized (i.e. stabilized) preparations of adenovirus can also be formulated in collagen for stabilization and sustained gene delivery to the wound bed [30]. If, as predicted, the proliferative induction by biomaterials is critical for gene expression, we reasoned that growth factor supplementation of the biomaterial would enhance gene delivery by providing a chemotactic gradient promoting host cell infiltration. Lyophilized GAMs consisting of bovine type I collagen and adenovirus were prepared with and without the growth and angiogenic factor, FGF2. When the grafted biomaterial is placed into a full thickness excisional wound immediately after grafting, we observed a 40-fold increase in gene delivery to the wound bed (Fig. 4, p < 0.05) that is directly attributable to FGF2 and confirms that the formulation of mixed biomaterials can be further designed for increased–and optimized–gene expression.
4. Discussion
Gene delivery is an emerging technology in the field of tissue repair and is being used to administer genes that promote the natural physiologic response to injury [31–33]. Unfortunately, because there are few technologies to evaluate the efficacy of biomaterials in gene delivery, there is very little information defining the kinetics and dosing of the gene payloads in graft microenvironments. To this end, we investigated whether noninvasive imaging techniques that can monitor gene expression in vivo could be deployed with a murine biomaterial grafting model of tissue repair to assess drug effectiveness, graft patency, and define the parameters of optimal gene delivery.
In previous work, we showed that the early phase of tissue repair (post-grafting days 0–3) was characterized by extensive vascular leak and minimal angiogenesis, while in the later phase (post-grafting days 7 and later), there was minimal vascular leak and extensive re-vascularization, re-epithelialization, and contraction [16]. We hypothesized that, although the cellular composition of the early phase appears highly unorganized, there were a high number of target cells accessible to gene delivery vectors. In the results reported here, we tested this hypothesis by delivering adenovirus containing the luciferase gene to grafts and quantifying the kinetics of expression, defining the optimal dosing, and establishing the optimal timing of delivery post-operatively. As predicted, adenovirus administered either immediately following the graft surgery or 3 days post-operatively was superior to delivery at later time points. The peak of expression was observed 3–7 days post-injection when cell proliferation is at its highest. To exploit this observation, we then showed that a stabilized adenovirus formulated in a collagen-based biomaterial can be significantly improved by the inclusion of a growth factor like FGF2 that is capable of stimulating tissue remodeling.
The application of noninvasive CCD imaging technologies to monitor gene expression in vivo has been applied in many experimental systems including oncology [34,35], stem cell tracking [36], infectious disease [37], and wound healing [38–40]. The sensitivity and reproducibility of noninvasive CCD imaging is well established in these model systems and enables the monitoring of disease progression over time in the same cohort of animals, generally requiring fewer animals per time point [41]. Here we apply bioluminescent imaging to examine a classic gene delivery system based on the well-characterized and generally well-tolerated Ad5 vector in a clinically-relevant model of a full thickness wound followed by grafting, a common surgical procedure in severe burns as well as in select cutaneous trauma and chronic wounds [42,43]. Although this study focused on grafts in healthy young animals, we define the parameters for gene delivery in a complex grafted wound bed and provide quantifiable endpoints for quantification of gene delivery over an extended time course. We predict that bioluminescence can be used to optimize gene delivery systems to the wound, such as the testing of new formulations that accelerate the normal wound healing process while minimizing scar formation.
To this end, we have explored the use of GAMs as a tool to deliver growth factors in a variety of formulations. Growth factors (i.e. generally FGF2 and PDGF) have been widely applied to the wound bed as a stand-alone therapeutic, either as a recombinant protein [44,45] or as a cDNA in an adenovirus vector [17,46]. Fewer studies have explored their utility when adenovirus delivery is targeted to the extracellular matrix of the wound [5], infiltrating fibroblasts [47,48], or in combination with matrix proteins [3,23]. Aqueous type I collagen can be used to enhance gene delivery of adenovirus [3]. For this reason, we explored whether we could enhance gene delivery by incorporating recombinant FGF2 into GAMs, not as a factor to accelerate wound closure in and of itself, but as an adjunct to gene delivery that would act as a chemo-attractant in the wound bed to enhance the recruitment of cells to the wound (i.e. angiogenic endothelium, fibroblasts, epithelium or inflammatory cells) [19]. In our comparison of FGF2-containing collagen:Ad-luc GAMs vs. collagen:Ad-luc GAMs lacking FGF2, we observed a significant increase in the efficacy of gene delivery over 7 days, a critical period in the kinetics of re-vascularization, re-epithelialization and wound closure in this and other models of wound healing in general [10,16,49]. This is further supported by recent in vitro research that showed collagen-based gelatin matrix particles bind exogenous FGF2 and release it slowly over a course of 14 days to stimulate proliferation [50]. Whereas appropriate formulations of biomaterials for tissue repair have always been expected to meet the spatial needs that characterize the injury response, their combination with gene delivery opens the possibility of introducing temporal and cell-specific control of drug delivery within these 3-dimensional matrices.
Finally, it is important to note that the noninvasive imaging of gene delivery can be extended to evaluate the efficacy of bio-therapeutics for wound healing in preclinical models of tissue repair. In these paradigms, the luciferase gene is controlled by specific promoters that are associated with angiogenesis (vascular endothelial growth factor receptor 2), inflammation (NF-κB), and scarring (TGF-β) for example, and introduced into transgenic mice [39,40]. The spatio-temporal effects of drug-candidates, including growth factors, new biomaterials, stem cells and small molecule drugs, can be directly assessed in terms of gene expression both within and distant to the wound. The deployment of these noninvasive methodologies to the study of tissue repair and wound healing has the promise to transform the preclinical assessment of potential therapeutics.
5. Conclusions
In this study we have shown that adenovirus-mediated delivery of luciferase to the wound bed has several characteristics: maximal gene transduction occurs at seven days after injection of the virus, and injection within three days after grafting results in the highest gene transduction. Furthermore, we have demonstrated that the addition of a collagen GAM containing FGF2 facilitates more efficient gene transduction to the target tissues. These studies establish the application of a straightforward and stable formulation of a gene delivery vector that is compatible with clinically established synthetic graft matrices (i.e. Integra™) used in the treatment of full thickness cutaneous wounds. We propose that this Ad-luc reporter model can be used to identify combinations of other growth factors and matrix proteins that can deliver genes of interest with better persistence of expression and control of spatio-temporal specificity in the wound bed. Additionally, the noninvasive imaging techniques measuring reporter gene expression in vivo presented here could be used to evaluate the efficacy of potential biotherapeutic agents and improve the treatment of cutaneous wounds.
Acknowledgments
We acknowledge Matthew Wang (UCSD) for technical support in the animal studies and Emelie Amburn (UCSD) and Tiffany Wang (UCSD) for the adenovirus preparations. There are no disclosures or conflicts of interest. Studies supported by an NIGMS P20 grant (A.B.).
Appendix
Figures with essential color discrimination. Certain figures in this article, in particular parts of Figs. 1 and 4, may be difficult to interpret in black and white. The full color images can be found in the on-line version, at doi:10.1016/j.biomaterials.2009.07.069.
References
- 1.Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305–13. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 2.Auger FA, Lacroix D, Germain L. Skin substitutes and wound healing. Skin Pharmacol Physiol. 2009;22(2):94–102. doi: 10.1159/000178868. [DOI] [PubMed] [Google Scholar]
- 3.Doukas J, Chandler LA, Gonzalez AM, Gu D, Hoganson DK, Ma C, et al. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum Gene Ther. 2001 May 1;12(7):783–98. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch T, von Peter S, Dubin G, Mittler D, Jacobsen F, Lehnhardt M, et al. Adenoviral gene delivery to primary human cutaneous cells and burn wounds. Mol Med. 2006 Sep–Oct;12(9–10):199–207. doi: 10.2119/2006-00031.Hirsch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoff A, Rivera AA, Banerjee NS, Mathis JM, Espinosa-de-los-Monteros A, Le LP, et al. Strategies to enhance transductional efficiency of adenoviral-based gene transfer to primary human fibroblasts and keratinocytes as a platform in dermal wounds. Wound Repair Regen. 2006 Sep–Oct;14(5):608–17. doi: 10.1111/j.1743-6109.2006.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoff A, Rivera AA, Mathis JM, Moore ST, Banerjee NS, Everts M, et al. Effect of adenoviral mediated overexpression of fibromodulin on human dermal fibroblasts and scar formation in full-thickness incisional wounds. J Mol Med. 2007 May;85(5):481–96. doi: 10.1007/s00109-006-0148-z. [DOI] [PubMed] [Google Scholar]
- 7.Lee JA, Conejero JA, Mason JM, Parrett BM, Wear-Maggitti KD, Grant RT, et al. Lentiviral transfection with the PDGF-B gene improves diabetic wound healing. Plast Reconstr Surg. 2005 Aug;116(2):532–8. doi: 10.1097/01.prs.0000172892.78964.49. [DOI] [PubMed] [Google Scholar]
- 8.Hansen SL, Myers CA, Charboneau A, Young DM, Boudreau N. HoxD3 accelerates wound healing in diabetic mice. Am J Pathol. 2003 Dec;163(6):2421–31. doi: 10.1016/S0002-9440(10)63597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006 May 23;113(20):2413–24. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 10.Macri L, Clark RA. Tissue engineering for cutaneous wounds: selecting the proper time and space for growth factors, cells and the extracellular matrix. Skin Pharmacol Physiol. 2009;22(2):83–93. doi: 10.1159/000178867. [DOI] [PubMed] [Google Scholar]
- 11.Hansen SL, Young DM, Boudreau NJ. HoxD3 expression and collagen synthesis in diabetic fibroblasts. Wound Repair Regen. 2003 Nov–Dec;11(6):474–80. doi: 10.1046/j.1524-475x.2003.11615.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999 Jul;5(7):753–9. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 13.Schillinger U, Wexel G, Hacker C, Kullmer M, Koch C, Gerg M, et al. A fibrin glue composition as carrier for nucleic acid vectors. Pharm Res. 2008 Dec;25(12):2946–62. doi: 10.1007/s11095-008-9719-8. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein SA, Bonadio J. Potential role for direct gene transfer in the enhancement of fracture healing. Clin Orthop Relat Res. 1998 Oct;(355 Suppl):S154–62. doi: 10.1097/00003086-199810001-00017. [DOI] [PubMed] [Google Scholar]
- 15.Bonadio J. Tissue engineering via local gene delivery. J Mol Med. 2000;78(6):303–11. doi: 10.1007/s001090000118. [DOI] [PubMed] [Google Scholar]
- 16.Shaterian A, Borboa A, Sawada R, Costantini TW, Potenza B, Coimbra R, et al. Real time analysis of the kinetics of angiogenesis and vascular permeability in an animal model of wound healing. Burns. 2009 Sep;35(6):811–7. doi: 10.1016/j.burns.2008.12.012. [Epub 2009 May 6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu PY, Wang XT, Badiavas E, Rieger-Christ K, Tang JB, Summerhayes I. Enhancement of ischemic flap survival by prefabrication with transfer of exogenous PDGF gene. J Reconstr Microsurg. 2005 May;21(4):273–9. doi: 10.1055/s-2005-871755. [DOI] [PubMed] [Google Scholar]
- 18.Jiang S, Zavitz CC, Wang J, Saraf A, Zielinski R, Ramsbottom JD, et al. Non-adenine based purines accelerate wound healing. Purinergic Signal. 2006 Nov;2(4):651–61. doi: 10.1007/s11302-006-9022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braund R, Hook S, Medlicott NJ. The role of topical growth factors in chronic wounds. Curr Drug Deliv. 2007 Jul;4(3):195–204. doi: 10.2174/156720107781023857. [DOI] [PubMed] [Google Scholar]
- 20.Wu JC, Sundaresan G, Iyer M, Gambhir SS. Noninvasive optical imaging of firefly luciferase reporter gene expression in skeletal muscles of living mice. Mol Ther. 2001 Oct;4(4):297–306. doi: 10.1006/mthe.2001.0460. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Iyer M, Annala AJ, Chappell S, Mauro V, Gambhir SS. Noninvasive monitoring of target gene expression by imaging reporter gene expression in living animals using improved bicistronic vectors. J Nucl Med. 2005 Apr;46(4):667–74. [PubMed] [Google Scholar]
- 22.De A, Lewis XZ, Gambhir SS. Noninvasive imaging of lentiviral-mediated reporter gene expression in living mice. Mol Ther. 2003 May;7(5 Pt 1):681–91. doi: 10.1016/s1525-0016(03)00070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu DL, Nguyen T, Gonzalez AM, Printz MA, Pierce GF, Sosnowski BA, et al. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Mol Ther. 2004 May;9(5):699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Yuan B, Zhao Z, Zhang YR, Wu CT, Jin WG, Zhao S, et al. Short-term safety and curative effect of recombinant adenovirus carrying hepatocyte growth factor gene on ischemic cardiac disease. In Vivo. 2008 Sep-Oct;22(5):629–32. [PubMed] [Google Scholar]
- 25.Keedy V, Wang W, Schiller J, Chada S, Slovis B, Coffee K, et al. Phase I study of adenovirus p53 administered by bronchoalveolar lavage in patients with bronchioloalveolar cell lung carcinoma: ECOG 6597. J Clin Oncol. 2008 Sep 1;26(25):4166–71. doi: 10.1200/JCO.2007.15.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doukas J, Blease K, Craig D, Ma C, Chandler LA, Sosnowski BA, et al. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol Ther. 2002 May;5(5 Pt 1):517–27. doi: 10.1006/mthe.2002.0579. [DOI] [PubMed] [Google Scholar]
- 27.Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004 Apr;9(4):519–26. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreiber RE, Blease K, Ambrosio A, Amburn E, Sosnowski B, Sampath TK. Bone induction by AdBMP-2/collagen implants. J Bone Joint Surg Am. 2005 May;87(5):1059–68. doi: 10.2106/JBJS.D.02025. [DOI] [PubMed] [Google Scholar]
- 29.Berry M, Gonzalez AM, Clarke W, Greenlees L, Barrett L, Tsang W, et al. Sustained effects of gene-activated matrices after CNS injury. Mol Cell Neurosci. 2001 Apr;17(4):706–16. doi: 10.1006/mcne.2001.0975. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez AM, Berlanga O, Leadbeater WE, Cooper-Charles L, Sims K, Logan A, et al. The deployment of adenovirus-containing gene activated matrices onto severed axons after CNS injury leads to transgene expression in target neuronal cell bodies. J Gene Med. 2009 Aug;11(8):679–88. doi: 10.1002/jgm.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson JM. Growth factors: the promise and the problems. Int J Low Extrem Wounds. 2007 Mar;6(1):8–10. doi: 10.1177/1534734607299180. [DOI] [PubMed] [Google Scholar]
- 32.Bonadio J. Tissue engineering via local gene delivery: update and future prospects for enhancing the technology. Adv Drug Deliv Rev. 2000 Nov 15;44(2–3):185–94. doi: 10.1016/s0169-409x(00)00094-6. [DOI] [PubMed] [Google Scholar]
- 33.Eming SA, Krieg T, Davidson JM. Gene therapy and wound healing. Clin Dermatol. 2007 Jan–Feb;25(1):79–92. doi: 10.1016/j.clindermatol.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouvet M, Wang J, Nardin SR, Nassirpour R, Yang M, Baranov E, et al. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002 Mar 1;62(5):1534–40. [PubMed] [Google Scholar]
- 35.Ke S, Wen X, Gurfinkel M, Charnsangavej C, Wallace S, Sevick-Muraca EM, et al. Near-infrared optical imaging of epidermal growth factor receptor in breast cancer xenografts. Cancer Res. 2003 Nov 15;63(22):7870–5. [PubMed] [Google Scholar]
- 36.Min JJ, Ahn Y, Moon S, Kim YS, Park JE, Kim SM, et al. In vivo bioluminescence imaging of cord blood derived mesenchymal stem cell transplantation into rat myocardium. Ann Nucl Med. 2006 Apr;20(3):165–70. doi: 10.1007/BF03027425. [DOI] [PubMed] [Google Scholar]
- 37.Engelsman AF, van der Mei HC, Francis KP, Busscher HJ, Ploeg RJ, van Dam GM. Real time noninvasive monitoring of contaminating bacteria in a soft tissue implant infection model. J Biomed Mater Res B Appl Biomater. 2009 Jan;88(1):123–9. doi: 10.1002/jbm.b.31158. [DOI] [PubMed] [Google Scholar]
- 38.Ryan PL, Youngblood RC, Harvill J, Willard ST. Photonic monitoring in real time of vascular endothelial growth factor receptor 2 gene expression under relaxin-induced conditions in a novel murine wound model. Ann N Y Acad Sci. 2005 May;1041:398–414. doi: 10.1196/annals.1282.061. [DOI] [PubMed] [Google Scholar]
- 39.Ho TY, Chen YS, Hsiang CY. Noninvasive nuclear factor-kappaB bioluminescence imaging for the assessment of host–biomaterial interaction in transgenic mice. Biomaterials. 2007 Oct;28(30):4370–7. doi: 10.1016/j.biomaterials.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Hsiang CY, Chen YS, Ho TY. Nuclear factor-kappaB bioluminescence imaging-guided transcriptomic analysis for the assessment of host–biomaterial interaction in vivo. Biomaterials. 2009 Jun;30(17):3042–9. doi: 10.1016/j.biomaterials.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Klerk CP, Overmeer RM, Niers TM, Versteeg HH, Richel DJ, Buckle T, et al. Validity of bioluminescence measurements for noninvasive in vivo imaging of tumor load in small animals. Biotechniques. 2007 Jul;43(1 Suppl):7–13. 30. doi: 10.2144/000112515. [DOI] [PubMed] [Google Scholar]
- 42.Jones I, Currie L, Martin R. A guide to biological skin substitutes. Br J Plast Surg. 2002 Apr;55(3):185–93. doi: 10.1054/bjps.2002.3800. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen DQ, Dickson WA. A review of the use of a dermal skin substitute in burns care. J Wound Care. 2006 Sep;15(8):373–6. doi: 10.12968/jowc.2006.15.8.26944. [DOI] [PubMed] [Google Scholar]
- 44.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998 May;21(5):822–7. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 45.Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990 Jun;136(6):1235–46. [PMC free article] [PubMed] [Google Scholar]
- 46.Wang XT, Liu PY, Tang JB. PDGF gene therapy enhances expression of VEGF and bFGF genes and activates the NF-kappaB gene in signal pathways in ischemic flaps. Plast Reconstr Surg. 2006 Jan;117(1):129–37. doi: 10.1097/01.prs.0000185609.07293.3e. [discussion: 138–129] [DOI] [PubMed] [Google Scholar]
- 47.Lanciotti J, Song A, Doukas J, Sosnowski B, Pierce G, Gregory R, et al. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol Ther. 2003 Jul;8(1):99–107. doi: 10.1016/s1525-0016(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 48.Chandler LA, Doukas J, Gonzalez AM, Hoganson DK, Gu DL, Ma C, et al. FGF2-targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Mol Ther. 2000 Aug;2(2):153–60. doi: 10.1006/mthe.2000.0102. [DOI] [PubMed] [Google Scholar]
- 49.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999 Sep 2;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 50.Huang S, Deng T, Wang Y, Deng Z, He L, Liu S, et al. Multifunctional implantable particles for skin tissue regeneration: preparation, characterization, in vitro and in vivo studies. Acta Biomater. 2008 Jul;4(4):1057–66. doi: 10.1016/j.actbio.2008.02.007. [DOI] [PubMed] [Google Scholar]