Abstract
To observe whether cyclin D1 siRNA-mediated inhibition of cyclin D1 represents a promising antigrowth and antimetastatic strategy for cancer gene therapy, particularly for non–small cell lung cancers. To stably transfect the A549 cell line with a cyclin D1–targeted siRNA to downregulate cyclin D1 expression and observe the effects on protein expression, and tumor growth in vitro and in vivo. Expression of cyclin D1–targeted siRNA resulted in a decrease in cyclin D1, MMP-2, RhoA, and Rac1 protein levels, as detected by Western blot and immunofluorescence studies. Transfected cells also exhibited a marked decrease in the rate of cell growth, and decreased invasive capacity, compared to cells transduced with a scrambled siRNA plasmid and untransduced A549 cells. siRNA-mediated inhibition of cyclin D1 expression represents a promising antigrowth and antimetastatic strategy for cancer gene therapy, particularly for non–small cell lung cancers. It is the reason for inhibiting tumor growth so that cyclin D1 siRNA can inhibit the cell cycle progression. In addition, the mechanism of inhibiting tumor metastasis was related to the decrease in the expression of MMP-2, RhoA, and Rac1 after cyclin D1 was decreased by cyclin D1 siRNA.
Introduction
The human cyclin D1 gene was cloned from a break point rearrangement discovered in parathyroid adenoma (Motokura et al., 1991). Cyclin D1 is the regulatory subunit of holoenzymes that phosphorylate the retinoblastoma protein (pRb), in combination with sequential phosphorylation by cyclin E/cyclin-dependent kinase 2 (CDK2), to inactivate the cell cycle inhibiting function of pRb. Following phosphorylation of pRb, the progression of the cell cycle from the G1 phase into DNA synthesis is allowed (Motokura and Arnold, 1993; SHERR, 2000). When cyclin D1 is overexpressed, an early onset of cancer and increased risk of tumor progression and metastasis has been observed (Zhang et al., 1993; Bartkova et al., 1994; Afar et al., 1995; Callanan et al., 1996; Gansauge et al., 1997; Barnes and Gillett, 1998; Amanatullah et al., 2001; DIEHL, 2002; STACEY, 2003; CHUNG, 2004). It is hypothesized that the overexpression of cyclin D1 occurs relatively early in the process of tumorigenesis (WEINSTEIN, 1996).
Cyclin D1 is a protein that is also essential in the processes of cell adhesion, motility, and guided migration demonstrated by primary bone marrow macrophages (Neumeister et al., 2003). The abundance of cyclin D1 has been shown to regulate the dynamics of cell adhesion, suggesting that cyclin D1 may contribute to cell growth by regulating cell substratum interactions, independent of its effects on cell cycle progression. Proteins such as RhoA, Rac1, and Cdc42 have been characterized for their effects on mediating changes in the cytoskeleton and cell adhesion to affect cell motility (HALL, 1998). Invasiveness and metastasis are events that require cell motility; therefore, changes in these cytoskeletal proteins would be hypothesized to be affected by changes in cyclin D1 expression. Activation of Rac1 promotes the formation of sheath-like protrusions, termed lamellipodia, to promote an increase in cell motility (Ridley et al., 1992; Nobes and Hall, 1995; Chen et al., 2004). In addition to the regulation of protrusive events, both Cdc42 and Rac1 promote the formation of smaller focal complexes. Activation of RhoA is recognized to mediate the formation of stress fibers and focal contacts that firmly anchor cells to their substrata, permitting retraction of cellular rear ends (Ridley and Hall, 1992; Hotchin and Hall, 1995). In addition to the process of cell motility requiring intracellular signaling, extracellular matrix degradation is required. Matrix metalloproteinases (MMP) are a group of proteinases that degrade and remodel the extracellular matrix (STAMENKOVIC, 2003), and have been shown to have multiple roles in different stages of tumor progression. Elevated levels of MMPs have been observed in many tumors, and these increases have a strong correlation with an invasive phenotype. MMP-2 expression has been associated with the invasive potential of cancer cell lines in vitro (Ura et al., 1989), and has been directly correlated with the aggressiveness of tumor cells (Birkedal-Hansen et al., 1993; Coussens and Werb, 1996). Its expression and activity is related to the invasiveness and metastatic potential of various cancer cells (Yu et al., 1996; Waas et al., 2002). In lung cancer, higher levels of MMP-2 have been shown to generate more invasive and metastatic tumors.
Many gene therapy strategies targeting cyclin D1 have been tested in vitro and in vivo, and have been shown to suppress tumor growth (Driscoll et al., 1997; Edward et al., 2000). In this study, we show efficient reduction of cell proliferation with the expression of a cyclin D1–targeted siRNA expressed from a plasmid. Transfection of cyclin D1–targeted siRNA significantly decreased in vitro invasion in a Boyden chamber assay, and in vivo, inhibited a xenograft model of A549 tumor growth and metastasis to lung and regional lymph nodes in nude mice.
Materials and Methods
Cell culture
The lung adenocarcinoma cell line, A549, was obtained from the Xinqiao Hospital Centre Lab. Cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Sigma, St. Louis, MO) at 37°C in 5% CO2 atmosphere. Cells were passaged regularly to maintain robust growth.
SiRNA expression plasmid
SiRNA constructs were synthesized by GenScript (Scotch Plains, NJ). The sequence of the cyclin D1–targeting siRNA and the scrambled control are (5′–3′) CCAGAGTGATCAAGTGTGA and GACTTCATAAGGCGCATGC, respectively. These sequences were chemically synthesized as part of a small, double-stranded 66-bp DNA insert containing the target sequence in the sense orientation, followed by a short-loop region, the target in the antisense orientation, and six thymidines at the 3′ end to provide a polymerase III transcription termination site. The synthesized insert was flanked by BamHI and HindIII restriction sites and cloned into the siRNA expression vector, Pgenesil-1, containing a RNA polymerase III promoter for the initiation of transcription of short hairpin RNA. The Pgenesil-1 plasmid contains the selectable marker neomycin to facilitate the selection of stably transfected cells.
Stable transfections
Twenty-four hours before transfection, human A549 cells were trypsinized, collected, and diluted with fresh medium without antibiotics. Transfections were performed with Lipofectamine™ 2000 (Invitrogen). Initial transfections to identify effective siRNAs were carried out in 24-well cell culture plates, while cell growth assays were carried out in 96-well cell culture microplates. Two micrograms of vector were used in all experiments with scrambled siRNA vector used as a control. Lipid-mediated transfection of cyclin D1 and scrambled siRNA plasmids was performed as described previously. After antibiotic selection (800 μg/mL G418; TIANGEN Biotechnology, Beijing, China), transfectants were pooled to avoid the effects of clonal selection and were expanded in 400 μg/mL G418.
Western blot analysis
A549 cells stably transfected with cyclin D1 siRNA, scrambled siRNA, or untreated cells were incubated in 10% FBS for the indicated time. Cells were harvested into RIPA buffer containing 150 mM NaCl, 50 mM Tris–HCl (pH 7.5), 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100, 5 mM EDTA, 1% aprotinin, and freshly added 2 mM phenylmethylsulfonyl fluoride (PMSF). After two freeze-thaw cycles and centrifugation at 13,000g for 5 minutes at 4°C, protein concentrations were quantified by using the Detergent-Compatible Protein Assay system (Bio-Rad) and absorbance was measured using a spectrophotometer at 595 nm. Twenty micrograms of total protein were resolved on 12% polyacrylamide gels and transferred on to nitrocellulose (NC) membranes (Roche, USA). Membranes were blocked for 1 hour in 5% dry milk in Tris-buffered saline (TBS-T: 100 mM NaCl, 50 mM Tris, and 0.05% Tween 20, pH 7.6). Membranes were incubated with mouse monoclonal antibodies to cyclin D1 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), MMP-2 (1:500; Santa Cruz Biotechnology), and β-actin (1:1000; Santa Cruz Biotechnology) in TPBS (0.05% Tween 20 in phosphate-buffered saline [PBS] [v/v]). Antibody binding was detected by incubation with horseradish peroxidase–conjugated swine-anti-mouse IgG (1:200; JINSHAN, China) and visualized using a Western DAB kit (JINSHAN, China). Bands were scanned and quantified using Chemilmager™ 5500. The densitometry readings of the bands were normalized to β-actin expression. Control lanes were normalized to 1.0.
Real-time PCR
A549 cells stably transfected with cyclin D1 siRNA, scrambled siRNA, or mock transfected were incubated in 10% FBS for the indicated time. Total RNA was prepared using Tripure reagent (Roche, USA). The quality of RNA collected was determined by electrophoresis through agarose gels and staining with ethidium bromide. RNA bands, 18S and 28S, were visualized using UV illumination. cDNA was generated in 20 μL reactions containing 1 μg total RNA using the Promega RT-PCR kit. Relative quantitative real-time PCR (qRT-PCR) analysis was performed in 20 μL reactions containing reverse-transcribed cDNA template, real-time PCR Master Mix, and 0.1 μM of forward and reverse primers (for cyclin D1: 40 cycles at 94°C for 30 seconds, 62.5°C for 30 seconds, and 72°C for 60 seconds; for MMP-2: 40 cycles at 94°C for 30 seconds, 54.2°C for 30 seconds, and 72°C for 60 seconds). The volume of cDNA template required to achieve equivalent levels of GAPDH (311-bp product) between samples was to amplify cyclin D1 cDNA (206-bp amplicon), MMP-2 (150-bp amplicon). Primers used include cyclin D1 (forward primer: 5′-CACCTAGCAAGCTGCCG AACC-3′; reverse primer: 5′-CGACAGACAAAGCGTCCCTC-3′), MMP-2 (forward primer: 5′-TGATGCCTTTGCTCGTGC-3′; reverse primer: 5′-TGGAGTCCGTCCTTACCGT-3′), and GAPDH (forward primer: 5′-CGGGAAACTGTGGCGTGAT-3′; reverse primer: 5′-CAAAGGTGGAGGAGTGGGT-3′). Resulting data were analyzed using 7500 system software.
Immunofluorescence analysis
Cells were grown on coverslips in 6-well plates. After treatment, cells were fixed in 3% paraformaldehyde for 30 minutes at room temperature, washed twice in PBS, and permeabilized with 0.5% Triton X-100 for 5 minutes at room temperature. After three washes in PBS, coverslips were incubated for 10 minutes in 1% fetal calf serum (FCS) in PBS at room temperature. Immunolabeling was performed as follows: coverslips were incubated with primary antibody (monoclonal antibody against RhoA/Rac1) for 1 hour at 37°C, washed twice in 1% FCS in PBS, and incubated with fluorochrome-conjugated secondary antibody (CY3 antibody against mouse Ig). Excess reagents were washed off with PBS-Tween, and cells were mounted and counterstained in 4′,6-diamidino-2-phenylindole–containing mounting solution and visualized by fluorescence microscopy imaged with an Olympus camera.
Cell proliferation assay
Equal numbers of stable transfectants were seeded on to 12-well plates at a density of 7500 cells/mL. Medium was changed after 3, 5, 7 days, and cells were counted using a standard hemocytometer. Each experiment was carried out in triplicate, and results were expressed as mean ± SE for each determination and then plotted on a log scale versus time.
Flow cytometric analysis
Cell cycle analysis was performed by flow cytometry (BD Biosciences). Cells were trypsinized, washed with ice-cold PBS, and fixed with ice-cold 70% ethanol at −20°C overnight. Cells were then washed twice with PBS, incubated with 20 μg/mL RNase A and 200 μg/mL propidium iodide in PBS at room temperature for 30 minutes in the dark. Flow cytometry was performed using a FACSCalibur flow cytometer. Data were analyzed using ModFit LT (Verity Software House, Inc., Topsham, ME).
Invasion assay
Standard Boyden chamber invasion assays were performed as described previously (Sun et al., 2001). Transwell Boyden chamber plates (6.4-mm diameter; Costar) with a 8-μm polycarbonate membrane pore size were seeded with stably transfected cells (6 × 105 cells per membrane) in medium containing 5% FCS. Cells were incubated for 3 hours at 37°C, at which time cells were fixed and stained using hematoxylin/eosin (H&E) stain. The upper layer of the membrane was scraped free of cells so that cells that had migrated to the lower surface of the membrane could be quantified. Four high-power fields per membrane were counted and experiments were performed in triplicate for three independently transfected groups of A549 cells.
Subcutaneous tumor growth model
A549 cells form rapidly growing primary tumors. Three sets of nude mice with six animals per group received injections of untransfected A549 cells, scrambled siRNA transfected A549 cells, and cyclin D1 siRNA transfected cells. Tumor volume was calculated as v = L × l2 × 0.52 every 10 days. Sixty days after the subcutaneous (s.c.) inoculation of tumor cells, when the primary tumor reached its maximum volume, the animals were killed. Tumor samples were collected and fixed in 10% neutral buffered formalin. Animal care was provided at the Xinqiao Lab Centre in Chongqing, China, according to the institutional guidelines.
Abdominal cavity tumor xenografts metastasis model
A549 cells formed lung and lymph node metastases after receiving abdominal cavity inoculation of cells. Three sets of nude mice with four animals per group received injections of untransfected A549 cells, scrambled siRNA transfected A549 cells, and cyclin D1 siRNA transfected cells. Sixty days after the s.c. inoculation of tumor cells, the animals were killed. Lymph node and lung samples were collected and fixed in 10% neutral buffered formalin.
Immunohistochemistry
Sections (5 μm) of tumor and lung and lymph node samples were prepared as described previously (Su et al., 2004). Before staining, the sections were deparaffinized in xylene, rehydrated, and subjected to pronase treatment (5-minute incubation at 37°C in 100 μg/mL pronase in 0.1% PBS. Sections were incubated overnight at 4°C with monoclonal mouse antibodies to cyclin D1, MMP-2, RhoA, and Rac1 (IgG) diluted 1:100 or 1:200 (Santa Cruz), followed by incubation with a biotin-conjugated goat anti-mouse secondary antibody (JINQIAO, China). Antibody binding was detected using streptavidin-conjugated horseradish peroxidase and staining with a DAB kit (Santa Cruz). Sections were also counterstained with haematine. As additional controls, the primary antibodies were neutralized by preabsorption with the blocking peptide available (Santa Cruz), or the blots were probed with irrelevant mouse or goat IgG monoclonal antibodies. Secondary antibodies were also tested for nonspecific binding. Cells were examined by two independent observers under a Leica microscope with a magnification of 200× or 400×.
Statistical analyses
Statistical comparisons were made of incidence of lung and lymph node metastasis using a χ2-test, tumor volume using the t-test, mRNA and protein expression using Student's t-test, and cell proliferation using Student's t-test.
Results
Cyclin D1–targeted siRNA reduces cyclin D1 mRNA and protein levels
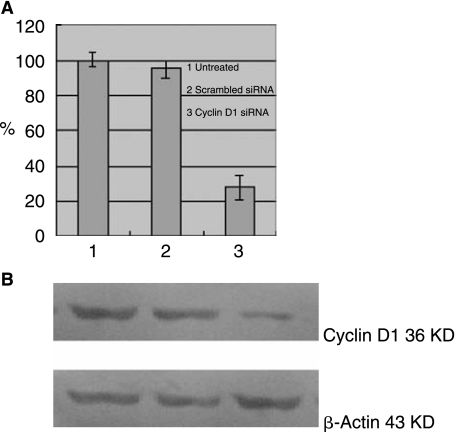
Expression of cyclin D1 has been previously characterized in the A549 lung cancer cell line (Khan et al., 2007). In this study, plasmids expressing cyclin D1–targeted siRNA or a scrambled control siRNA were introduced into A549 cells and stable transfectants were selected. Transfectants were pooled to avoid clonal selection effects and were expanded. Analysis of cyclin D1 mRNA levels in cells transfected with cyclin D1–targeted siRNA showed reduced steady-state levels up to 72% compared with untreated A549 cells, or to cells transfected with the scrambled siRNA control (P = 0.000 < 0.01) (Fig. 1A). To determine whether the reduction in the levels of cyclin D1 mRNA was accompanied by a decrease in the levels of cyclin D1 protein, Western blotting of cell extracts was performed. Cyclin D1 protein levels were lower 65% in cells transfected with cyclin D1–targeted siRNA (P = 0.000 < 0.01), whereas transfection of a scrambled siRNA control did not affect cyclin D1 protein levels (Fig. 1B).
FIG. 1.
Cyclin D1 siRNA inhibits cyclin D1 mRNA and protein expression. A549 cells were stably transfected with cyclin D1 siRNA, scrambled siRNA, or untreated cells. (A) Relative expression levels of cyclin D1 mRNA were quantified by qRT-PCR. GAPDH mRNA was presented as controls for internal mRNA loading. To compare the relative expression levels of cyclin D1 of three different cells, values were normalized to that of untreated cells. Each experiment was performed in triplicate. Columns, mean of three different experiments; bars, SD. (B) Western blot analysis of cyclin D1 protein expression relative levels in cell lysates from three different cells. Protein from these samples was mixed with loading buffer and run on 12% SDS–PAGE. Densitometric quantification of cyclin D1 was performed. β-Actin protein was presented as controls for internal protein loading. Values were normalized to that of untreated cells.
Decreased intracellular MMP-2, RhoA, and Rac1 expression
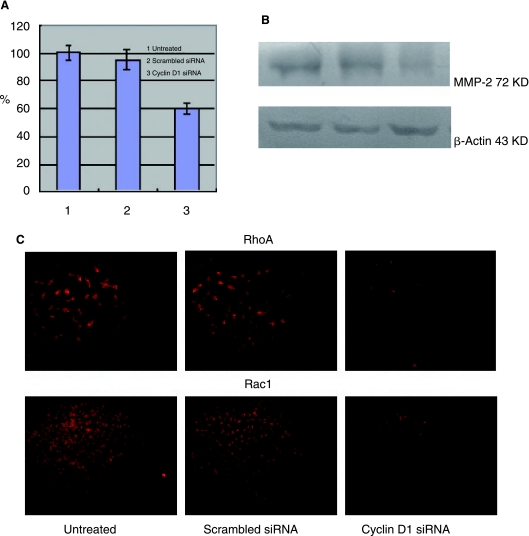
To evaluate whether targeting of cyclin D1 mRNA by siRNA affects the intracellular level of MMP-2, RhoA, and Rac1 proteins, mRNA and protein levels for each of these proteins were detected. Using qRT-PCR, A549 cells transfected with cyclin D1–targeted siRNA were found to have a substantial decrease in MMP-2 mRNA (P = 0.002 < 0.01) (Fig. 2A). Western blotting of transfected cell extracts also showed a decrease in MMP-2 protein (P = 0.000 < 0.01) (Fig. 2B). Indirect immunofluorescence studies were used to compare the expression of RhoA and Rac1 proteins (Fig. 2C) and both showed decreased expression in A549 cells transfected with cyclin D1–targeted siRNA.
FIG. 2.
Transfection with cyclin D1 siRNA decreases intracellular MMP-2, RhoA, and Rac1 expression. A549 cells were stably transfected with cyclin D1 siRNA, scrambled siRNA, or untreated cells. (A) Relative expression levels of MMP-2 mRNA were quantified by qRT-PCR. GAPDH mRNA was presented as controls for internal mRNA loading. To compare the relative expression levels of MMP-2 of three different cells, values were normalized to that of untreated cells. Each experiment was performed in triplicate. Columns, mean of three different experiments; bars, SD. (B) Western blot analysis of MMP-2 protein expression relative levels in cell lysates from three different cells. β-Actin protein was presented as controls for internal protein loading. Values were normalized to that of untreated cells. (C) RhoA and Rac1 expressions were detected by indirect immunofluorescence. Fluorescence emission images were obtained using a Leica fluorescence microscope system.
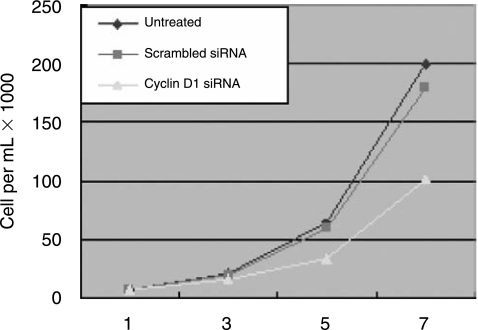
Reduced growth rate of cyclin D1 siRNA transfected cells
To determine the in vitro growth rates of cyclin D1 down-regulated cells, cell proliferation assay analysis was performed at 1, 3, 5, and 7 day time points (Fig. 3). Compared to untreated A549 cells, cells transfected with the scrambled siRNA control did not affect cellular proliferation; however, cells transfected with cyclin D1–targeted siRNA exhibited a significant decrease in cellular proliferation (P < 0.05). These results suggest that cyclin D1 expression is important for the proliferation of A549 cells.
FIG. 3.
Reduced growth rate of cyclin D1 siRNA transfected cells. Cell members were determined after 3, 5, 7 days as described in Materials and Methods. Significant inhibition of cell proliferation was observed in the graph. Points, means of three replicate determinations for each treatment group; bars, SE.
Cyclin D1 siRNA causes cell cycle arrest
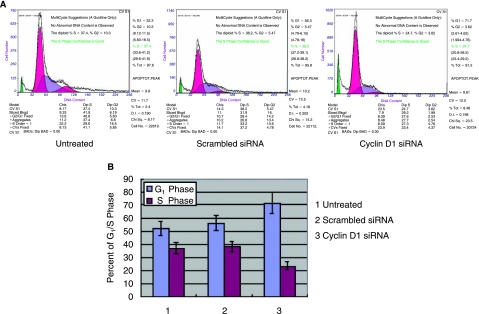
To identify which phase of the cell cycle is associated with the reduced growth rate for A549 cells transfected with cyclin D1–targeted siRNA, cell cycle analysis was performed using flow cytometry (Fig. 4A). For cells stably transfected with cyclin D1 siRNA, an increased percentage of cells were detected in the G0/G1 phase and a decreased percentage of cells were detected in the S phase compared with the cell cycle distribution profiles of untreated and control cells (P < 0.05) (Fig. 4B). These data indicate that cyclin D1–targeted siRNA affects the progression of A549 cells from the G0/G1 phase to the S phase of the cell cycle.
FIG. 4.
Cyclin D1 siRNA causes G1/S phase arrest in A549 cells. (A) Cell cycle analysis was performed by flow cytometry as described in Materials and Methods. (B) Columns, average values from three separate experiments; bars, SE.
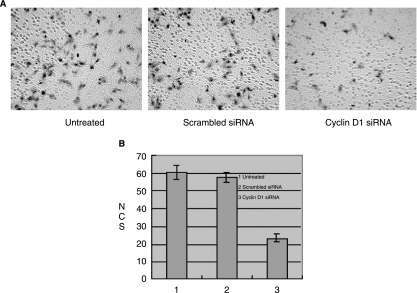
Inhibition of the invasive capacity of A549 cells in vitro
The invasive potential of A549 cells transfected with cyclin D1–targeted siRNA was determined using a Matrigel invasion assay. Figure 5A shows the staining of A549 cells that migrated through the Matrigel assay. Cells treated with cyclin D1–targeted siRNA showed decreased migration through the Matrigel compared with the untransfected and control siRNA transfected cells (P = 0.000 < 0.01). Quantitative analysis of the invasion assay data show that tumor cell invasion decreased 62% when A549 cells were treated with cyclin D1–targeted siRNA (Fig. 5B).
FIG. 5.
Cyclin D1 siRNA infection could remarkably inhibit the invasive capacity of A549 cells in vitro. Invasion assays were carried out in 24-well Transwell units (6 × 105 cells per treatment condition in triplicate). After a 24-hour incubation period, the cells that had passed through the filter into the bottom wells were fixed and photographed (A). The number of invasions was quantified as described in Materials and Methods (B). Columns, mean of three different experiments; bars, SE.
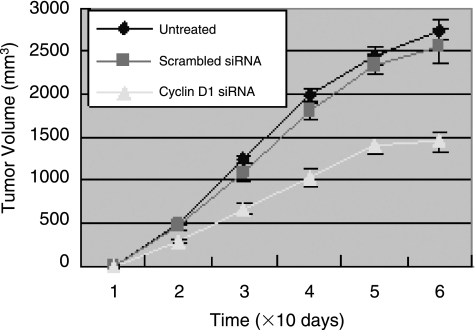
Decreased tumor growth and metastasis
To determine whether cyclin D1 plays a causal role in tumor growth and metastasis, three groups of nude mice were injected with A549 cells transfected with cyclin D1 siRNA, scrambled siRNA, or mock transfected cells. Injections were made subcutaneously into the right flank and measurements of primary tumor volume were recorded every 10 days. As shown in Table 1, the three different treatment groups formed primary tumors at different times, with the mice receiving cyclin D1 siRNA transfected A549 cells having the most delayed tumor growth (P < 0.05). Tumor growth rate was also significantly slower for the mice injected with A549 cells transfected with cyclin D1–targeted siRNA (P < 0.05) (Fig. 6). These results suggest that high expression levels of cyclin D1 are required for primary tumor formation by A549 cells.
Table 1.
The Time in Which Three Groups Formed Primary Tumors (Days, x ± s, n = 6)
| Group | Untreated | Scrambled siRNA | Cyclin D1 siRNA |
|---|---|---|---|
| Days | 7.3 ± 0.9 | 7.7 ± 0.7 | 12.1 ± 0.8 |
|
Tumor volume (mm, x ± s, n = 6) | |||
|---|---|---|---|
| Time (day) | Untreated | Scrambled siRNA | Cyclin D1 siRNA |
| 10 | 5 ± 1 | 5 ± 1 | 0 |
| 20 | 511 ± 44 | 469 ± 38 | 298 ± 20 |
| 30 | 1238 ± 162 | 1096 ± 105 | 671 ± 63 |
| 40 | 1983 ± 179 | 1797 ± 92 | 1026 ± 110 |
| 50 | 2439 ± 185 | 2343 ± 120 | 1395 ± 1048 |
| 60 | 2745 ± 216 | 2551 ± 198 | 1451 ± 117 |
|
The rates of three groups formed metastatic nodules | |||
|---|---|---|---|
| Group | Untreated | Scrambled siRNA | Cyclin D1 siRNA |
| Lung | 4/4 | 4/4 | 0/4 |
| Lymph node | 4/4 | 3/4 | 0/4 |
FIG. 6.
Significant reduction of experimental tumor growth and metastasis after treatment with cyclin D1 siRNA. Growth curves of mice carrying s.c. xenografted tumors derived from A549 cells. Points, mean of measurements for primary tumors; bars, SE.
To determine whether the loss of cyclin D1 expression affected the ability of A549 cells to metastasize lung and lymph node samples were collected at the end point of the study when primary tumor volume had reached its maximum. Although untreated cells and control siRNA transfected A549 cells formed many microscopically visible metastases in lung and lymph node, lymph node samples from mice injected with cyclin D1 siRNA transfected A549 cells were found to have no metastases. 4/4 and 3/4 nude mice having metastatic lung and lymph node were obtained for mice injected with untreated or control cells, whereas 0/4 nude mice having metastatic lung and lymph node were obtained for mice injected with A549 transfected with cyclin D1–targeted siRNA (P = 0.01 < 0.05). On the basis of these data, summarized in Table 1, we conclude that the decreased expression of cyclin D1 leads to reduced primary tumor growth and metastasis to lung and lymph node tissue.
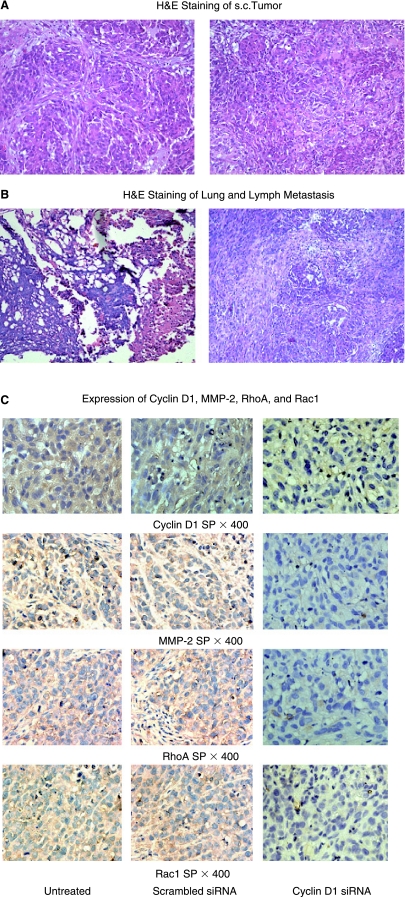
Expression of cyclin D1, MMP-2, RhoA, and Rac1 was diminished in tumor samples
Primary tumor samples collected from each of the three treatment groups were analyzed for the expression of cyclin D1, MMP-2, RhoA, and Rac1. Initially, H&E staining of both primary tumor and lung and lymph node tissue samples was performed to confirm neoplastic growth and metastasis (Fig. 7A and 7B). Cyclin D1 expression was analyzed in the primary tumor tissue collected for each treatment group (Fig. 7C). Sections of tissue collected from mice that received untreated cells or scrambled siRNA treated cells showed high levels of cyclin D1 expression. In contrast, decreased expression of cyclin D1 was detected in tumor samples from mice injected with cyclin D1–targeted siRNA cells. To determine whether the inhibition of cyclin D1 affected other protein expression profiles in vivo, primary tumor tissue sections were also stained for the expression of MMP-2, RhoA, and Rac1 (Fig. 7C). In comparison with control sections, a significant decrease in staining for MMP-2, RhoA, and Rac1 was observed for tissue samples from mice receiving the cyclin D1 siRNA transfected A549 cells.
FIG. 7.
(A) H&E staining of s.c. tumor showing neoplastic growth. Magnification, ×200. (B) H&E staining of s.c. tumor showing lung and lymph metastasis. Magnification, ×200. (C) Cyclin D1, MMP-2, RhoA, and Rac1(×400) visualized with their antibodies are significantly inhibited in tumors from mice injected with cyclin D1 siRNA stable transfected A549 cells compared with untreated and scramble siRNA controls.
Discussion
Cyclin D1 is critical for the G1–S transition of the cell cycle, and in recent years, much attention has been focused on identifying the mechanistic details of cyclin D1's function. However, cyclin D1 has also been shown to have roles in cellular metabolism, cell differentiation, transcription, cell migration, and metastasis. Overexpression of cyclin D1 mRNA and protein have been found in many solid tumors (Sun et al., 2001; Dworakowska et al., 2005; Ishii et al., 2006), including non–small cell lung cancer (NSCLC). Increased expression of cyclin D1 correlates with the early onset of cancer and an increased risk of tumor progression and metastasis that negatively affect patient survival (Capaccio et al., 2000; Guo et al., 2003; Langsenlehner et al., 2005; Yu and Weinberger, 2005), especially for patients with lung cancer (Ratschiller et al., 2003; Dobashi et al., 2004; DWORAKOWSKA, 2005).
We analyzed the efficiency of expressing siRNA sequences targeted to cyclin D1 in reducing the invasive capacity, tumor growth, and metastasis of A549 cells in vitro and in vivo. In vitro studies demonstrated that the downregulation of cyclin D1 reduced not only proliferation rates but also cell migration and cell invasion. Subcutaneous injection of A549 cells with downregulated cyclin D1 expression significantly reduced tumor formation, delayed tumor growth, and inhibited the development of metastases to the lung and regional lymph nodes. In combination, these data suggest that the overexpression of cyclin D1 in A549 cells plays an important role in the ability of A549 cells to proliferate. It is difficult to determine whether the downregulation of cyclin D1 expression significantly inhibited the metastatic potential of the transfected A549 cells, or whether metastasis was reduced due to decreased tumorigenicity and tumor burden (delayed growth). However, considering the in vitro data for the transfected A549 cells that showed reduced migration and invasive capacity, we hypothesize that the mechanisms of metastasis were directly affected by the downregulation of cyclin D1 expression.
We originally hypothesized that the suppression of cyclin D1 would decrease the expression of metastasis-promoting proteins such as RhoA, Rac1, and MMP-2. The results from this study indicate that the Rho family GTPases, RhoA and Rac1, as well as the metalloproteinase, MMP-2, are required for cyclin D1-mediated invasion and metastasis of human lung adenocarcinoma in our A549 model. Stable expression of cyclin D1–targeted siRNA decreased protein expression of RhoA, Rac1, and MMP-2 in A549 cells both in vitro and in vivo. On the basis of these data, as well as previous work describing the ability of cyclin D1 to regulate transcription and enhance transcription independent of CDK-binding activity, we hypothesize that the suppression of cyclin D1, and the associated decrease in the expression of RhoA, Rac1, and MMP-2, may be the result of transcriptional events. Additional experiments would be required to characterize these proposed mechanism(s); however, cyclin D1 has been shown to regulate and enhance the transcription of estrogen response element (ERE)-responsive genes independent of CDK-binding activity. Cyclin D1 upregulates the transcriptional activity of ERα through increased binding of both liganded and unliganded receptor to ERE sequences and increases the association of ERα with P/CAF (Zwijsen et al., 1998; McMahon et al., 1999). P/CAF in turn potentiates cyclin D1 and ERα activity, and this effect is largely dependent on the acetyltransferse activity of P/CAF. Growing evidence suggests that cyclin D1 physically associates with transcriptional factors or coactivators including histone acetyltransferases (HATs) and histone deacetylases (HDACs) to regulate transcription and mediate epigenetic changes (Zwijsen et al., 1998; Chan et al., 2001; Lin et al., 2002).
In vitro studies have also shown that cyclin D1 can enhance oncogenic transformation. For example, many studies have shown that the expression of cyclin D1 can induce mammary tumors (Hinds et al., 1994; Lovec et al., 1994; Wang et al., 1994; Bortner and Rosenberg, 1997; Robles et al., 1998). In vivo studies using the Eu-cyclin D1–induced lymphomagenesis model showed an enhanced rate of onset and progression when mated with an Eu-Myc transgenic mouse (Bodrug et al, 1994). Conversely, cyclin D1–/– mice were resistant to mammary tumorigenesis induced by either ErbB2 or Ras (Yu et al., 2001), consistent with earlier studies using cyclin D1 antisense to decrease the expression of cyclin D1 (Lee et al., 2000). Data from the studies of cyclin D1 knockout mice also indicate that the role of cyclin D1 may be tissue and oncogene specific. For example, cyclin D1–deficient mice showed enhanced mammary tumorigenesis in response to activation of the B-catenin signaling pathway (Hatsell et al., 2003), yet were resistant to gastrointestinal tumor induction by mutation of the ApcMin gene (Hulit et al., 2004).
Previous studies have shown that cyclin D1 is differentially expressed in tumor tissues, and its expression can be directly correlated with tumor stage, lymph node metastasis, and patient survival. Our data are consistent with these studies and identify a role for cyclin D1 in the invasion and metastasis of lung adenocarcinoma cells, in combination with RhoA, Rac1, and MMP-2. These findings provide additional insight into the mechanisms by which lung adenocarcinoma proliferates and metastasizes, and suggests potential gene therapy targets.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 30772144) and the National Natural Science Foundation of ChongQing (No. c51c-2005BB 52687).
References
- AFAR D.E. MCLAUGHLIN J. SHERR C.J. WITTE O.N. ROUSSEL M.F. Signaling by ABL oncogenes through cyclin D1. Proc. Natl. Acad. Sci. USA. 1995;92:9540–9544. doi: 10.1073/pnas.92.21.9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMANATULLAH D.F. ZAFONTE B.T. ALBANESE C. FU M. MESSIERS C. HASSELL J. PESTELL R.G. Ras regulation of cyclin D1 promoter. Methods Enzymol. 2001;333:116–127. doi: 10.1016/s0076-6879(01)33050-1. [DOI] [PubMed] [Google Scholar]
- BARNES D.M. GILLETT C.E. Cyclin D1 in breast cancer. Breast Cancer Res. Treat. 1998;52:1–15. doi: 10.1023/a:1006103831990. [DOI] [PubMed] [Google Scholar]
- BARTKOVA J. LUKAS J. MULLER H. LUTZHOFT D. STRAUSS M. BARTEK J. Cyclin D1 protein expression and function in human breast cancer. Int. J. Cancer. 1994;57:353–361. doi: 10.1002/ijc.2910570311. [DOI] [PubMed] [Google Scholar]
- BIRKEDAL-HANSEN H. MOORE W.G. BODDEN M.K. WINDSOR L.J. BIRKEDAL-HANSEN B. DECARLO A. ENGLER J.A. Matrix metalloproteinases: a review. Crit. Rev. Oral. Biol. Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- BODRUG S.E. WARNER B.J. BATH M.L. LINDEMAN G.J. HARRIS A.W. ADAMS J.M. Cyclin D1 transgene impedes lymphocyte maturation and collaborates in lymphomagenesis with the myc gene. EMBO J. 1994;13:2124–2130. doi: 10.1002/j.1460-2075.1994.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORTNER D.M. ROSENBERG M.P. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol. Cell. Biol. 1997;17:453–459. doi: 10.1128/mcb.17.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLANAN M. LEROUX D. MAGAUD J.P. RIMOKH R. Implication of cyclin D1 in malignant lymphoma. Crit. Rev. Oncog. 1996;7:191–203. doi: 10.1615/critrevoncog.v7.i3-4.30. [DOI] [PubMed] [Google Scholar]
- CAPACCIO P. PRUNERI G. CARBONI N. PAGLIARI A.V. QUATELA M. CESANA B.M. PIGNATARO L. Cyclin D1 expression is predictive of occult metastases in head and neck cancer patients with clinically negative cervical lymph nodes. Head Neck. 2000;22:234–240. doi: 10.1002/(sici)1097-0347(200005)22:3<234::aid-hed5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- CHAN H.M. KRSTIC-DEMONACOS M. SMITH L. DEMONACOS C. LA THANGUE N.B. Acetylation control of the retinoblastoma tumour-suppressor. Mol. Biol. Cell. 2001;14:2005–2015. doi: 10.1038/35083062. [DOI] [PubMed] [Google Scholar]
- CHEN H.Y. SHEN C.H. TSAI Y.T. LIN F.C. HUANG Y.P. CHEN R.H. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol. Cell. Biol. 2004;24:10558–10572. doi: 10.1128/MCB.24.24.10558-10572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG D.C. Cyclin D1 in human neuroendocrine: tumorigenesis. Ann. NY Acad. Sci. 2004;1014:209–217. doi: 10.1196/annals.1294.022. [DOI] [PubMed] [Google Scholar]
- COUSSENS L.M. WERB Z. Matrix metalloproteinases and the development of cancer. Chem. Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- DIEHL J.A. Cycling to cancer with cyclin D1. Cancer Biol. Ther. 2002;1:226–231. doi: 10.4161/cbt.72. [DOI] [PubMed] [Google Scholar]
- DOBASHI Y. GOTO A. FUKAYAMA M. ABE A. OOI A. Overexpression of cdk4/cyclin D1, a possible mediator of apoptosis and an indicator of prognosis in human primary lung carcinoma. Int. J. Cancer. 2004;110:532–541. doi: 10.1002/ijc.20167. [DOI] [PubMed] [Google Scholar]
- DRISCOLL B. WU L. BUCKLEY S. HALL F.L. ANDERSON K.D. WARBURTON D. Cyclin D1 antisense RNA destabilizes pRb and retards lung cancer cell growth. Am. J. Physiol. 1997;273:L941–L949. doi: 10.1152/ajplung.1997.273.5.L941. [DOI] [PubMed] [Google Scholar]
- DWORAKOWSKA D. Clinical significance of cyclin D1 expression in non-small cell lung cancer. Pneumonol. Alergol. Pol. 2005;73:297–330. [PubMed] [Google Scholar]
- DWORAKOWSKA D. JASSEM E. JASSEM J. BOLTZE C. WIEDORN K.H. DWORAKOWSKI R. SKOKOWSKI J. JASKIEWICZ K. CZESTOCHOWSKA E. Prognostic value of cyclin D1 over-expression in correlation with pRb and p53 status in non-small cell lung cancer (NSCLC) J. Cancer Res. Clin. Oncol. 2005;131:479–485. doi: 10.1007/s00432-004-0661-9. [DOI] [PubMed] [Google Scholar]
- EDWARD R.S. MEENHARD H. LIU S.C. Prolonged response to antisense Cyclin D1 in a human squamous cancer xenograft model. Clin. Cancer Res. 2000;6:654–660. [PubMed] [Google Scholar]
- GANSAUGE S. GANSAUGE F. RAMADANI M. STOBBE H. RAU B. HARADA N. BEGER H.G. Overexpression of cyclin D1 in human pancreatic carcinoma is associated with poor prognosis. Cancer Res. 1997;57:1634–1637. [PubMed] [Google Scholar]
- GUO S.S. WU X. SHIMOIDE A.T. WONG J. MOATAMED F. SAWICKI M.P. Frequent overexpression of cyclin D1 in sporadic pancreatic endocrine tumours. J. Endocrinol. 2003;179:73–79. doi: 10.1677/joe.0.1790073. [DOI] [PubMed] [Google Scholar]
- HALL A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- HATSELL S. ROWLANDS T. HIREMATH M. COWIN P. β-Catenin and Tcfs in mammary development and cancer. J. Mammary Gland Biol. Neoplasia. 2003;8:145–158. doi: 10.1023/a:1025944723047. [DOI] [PubMed] [Google Scholar]
- HINDS P.W. DOWDY S.F. EATON E.N. ARNOLD A. WEINBERG R.A. Function of a human cyclin gene as an oncogene. Proc. Natl. Acad. Sci. USA. 1994;91:709–713. doi: 10.1073/pnas.91.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTCHIN N.A. HALL A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J. Cell. Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HULIT J. WANG C. ALABANESE C. DI VIZIO D. MAHMOOD R. AUGENLICHT L.H. RUSSELL R. PESTELL R.G. Cyclin D1 genetic heterozygosity regulates colonic epithelial cell differentiation and tumor number in ApcMin mice. Mol. Cell. Biol. 2004;24:7598–7611. doi: 10.1128/MCB.24.17.7598-7611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII Y. PIRKMAIER A. ALVAREZ J.V. FRANK D.A. KESELMAN I. LOGOTHETIS D. MANDELI J. O'CONNELL M.J. WAXMAN S. GERMAIN D. Cyclin D1 over-expression and response to bortezomib treatment in a breast cancer model. J. Natl. Cancer Inst. 2006;98:1238–1247. doi: 10.1093/jnci/djj334. [DOI] [PubMed] [Google Scholar]
- KHAN N. HADI N. AFAQ F. SYED D.N. KWEON M. MUKHTAR H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28:163–173. doi: 10.1093/carcin/bgl145. [DOI] [PubMed] [Google Scholar]
- LANGSENLEHNER U. HOFMANN G. SAMONIGG H. KRIPPL P. RENNER W. CLAR H. Cyclin D1 genotype and breast cancer metastasis. Cancer Epidemiol. Biomarkers Prev. 2005;14:1844–1845. doi: 10.1158/1055-9965.EPI-05-0204. [DOI] [PubMed] [Google Scholar]
- LEE R.J. ALBANESE C. FU M. D'AMICO M. LIN B. WATANABE G. HAINES G.K., III SIEGEL P.M. HUNG M.C. YARDEN Y. HOROWITZ J.M. MULLER W.J. PESTELL R.G. Cyclin D1 is required for transformation by activated Neu and is induced through an E2F-dependent signaling pathway. Mol. Cell. Biol. 2000;20:672–683. doi: 10.1128/mcb.20.2.672-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN H.M. ZHAO L. CHENG SY. Cyclin D1 is a ligand-independent co-repressor for thyroid hormone receptors. J. Biol. Chem. 2002;277:28733–28741. doi: 10.1074/jbc.M203380200. [DOI] [PubMed] [Google Scholar]
- LOVEC H. SEWING A. LUCIBELLO F.C. MULLER R. MOROY T. Oncogenic activity of cyclin D1 revealed through cooperation with Ha-ras: link between cell cycle control and malignant transformation. Oncogene. 1994;9:323–326. [PubMed] [Google Scholar]
- MCMAHON C. SUTHIPHONGCHAI T. DIRENZO J. EWEN M.E. P/CAF associates with cyclin D1 and potentiates its activation of the estrogen receptor. Proc. Natl. Acad. Sci. USA. 1999;96:5382–5387. doi: 10.1073/pnas.96.10.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTOKURA T. ARNOLD A. PRAD1/cyclin D1 proto-oncogene: genomic organization, 5′ DNA sequence, and sequence of a tumor-specific rearrangement breakpoint. Genes Chromosomes Cancer. 1993;7:89–95. doi: 10.1002/gcc.2870070205. [DOI] [PubMed] [Google Scholar]
- MOTOKURA T. BLOOM T. KIM H.G. JUPPNER H. RUDERMAN J.V. KRONEBERG H.M. ARNOLD A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991;350:512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- NEUMEISTER P. PIXLEY F.J. XIONG Y. XIE H. WU K. ASHTON A. CAMMER M. CHAN A. SYMONS M. STANLEY E.R. PESTELL R.G. Cyclin d1 governs adhesion and motility of macrophages. Mol. Biol. Cell. 2003;14:2005–2015. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOBES C.D. HALL A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- RATSCHILLER D. HEIGHWAY J. GUGGER M. KAPPELER A. PIRNIA F. SCHMID R.A. BORNER M.M. BETTICHER D.C. Cyclin D1 overexpression in bronchial epithelia of patients with lung cancer is associated with smoking and predicts survival. J. Clin. Oncol. 2003;21:2085–2093. doi: 10.1200/JCO.2003.03.103. [DOI] [PubMed] [Google Scholar]
- RIDLEY A.J. HALL A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;7:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- RIDLEY A.J. PATERSON H.F. JOHNSTON C.L. DIEKMANN D. HALL A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- ROBLES A.I. RODRIGUEZ-PUEBLA M.L. GLICK A.B. TREMPUS C. HANSEN L. SICINSKI P. TENNANT R.W. WEINBERG R.A. YUSPA S.H. CONTI C.J. Reduced skin tumor development in cyclin D1-deficient mice highlights the oncogenic ras pathway in vivo. Genes Dev. 1998;12:2469–2474. doi: 10.1101/gad.12.16.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERR C.J. The Pezcoller Lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- STACEY D.W. Cyclin D1 serves as a cell cycle regulatory switch in actively proliferating cells. Curr. Opin. Cell. Biol. 2003;15:158–163. doi: 10.1016/s0955-0674(03)00008-5. [DOI] [PubMed] [Google Scholar]
- STAMENKOVIC I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J. Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- SU J.L. SHIH J.Y. YEN M.L. JENG Y.M. CHANG C.C. HSIEH C.Y. WEI L.H. YANG P.C. KUO M.L. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 2004;64:554–564. doi: 10.1158/0008-5472.can-03-1301. [DOI] [PubMed] [Google Scholar]
- SUN S. ZIMMET J.M. TOSELLI P. THOMPSON A. JACKSON C.W. RAVID K. Overexpression of cyclin D1 moderately increases ploidy in megakaryocytes. Haematologica. 2001;86:17–23. [PubMed] [Google Scholar]
- URA H. BONFIL R.D. REICH R. REDDEL R. PFEIFER A. HARRIS C.C. KLEIN-SZANTO A.J. Expression of type IV collagenase and procollagen genes and its correlation with the tumorigenic, invasive, and metastatic abilities of oncogene-transformed human bronchial epithelial cells. Cancer Res. 1989;49:4615–4621. [PubMed] [Google Scholar]
- WAAS E.T. LOMME R.M. DEGROOT J. WOBBES T. HENDRIKS T. Tissue levels of active matrix metalloproteinase-2 and −9 in colorectal cancer. Br. J. Cancer. 2002;86:1876–1883. doi: 10.1038/sj.bjc.6600366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG T.C. CARDIFF R.D. ZUKERBERG L. LEES E. ARNOLD A. SCHMIDT E.V. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature. 1994;369:669–671. doi: 10.1038/369669a0. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN I.B. Relevance of cyclin D1 and other molecular markers to cancer chemoprevention. J. Cell. Biochem. Suppl. 1996;25:23–28. [PubMed] [Google Scholar]
- YU A.E. HEWITT R.E. KLEINER D.E. STETLER-STEVENSON W.G. Molecular regulation of cellular invasion—role of gelatinase A and TIMP-2. Biochem. Cell. Biol. 1996;74:823–831. doi: 10.1139/o96-088. [DOI] [PubMed] [Google Scholar]
- YU Q. GENG Y. SICINSKI P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- YU Z. WEINBERGER P.M. Cyclin D1 is a valuable prognostic marker in oropharyngeal squamous cell carcinoma. Clin. Cancer Res. 2005;11:1160–1166. [PubMed] [Google Scholar]
- ZHANG Y.J. JIANG W. CHEN C.J. LEE C.S. KAHN S.M. SANTELLA R.M. WEINSTEIN I.B. Amplification and overexpression of cyclin D1 in human hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 1993;196:1010–1016. doi: 10.1006/bbrc.1993.2350. [DOI] [PubMed] [Google Scholar]
- ZWIJSEN R.M. BUCKLE R.S. HIJMANS E.M. LOOMANS C.J. BERNARDS R. Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev. 1998;12:3488–3498. doi: 10.1101/gad.12.22.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]