Abstract
Background
Repeated stress or administration of corticotropin-releasing factor (CRF) prior to ethanol exposure sensitizes anxiety-like behavior in adult rats. Current experiments determined whether adolescent rats were more sensitive to these challenges in sensitizing ethanol withdrawal-induced anxiety and altering CRF levels in brain during withdrawal.
Methods
Male adult and adolescent Sprague–Dawley rats were restraint stressed (1 hour) twice 1 week apart prior to a single 5-day cycle of ethanol diet (ED; stress/withdrawal paradigm). Other rats received control diet (CD) and three 1-hour restraint stress sessions. Rats were then tested 5, 24, or 48 hours after the final withdrawal for anxiety-like behavior in the social interaction (SI) test. In other experiments, adolescent rats were given two microinjections of CRF icv 1 week apart followed by 5-days of either CD or ED and tested in social interaction 5 hours into withdrawal. Finally, CRF immunoreactivity was measured in the central nucleus of the amygdala (CeA) and paraventricular nucleus (PVN) after rats experienced control diet, repeated ethanol withdrawals, or stress/withdrawal.
Results
Rats of both ages had reduced SI following the stress/withdrawal paradigm, and this effect recovered within 24 hours. Higher CRF doses were required to reduce SI in adolescents than previously reported in adults. CRF immunohistochemical levels were higher in the PVN and CeA of CD-exposed adolescents. In adolescent rats, repeated ethanol withdrawals decreased CRF in the CeA but was not associated with decreased CRF cell number. There was no change in CRF from adult treatments.
Conclusions
In the production of anxiety-like behavior, adolescent rats have equal sensitivity with stress and lower sensitivity with CRF compared to adults. Further, adolescents had higher basal levels of CRF within the PVN and CeA and reduced CRF levels following repeated ethanol withdrawals. This reduced CRF within the CeA could indicate increased release of CRF, and future work will determine how this change relates to behavior.
Keywords: Repeated Ethanol Withdrawal, Corticotropin-Releasing Factor, Anxiety, Stress, Adolescents
Adolescence is known to be a time of increased use of alcohol, and this use is the strongest predictor of alcohol dependence in adulthood (Grant, 1998; Grant and Dawson, 1997). Additionally, the dependency course (time between initial use and dependence) is more rapid in adolescents than in adults (Clark et al., 1998). Further, it has been suggested that stress during adolescence is also a contributing factor for the development of alcohol dependence (Arnsten and Shansky, 2004; Enoch, 2006; Wagner, 1993). Studies in adolescent rodents have generated mixed findings regarding the consequences of stress on ethanol consumption (Brunell and Spear, 2005; Chester et al., 2008; Doremus et al., 2005). While these studies have demonstrated that the interactions between stress and ethanol intake during adolescence are likely complex, minimal work has been performed to evaluate how stress impacts ethanol withdrawal-related behaviors (i.e., anxiety).
Studies in rodents showed that repeated episodes of ethanol withdrawal lead to increased sensitivity for seizure sensitization or “kindling” following repeated ethanol withdrawals (Becker and Hale, 1993; Kokka et al., 1993; McCown and Breese, 1990). Further investigations demonstrated that other symptoms of withdrawal (i.e., anxiety) could also undergo a kindling-like process in adolescent and adult rodents (Breese et al., 2004; Overstreet et al., 2002; Wills et al., 2009). In these experiments, it was shown that withdrawal from three 5-day cycles of ethanol diet (see Fig. 1A) induced anxiety-like behavior (measured by a reduction in social interaction), which was not produced by withdrawal from 15 continuous days of ethanol exposure (Overstreet et al., 2002; Wills et al., 2009). This anxiety-like behavior only following repeated ethanol withdrawals illustrated that the pattern of ethanol exposure was important in sensitizing anxiety-like behavior (Breese et al., 2005a). Later work set out to determine whether there were sensitivity differences between adolescent and adult rats in the anxiety produced from repeated ethanol withdrawals. While there were no differences between ages in the amount of anxiety (% decrease in SI from controls) 5 hours into withdrawal, there were differences in the duration of this anxiety (length of time present after final withdrawal). In adolescent rats, anxiety-like behavior was present 7 days after the final withdrawal, while in adult rats anxiety had returned to baseline with 2 days. These data indicated increased sensitivity to the effects of repeated withdrawals in adolescent rats.
Fig. 1.
Procedure for repeated ethanol withdrawal, stress/withdrawal, and CRF/withdrawal paradigms (Panels A–C). (A) In the repeated ethanol withdrawal paradigm, rats are given three 5-day cycles of ethanol diet separated by two 2-day withdrawal period (rats receive CD). (B) In the stress/withdrawal paradigm, stress (1-hour restraint stress) is substituted for first two withdrawal periods (in repeated ethanol withdrawals) followed by a single cycle of 5-day ethanol diet. (C) In the CRF/withdrawal paradigm, CRF is administered to substitute for first two withdrawal periods (in repeated withdrawals) followed by a single cycle of 5-day ethanol diet.
Other studies (mainly in adults) have also established an interaction between stress and CRF in the development of this anxiety-like behavior during ethanol withdrawal. Initial work found pretreatment with a CRF1 receptor antagonist during repeated ethanol withdrawals prevented the development of anxiety-like behavior in both adolescent and adult rats (Breese et al., 2004; Knapp et al., 2004; Wills et al., 2009). Additional work in adult rats has shown that two weekly 1-hour restraint stress sessions followed by a single 5-day cycle of ethanol diet (not anxiogenic alone) could sensitize anxiety-like behavior (see Fig. 1B; Breese et al., 2004). Further work in adult rats found that CRF administered intraventricularly also substituted for either withdrawals or stress (see Fig. 1C) to produce anxiety-like behavior (Overstreet et al., 2004a).
Because adolescent rats were shown to be more sensitive than adults to the effects of repeated ethanol withdrawals, the present work determined whether adolescent rats were also more sensitive to the effects of stress and CRF on ethanol withdrawal-induced anxiety-like. First, it was evaluated whether stress can substitute for withdrawals (stress/withdrawal paradigm) to produce anxiety-like behavior in adolescent rats, as it has been shown to do in adult rats. Further, it was evaluated whether the duration of this anxiety-like behavior (measured as a reduction in social interaction) following stress/withdrawal differs between adolescents and adults, as this metric revealed a sensitivity differences in the repeated withdrawal paradigm. Additional studies were performed to determine whether CRF substituted for withdrawals or stress to produce anxiety-like behavior in adolescent rats and whether the effective doses were similar to those used in adult rats. Finally, experiments were performed to assess the levels of CRF in stress- and anxiety-responsive nuclei (the central nucleus of the amygdale; CeA and paraventricular nucleus of the hypothalamus; PVN) following stress/withdrawal and repeated withdrawal paradigms in both adolescent and adult rats.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (Charles-River, Raleigh, NC) were obtained at 7 weeks of age for the adult groups and 3 weeks of age for the adolescent groups. Animals were group-housed for 1 day to adapt to the local conditions (light/dark cycle of 12:12, with lights on between 0700 and 1900 hour). Rats were then individually housed for the remainder of the experiments with food and fluids monitored as described in the following sections. The experiments described were approved by the University of North Carolina Institutional Animal Care and Use Committee.
Ethanol and Control Diets
Following a day of adaptation to isolate housing, all rats were placed on nutritionally complete liquid diets that have been used routinely in this laboratory (e.g., Frye et al., 1983; Knapp et al., 1998; Moy et al., 1997; Overstreet et al., 2002). The diet is lactalbumin/dextrose based with vitamins, minerals, and other nutrients from Dyets (Bethlehem, PA). The number of calories from dextrose was equated with calories from the ethanol so that both control diet (CD) and ethanol diet (ED) were calorically balanced. Rats were habituated for 3 days on CD and then placed into one of three treatment groups. One-third of the rats received 19 days of CD, and the other two-thirds received ethanol diet (3.5% or 2.5% ED; in a manner described in the following sections). Rats were given CD in a volume equivalent to that consumed by the ED group on the previous day. Rats were also weighed weekly, and volumes of CD were adjusted to minimize weight gain differences between groups. ED and CD volumes were measured daily at the end of the dark cycle to determine g/kg intake/day. Behavioral measures were obtained at various times into the final withdrawal and will be discussed in the following sections.
Social Interaction Test
The social interaction test (SI) was first described by File and Hyde (1978), and a modified version of this test has been used regularly in our laboratory to assess anxiety-like behavior. The results of the social interaction test have also been confirmed with the use of the elevated plus maze (Overstreet et al., 2002, 2004b). In the social interaction test, rats were placed into a 60 × 60 cm square open field with 15 × 15 cm squares marked on the floor under low lighting conditions (30 lx). Two rats naïve to the testing environment were paired according to body weight and monitored for 5 minutes. An observer blind to the treatment condition measured the amount of time (in seconds) that each rat was engaged in social behavior (conspecific grooming, sniffing, following, crawling over/under) with its partner. Line crosses were also recorded as a measure of locomotor activity. Ethanol withdrawal has been repeatedly shown to reduce social interaction and sometimes locomotor activity as well (Breese et al., 2004; Overstreet et al., 2002, 2003, 2004a,b). However, it is important to note that reductions in social interaction and locomotor activity seem to be independently manipulatable (Breese et al., 2004, 2005b; Knapp et al., 2005; Overstreet et al., 2002). Additionally, previous work illustrated that the social behavior of one member of a testing pair is independent of the other rat’s behavior (Breese et al., 2004; Overstreet et al., 2002, 2003, 2004a). It is therefore possible to use the data from individual animals rather than the average performance of the pair (Overstreet et al., 2003). At the time of social interaction test (5 hours into withdrawal), all adolescents are postnatal day (P) 45 and adult rats are P69. In duration experiments (described later), adolescent rats were P46 and 47 while adult rats were P70 and 71 at the 24 and 48 hour time points, respectively.
Stress/Withdrawal Paradigm
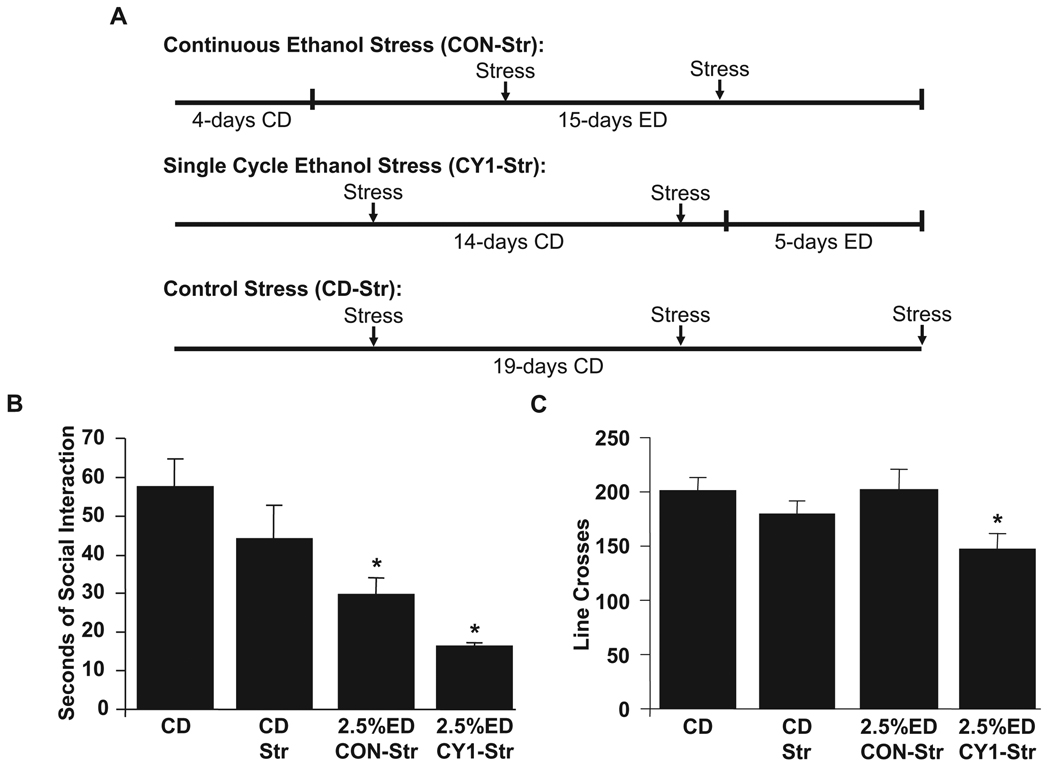
The stress/withdrawal paradigm involves the substitution of stress for the first two early withdrawals (compare Fig. 1A,B). The stress used in these experiments is 1 hour of restraint stress in a plastic cone. Adolescents were placed in one of the following groups: 19 days of control diet (CD) (no stress; CD), 4 days of CD followed by 15 days of continuous (CON) 2.5% ethanol diet (ED) with stress (given on Day 6 and 11 of ED; 2.5% CON-Str; Fig. 2A), 14 days of CD with stress (given on Day 6 and 13 of CD) followed by one 5-day cycle of 2.5% ED (2.5% CY1-Str; Fig. 2A), or 19 days of CD with stress (given on Day 6, 13, 19 of CD; CD-Str; Fig. 2A). Stress was administered on different days in CON-Str (day 11) compared to CD-Str and CY1-Str (day 13) to equally disperse stress episodes through continuous exposure. In this way, stress/withdrawal paradigms more closely resemble the repeated withdrawal paradigm that is being modeled. Social interaction was performed 5 hours after the final withdrawal or stress.
Fig. 2.
Procedure for diet and stress, social interaction, and locomotor activity in adolescent rats (Panels A–C). Male adolescent rats were given either control diet (CD), CD with stress (CD-Str), 15 days of continuous 2.5% ethanol diet (ED) with stress (2.5% Con-Str), or one 5-day cycle of 2.5% ED with stress (2.5% CY1-Str). Stress was one hour of restraint stress given in weekly intervals. All adolescent rats were tested 5 hours into the final ethanol withdrawal or final stress (for CD-Str group). Data represent means ± SEM for 8 rats/group. “*” is significantly different from control (p < 0.05).
Duration of Reduced Social Interaction Following the Stress/Withdrawal Paradigm
These experiments were conducted to determine the duration of anxiety-like behavior (measured by reduction in social interaction) following the stress/withdrawal paradigm in adolescent and adult rats. The stress/withdrawal paradigm used in these experiments involved giving a group two stress sessions (on Day 6 and 13) followed by 5 days of 2.5% ED (adolescents) or 3.5% ED (adults). Different ethanol diet concentrations where used in adolescent and adult rats because of previous data showing that adolescent rats have higher g/kg ethanol intake than their adult counterparts (Wills et al., 2008, 2009). In previous experiments, it was shown that treating adolescent rats with 2.5% ED and adult rats with 3.5% ED produces similar ethanol intakes (Wills et al., 2009). For comparison, other rats were maintained on CD throughout the experiment. Following these treatments, separate groups of rats were given social interaction tests at either 5, 24, or 48 hours following the final withdrawal. All rats are maintained on CD from the final withdrawal until the time of testing.
Surgery
Rats were anesthetized with 2.5%isoflurane and then placed in a stereotaxic instrument (Kopf Instruments, Tujunga, CA) for cannulae placement. After exposing the dorsal surface of the skull, holes were drilled in the skull at the appropriate locations and cannulae were inserted at the appropriate depth. Jeweler’s screws were implanted into the skull, and dental acrylic was applied to secure the cannulae to the skull. All cannulae were made from 26-gauge stainless steel tubing. Once recovered from anesthesia, the rats were given acetaminophen (children’s Q-Pap, cherry flavor, 6 mg/ml) in the drinking water for 48 hours.
CRF Administration
Adolescent rats were microinjected with 5.0 or 7.5 µg/5 µl of CRF into the lateral ventricle (AP = −0.6, ML = −1.2, DV = −1.5). Two injections of CRF or Veh (artificial cerebral spinal fluid) were administered 1 week apart starting 1 week after recovery from surgery. Rats in these experiments were exposed to one of the following diet conditions in combination with CRF or Veh administration. Rats combined into the control group received either 19 days of CD or 14 days of CD followed by a single 5-day cycle of 2.5% ED and Veh microinjections. For each dose of CRF, rats were given either 19 days of CD or 14 days of CD followed by a single 5-day cycle of 2.5% ED (see Fig. 1C).
Immunohistochemistry
For analysis of CRF immunohistochemistry, adolescent and adult rats were divided into three treatment groups: repeated ethanol withdrawal groups that received three 5-day cycles of either 2.5% ED (adolescents; 2.5% CY3) or 3.5% ED (adults; 3.5% CY3) interspersed with two 2-day withdrawal periods (given CD; see Fig. 1A); stress/withdrawal groups that received 14 days of CD with stress (given on Day 6 and 13) followed by one 5-day cycle of 2.5% ED (adolescents; 2.5% CY1-Str) or 3.5%ED(adults; 3.5%CY1-Str; see Fig. 1B), or control groups that received 19 day of ethanol diet (CD). Again, these ethanol concentrations were used to equalize differences in ethanol intake that occur between adolescent and adult rats (Wills et al., 2008, 2009). All of these rats were tested for social interaction 5 hours into the final ethanol withdrawal or on the 20th day of CD.
Immediately following the social interaction test, rats were perfused with 0.1M PBS and then 4% paraformaldehyde/0.1 M PB pH 7.4. After postfixing brains for at least 24 hours, free floating 40 µ coronal sections throughout the brain were collected with a vibrating microtome at 4°C. Immunohistochemical assays of CRF (rabbit anti-CRF human, rat; Peninsula laboratories; 1:5000) were conducted using a modification of a standard avidin-biotin/horseradish peroxidase method described previously (Knapp et al., 1998, 2001). For the brain areas of interest, every fourth section was analyzed producing 120 µ between quantified sections. Four sections of the central nucleus of amygdala (CeA; approximately −2.30 thru −2.80 from bregma) and two sections of the paraventricular nucleus of the hypothalamus (PVN; approximately −1.80 from bregma) for each rat were photographed digitally at 10×, captured with Bioquant Life Sciences (Ver. 8.0), and then analyzed using the ImageJ program. In the ImageJ program, images were converted to grayscale and a threshold was created that contained all CRF immunoreactivity. Once this threshold was determined, the area of CRF immunoreactivity was calculated in the defined areas (CeA or PVN). Counts of CRF immune-positive cell bodies were also performed at 20× magnification on the same four representative sections of the CeA in adolescent rats that received control diet and repeated ethanol withdrawals.
Statistics
Analyses of social interaction, locomotor activity, and immunohistochemistry were conducted with one-way ANOVAs. Comparisons between two groups in immunohistochemical studies used t-tests. Differences between groups were determined with Fisher’s post hoc tests.
RESULTS
Stress/Withdrawal Paradigm in Adolescent Rats (Social Interaction and Locomotor Activity)
Analysis of social interaction in adolescent rats given the continuous ethanol diet with stress (2.5% Con-Str) or a single ethanol cycle with stress (2.5% CY1-Str) showed significant differences across the treatment groups [F(3,27) = 8.26, p < 0.0005; Fig. 2B]. Specifically, adolescent rats experiencing the stress/withdrawal paradigm (2.5% Con-Str and 2.5% CY1-Str) had lower social interaction compared to rats given CD. Additionally, there was no significant difference in social interaction between control diet rats that did or did not experience stress (CD-Str and CD).
During the social interaction test, locomotor activity was also simultaneously measured. Analysis of locomotor activity in these groups also showed modest differences across the groups [F(3,27) = 3.11, p < 0.05; Fig. 2C]. That is, rats given stress with a single ethanol cycle (2.5% CY1-Str) showed significantly lower locomotor activity compared to CD- and 2.5% Con-Str-treated rats. There were no significant differences between other treatment groups.
Duration of Reduced Social Interaction Following Stress/Withdrawal Paradigm in Adolescent and Adult Rats
Further experiments determined the duration of reduced social interaction (measured at various times after the final withdrawal) in adolescent rats following stress/withdrawal paradigm (2.5% CY1-Str). Evaluation of social interaction in adolescent rats showed a significant difference between groups tested at different durations [F(3,36) = 3.96, p < 0.05; Fig. 3A]. Specifically, adolescent rats tested 5 hours into the withdrawal showed reduced social interaction compared to CD-treated rats (as shown previously above). However, adolescent rats tested 24 and 48 hours into the final withdrawal were different from rats tested at 5 hours but not from CD-treated rats. Additionally, there were no significant differences in locomotor activity [F(3,36) = 0.25, NS; Fig. 3B].
Fig. 3.
Duration of reduced social interaction in adolescent and adult rats exposed to stress/withdrawal paradigm. Male adolescent (Panels A & B) and adult (Panels C & D) rats were given either control diet (CD) or 14-days of CD, in which two stress episodes were given 1 week apart, followed by a single 5-day cycle of ethanol diet (ED; 2.5% Str—adolescents; 3.5% Str—adults). Stress was 1 hour of restraint stress. Rats were tested 5 hours, 1, or 2 days after the removal of ethanol during the final withdrawal. Data represent means ± SEM for 8 rats/group. “*” is significantly different from control (p < 0.05).
In adult rats, the duration of reduced social interaction from stress/withdrawal paradigms (3.5% CY1-Str) was also evaluated. There was a significant difference between groups tested at different durations in adult rats [F(3,38) = 3.14, p < 0.05; Fig. 3C]. In particular, social interaction was decreased 5 hours into withdrawal compared to CD-treated rats but was not different from controls at either 24 or 48 hours. Further, there were no significant differences in locomotor activity [F(3,38) = 0.42, NS; Fig. 3D].
Additionally, these reductions in SI from the stress/withdrawal paradigm were also converted into a % decrease from controls to correct for the differences in baseline (controls) SI between adolescent and adult rats. There were no significant differences between adolescent and adult rats in % decrease from baseline (t(14) = 0.51, p > 0.05).
CRF/Withdrawal Paradigm in Adolescent Rats (Social Interaction and Locomotor Activity)
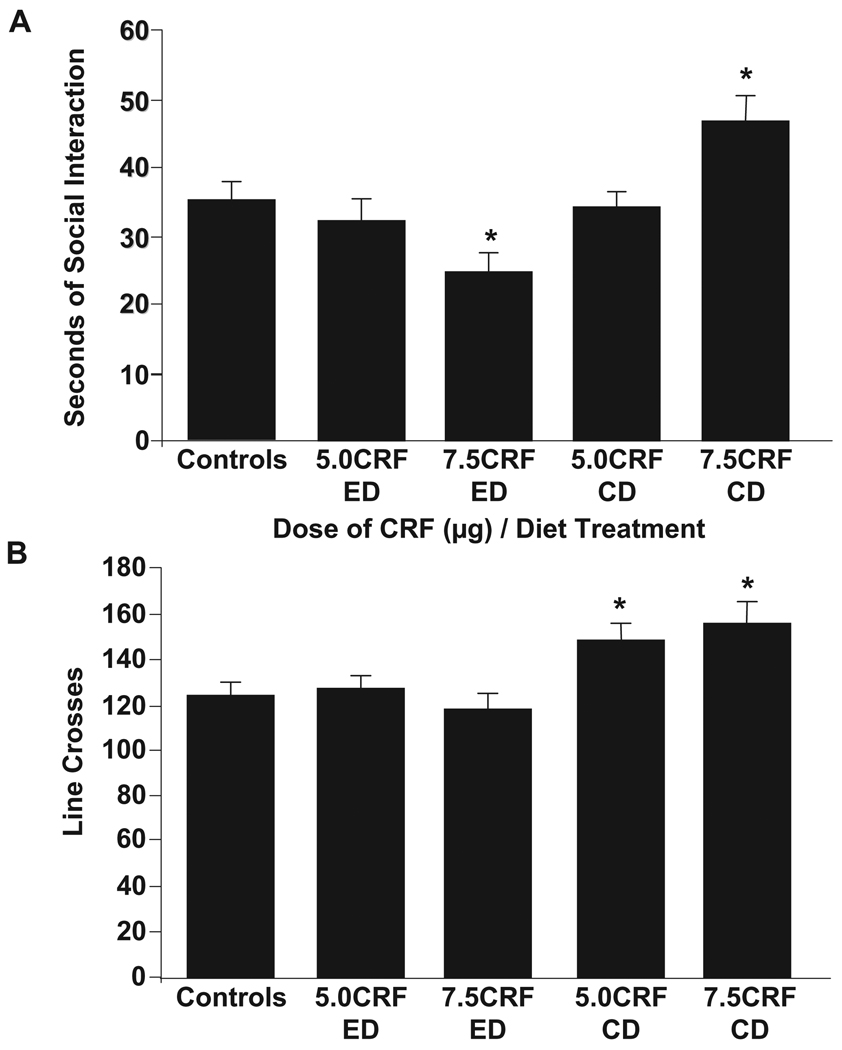
These experiments determined whether CRF could also substitute for early withdrawals in adolescents to produce anxiety, as it does in adults. The controls are a combination of vehicle-injected rats given either CD or a single cycle of 2.5% ED because there were no significant differences in social interaction between these groups [t(22) = 1.6, NS]. Analysis of social interaction in adolescent rats given the CRF/withdrawal paradigm revealed a differences across the CRF/diet treatments [F(4,82) = 4.08, p < 0.005; Fig. 4A]. Specifically, adolescent rats given 7.5 µg of CRF and ethanol diet (7.5 CRF-ED) had lower social interaction compared to controls, 5 µg dose with CD, or 7.5 µg dose with CD. The 5 µg dose of CRF combined with ED was not significantly different from controls. In rats that received CRF with CD, social interaction compared to controls was increased with 7.5 µg and unchanged with 5 µg of CRF. Additionally, there was a modest effect of CRF/diet treatment on locomotor activity [F(4,82) = 3.42, p < 0.05; Fig. 4B]. This effect was a result of increased locomotor activity in 7.5 µg dose with CD group compared to all other groups except the 5 µg dose with CD group. Additionally, rats given 5 µg dose with CD had higher locomotor activity compared to controls and rats given 7.5 µg dose with ED.
Fig. 4.
Dose-response of intracerebroventricular (icv) corticotropin-releasing factor (CRF) in adolescent rats (Panels A & B). Rats were given 14 days of control diet (CD) and two weekly microinjections of CRF icv (5.0 µg or 7.5 µg/5 µl) or artificial cerebral spinal fluid (ACSF; given to controls). Rats were then given either 5 days of CD (CRF-CD) or 2.5% ethanol diet (CRF-ED). Controls represent rats that received either CD-ACSF or ED-ACSF. Social interaction and locomotor activity were assessed 5 hours into the ethanol withdrawal. Data represent means ± SEM for 8 rats/group. “*” is significantly different from control (p < 0.05).
CRF Immunohistochemistry: Baseline Differences between Adolescent and Adult Rats in Liquid Diet Controls (CD)
Further experiments were conducted to evaluate baseline levels of CRF between adolescent and adult rats receiving control diet throughout the experiment. In the central nucleus of the amygdala (CeA), there were significantly higher levels of CRF in adolescents compared to adults [t(16) = 2.90, p < 0.05; Fig. 5A]. There were also differences in total density of CRF between age groups in the paraventricular nucleus of hypothalamus [PVN; t(15) = 2.54, p < 0.05; Fig. 5B].
Fig. 5.
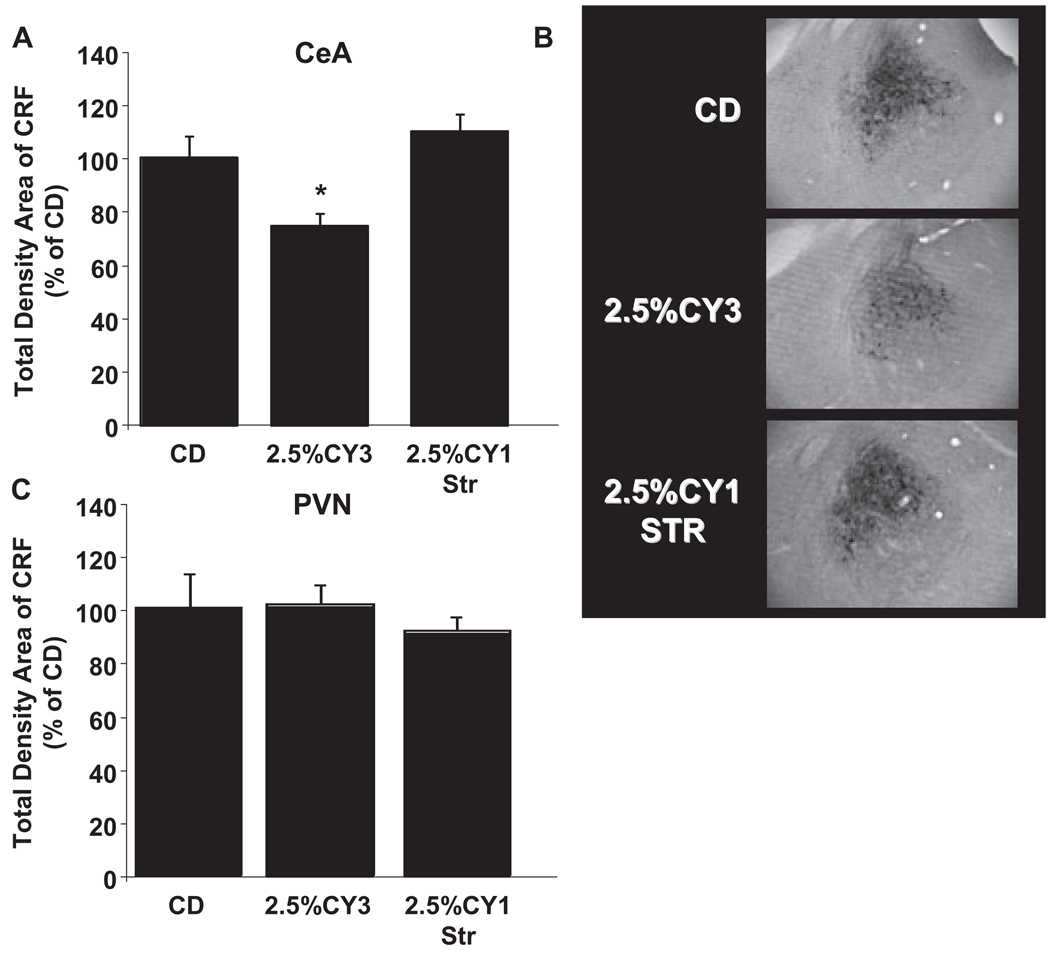
Corticotropin-releasing factor (CRF) immunoreactivity within the central nucleus of the amygdala (CeA) and paraventricular nucleus of the hypothalamus (PVN) of liquid diet control adolescent and adult rats (Panels A–C). Rats were given 19 days of control diet (CD), and brain tissue was collected immediately following social interaction. Total density measurements were taken using ImageJ program for CeA (Panel A) and PVN (Panel C). Panel B shows a representative image of CRF immunoreactivity in the CeA. Data represent means ± SEM for 8 rats/group. “*” is significantly different from control (p < 0.05).
Cell counts were also conducted in adolescent and adult rats that received CD. In the CeA, there was a significant difference in the number of cell bodies with CRF immunoreactivity between ages [t(16) = 3.16, p < 0.01; data not shown]. This was a result of more CRF-immunoreactive cells in the CeA of adolescent rats compared to adults. In the PVN, there was also a significant difference in the number of cell bodies with CRF immunoreactivity between ages [t(15) = 3.05, p < 0.01; data not shown]. There were more CRF immunoreactive cells in the PVN of adolescent rats compared to adults.
CRF Immunohistochemistry: Repeated Ethanol Withdrawal, Stress/Withdrawal, or CD in Adolescent and Adult Rats
These experiments also compared changes in CRF levels in the CeA or PVN during withdrawal from repeated withdrawals and stress/withdrawal paradigms in adolescent and adult rats. These levels were converted to a % decrease from controls to correct for the differences in baseline (treated with control diet) CRF levels between adolescent and adult rats (see Fig. 5). There were significant differences between treatment groups in the CeA in adolescent rats [F(2,27) = 7.82, p < 0.005, Fig. 6A,B]. Specifically, adolescent rats that experienced repeated ethanol withdrawals showed decreased total density of CRF immunoreactivity in the CeA compared to stress/withdrawal and control rats. However, there was no difference between treatment groups in total density of CRF immunoreactivity in the PVN[F(2,26) = 0.34, NS; Fig. 6C].
Fig. 6.
Adolescent corticotropin-releasing factor (CRF) immunoreactivity within the central nucleus of the amygdala (CeA) and paraventricular nucleus of the hypothalamus (PVN) following repeated ethanol withdrawal and stress/withdrawal paradigms (Panels A–C). Adolescent rats were given either 19 days of control diet (CD), three 5-day cycles of 2.5% ethanol diet (ED) interspersed with two 2-day withdrawal period (2.5% CY3), or 14 days of CD followed by 5 days of 2.5% ED with stress (2.5% CY1-Str). Stress was one hour of restraint stress given at weekly intervals. Brain tissue was collected immediately following social interaction 5 hours into ethanol withdrawal. Total density measurements were taken using ImageJ program for CeA (Panel A) and PVN (Panel C). Panel B shows a representative image of CRF immunoreactivity in the CeA. Data represent means ± SEM for 8 rats/group. “*” is significantly different from control (p < 0.05).
Cell counts were also performed in the CeA to determine whether the decreased density in CRF following repeated ethanol withdrawals was a result of decreased CRF-containing cell bodies. There were no significant differences between treatment groups in the CeA of adolescent rats [F(2,27) = 0.25, NS; data not shown].
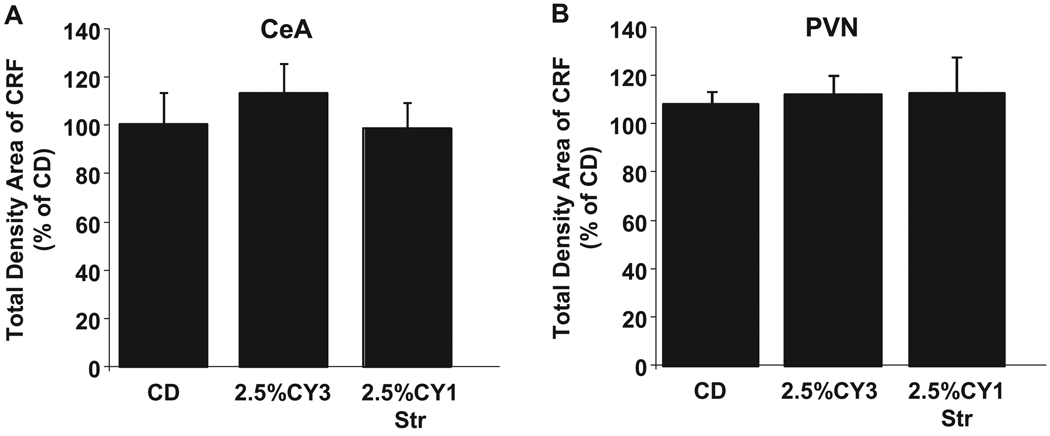
Similar treatments were also investigated in adult rats. There were no significant differences between treatment groups in the CeA [F(2,21) = 0.44, NS; Fig. 7A] or PVN [F(2,20) = 0.05, NS; Fig. 7B]. Even increasing the dietary ethanol concentration to 7% in adults resulted in no significant differences in CRF levels (p > 0.05; data not shown) in ethanol-exposed or stress-ethanol-exposed adult rats relative to controls.
Fig. 7.
Adult corticotropin-releasing factor (CRF) immunoreactivity within the central nucleus of the amygdala (CeA) and paraventricular nucleus of the hypothalamus (PVN) following repeated ethanol withdrawal and stress/withdrawal paradigms (Panels A & B). Adult rats were given either 19 days of control diet (CD), three 5-day cycles of 3.5% ethanol diet (ED) interspersed with two 2-day withdrawal periods (3.5% CY3), or 14 days of CD followed by 5 days of 3.5% ED with stress (3.5% CY1-Str). Stress was one hour of restraint stress given at weekly intervals. Brain tissue was collected immediately following social interaction 5 hours into ethanol withdrawal. Total density measurements were taken using ImageJ program for CeA and PVN. Data represent means ± SEM for 8 rats/group. “*” is significantly different from control (p < 0.05).
DISCUSSION
This work illustrates that stress coupled with a single ethanol withdrawal can reduce social interaction in adolescent rats. This reduction in social interaction is a validated measure of increased anxiety-like behavior (File and Seth, 2003). Importantly, it was demonstrated that the combination of both stress and ethanol withdrawal was critical, as neither stress nor a single ethanol withdrawal alone produced this anxiety-like behavior (Wills et al., 2009). Similar findings were previously found in adult rats, where stress substituted for early withdrawals to produce anxiety-like behavior (Breese et al., 2004). Further, the changes in locomotor activity following the stress/withdrawal paradigm in adolescent rats were also similar to data in adults. In both age groups, reduced locomotor activity was found for rats given two stress episodes followed by a single 5-day cycle of ethanol diet (Breese et al., 2004), although this effect was modest and unlikely to impact on the social interaction of this group. Additional support can be found in the duration experiment, where this same stress/withdrawal treatment (2.5% CY1-Str at 5 hours) elicited a similar reduction in social interaction without a reduction in activity. These results reconfirm the idea that social interaction and locomotor activity are independently manipulatable and not necessarily contingent on one another through changes in ethanol treatment and drug treatments (Breese et al., 2004, 2005b; Knapp et al., 2005; Overstreet et al., 2002).
In other experiments, the anxiety-like behavior produced from the stress/withdrawal paradigm was present at 5 hours but had returned to baseline by 24 hours into withdrawal in both adolescent and adult rats. This profile of recovery is similar to that seen with repeated ethanol withdrawals in adult rats (Wills et al., 2009). However, this duration is very different from that found with repeated ethanol withdrawals in adolescent rats (Wills et al., 2009). In these previous results, it was illustrated that anxiety-like behavior after repeated ethanol withdrawals could be detected for up to 1 week following the final withdrawal. The reason for differences in the duration of reduced social interaction between these paradigms may be because of a lower sensitivity to the anxiogenic effects of acute restraint stress in adolescent rats. This behavioral divergence between paradigms (repeated ethanol withdrawal vs. stress/withdrawal) suggests that different mechanisms likely contribute to the development of this anxiety-like behavior. Adolescent rats were also shown to display higher baseline levels of activity and social interaction than adult rats. This result was consistent with previous reports of elevated exploration and social behavior in adolescent rats (Adriani et al., 1998; Primus and Kellogg, 1989; Vanderschuren et al., 1997). Corrections for these baseline differences were made (converted to % decrease from baseline) and illustrated that adolescent and adult rats showed equivalent reduction in social interaction (45 to 50%). The next set of experiments provided evidence for a role of CRF in the sensitization of anxiety-like behavior. Adolescent rats given 7.5 µg of CRF in combination with a single ethanol withdrawal exhibited anxiety-like behavior (reduction in social interaction), which was not found with either of these treatments alone. This dose of CRF required to elicit sensitization of withdrawal-induced anxiety in adolescents was higher than doses effective in producing this anxiety in adult studies (5 µg; Overstreet et al., 2004a). Therefore, it seems that adolescent rats may also be less sensitive to the anxiogenic effects of CRF. There were modest effects on locomotor activity in these experiments caused by an increased in activity from CRF-CD groups (7.5 and 5 µg). It is unlikely that these differences in activity had any effect on social interaction for the reasons described earlier. Additionally, the differences do not affect the primary comparison between 7.5 µg-ED and controls as these groups were not different.
Analyses were also made of CRF levels in adolescent and adult rats that received repeated withdrawals, stress/withdrawals, or control diet. Results illustrated that CRF immunoreactivity was not significantly changed by any of the adult treatments in any of the regions that were evaluated (CeA or PVN). Even higher (7%) ethanol concentrations in one experiment failed to modify CRF levels in adults. However, in adolescent rats that were given repeated ethanol withdrawal, there was a difference in CRF immunoreactivity in the CeA, but not PVN. This difference was caused by a decrease in density of CRF in the CeA without a change in CRF-immunopositive cell number. We hypothesize that this decrease in CRF density levels from repeated ethanol withdrawal relates to an increase in CRF release during withdrawal. An increase in CRF release in this group would presumably deplete the supply of CRF contained within the cell body and would lead to the decreased immuno-density that was found. This idea can be further supported by microdialysis studies which showed that CRF levels increased and peaked 11 to 12 hours into withdrawal from chronic ethanol exposure (Merlo Pich et al., 1995). Similar results have been found under more chronic ethanol conditions. For example, Zorrilla et al. (2001) illustrated that during the first day of withdrawal there was a decrease in CRF immunoreactivity in the amygdala. Later studies demonstrated this reduction in CRF immunoreactivity specifically in the CeA during withdrawal of dependent rats (Funk et al., 2006). In agreement with our results, this study also found no change in CRF-positive cell number (Funk et al., 2006). Both authors also speculate that this reduction in immunoreactivity was an indication of increased CRF release during this period. This reduction in CRF immunoreactivity in the CeA was only found in adolescent rats that experienced repeated ethanol withdrawals, not following stress/withdrawal procedure or following either treatment in adult rats. It is important to note that both of these treatments in adolescent and adults produced anxiety-like behavior; therefore, this change in CRF is most likely not universally related to this acute (5 hours into ethanol withdrawal) anxiety-like behavior. Further, previous work has demonstrated that CRF1 antagonists are able to prevent the sensitization of anxiety-like behavior in the stress/withdrawal and repeated withdrawal paradigm in adult rats (Breese et al., 2004; Knapp et al., 2004). This work illustrates a role of CRF in the development of anxiety-like behavior under these paradigms in adult rats. Thus, the reduction in CRF immunoreactivity seen with repeated withdrawals in adolescent rats is likely related to the greater sensitivity illustrated by an extended anxiety-like behavior (up to 1 week into withdrawal) found only in this group. These results could also suggest that different mechanisms underlie repeated ethanol withdrawal and stress/withdrawal in adolescent rats.
The immunohistochemical experiments also indicated that adolescent rats have higher basal immunoreactivity of CRF (increased density and cell number) in the PVN and CeA compared to adult rats. This result provides unique evidence of increased CRF protein levels and cell number within the PVN and CeA in adolescent rats. An earlier study measuring mRNA levels of CRF found no difference in the PVN and an increase in the CeA from 30- to 60-day-old male rats (Viau et al., 2005). This study differs from the current results presented here in that only changes in CRF mRNA levels were quantified and somewhat younger animals were assayed (their ages 30 and 60; our ages P45 and P73). Therefore, our results indicate that at least at P45 there are increased levels of CRF protein within the PVN and CeA under our control conditions (liquid diet and isolate housing). The implications of this observation would benefit from further examinations of CRF levels across age and challenge conditions. Additionally, these baseline differences in CRF between adolescent and adult rats in the current experiments might have been responsible for reduced behavioral sensitivity to the stress and CRF withdrawal paradigms.
It is also important to note that these studies contain numerous variables that can be considered stressful aside from the experimentally administered restraint stress. These variables include liquid diet exposure, isolate housing, and ethanol exposure, which could all interact in an age-specific manner to effect social interaction behavior in the current studies. While this current work does not address how these paradigms may be impacted by other control conditions (chow-fed and group-housed), the controls employed facilitate appropriate comparisons that support our conclusions.
In summary, these experiments have illustrated that both stress and CRF substitute for early withdrawals and sensitize anxiety-like behavior. Further, there were higher levels of basal CRF levels with the PVN and CeA in adolescent rats. Finally, it was demonstrated that repeated ethanol withdrawals in adolescent rats caused a decrease in CRF density but not cell number within the CeA. Future studies will extend this work to evaluate the role of increased basal CRF in adolescents and how changes in CRF following repeated withdrawals might be tied to the extended anxiety-like behavior found in this group.
ACKNOWLEDGMENTS
These studies were supported by AA11605, AA14949, and NRSA Predoctoral Fellowship AA16704. We thank Robert Angel and Kui-Ling Huang for technical assistance.
REFERENCES
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d- amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Shansky RM. Adolescence: vulnerable period for stress-induced prefrontal cortical function? Introduction to part IV. Ann N Y Acad Sci. 2004;1021:143–147. doi: 10.1196/annals.1308.017. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal- induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology (Berl) 2005a;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine- receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005b;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res. 2008;32:1782–1794. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE. Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend. 1998;49:115–121. doi: 10.1016/s0376-8716(97)00154-3. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Enoch MA. Genetic and environmental influences on the development of alcoholism: resilience vs. risk. Ann N Y Acad Sci. 2006;1094:193–201. doi: 10.1196/annals.1376.019. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Characterization of susceptibility to audiogenic seizures in ethanol-dependent rats after microinjection of gamma-aminobutyric acid (GABA) agonists into the inferior colliculus, substantia nigra or medial septum. J Pharmacol Exp Ther. 1983;227:663–670. [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. The impact of a family history of alcoholism on the relationship between age at onset of alcohol use and DSM-IV alcohol dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Alcohol Health Res World. 1998;22:144–147. [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Braun CJ, Duncan GE, Qian Y, Fernandes A, Crews FT, Breese GR. Regional specificity of ethanol and NMDA action in brain revealed with FOS-like immunohistochemistry and differential routes of drug administration. Alcohol Clin Exp Res. 2001;25:1662–1672. [PubMed] [Google Scholar]
- Knapp DJ, Duncan GE, Crews FT, Breese GR. Induction of Fos-like proteins and ultrasonic vocalizations during ethanol withdrawal: further evidence for withdrawal- induced anxiety. Alcohol Clin Exp Res. 1998;22:481–493. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/gamma-aminobutyric acid ligands. Alcohol Clin Exp Res. 2005;29:553–563. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology (Berl) 1997;131:354–360. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal- induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004a;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004b;78:459–464. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2c antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology (Berl) 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress- induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Wagner EF. Delay of gratification, coping with stress, and substance use in adolescence. Exp Clin Psychopharmacol. 1993;1:27–43. [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Differential dietary ethanol intake and blood ethanol levels in adolescent and adult rats: effects on anxiety-like behavior and seizure thresholds. Alcohol Clin Exp Res. 2008;32:1350–1360. doi: 10.1111/j.1530-0277.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Sensitization, Duration, and Pharmacological Blockade of Anxiety-Like Behavior Following Repeated Ethanol Withdrawal in Adolescent and Adult Rats. Alcohol Clin Exp Res. 2009;33:455–463. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like- immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]