Abstract
Turtles use their limbs during both aquatic and terrestrial locomotion, but water and land impose dramatically different physical requirements. How must musculoskeletal function be adjusted to produce locomotion through such physically disparate habitats? We addressed this question by quantifying forelimb kinematics and muscle activity during aquatic and terrestrial locomotion in a generalized freshwater turtle, the red-eared slider (Trachemys scripta), using digital high-speed video and electromyography (EMG). Comparisons of our forelimb data to previously collected data from the slider hindlimb allow us to test whether limb muscles with similar functional roles show qualitatively similar modulations of activity across habitats. The different functional demands of water and air lead to a prediction that muscle activity for limb protractors (e.g. latissimus dorsi and deltoid for the forelimb) should be greater during swimming than during walking, and activity in retractors (e.g. coracobrachialis and pectoralis for the forelimb) should be greater during walking than during swimming. Differences between aquatic and terrestrial forelimb movements are reflected in temporal modulation of muscle activity bursts between environments, and in some cases the number of EMG bursts as well. Although patterns of modulation between water and land are similar between the fore- and hindlimb in T. scripta for propulsive phase muscles (retractors), we did not find support for the predicted pattern of intensity modulation, suggesting that the functional demands of the locomotor medium alone do not dictate differences in intensity of muscle activity across habitats.
Keywords: electromyography, EMG, muscle, biomechanics, kinematics, locomotion, swimming, walking, aquatic, terrestrial, turtle
INTRODUCTION
Animals move through their environment to perform a wide range of crucial tasks, ranging from acquiring food, to finding mates, to avoiding and escaping predators. The physical characteristics of locomotor environments strongly influence the functional demands that the musculoskeletal systems of animals must satisfy (Gillis, 1998a; Gillis and Biewener, 2000; Gillis and Blob, 2001; Higham and Jayne, 2004; Blob et al., 2008; Pace and Gibb, 2009). Species that live in a restricted range of habitats may show specializations that facilitate locomotor performance under specific physical conditions, whereas species that live in or traverse multiple habitats must use a single set of locomotor structures to meet potentially disparate functional requirements (Gillis and Biewener, 2002; Daley and Biewener, 2003; Biewener and Daley, 2007).
One of the most common ways in which animals encounter locomotor environments with divergent demands is through the use of both aquatic and terrestrial habitats. Species that regularly move both through water and over land occur in every major group of vertebrates (i.e. fishes, amphibians, mammals, non-avian reptiles and birds). Given the differences in viscosity, density and the effects of gravity between these habitats, the functional demands placed on the musculoskeletal system are expected to be very different between aquatic and terrestrial locomotion (Horner and Jayne, 2008). How do animals adjust musculoskeletal function to meet the differing demands of water and land?
Previous studies have highlighted three general neuromuscular strategies for accommodating divergent demands (Biewener and Gillis, 1999; Gillis and Blob, 2001; Blob et al., 2008). First, there might be no change in muscle activation patterns between behaviors. This pattern seems unlikely for locomotion in water and on land given the dramatically different physical characteristics of aquatic and terrestrial habitats (Biewener and Gillis, 1999; Gillis and Blob, 2001), and because such fixed motor patterns might actually impede performance of some behaviors (Biewener and Gillis, 1999; Blob et al., 2008). However, such motor stereotypy might be found if a central pattern generator were the dominant source of control for the muscles in question (Buford and Smith, 1990; Pratt et al., 1996; Blob et al., 2008), possibly simplifying locomotor control in systems with serially homologous appendages. A second possible strategy is that the same set of muscles might be recruited across behaviors, but with differences in timing or intensity of activity (Gruner and Altman, 1980; Roy et al., 1985; Macpherson, 1991; Roy et al., 1991; Johnston and Bekoff, 1996; Kamel et al., 1996; Gillis and Biewener, 2000; Reilly and Blob, 2003; Blob et al., 2008). Depending on the functional demands and requirements of the motion in question, some general patterns of coactivation may be maintained with only small differences in the intensity or timing of muscle activity (Gruner and Altman, 1980; Johnston and Bekoff, 1996). In other cases the timing of muscle activity might change so drastically between motor tasks that synergistic muscles in one task could act as antagonists in another (Buchanan et al., 1986). A third possibility is that different motor tasks might be accomplished through the actions of different muscles, or through the recruitment of specific muscles only during the performance of specific tasks (Gatesy, 1997). Because vertebrate limb musculature is highly redundant, with a number of muscles able to contribute to movement in each direction, these three possibilities are not mutually exclusive (Biewener and Gillis, 1999; Gillis and Blob, 2001; Blob et al., 2008). Several previous examinations of limb muscle motor patterns during aquatic versus terrestrial locomotion have found that modifications of at least some aspects of muscle activity are required to produce effective locomotion through both aquatic and terrestrial environments (Biewener and Gillis, 1999; Gillis and Biewener, 2001; Gillis and Blob, 2001; Blob et al., 2008). However, these studies, like the majority that have compared limb muscle motor patterns across disparate tasks (Ashley-Ross, 1995; Kamel et al., 1996; Ashley-Ross and Lauder, 1997; Gatesy, 1997; Gatesy, 1999; Gillis and Biewener, 2000; Gillis and Biewener, 2001; Higham and Jayne, 2004), have focused on the hindlimb. How similar are the modulation of fore- and hindlimb motor patterns across locomotor behaviors with different demands? Are modulation patterns observed in one set of limbs a good predictor of those in the other?
Turtles are an excellent group in which to examine questions about environmentally correlated modulation of motor patterns for several reasons. First, many species of turtle regularly perform both aquatic and terrestrial locomotion as part of their natural behaviors, with many species spending substantial amounts of time in both types of environments (Cagle, 1944; Bennett et al., 1970; Gibbons, 1970; Zug, 1971; Davenport et al., 1984; Ernst et al., 1994; Gillis and Blob, 2001; Blob et al., 2008). Second, because the rigid body design of turtles involves fusion of most of the body axis to a bony shell, propulsive forces are generated almost exclusively by the limbs in any habitat (Blob et al., 2008). Thus, evaluations of differences in limb muscle motor patterns across habitats will not be confounded by changes in the contribution of other structures to propulsion, such as flexible bodies, tails, or specialized fins (Blake et al., 1995; Walker, 2000; Fish, 2002; Fish and Nicastro, 2003; Rivera et al., 2006). Additionally, because freshwater turtles (with the exception of the pig-nosed turtle, Carettochelys insculpta) use fore- and hindlimbs for locomotion, it makes them ideal for studying both sets of limbs. Locomotor activity of the hindlimb muscles has been examined in two species of turtle, the slider (Trachemys scripta) and the spiny softshell (Apalone spinifera) (Gillis and Blob, 2001; Blob et al., 2008), but the forelimb has not been examined.
In this study, we examined how muscle function is modulated to accommodate different performance demands by comparing the motor patterns of forelimb muscles in a generalized freshwater turtle, Trachemys scripta (Schoepff) (red-eared slider turtle), during aquatic and terrestrial locomotion. Like many freshwater turtles, sliders spend considerable time in the water, but also move over land to perform vital tasks such as nesting, basking or moving between aquatic habitats (Gibbons, 1970; Gibbons, 1990; Ernst et al., 1994; Bodie and Semlitsch, 2000). Sliders must use the same set of muscles to produce these movements under the different performance demands of both habitats. These differing demands provide a basis for several predictions of how slider forelimb muscle activity might be modulated between water and land. First, because water is much more dense and viscous than air, turtles may show elevated activity in limb protractors during swimming versus walking in order to overcome the greater drag incurred during the recovery phase in water compared with on land (Gillis and Blob, 2001). Conversely, the limb retractors may show elevated activity on land relative to water in order to counteract gravitational loads and support the body without the benefit of buoyancy (Gillis and Blob, 2001). Such differences in activity between habitats could be produced through changes in the duration of muscle bursts, the intensity of muscle activity, or both. Yet, though attractive to apply to the forelimb, EMG data from the hindlimb of T. scripta (and a second turtle species, the spiny softshell, Apalone spinifera) during swimming and walking do not uniformly support these predicted modulations of motor pattern based on differences in the physical characteristics of the locomotor environment (Gillis and Blob, 2001; Blob et al., 2008). For example, the mean amplitudes of bursts by two stance/thrust phase muscles, the hip retractor, flexor tibialis internus (FTI) and the knee extensor, femorotibialis (FT), are both greater in water than on land in T. scripta (Gillis and Blob, 2001; Blob et al., 2008). In addition, although one hindlimb protractor, iliofemoralis (ILF), showed bursts of greater mean amplitude, as predicted, during swimming compared with walking, a second hindlimb protractor with activity nearly synchronous with ILF, the puboischiofemoralis internus (PIFI), showed the opposite pattern of modulation, with higher amplitude bursts on land (Gillis and Blob, 2001; Blob et al., 2008). It is uncertain whether forelimb muscles should be expected to show patterns of modulation that follow predictions based on physical differences in locomotor environment, or whether they might show patterns similar to those of the serially homologous hindlimb. Our EMG data from slider forelimbs will allow us to address this question, helping to build understanding of how animals modulate muscle activity to accommodate different environments and potentially contributing insights into how new forms of quadrupedal locomotion evolve.
MATERIALS AND METHODS
Experimental animals
Seven juvenile slider turtles (4 years old) that were similar in carapace length (average 14.5±0.6 cm) and body mass (average 450±42 g) were purchased from a commercial vendor (Concordia Turtle Farm, Wildsville, LA, USA). Turtles were housed in groups in 600 liter (150 gallon) stock tanks equipped with pond filters and dry basking platforms. Tanks were located in a temperature-controlled greenhouse facility, thus exposing turtles to ambient light patterns during the course of experiments (February to May). Turtles were fed a diet of commercially available reptile food (ReptoMin®, Tetra®, Blacksburg, VA, USA), supplemented with earthworms. All animal care and experimental procedures were conducted in accordance with Clemson University IACUC guidelines (protocol 50110).
Collection and analysis of kinematic data
Kinematic data were collected simultaneously in lateral and ventral views (100 Hz) using two digitally synchronized high-speed video cameras (Phantom V4.1, Vision Research, Inc.; Wayne, NJ, USA). Locomotor trials (swimming and walking: see supplementary material Table S1) were conducted in a custom-built recirculating flow tank with a transparent glass side and bottom. Ventral views were obtained by directing the ventral camera at a mirror oriented at a 45 deg angle to the transparent bottom of the tank. For aquatic trials, the tank was filled with water and flow was adjusted to elicit forward swimming behavior (Pace et al., 2001). Once the turtle was swimming, flow was adjusted to keep pace with the swimming speed of the animal. For terrestrial trials, water was drained from the tank, the glass was dried thoroughly, and turtles were encouraged to walk forward by gently tapping the back of the shell and providing them with a dark hiding spot at the far end of the tank. Although dried glass clearly differs from the substrate the turtles would encounter in nature, a transparent surface through which we could film was required. Because the glass and turtle were thoroughly dried prior to terrestrial trials the surface was not slippery, and all animals walked normally. Aquatic and terrestrial locomotor sequences were collected from each turtle, yielding 16–20 limb cycles for each habitat, from each turtle.
To facilitate digitization of animal movement from videos, a combination of white correction fluid and black marker pen were used to draw high-contrast points on the following 13 anatomical landmarks (Fig. 1): tip of the nose, shoulder, elbow, wrist, digits 1, 3 and 5, an anterior and posterior point on the bridge of the shell (visible in lateral and ventral view), and right, left, anterior and posterior points on the plastron (plastral points visible in ventral view only). Landmark positions were digitized frame by frame in each video using DLTdataViewer2 (Hedrick, 2008). The three-dimensional coordinate data generated were then processed using custom Matlab (Student Ver. 7.1, MathWorks, Inc.; Natick, MA, USA) routines to calculate limb kinematics during swimming and walking, including protraction and retraction of the humerus, elevation and depression of the humerus, and extension and flexion of the elbow. Calculated values for kinematic variables from each limb cycle were fitted to a quintic spline (Walker, 1998) to smooth the data, and interpolated to 101 values, representing 0–100% of the limb cycle. Transformation of the duration of each cycle to a percentage allowed us to compare locomotor cycles of different absolute durations and calculate average kinematic profiles and standard errors for each variable through the course of walking and swimming trials. A humeral protraction/retraction angle of 0 deg indicates that the humerus is perpendicular to the midline of the turtle, whereas an angle of 90 deg indicates a fully protracted forelimb with the distal end of the humerus directed anteriorly (an angle of –90 deg would indicate a fully retracted forelimb with the distal tip of the humerus directed posteriorly). A humeral elevation/depression angle of 0 deg indicates that the humerus is in the turtle's horizontal plane. Angles greater than zero indicate elevation above the horizontal (distal end above proximal end) whereas negative angles indicate depression of the humerus (distal end lower than proximal end). Extension of the elbow is indicated by larger flexion/extension angles and flexion is indicated by smaller values. An elbow angle of 0 deg indicates the hypothetical fully flexed (i.e. humerus perfectly parallel to radius and ulna) elbow, 180 deg indicates a fully extended elbow, and 90 deg indicates that the humerus is perpendicular to the radius and ulna. Forefoot orientation angle was also calculated as the angle between a vector pointing forwards along the anteroposterior midline (also the path of travel) and a vector emerging from the palmar surface of a plane defined by the tips of digits 1 and 5 and the wrist; this angle was transformed by subtracting 90 deg from each value (Pace et al., 2001). A high-drag orientation of the forefoot paddle with the palmar surface of the paddle directed opposite the direction of travel (and in the same direction as the flow of water) is indicated by an angle of 90 deg, and a perfect low-drag orientation of the forefoot paddle is indicated by an angle of 0 deg.
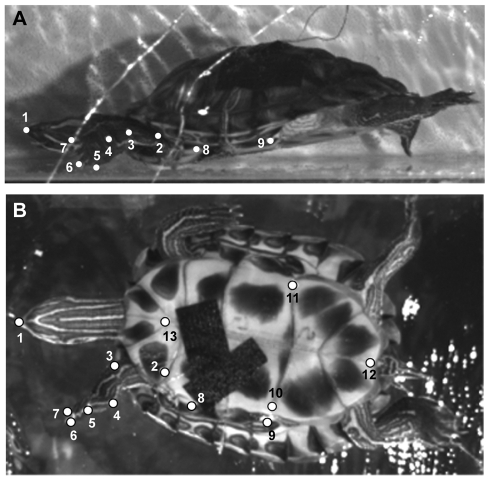
Fig. 1.
Representative still images from lateral (A) and ventral (B) videos showing landmarks digitized for kinematic analysis. Points 1–9 are the same in lateral and ventral view; points 10–13 are only visible in ventral view. Landmarks include: 1, tip of the nose; 2, shoulder; 3, elbow; 4, wrist; 5, digit 1; 6, digit 3; 7, digit 5; 8, anterior point on bridge; 9, posterior point on bridge; 10, point on left side of plastron; 11, point on right side of plastron; 12, posterior point on plastron; and 13, anterior point on plastron.
Kinematics were tested at speeds chosen by the animals (Pace et al., 2001) which, for terrestrial locomotion in particular, were difficult to control. Additionally, freshwater turtles typically swim faster than they walk (Blob et al., 2008). Because we sought to compare motor patterns for typical swimming and walking behaviors, we therefore collected data over a range of speeds for both behaviors. Swimming T. scripta completed limb cycles in 0.46±0.01 s (mean ± s.e.m.), whereas walking limb cycle durations averaged 1.03±0.04 s. Although there was greater variability in the time required to complete walking cycles (0.36–2.88 s) than swimming cycles (0.25–0.80 s) these ranges showed extensive overlap. No differences in kinematics (or muscle activity) were evident across the relatively broader range of speeds exhibited during walking.
Collection and analysis of electromyographic data
Concurrent with video acquisition, electromyography (EMG) was used to measure muscle firing patterns of target forelimb muscles (Loeb and Gans, 1986). Following previously established protocols (Loeb and Gans, 1986; Westneat and Walker, 1997; Gillis and Blob, 2001; Blob et al., 2008), turtles were anesthetized with intramuscular injections of ketamine HCl (90–100 mg kg–1) and bipolar fine-wire electrodes (0.05 mm diameter; insulated stainless steel; 0.5 mm barbs; California Fine Wire Co., Grover Beach, CA, USA) were implanted percutaneously into target muscles in the left forelimb using hypodermic needles. External landmarks for implants were determined prior to experiments through dissection, helping to ensure accurate placement of electrodes. Up to 12 implants were performed for each experiment, with target muscles receiving more than one electrode (typically 2 or 3, but occasionally up to 4) to help ensure successful recordings even if some electrodes failed. Electrode wires exiting the forelimb were allowed several centimeters of slack before being bundled together and glued into two separate cables that were directed ventrally and posteriorly to run along a segment of the plastron, and then dorsally along the curve of the bridge before being secured to the carapace using waterproof tape (Fig. 1). The anterior cable bundle contained electrodes from the medial side of the forelimb, and the posterior cable contained electrodes from the lateral side. Following electrode implantation, the locations of digitizing landmarks were marked (as described above) and turtles were allowed to recover overnight. During locomotor trials, EMG signals were relayed from the electrodes in each turtle to a Grass 15LT amplifier system (West Warwick, RI, USA) for amplification (usually 10,000 times, but occasionally set to 5000 times) and filtering (60 Hz notch filter, 30 Hz–6 kHz bandpass). Analog EMG signals were converted to digital data and collected at 5000 Hz using custom LabVIEW (v.6.1; National Instruments Corp., Austin, TX, USA) routines. Kinematic data were synchronized with electromyographic data by triggering a signal generator that simultaneously produced a light pulse visible in the video and a square wave in the EMG data. Following data collection, turtles were killed by intraperitoneal injection of sodium pentobarbital (200 mg kg–1) and electrode positions were verified by dissection.
We focused on five target muscles for this study, covering all major planes of motion of the forelimb during swimming and walking (Fig. 2). Predicted actions for each muscle were based on anatomical position (Walker, 1973). The coracobrachialis is positioned posterior to the humerus and expected to retract the forelimb. The pectoralis is a large, triangular sheet that extends widely from approximately the plastral midline to converge and insert on the flexor border of the lateral process of the humerus, and is predicted to retract and depress the humerus. The latissimus dorsi is positioned anterior and dorsal to the humerus and is predicted to protract and elevate the limb. The deltoid is located more ventrally, attaching to the plastron close to its midline and running to the shoulder joint, but also with predicted actions of humerus protraction and elevation. Finally, the triceps complex is located on the extensor surface of the arm, running from the shoulder joint to the elbow, and is predicted to act in elbow extension. Data were incidentally collected from two additional muscles: the supracoracoideus, a large ventral muscle deep to the pectoralis with anterior and posterior heads, is predicted to retract and depress the humerus [although some anterior fibers might aid protraction (Walker, 1973)]; and the subscapularis, the largest dorsal muscle on the pectoral girdle, covering the lateral, posterior and much of the medial surface of the scapula and predicted to elevate the humerus. The subscapularis was sampled using two different approaches; in a ‘cor approach’ the electrode was implanted into the muscle by inserting it more posteriorly and laterally (as if approaching the coracobrachialis), whereas in a ‘lat approach’ the electrode was implanted into the muscle by aiming more anteriorly (as if approaching the latissimus dorsi). These two approaches, and therefore separate segments of muscle, are henceforth, referred to as the subscapularis (cor approach) and subscapularis (lat approach).
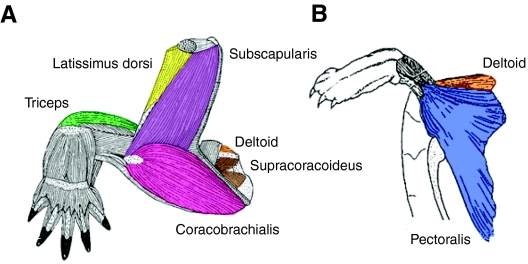
Fig. 2.
Illustration showing the five target muscles and two supplemental muscles from which electromyographic data were collected. (A) Posterior view of the left forelimb musculature of Trachemys scripta; modified from Walker (Walker, 1973). (B) Ventral view of the forelimb musculature of Trachemys scripta; modified from Wyneken (Wyneken, 1997). Predicted muscle actions are based on their anatomical positions. The coracobrachialis (pink) is situated posterior to the humerus and is expected to retract the forelimb. The most ventral target muscle, the pectoralis (blue) extends from the plastral midline towards the anterior margin of the bridge to a tendon that inserts on the lateral process of the humerus, and is predicted to retract and depress the humerus. The latissimus dorsi (yellow) is anterior and dorsal to the humerus and is predicted to protract and elevate the forelimb. More ventrally is the deltoid (orange), which runs from the plastron to the shoulder joint and is predicted to protract and elevate the humerus. The triceps (green) is located on the extensor surface of the arm, running from the shoulder joint distally to the elbow, and is predicted to act in elbow extension. The subscapularis (purple) is the largest of the dorsal pectoral girdle muscles, occupying much of the posterior, lateral and medial surfaces of the scapular prong, and is predicted to elevate the humerus. Supracoracoideus (brown) is deep to the pectoralis, divided into anterior and posterior heads, and predicted to retract the humerus.
EMG data were analyzed using custom LabVIEW software routines to identify bursts of muscle activity. EMG variables calculated included onset, offset and duration of muscle bursts, as well as mean amplitude of each burst (to provide a measure of intensity). The mean amplitude recorded from different electrodes should not be compared because minor differences in electrode construction can affect signal strength (Loeb and Gans, 1986). For this reason, burst intensities were normalized for each electrode by dividing the mean amplitude for each burst by the maximum value for mean amplitude recorded from that electrode throughout aquatic and terrestrial trials (Gillis and Biewener, 2000; Konow and Sanford, 2008). This enables the comparison of burst intensity across individuals, allowing us to determine if there are consistent patterns of intensity change between swimming and walking.
Statistical analysis
To assess general patterns of movement and muscle function, the overall mean and standard error of each variable was calculated for all terrestrial and aquatic trials. Muscle activity variables include for each muscle: (i) onset, (ii) offset, (iii) duration and (iv) normalized mean amplitude. Kinematic variables include: (i) maximum protraction, retraction, elevation and depression of the humerus, (ii) maximum elbow extension and flexion, (iii) anteroposterior and dorsoventral excursion of the humerus, (iv) elbow excursion, (v) percentage of the cycle at which maximum elbow extension occurs, (vi) the percentage of the limb cycle at which a switch from protraction to retraction occurs, and (vii) the degree of feathering of the forefoot during protraction. Because the maximum values for each limb cycle do not always occur at the same percentage of the limb cycle, it is possible that the average of the maximum values calculated for all limb cycles may be masked (appear lower) in average kinematic profiles. We used Systat (v.12) for all statistical analyses, and P<0.05 as the criterion for significance.
To determine the effect of environment on variables characterizing forelimb kinematics and muscle function, we conducted two-way, mixed-model analyses of variance (ANOVA), with environment as a fixed factor and individual as a random factor. Two-way mixed model ANOVAs (corrected for unbalanced sampling) were performed separately on each variable, except for the coracobrachialis, the supracoracoideus (anterior head) and the subscapularis (lat approach), which were sampled in an insufficient number of individuals, or incompletely within individuals, and which were, therefore, analyzed separately using one-way ANOVAs with habitat as the independent factor and values for each habitat pooled together. Two-way mixed model ANOVAs were calculated using individual variation as the error term, whereas one-way ANOVAs were calculated using cycle to cycle variation as the error term. One set of ANOVAs was performed on data from each muscle and on each kinematic variable; kinematic and timing variables include data from all recordings, but intensity comparisons only include data from individuals for which we successfully recorded both swimming and walking from the same electrode. In tabular data summaries we provide degrees of freedom and F-values, in addition to results of sequential Bonferroni corrections (Holm, 1979; Rice, 1989), to clarify the potential effects of making multiple comparisons. For statistical analyses of EMG timing variables (onset, offset, duration), only data from individuals with both aquatic and terrestrial EMG data were used (see supplementary material Table S2). For statistical analyses of EMG intensity variables, only data from individuals in which the same electrode successfully recorded during both aquatic and terrestrial trials were used (see supplementary material Table S2).
RESULTS
For kinematic analyses, 16–20 swimming and walking trials were obtained from each of six turtles, with a seventh providing a similar number of swimming trials but fewer walking trials (see supplementary material Table S1). The number of trials from which EMG data were collected is variable across individuals and muscles because of differences in the success of electrode implants. Plots depicting the general pattern of muscle activation during swimming and walking were constructed using all collected and verified EMG data (see supplementary material Table S3). A general summary of sample sizes from each individual, and from each environmental condition, are given for statistical analyses (see supplementary material Tables S1 and S2) and EMG timing variables (see supplementary material Table S3).
Kinematics of swimming and walking
Previously published descriptions of forelimb kinematics in swimming T. scripta (in the context of a comparison with an aquatic specialist Apalone spinifera) (Pace et al., 2001) were for larger individuals than those used in our study; we describe aquatic forelimb kinematics here with a focus on comparison with terrestrial kinematics and synchronization with EMG data. For both swimming and walking, the limb cycle is defined as starting at the beginning of humeral protraction and ending at the start of the next protraction cycle. The limb cycle can be divided into two separate phases; humeral protraction represents the ‘recovery’ phase in water or the ‘swing’ phase on land, followed by retraction of the humerus through the ‘thrust’ phase in water or the ‘stance’ phase on land.
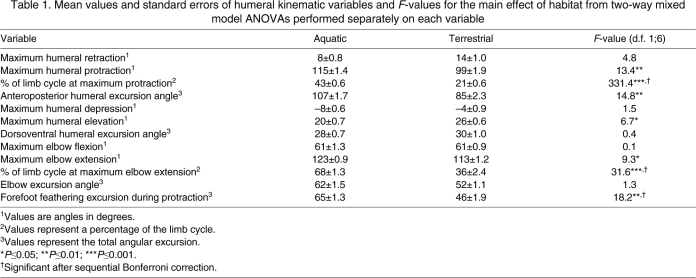
In both aquatic and terrestrial locomotion there is a single peak of humeral protraction. The duration of protraction differs significantly between swimming and walking, with protraction comprising the first 43±0.6% (mean ± s.e.m.) of the limb cycle in swimming, and only the first 21±0.6% of the cycle during walking (Fig. 3A; Table 1). The humerus is protracted significantly more during swimming (115±1.4 deg) than in walking (99±1.9 deg), although both locomotor behaviors are characterized by roughly similar humeral retraction (Fig. 3A). Total anteroposterior excursion of the humerus also differs significantly between the two environments, with the humerus experiencing a much larger range of motion during swimming (107±1.7 deg) than during walking (85±2.3 deg; Table 1; Fig. 3A).
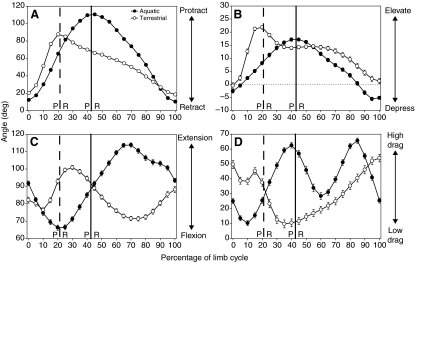
Fig. 3.
Mean kinematic profiles for Trachemys scripta during swimming (filled symbols) and walking (open symbols). Each trial was normalized to the same duration and angle values interpolated to 101 points representing 0–100% of the limb cycle. The limb cycle is defined as protraction of the humerus followed by retraction. Mean angle values ± s.e.m. are plotted for every fifth increment (every 5% through the cycle) for all individuals. Vertical lines demarcate the switch from protraction (P) to retraction (R) for swimming (solid line) and walking (dashed line). (A) Humeral protraction and retraction (i.e. angle from the transverse plane). An angle of 0 deg indicates that the humerus is perpendicular to the midline of the turtle, whereas an angle of 90 deg indicates a fully protracted forelimb with the distal end of the humerus directed anteriorly (an angle of –90 deg would indicate a fully retracted forelimb with the distal tip of the humerus directed posteriorly). (B) Humeral elevation and depression (i.e. angle from the horizontal plane). An angle of 0 deg indicates that the humerus is in the horizontal plane. Angles greater than zero indicate elevation above the horizontal (distal end above proximal end) and negative angles indicate depression of the humerus (distal end lower than proximal end). Peak elevation is coincident with peak protraction for both swimming and walking, meaning that limb protraction happens at the same time as elevation and retraction is concurrent with depression. (C) Elbow flexion and extension. Extension is indicated by larger angles and flexion is indicated by smaller angles. An angle of 0 deg indicates complete flexion, 180 deg indicates a fully extended elbow, and 90 deg indicates that the humerus is perpendicular to the radius and ulna. (D) Forefoot orientation angle is calculated as the angle between a vector pointing forwards along the anteroposterior midline (also the path of travel) and a vector emerging from the palmar surface of a plane defined by the tips of digits 1 and 5 and the wrist; this angle is transformed by subtracting 90 deg from each value. A high-drag orientation of the forefoot paddle with the palmar surface of the paddle directed opposite the direction of travel (and in the same direction as the flow of water) is indicated by a feathering angle of 90 deg, and a perfect low-drag orientation of the forefoot paddle is indicated by a feathering angle of 0 deg. Feathering of the forefoot paddle during retraction is obscured during walking because the foot is on the substrate and the limb is supporting the body.
Table 1.
Mean values and standard errors of humeral kinematic variables and F-values for the main effect of habitat from two-way mixed model ANOVAs performed separately on each variable
Peak humeral elevation (Fig. 3B) differs significantly between swimming (20±0.7 deg) and walking (26±0.6 deg; Table 1), and is roughly coincident with the switch from protraction to retraction (Table 1; Fig. 3A), indicating that the limb reaches maximum elevation in both swimming and walking at or near the end of recovery, or swing, phase. The humerus is greatly elevated during the recovery phase (i.e. swing phase; Fig. 3B) of walking as the limb is swung up and forward (Fig. 3A,B). Elevation of the humerus during the recovery phase of swimming is more gradual than that during the swing phase of walking (Fig. 3A,B). In both swimming and walking, the limb reaches its greatest anterior extent and elevation just before the beginning of retraction. At this point, the extreme angle of protraction of the humerus (115±1.4 deg for swimming and 99±1.9 deg for walking), shifts the position of the elbow medial to the shoulder and above the head [a result also found by Pace et al. (Pace et al., 2001) for swimming]. Maximum humeral depression and dorsoventral excursion of the humerus do not differ significantly between swimming and walking (Table 1). During retraction, the humerus is depressed while it is moved posteriorly until maximal retraction and depression are reached nearly simultaneously (Fig. 3A,B).
Elbow extension patterns differed between swimming and walking (Fig. 3C). During swimming, T. scripta flex the elbow for the first half of protraction and then begin elbow extension, reaching maximum extension midway through retraction, and then flexing the elbow for the remainder of the limb cycle to return to the starting position (Fig. 3C). During walking, as in swimming, the elbow is flexed until midway through protraction when extension begins (Fig. 3C). However, unlike swimming, maximum elbow extension is reached very early during terrestrial retraction, followed quickly by a period of elbow flexion as the limb begins to support the weight of the body, and a second phase of elbow extension follows as the body is propelled anteriorly relative to the supporting limb (Fig. 3C). Although maximum elbow flexion and excursion did not differ between swimming and walking, maximum elbow extension was significantly greater in swimming than in walking (123±0.9 deg versus 113±1.2 deg; Table 1) and occurred significantly later in the limb cycle (68±1.3% swimming versus 36±2.4% walking; Table 1).
The orientation of the forefoot relative to the direction of travel (or the direction of water flow) differs between swimming and walking (Fig. 3D). In water, this variable indicates whether the forefoot is in a high drag orientation with the plane of the forefoot perpendicular to the direction of travel, or a low drag (feathered) orientation (Pace et al., 2001). Similar to results of Pace et al. (Pace et al., 2001), the forefoot of T. scripta is feathered in a low-drag orientation early in protraction and reaches a first peak of high-drag orientation (nearly perpendicular to the flow of water) very near the end of protraction; this is followed by a second, high-drag peak at roughly two-thirds through the retraction phase, and ends with the palmar surface of the forefoot directed dorsally (Fig. 3D). During the protraction phase of walking, the forefoot is held in a less feathered orientation than in swimming, and the total feathering excursion angle experienced by the forefoot during protraction is significantly greater during swimming than walking (65±1.3 deg versus 46±1.9 deg; Fig. 3D; Table 1). During the stance phase of walking, the forefoot is placed flat relative to the ground, as it must support the weight of the body, but then gradually peels off the substrate to an angle more perpendicular to the ground.
In summary, although both swimming and walking are characterized by the same general motions of the forelimbs in T. scripta, there are several striking differences (Table 1). The timing of protraction and retraction differs greatly between swimming and walking, as does the maximum angle of humeral protraction and the anteroposterior excursion angle of the humerus, although the humerus is retracted to nearly the same degree in both environments. Peak elevation of the humerus is coincident with peak protraction in both environments, but while there is significantly greater elevation during walking, the level of humeral depression does not differ between habitats. The elbow is held straighter during walking, but with peak extension occurring significantly later in the limb cycle than during swimming. Finally, during protraction, sliders showed a much greater angular excursion range for orientation of the forefoot during swimming than during walking.
Patterns of muscle activation during swimming and walking
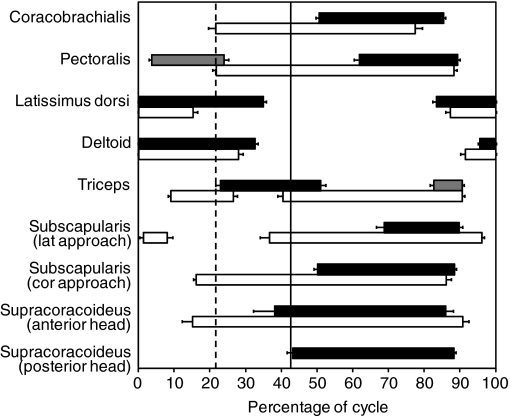
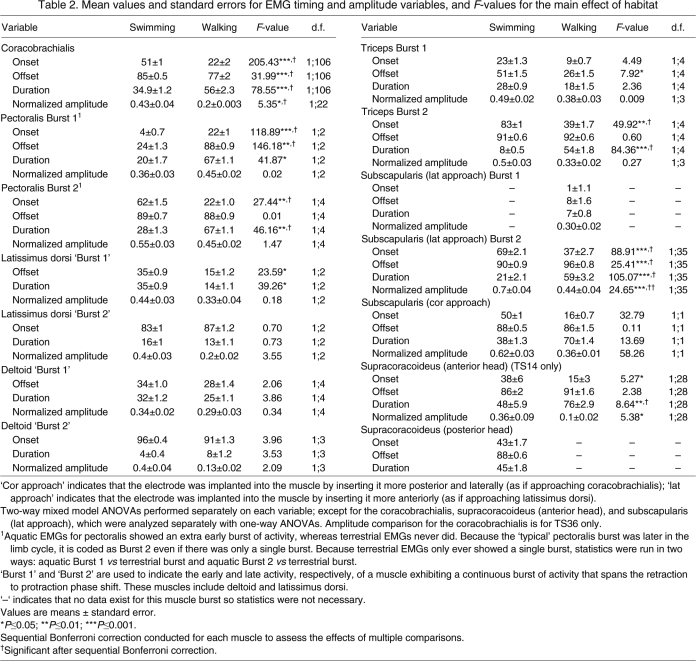
Among predicted humeral retractors, the coracobrachialis exhibits a single burst of activity during most of retraction phase in both swimming and walking, although onset, offset and duration of activity relative to the entire limb cycle differ significantly between environments for this muscle (Fig. 4, Table 2). By contrast, the other predicted humeral retractor, the pectoralis, exhibits two bursts of activity in swimming but only one during walking (Fig. 4). The early burst of activity seen in the pectoralis during swimming is variable, in that it was not present in every swimming cycle; two of five turtles never showed this early burst, one individual (TS09) always did, another did most of the time (TS11, 18 of 20 cycles), and the final turtle (TS99) seldom did (2 of 20 cycles). Verification dissections revealed no detectable differences in placement of the electrodes across turtles that varied with regard to the presence of this variable burst, and kinematics did not clearly differ in relation to whether the burst was present or absent. This early variable burst of pectoralis activity during swimming occurs fully during protraction when present, whereas the later burst of activity for pectoralis that was always present occurred nearly entirely during retraction in both environments. Because there is only one burst of activity in walking, this single burst was compared to both bursts of activity seen during swimming (Table 2). Comparison with the early burst seen in swimming shows significant differences for onset, duration and offset (Table 2) whereas comparison with the later burst during swimming shows significant differences in onset and duration, but not offset (Table 2).
Fig. 4.
Bar plot showing patterns of forelimb muscle activation during swimming and walking in Trachemys scripta. Bars represent the mean and standard error for the period of activity for each muscle. Solid bars represent swimming, open bars represent walking, and gray bars represent variable bursts of muscle activity observed during swimming that were not present in every trial. Vertical lines demarcate the switch from protraction to retraction for walking (dashed line) and swimming (solid line). The x-axis shows the percentage of the limb cycle from 0 to 100%. ‘Cor approach’ indicates that the electrode was implanted into the muscle by inserting it more posterior and laterally (as if approaching the coracobrachialis). ‘Lat approach’ indicates the electrode was implanted into the muscle by inserting it more anteriorly (as if approaching the latissimus dorsi). Note that data from the posterior head of the supracoracoideus were only obtained during swimming; this does not, however, indicate that there was no activity during walking.
Table 2.
Mean values and standard errors for EMG timing and amplitude variables, and F-values for the main effect of habitat
Among humeral protractors, the latissimus dorsi and deltoid both show one long continuous burst of activity in both environments, starting shortly before the end of retraction and continuing into the protraction phase (Fig. 4). Because our definition of the limb cycle divides these continuous bursts into two portions for graphic presentation, we will use quotation marks to distinguish references to the ‘early‘ and ‘late’ bursts (or ‘Burst 1’ and ‘Burst 2’) for these muscles, in contrast to references to separate, non-continuous bursts of activity in other muscles. Thus, for the latissimus dorsi and deltoid, onset refers to the beginning of activity observed for ‘Burst 2’ and offset refers to the end of activity observed for ‘Burst 1’. The onset of ‘Burst 1’ and the offset of ‘Burst 2’ always occur at 0% and 100% of the limb cycle, respectively. Offset and duration differ significantly between swimming and walking for latissimus dorsi ‘Burst 1’, with activity ceasing later (and duration longer) in swimming; however, there were no differences between environment in the onset or duration of ‘Burst 2’ (Fig. 4; Table 2). Unlike latissimus, timing variables did not differ significantly between swimming and walking for either the ‘early’ or ‘late’ deltoid ‘bursts’.
The triceps is characterized by two bursts for both swimming and walking; one burst straddling the switch from protraction to retraction and the other occurring during the retraction phase of the limb cycle (Fig. 4). The later triceps burst was always present during walking, but was variably present during swimming (Fig. 4), always occurring in two turtles (TS02 and TS99) and in between 50 and 75% of cycles in the remaining three (11 of 20 for TS11, 10 of 20 for TS14, and 15 of 20 for TS31) (supplementary material Table S3). Offset of Burst 1 of triceps activity occurs significantly later during swimming, with no significant differences in onset or duration of Burst 1 triceps activity, although, the timing of onset is visibly later during swimming (Fig. 4; Table 2). During swimming, onset of triceps Burst 2 occurs significantly earlier, and therefore duration is significantly longer; offset does not differ between habitats (Fig. 4; Table 2).
Among incidentally sampled muscles, subscapularis activity was recorded using electrodes implanted from two different approaches. The more posterior (cor approach) electrode of subscapularis detected a single burst for both swimming and walking, occurring mostly during retraction (Fig. 4). Although the offset of activity is not significantly different, the duration of activity is significantly longer during walking, with onset occurring visibly (but not significantly) earlier in the limb cycle (Table 2). The more anterior (lat approach) implantation of subscapularis shows differing patterns, with two bursts of activity during walking and only one during swimming (Fig. 4). The early burst of subscapularis (lat approach) during walking occurs early in the protraction phase (Fig. 4). The second burst of walking subscapularis (lat approach) activity and the single swimming burst occur during retraction, with the walking burst starting significantly earlier and ending significantly later (Fig. 4; Table 2). The anterior head of the supracoracoideus presents a single burst of activity in both swimming and walking, beginning just before the switch from protraction to retraction and lasting for most of retraction. Although the offset of activity for this muscle did not differ between environments, onset occurs significantly earlier in walking, resulting in a significantly longer duration (Fig. 4; Table 2). The posterior head of the supracoracoideus was only sampled successfully during swimming, when it showed one burst of activity starting just before, and continuing through, most of the retraction phase (Fig. 4).
Comparisons of the intensity of muscle activity (normalized mean amplitude) between habitats for the pectoralis (each aquatic burst versus the terrestrial burst), latissimus dorsi and deltoid (both ‘early’ and ‘late bursts’ of activity), triceps and subscapularis (cor approach) indicated no significant differences between water and land (Table 2). By contrast, swimming was characterized by greater intensity bursts for the coracobrachialis, subscapularis (lat approach) and supracoracoideus (anterior head; Table 2).
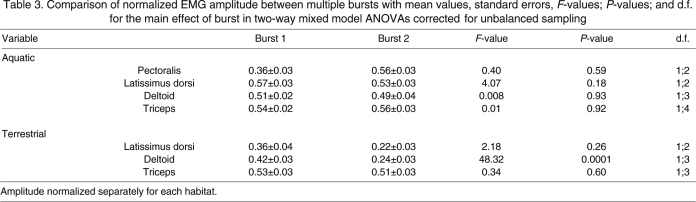
In cases where two bursts of activity were present for a muscle we tested for differences in intensity (Table 3). Two-way mixed-model ANOVAs detected no significant differences between bursts for the deltoid, latissimus dorsi, pectoralis or triceps during swimming or for latissimus dorsi or triceps during walking. The early period of deltoid activity during walking showed significantly higher mean amplitude than the later period (Tables 2 and 3).
Table 3.
Comparison of normalized EMG amplitude between multiple bursts with mean values, standard errors, F-values; P-values; and d.f. for the main effect of burst in two-way mixed model ANOVAs corrected for unbalanced sampling
DISCUSSION
We identified several differences in the kinematics of swimming and walking in Trachemys scripta, including a longer duration of protraction, greater maximum humeral protraction, less humeral elevation, and a feathered forefoot orientation during the protraction phase of swimming. Although most muscles examined were active when we predicted they would be, the triceps, pectoralis and subscapularis all showed additional bursts of activity. Contrary to our predictions, we found no difference in the intensity of protractor activity during swimming versus walking and several retractors actually exhibited higher intensity bursts during swimming. Motor patterns for forelimb protractors are not consistent with those observed in functionally analogous hindlimb muscles, but motor pattern modulations for forelimb retractors between water and land are largely parallel between the fore- and hindlimb.
Kinematic comparison of swimming and walking
Several key differences emerged in the forelimb kinematics of T. scripta between aquatic and terrestrial locomotion. First, the protraction (or recovery) phase during swimming lasts almost twice as long as swing phase during walking (43±0.6% versus 21±0.6% of the limb cycle). This means that roughly equal time is spent in recovery and thrust phase in swimming, but only about a fifth of the limb cycle is spent in swing during walking. With regard to angular excursions, a general pattern that emerged is that one extreme of a range of motion differs between environments but the other does not. For example, maximum humeral retraction does not differ between swimming and walking, but the forelimb is protracted significantly more during swimming, resulting in vastly different ranges of anteroposterior humeral excursion in the two behaviors (Fig. 3A; Table 1). Similar maximal retractions between habitats could reflect a limit to the amount of retraction that is possible for the humerus of T. scripta because of the presence of the bridge of the shell posterior to the shoulder. By contrast, greater protraction of the forelimb during swimming would allow greater posterior excursion of the forelimb during retraction relative to that during walking, a pattern that might affect aquatic thrust production (Pace et al., 2001), although specific functional benefits to such differences in motion patterns between habitats remain to be tested. Maximal humeral depression is also similar during swimming and walking, but the swing phase of walking is characterized by a much greater maximum elevation angle than the recovery phase of swimming (Fig. 3B; Table 1). This distinction also might reflect the different demands placed on the musculoskeletal system between aquatic and terrestrial locomotion. Because turtle limbs need to clear the substrate during swing phase on land, substantial humeral elevation might be needed during walking. However, in freshwater turtles, forward thrust during swimming is generated primarily through anteroposterior movements of the limbs, so extraneous dorsoventral motions might be detrimental to thrust production and would be expected to be limited (Pace et al., 2001).
Elbow kinematics also differ between swimming and walking (Fig. 3C). During swimming, the elbow flexes for the first half of protraction as the forelimb moves towards the level of the shoulder, then extends through the remainder of protraction until about halfway through humeral retraction (i.e. thrust phase), when the elbow starts to flex again to move the forelimb paddle through the greatest arc possible to generate thrust for swimming. During walking, the elbow is also flexed for the first half of protraction, until the forelimb is moved to the level of the shoulder. However, the elbow then extends only until it reaches a maximum shortly after the start of the retraction phase, during which a second flexion–extension cycle is performed as the limb receives the weight of the body and pushes off to complete the step. As in movements at the shoulder, only one extreme of the range of elbow motion differs between swimming and walking. Maximum elbow flexion is almost identical in the two behaviors (61±1.3 deg in swimming and 61±0.9 deg in walking), perhaps indicating a limit to the degree of elbow flexion possible. In contrast, maximum elbow extension is significantly greater during retraction in swimming, potentially facilitating aquatic thrust production (Pace et al., 2001). It is also possible that the restricted range of elbow extension during terrestrial locomotion would help to minimize vertical fluctuations of the center of mass, potentially minimizing energy loss during walking. A more terrestrial emydid, the ornate box turtle (Terrapene ornata), has recently been identified as an economical walker (Zani and Kram, 2008), although contributing limb kinematic mechanisms have not been addressed.
Foot kinematics also differ significantly between water and land. In swimming, foot movements lead to a feathered orientation for much of humeral protraction (recovery phase), helping to minimize drag as the foot is drawn forwards through the water (Fig. 3D). During walking, however, such a feathered forefoot orientation is not maintained during humeral protraction, perhaps in part because drag is not a substantial factor during swing phase on land.
Effect of habitat on forelimb muscle activation patterns
The majority of the pectoral girdle muscles examined are active at the portions of the limb cycle predicted from their anatomical positions. Coracobrachialis, pectoralis, and supracoracoideus (both heads) were confirmed to be active during humeral retraction and depression, whereas the latissimus dorsi and deltoid were confirmed to be active during humeral protraction and elevation (Fig. 4). The triceps, a predicted elbow extensor, was likewise found to be active during elbow extension.
However, our EMG data yielded some surprising findings. For example, with regard to burst intensity, we had predicted that limb protractors might show higher mean amplitude bursts during swimming to overcome the greater resistance to movement through water than air, whereas limb retractors might show greater activity on land in order to support the body without the benefit of buoyancy. Instead, most muscles did not exhibit significant differences in mean burst amplitude between habitats, and the few that did, including the coracobrachialis, subscapularis (lat approach), and the anterior head of supracoracoideus, ran contrary to our predictions, with all of these retractors exhibiting significantly higher mean amplitudes during swimming (Table 2).
Differences in the timing of activity patterns between habitats were more common than differences in burst intensity. Some of these seem to be straightforward reflections of differences in the durations of limb cycle phases between swimming and walking. For example, the later onset of coracobrachialis in water probably reflects the later initiation of humeral retraction during swimming, whereas the earlier offset of the latissimus dorsi on land matches the earlier end of protraction during walking (Fig. 4). However, some differences in the timing of muscle activity between habitats are more surprising. For instance, although the pectoralis was confirmed to be active during retraction in both habitats, swimming T. scripta display an additional early burst of activity that occurs during protraction (Fig. 4). This early burst in swimming is not present in all swimming cycles, but may act to stabilize the shoulder during humeral protraction when the limb is being moved through the dense aquatic medium. The lack of this stabilizing burst during walking may relate to the different demands being placed on the limbs during locomotion in water versus air. The ventrally situated pectoralis is in a position to depress the forelimb when it contracts. The timing of the early stabilizing activity seen during swimming would, during walking, occur during swing phase. During swing phase the forelimb is quite literally ‘swung’ forward and upward, with walking characterized by much greater humeral elevation than swimming (Fig. 3B; Table 1). In addition to the shoulder probably not requiring much stabilization while moving through less resistant air than through water, additional pectoralis activity during terrestrial swing phase would not only act counter to the forward movement of the limb but also counter to its elevation required to clear the ground.
Another unexpected finding, and difference in pattern between swimming and walking, is in the activity of the subscapularis. Although the posterior ‘cor approach’ shows a single burst of activity for both habitats, the more anterior ‘lat approach’ shows two bursts during walking and only a single burst during swimming (Fig. 4). In addition, this muscle is predicted to act during humeral elevation based on anatomical position (Walker, 1973), but most of its activity occurs during humeral retraction and depression. Walking T. scripta exhibit significantly greater humeral elevation, which may account for the early burst from the anterior (‘lat approach’) regions of subscapularis on land. Although our sample size for this muscle is limited (N=2 for ‘cor approach’, N=1 for ‘lat approach’), this muscle may be acting as a brake to reduce the amount of humeral depression during the thrust-producing power stroke.
The triceps also shows patterns that were not initially predicted. The triceps shows two bursts of activity in walking and swimming; although the early burst is always present in swimming, the later burst was variable, and both bursts were always present in walking. During walking, two periods of elbow extension occur roughly coincident with the two bursts of triceps activity (Fig. 3C, Fig. 4). During swimming, however, elbow extension only occurs from approximately 20–70% of the limb cycle, coinciding with the early burst of triceps activity. The later triceps activity during swimming may act to stabilize the elbow as the limb is brought closer to the body during thrust phase. Thus, identification of kinematic differences between environments was insufficient to predict the full range of differences in the motor patterns of the slider forelimb between water and land.
Comparison of forelimb and hindlimb motor patterns
Functional requirements for moving through an aquatic environment are quite different from those for moving on land. Predictions for the modulation of limb muscle motor patterns between these different habitats suggest that limb protractors might show more intense activity during swimming than in walking in order to accommodate the greater viscosity of water compared with air, while limb extensors might show more intense activity on land because bearing weight while moving could require higher forces than aquatic propulsion. However, these predictions are not universally borne out for the forelimb muscles we examined. Our data for T. scripta show no significant differences in intensity between swimming and walking for protractors. In fact, in most cases amplitude is very similar between swimming and walking for the two main forelimb protractors, latissimus dorsi and deltoid. Although not matching expectations based on physical differences between environments, EMG modulations for T. scripta forelimb protractors also differ from those seen in functionally analogous hindlimb protractors. The femoral protractors iliofemoralis (ILF) and puboischiofemoralis internus (PIFI), showed similar burst timing between swimming and walking in T. scripta, but different patterns of intensity modulation, with ILF showing greater amplitude in swimming as expected, but PIFI showing greater amplitude in walking (Gillis and Blob, 2001; Blob et al., 2008).
Modulation patterns exhibited by forelimb retractors and extensors also differed from predictions based on physical differences between the environments, as we found no differences in amplitude between swimming and walking for triceps or pectoralis, and coracobrachialis, subscapularis and supracoracoideus exhibited higher amplitude bursts during swimming than walking. However, although counter to our expectations based on physical differences between environments, patterns for the latter forelimb muscles do match patterns observed for functionally analogous hindlimb retractors femorotibialis (FT) and flexor tibialis internus (FTI) in T. scripta (Gillis and Blob, 2001; Blob et al., 2008), which also showed greater amplitude bursts during swimming. Thus, at least for propulsive phase muscles, motor pattern modulations between water and land in T. scripta are largely parallel in the fore- and hindlimb. It is possible that despite support of the body by buoyancy, the intensity of muscular effort required for propulsive rowing strokes through a viscous aquatic medium is greater than has previously been appreciated, perhaps because force transmission may be less efficient in water than on land. As a result, it might be reasonable to expect propulsive phase muscles (retractors) to show increased activity during swimming. Increased EMG amplitude does not necessarily correlate with higher force, because the force exerted by a muscle is dependent on both velocity and length (Loeb and Gans, 1986; Lieber, 2002), and differences in kinematics between environments could contribute to changes in both parameters. However, the potential for higher muscular forces during swimming might elevate expectations for the loads that would be placed on the limb skeleton during aquatic locomotion (Butcher and Blob, 2008; Butcher et al., 2008), although the direction of bone loading may differ substantially between the two habitats.
Comparisons with environmental modulations of motor patterns in other taxa
In most species examined to date, locomotion in different environments seems to consistently be accompanied by alterations in activity of major locomotor muscles (Ashley-Ross and Lauder, 1997; Gillis, 1998a; Gillis, 1998b; Gillis, 2000; Gillis and Biewener, 2000; Gillis and Biewener, 2001; Gillis and Blob, 2001; Higham and Jayne, 2004; Blob et al., 2008). These differences, which may be in the form of intensity, duration, timing or some combination of these variables, can even change the functional role of muscles between environments (Gillis and Blob, 2001). However, differences in the timing of muscle activity more commonly correlate with kinematic differences between habitats, and although changes in EMG amplitude between land and water are widespread, predicted differences based on the differing functional requirements of these environments are not always seen (Gillis and Blob, 2001; Blob et al., 2008).
A question that has received attention in many studies is which components of functional systems change during the evolution of new functions or behaviors (Westneat and Wainwright, 1989; Reilly and Lauder, 1992; Lauder and Reilly, 1996). The idea that new patterns of movement can be achieved while conserving the patterns of muscle activity is commonly described as the neuromotor conservation hypothesis (Peters and Goslow, 1983; Smith, 1994). Despite the drastic diversity in structure and locomotion across vertebrate taxa, remarkably similar patterns of limb muscle activation have been documented across behaviors ranging from sprawling and upright terrestrial locomotion to flight (Peters and Goslow, 1983; Goslow et al., 1989; Dial et al., 1991; Fish, 1996; Goslow et al., 2000). This has led to the hypothesis that patterns of neuromotor control for homologous tetrapod muscles are evolutionarily conserved, despite modifications to the limb muscles and skeleton for different uses (Jenkins and Goslow, 1983; Peters and Goslow, 1983; Smith, 1994).
Although T. scripta definitely exhibit some differences in muscle activity between swimming and walking (timing, intensity and number of bursts), the basic motor patterns involved in these behaviors are, in many ways, more similar than might be expected based on the dramatically different environmental conditions in which they are used. The differences that do exist typically correlate well with the required differences in kinematics between water and air. Examination of additional species could test if such patterns hold more broadly across turtles between environments. Additionally, with the presence of two distinct patterns of forelimb motion in lineages of swimming turtles – dorsoventral flapping in sea turtles (Davenport et al., 1984; Wyneken, 1997) and the anteroposterior rowing typical of most aquatic turtle species (Pace et al., 2001), evaluation of the conservation of swimming motor patterns across turtle species could provide a fruitful test of how muscle actions may evolve in concert with novel functions.
Supplementary Material
Acknowledgments
We would like to thank Gabriel Rivera, Jenn Seda, Shala Hankison, Margaret Ptacek, Erin Dougherty, Casey Gosnell and Jessica Hanson for help with animal care, and Casey Gosnell, Jessica Hanson and Jill Lutz for assistance with video analysis. We also thank Gabriel Rivera, Jeanette Wyneken, Tim Higham, Margaret Ptacek and two anonymous reviewers for comments on manuscript drafts. For statistical advice we thank Tim Higham, William Bridges and Michael Childress. Additionally, we are grateful to Warren F. Walker, Jr and Jeanette Wyneken for allowing us to modify their illustrations.
This project was supported by a Sigma Xi Grant-in-Aid-of-Research to A.R.V.R., NSF (IOS-0517340 to R.W.B.), and NIH (2 R01 DC005063-06A1 to E. Peterson, subaward UT10853 to R.W.B). Deposited in PMC for release after 12 months.
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/213/20/3515/DC1
REFERENCES
- Ashley-Ross M. A. (1995). Patterns of hindlimb motor output during walking in the salamander Dicamptodon tenebrosus, with comparisons to other tetrapods. J. Comp. Physiol. A 177, 273-285 [Google Scholar]
- Ashley-Ross M. A., Lauder G. V. (1997). Motor patterns and kinematics during backward walking in the Pacific giant salamander: Evidence for novel motor output. J. Neurophysiol. 78, 3047-3060 [DOI] [PubMed] [Google Scholar]
- Bennett D. H., Gibbons J. W., Franson J. C. (1970). Terrestrial activity in aquatic turtles. Ecology 51, 738-740 [Google Scholar]
- Biewener A. A., Daley M. A. (2007). Unsteady locomotion: integrating muscle function with whole body dynamics and neuromuscular control. J. Exp. Biol. 210, 2949-2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener A. A., Gillis G. B. (1999). Dynamics of muscle function during locomotion: accommodating variable conditions. J. Exp. Biol. 202, 3387-3396 [DOI] [PubMed] [Google Scholar]
- Blake R. W., Chatters L. M., Domenici P. (1995). Turning radius of yellowfin tuna (Thunnus albacares) in unsteady swimming manoeuvres. J. Fish Biol. 46, 536-538 [Google Scholar]
- Blob R. W., Rivera A. R. V., Westneat M. W. (2008). Hindlimb function in turtle locomotion: limb movements and muscular activation across taxa, environment, and ontogeny. In Biology of Turtles (ed. Wyneken J., Godfrey M. H., Bels V.), pp. 139-162 Boca Raton: CRC Press; [Google Scholar]
- Bodie J. R., Semlitsch R. D. (2000). Spatial and temporal use of floodplain habitats by lentic and lotic species of aquatic turtles. Oecologia 122, 138-146 [DOI] [PubMed] [Google Scholar]
- Buchanan T. S., Almdale D. P. J., Lewis J. L., Rymer W. Z. (1986). Characteristics of synergic relations during isometric contractions of human elbow muscles. J. Neurophysiol. 56, 1225-1241 [DOI] [PubMed] [Google Scholar]
- Buford J. A., Smith J. L. (1990). Adaptive control for backward quadrupedal walking. II. Hindlimb muscle synergies. J. Neurophysiol. 64, 756-766 [DOI] [PubMed] [Google Scholar]
- Butcher M. T., Blob R. W. (2008). Mechanics of limb bone loading during terrestrial locomotion in river cooter turtles (Pseudemys concinna). J. Exp. Biol. 211, 1187-1202 [DOI] [PubMed] [Google Scholar]
- Butcher M. T., Espinoza N. R., Cirilo S. R., Blob R. W. (2008). In vivo strains in the femur of river cooter turtles (Pseudemys concinna) during terrestrial locomotion: tests of force-platform models of loading mechanics. J. Exp. Biol. 211, 2397-2407 [DOI] [PubMed] [Google Scholar]
- Cagle F. R. (1944). Home range, homing behavior, and migration in turtles. Miscellaneous Publications, Museum of Zoology, University of Michigan 61, 1-34 [Google Scholar]
- Daley M. A., Biewener A. A. (2003). Muscle force-length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 206, 2941-2958 [DOI] [PubMed] [Google Scholar]
- Davenport J., Munks S. A., Oxford P. J. (1984). A comparison of the swimming in marine and freshwater turtles. Proc. R. Soc. Lond. B. Biol. Sci. 220, 447-475 [Google Scholar]
- Dial K. P., Goslow G. E., Jenkins F. A. (1991). The functional anatomy of the shoulder in the European starling (Sturnus vulgaris). J. Morphol. 207, 327-344 [DOI] [PubMed] [Google Scholar]
- Ernst C. H., Lovich J. E., Barbour R. W. (1994). Turtles of the United States and Canada. Washington: Smithsonian Institution Press; [Google Scholar]
- Fish F. E. (1996). Transitions from drag-based to lift-based propulsion in mammalian swimming. Am. Zool. 36, 628-641 [Google Scholar]
- Fish F. E. (2002). Balancing requirements for stability and maneuverability in cetaceans. Integr. Comp. Biol. 42, 85-93 [DOI] [PubMed] [Google Scholar]
- Fish F. E., Nicastro A. J. (2003). Aquatic turning performance by the whirligig beetle: constraints on maneuverability by a rigid biological system. J. Exp. Biol. 206, 1649-1656 [DOI] [PubMed] [Google Scholar]
- Gatesy S. M. (1997). An electromyographic analysis of hindlimb function in Alligator during terrestrial locomotion. J. Morphol. 234, 197-212 [DOI] [PubMed] [Google Scholar]
- Gatesy S. M. (1999). Guineafowl hindlimb function. II: Electromyographic analysis and motor pattern evolution. J. Morphol. 240, 127-142 [DOI] [PubMed] [Google Scholar]
- Gibbons J. W. (1970). Terrestrial activity and the population dynamics of aquatic turtles. Am. Midl. Nat. 83, 404-414 [Google Scholar]
- Gibbons J. W. (1990). Life History and Ecology of the Slider Turtle. Washington: Smithsonian Institution Press; [DOI] [PubMed] [Google Scholar]
- Gillis G. B. (1998a). Environmental effects on undulatory locomotion in the American eel (Anguilla rostrata): kinematics in water and on land. J. Exp. Biol. 201, 949-961 [Google Scholar]
- Gillis G. B. (1998b). Neuromotor control of anguilliform locomotion: patterns of red and white muscle activity during swimming in the American eel (Anguilla rostrata). J. Exp. Biol. 201, 3245-3256 [DOI] [PubMed] [Google Scholar]
- Gillis G. B. (2000). Patterns of white muscle activity during terrestrial locomotion in the American eel (Anguilla rostrata). J. Exp. Biol. 203, 471-480 [DOI] [PubMed] [Google Scholar]
- Gillis G. B., Biewener A. A. (2000). Hindlimb extensor muscle function during jumping and swimming in the toad (Bufo marinus). J. Exp. Biol. 203, 3547-3563 [DOI] [PubMed] [Google Scholar]
- Gillis G. B., Biewener A. A. (2001). Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus). J. Exp. Biol. 204, 2717-2731 [DOI] [PubMed] [Google Scholar]
- Gillis G. B., Biewener A. A. (2002). Effects of surface grade on proximal hindlimb muscle strain and activation during rat locomotion. J. Appl. Physiol. 93, 1731-1743 [DOI] [PubMed] [Google Scholar]
- Gillis G. B., Blob R. W. (2001). How muscles accommodate movement in different physical environments: aquatic vs. terrestrial locomotion in vertebrates. Comp. Biochem. Physiol. 131A, 61-75 [DOI] [PubMed] [Google Scholar]
- Goslow G. E., Dial K. P., Jenkins F. A. (1989). The avian shoulder: an experimental approach. Am. Zool. 29, 287-301 [Google Scholar]
- Goslow G. E., Wilson D., Poore S. O. (2000). Neuromuscular correlates to the evolution of flapping flight in birds. Brain Behav. Evol. 55, 85-99 [DOI] [PubMed] [Google Scholar]
- Gruner J. A., Altman J. (1980). Swimming in the rat: analysis of locomotor performance in comparison to stepping. Exp. Brain Res. 40, 374-382 [DOI] [PubMed] [Google Scholar]
- Hedrick T. L. (2008). Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 3, 034001 [DOI] [PubMed] [Google Scholar]
- Higham T. E., Jayne B. C. (2004). In vivo muscle activity in the hindlimb of the arboreal lizard, Chamaeleo calyptratus: general patterns and the effects of incline. J. Exp. Biol. 207, 249-261 [DOI] [PubMed] [Google Scholar]
- Holm S. (1979). A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65-70 [Google Scholar]
- Horner A. M., Jayne B. C. (2008). The effects of viscosity on the axial motor pattern and kinematics of the African lungfish (Protopterus annectens) during lateral undulatory swimming. J. Exp. Biol. 211, 1612-1622 [DOI] [PubMed] [Google Scholar]
- Jenkins F. A., Goslow G. E. (1983). The functional anatomy of the shoulder of the Savannah monitor lizard (Varanus ecanthematicus). J. Morphol. 175, 195-216 [DOI] [PubMed] [Google Scholar]
- Johnston R. M., Bekoff A. (1996). Patterns of muscle activity during different behaviors in chicks: implications for neural control. J. Comp. Physiol. 179A, 169-184 [DOI] [PubMed] [Google Scholar]
- Kamel L. T., Peters S. E., Bashor D. P. (1996). Hopping and swimming in the leopard frog, Rana pipiens: II. A comparison of muscle activities. J. Morphol. 230, 17-31 [DOI] [PubMed] [Google Scholar]
- Konow N., Sanford C. P. J. (2008). Is a convergently derived muscle-activity pattern driving novel raking behaviours in teleost fishes? J. Exp. Biol. 211, 989-999 [DOI] [PubMed] [Google Scholar]
- Lauder G. V., Reilly S. M. (1996). The mechanistic bases of behavioral evolution: a multivariate analysis of musculoskeletal function. In Phylogenies and the Comparative Method in Animal Behavior (ed. Martins E. P.), pp. 104-137 New York: Oxford University Press; [Google Scholar]
- Lieber R. L. (2002). Skeletal Muscle Structure, Function, and Plasticity. Philadelphia: Lippencott Williams and Wilkins; [Google Scholar]
- Loeb G. E., Gans C. (1986). Electromyography for Experimentalists. Chicago: The University of Chicago Press; [Google Scholar]
- Macpherson J. M. (1991). How flexible are muscle synergies? In Motor Control: Concepts and Issues (ed. Humphrey D. R., Freund H. J.), pp. 33-47 Chichester, UK: John Wiley and Sons; [Google Scholar]
- Pace C. M., Gibb A. C. (2009). Mudskipper pectoral fin kinematics in aquatic and terrestrial environments. J. Exp. Biol. 212, 2279-2286 [DOI] [PubMed] [Google Scholar]
- Pace C. M., Blob R. W., Westneat M. W. (2001). Comparative kinematics of the forelimb during swimming in red-eared slider (Trachemys scripta) and spiny softshell (Apalone spinifera) turtles. J. Exp. Biol. 204, 3261-3271 [DOI] [PubMed] [Google Scholar]
- Peters S. E., Goslow G. E. (1983). From salamanders to mammals: continuity in musculoskeletal function during locomotion. Brain Behav. Evol. 22, 191-197 [DOI] [PubMed] [Google Scholar]
- Pratt C. A., Buford J. A., Smith J. L. (1996). Adaptive control for backward quadrupedal walking: V. Mutable activation of bifunctional thigh muscles. J. Neurophysiol. 75, 832-842 [DOI] [PubMed] [Google Scholar]
- Reilly S. M., Blob R. W. (2003). Motor control of locomotor hindlimb posture in the American alligator (Alligator mississippiensis). J. Exp. Biol. 206, 4341-4351 [DOI] [PubMed] [Google Scholar]
- Reilly S. M., Lauder G. V. (1992). Morphology, behavior, and evolution: comparative kinematics of aquatic feeding in salamanders. Brain Behav. Evol. 40, 182-196 [DOI] [PubMed] [Google Scholar]
- Rice W. R. (1989). Analyzing tables of statistical tests. Evolution 43, 223-225 [DOI] [PubMed] [Google Scholar]
- Rivera G., Rivera A. R. V., Dougherty E. E., Blob R. W. (2006). Aquatic turning performance of painted turtles (Chrysemys picta) and functional consequences of a rigid body design. J. Exp. Biol. 209, 4203-4213 [DOI] [PubMed] [Google Scholar]
- Roy R. R., Hirota W. K., Kuehl M., Edgerton V. R. (1985). Recruitment patterns in the rat hindlimb muscle during swimming. Brain Res. 337, 175-178 [DOI] [PubMed] [Google Scholar]
- Roy R. R., Hutchinson D. L., Pierotti D. J., Hodgson J. A., Edgerton V. R. (1991). EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J. Appl. Physiol. 70, 2522-2529 [DOI] [PubMed] [Google Scholar]
- Smith K. K. (1994). Are neuromotor systems conserved in evolution? Brain Behav. Evol. 43, 293-305 [DOI] [PubMed] [Google Scholar]
- Walker J. A. (1998). Estimating velocities and accelerations of animal locomotion: a simulation experiment comparing numerically different algorithms. J. Exp. Biol. 201, 981-995 [Google Scholar]
- Walker J. A. (2000). Does a rigid body limit maneuverability? J. Exp. Biol. 203, 3391-3396 [DOI] [PubMed] [Google Scholar]
- Walker W. F., Jr (1973). The locomotor apparatus of Testudines. In Biology of the Reptilia, Vol. 4, Morphology D (ed. Gans C., Parsons T. S.), pp. 1-100 London: Academic Press; [Google Scholar]
- Westneat M. W., Wainwright P. C. (1989). Feeding mechanism of Epibulus insidiator (Labridae, Teleostei): Evolution of a novel functional system. J. Morphol. 202, 129-150 [DOI] [PubMed] [Google Scholar]
- Westneat M. W., Walker J. A. (1997). Motor patterns of labriform locomotion: Kinematic and electromyographic analysis of pectoral fin swimming in the labrid fish Gomphosus varius. J. Exp. Biol. 200, 1881-1893 [DOI] [PubMed] [Google Scholar]
- Wyneken J. (1997). Sea turtle locomotion: mechanisms, behavior, and energetics. In The Biology of Sea Turtles (ed. Lutz P. L., Musick J. A.), pp. 165-198 Boca Raton, FL: CRC Press; [Google Scholar]
- Zani P. A., Kram R. (2008). Low metabolic cost of locomotion in ornate box turtles, Terrapene ornata. J. Exp. Biol. 211, 3671-3676 [DOI] [PubMed] [Google Scholar]
- Zug G. R. (1971). Buoyancy, locomotion, morphology of the pelvic girdle and hind limb, and systematics of cryptodiran turtles. Miscellaneous Publications, Museum of Zoology, University of Michigan 142, 1-98 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.