Abstract
Purpose
The intrinsic drug resistance of colorectal cancers is related in part to overexpression of prosurvival Bcl-2 family proteins. We determined the effects of ABT-737, a small-molecule inhibitor of Bcl-2/Bcl-xL but not Mcl-1, on apoptosis induction alone and in combination with CPT-11 and explored mechanisms underlying their cooperativity.
Experimental Design
Human colorectal carcinoma cell lines (HCT116 wild-type and Bax−/−, HT-29, and RKO) were incubated with ABT-737 alone and combined with CPT-11or bortezomib, and cell viability, caspase cleavage, and Annexin V labeling were measured. In drug-treated cell lines, protein-protein interactions were analyzed by immunoprecipitation. Lentiviral short hairpin RNA was used to knockdown Noxa expression.
Results
ABT-737 induced apoptosis in a dose-dependent manner and its coadministration with the topoisomerase I inhibitor, CPT-11, resulted in a synergistic cytotoxic effect. Apoptosis induction by the drug combination was associated with enhanced caspase-8, caspase-9, and cas-pase-3 activation and poly(ADP-ribose) polymerase cleavage that were completely abrogated in Bax knockout cells. ABT-737 unsequestered the BH3-only protein Bim from its complex with Bcl-xL or Bcl-2 and disrupted the interaction of Bcl-xL with Bak. CPT-11treatment up-regulated Noxa expression, as did bortezomib, and enhanced Noxa/Mcl-1complexes. CPT-11also disrupted the Mcl-1/Bak interaction. Knockdown of Noxa using short hairpin RNA lentiviral constructs was shown to significantly attenuate the cytotoxic effect of CPT-11 or bortezomib combined with ABT-737 and inhibited caspase-3 cleavage.
Conclusions
Induction of Noxa by CPT-11or bortezomib can sensitize colorectal cancer cells expressing Mcl-1to ABT-737. Up-regulation of Noxa may therefore represent an important strategy to enhance the therapeutic efficacy of ABT-737 against colorectal cancer and other solid tumors.
Colorectal cancer is the third most common cancer and second leading cause of cancer-related mortality in the United States (1). This malignancy displays intrinsic apoptosis resistance related to the overexpression of prosurvival Bcl-2 proteins (2). Accordingly, new drugs are needed to circumvent Bcl-2-mediated resistance and to increase therapeutic efficacy. Proapoptotic BH3-only proteins are sensors of cellular stresses, including chemotherapeutic drugs, which bind to the hydrophobic cleft in prosurvival Bcl-2 family proteins to neutralize them, thereby shifting the balance in favor of proapoptotic molecules. Disabling prosurvival Bcl-2 proteins removes their restraint of downstream Bax and Bak. At least eight BH3-only members have been identified and these include Bad, Bid, Bik, Bim, Bmf, Hrk, Noxa, and Puma (3). The BH3-only proteins can be further divided into two subclasses: “activators” (e.g., Bim and tBid), which directly activate Bax/Bak to induce mitochondria outer membrane permeabilization, and “sensitizers” (e.g., Bad, Bik, Bmf, Hrk, Noxa, and Puma), which do not activate Bax/Bak directly but instead neutralize prosurvival proteins (4). Studies indicate that BH3-only proteins bind promiscuously or selectively to prosurvival Bcl-2 proteins (5–7). Bim and Puma have been shown to target all prosurvival proteins and, accordingly, are more potent inducers of apoptosis in vitro than are Bad and Noxa which target only a subset (6).
Recently, BH3 mimetics have been developed as a new and novel class of anticancer drugs. ABT-737 is a BH3 mimetic and potent small-molecule antagonist that binds with high affinity to Bcl-2, Bcl-xL, and Bcl-w but not Mcl-1 (8). ABT-737 has been shown to lower the apoptotic threshold for certain chemotherapeutic agents and also showed impressive preclinical activity against lymphoma in a murine model (8, 9). ABT-737 has shown single-agent activity against leukemia, lymphoma, and small cell lung cancer in preclinical models where high levels of Bcl-2 and/or Bcl-xL and low levels or absent Mcl-1 were found (10–12). Given that ABT-737 binds to Mcl-1 with low affinity, tumor cell Mcl-1 expression has been shown to represent an important mechanism of resistance to this agent (10, 11, 13).
Colorectal cancers have been shown to frequently express prosurvival members of the Bcl-2 protein family (2). Although sparse data exist as to the activity of ABT-737 against solid tumor cell lines, it is likely that significant antitumor efficacy against colorectal cancers may require ABT-737 in combination with cytotoxic chemotherapy (12). CPT-11 (irinotecan) is widely used for the treatment of advanced colorectal cancer in humans. CPT-11 is a camptothecin derivative and DNA topoisomerase I inhibitor that is metabolized by carboxylesterases in vivo to SN-38 and is believed to block DNA transcription and replication (14). However, apoptosis resistance related to prosurvival Bcl-2 proteins (15) and p53 inactivation (16) limit the therapeutic efficacy of CPT-11, resulting in treatment failure and disease progression. Therefore, strategies to enhance the apoptotic susceptibility of tumor cells to CPT-11 and other chemotherapeutic agents is a major objective of cancer treatment.
In this study, we determined the ability of ABT-737 to enhance CPT-11-mediated cell killing in human colorectal cancer cells. We found that the ABT-737 and CPT-11 produced a synergistic cytotoxicity that was due to a Bax-dependent induction of apoptosis. Specifically, ABT-737 released Bim from its sequestration by Bcl-2 or Bcl-xL and released Bak from Bcl-xL. Furthermore, CPT-11 treatment markedly induced Noxa expression, which complexed with Mcl-1, and was associated with Bak release from Mcl-1.
Materials and Methods
Cell culture, drugs, and reagents
Human colorectal cancer cell lines HCT116, HT-29, RKO, and HCT116 Bax−/− knockout cells (gift of Dr. B. Vogelstein, Johns Hopkins University) were used. Cell lines were cultured in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum and with 1% penicillin/streptomycin, 10 mmol/L HEPES, and 1% sodium pyruvate. ABT-737 (Abbott), CPT-11 (Sigma), SN-38 (United States Biological), or bortezomib (PS-341; Millennium) were dissolved in DMSO to produce 20 or 10 mmol/L stock solutions that were aliquoted and stored at −20°C.
Cell viability assay
Cell viability was determined in the presence or absence of drug treatment using the MTS reduction assay per the manufacturer’s protocol (Promega), as previously described (17). To exclude interference by CPT-11 or SN-38 in the MTS assay, we added each drug individually in the absence of cells and showed no change in absorbance.
Annexin V staining
After drug treatment, adherent cells were detached from culture dishes by treating with Accutase (Innovative Cell Technologies) for 5 to 15 min and combined with floating cells. Total cells were then washed with cold PBS twice and resuspended in 1× Annexin V binding buffer (BD Bioscience) at a concentration of 1 × 106 to 1 × 107 cells/mL. A single-cell suspension (100 μL) was stained with 10 μL Annexin V-FITC (BD Bioscience) for 15 min at room temperature in the dark. Propidium iodide (10 μL; Sigma) and 1× Annexin V binding buffer (380 μL) were then added to each tube. Two-color (FL1 and FL2) flow cytometry analysis was then done on a FACScan (Becton Dickinson). A minimum of 10,000 cells per sample were analyzed.
Immunoprecipitation and Western blotting
Mock-treated or drug-treated cells were harvested by scraping and then washed in cold PBS. After centrifugation, the cell pellet was lysed in CHAPS buffer [5 mmol/L MgCl2, 137 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% CHAPS, 10 mmol/L HEPES (pH 7.5)] for 30 min on ice with protease inhibitors (5 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 10 μg/mL pepstatin). The cell lysate was subjected to IP as previously described (17). The antibodies used were as follows: Bim (Santa Cruz Biotechnology), Bak (Upstate Biotechnology), and Mcl-1 (BD Biosciences). Western blotting was performed as previously described (17). The antibodies used were as follows: Bcl-2 (BD Biosciences); Bcl-xL (Cell Signaling); Mcl-1 (BD Biosciences); Bax (Cell Signaling); Bak (Upstate Biotechnology); β-tubulin (Sigma); caspase-8 (BD Biosciences); Bid, caspase-9, and cleaved caspase-3 (all from Cell Signaling); poly(ADP-ribose) polymerase (Biomol); Bim (Santa Cruz Biotechnology); Puma (Cell Signaling); Noxa (Calbiochem); and p53 (Cell Signaling).
Knockdown of Noxa using lentiviral short hairpin RNA
Target sequences for Noxa and a control sequence were selected and short hairpin RNA (shRNA) template oligonucleotides (synthesized by the Mayo Clinic Molecular Biology Core Facility) were ligated into the lentiviral shRNA cloning and expression vector pSIH1-H1 (System Bioscience) using a quick ligation kit (New England Biolabs). Insertion sequence at the intended site was confirmed by sequencing. The control shRNA sequence was AATAGCGACTAAACACATCAA (18). The targeting sequences for Noxa were GTAATTATTGACACATTTCTT (Noxa) and GAAGGTGCATTCATGGGTG (Noxa 3; refs. 19, 20). For lentivirus production, 2 μg endotoxin-free lentiviral shRNA expression construct DNA was mixed with 20 μg lentivector packaging plasmid DNA mix (System Bioscience) and diluted in 400 μL serum reduction medium (Opti-MEM; Invitrogen) containing 20 μL Plus reagent (Invitrogen). After incubation at room temperature for 15 min, 30 μL Lipofectamine reagent mixed with 400 μL Opti-MEM was added dropwise into the above DNA/Plus complex and incubated for another 15 min. The lentivirus producer cell line 293T was transfected with the DNA/Lipofectamine/Plus complex overnight in Opti-MEM in the 5% CO2 incubator at 37°C. The next day, the medium was replaced with fresh DMEM containing 2% heat-activated fetal bovine serum and incubation at 37°C was continued. At 48 h post-transfection, the supernatants were collected, clarified, and filtered through Millex-HV 0.45 μm polyvinylidene difluoride filters (Millipore). The supernatants were concentrated by adding 10% (final concentration) of PEG-8000 (Sigma), incubating at 4°C overnight for no less than 12 h, and centrifuged at 1,500 × g for 10 min at 4°C. For transduction of the lentiviral shRNA expression construct (packaged in pseudotyped viral particles) into target cells, the growth medium of target cells was replaced with Opti-MEM containing 8 μg/mL polybrene (Sigma) and appropriate amounts of lentivirus. The cells were incubated overnight at 37°C. The medium was replaced with normal growth medium the following day. The efficiency of knockdown was tested 72 h post-transduction. Alternatively, 2 to 4 μg/mL puromycin (Sigma) was added at 48 h post-transduction and the puromycin-resistant pool of cells was used for subsequent experiments.
Calculation of combination index
The effect of combination between ABT-737 and CPT-11 at a fixed ratio (1:1) was analyzed using Calcusyn software (Biosoft) as reported previously (21).
Statistical analysis
The values shown represent the mean ± SD for triplicate experiments. The statistical significance of the differences between experimental variables was determined using the Student’s t test. P < 0.05 was considered statistically significant.
Results
ABT-737 and CPT-11 cooperatively enhance cytotoxicity due to apoptosis
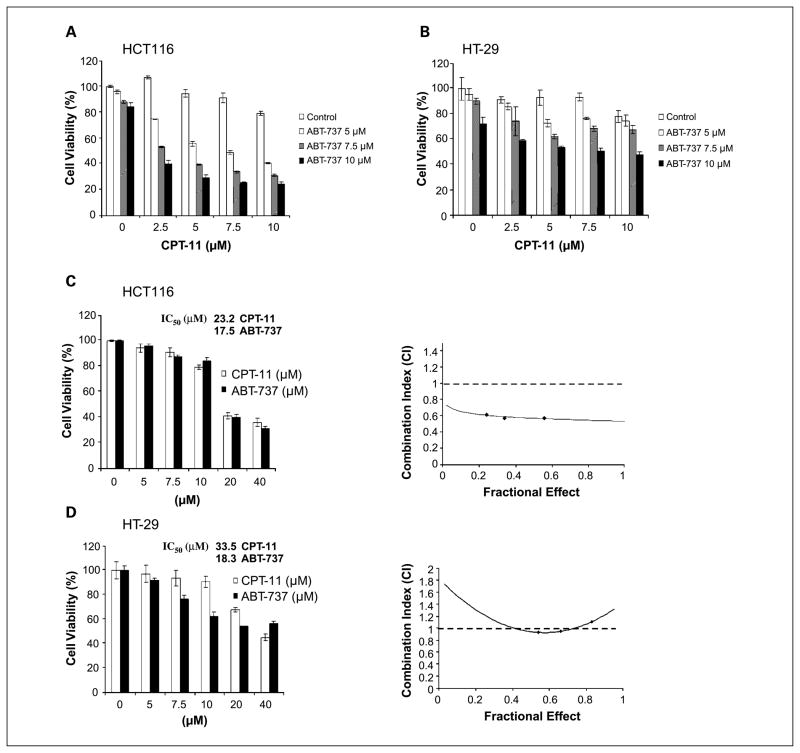
Treatment of HCT116 and HT-29 cell lines with ABT-737 (5–10 μmol/L) alone produced a modest reduction in cell viability. Furthermore, coadministration of ABT-737 (5–10 μmol/L) and CPT-11 (2.5–10 μmol/L) was shown to reduce cell viability to a greater extent than did either drug alone (Fig. 1A and B). To determine whether the cytotoxic effect of the drug combination was synergistic or additive, we performed an analysis using the median effect method. HCT116 and HT-29 cell lines were treated with ABT-737 or CPT-11 for 48 h and their IC50 values were calculated (Fig. 1C and D). The cell lines were treated with different concentrations of ABT-737 and CPT-11 at a fixed ratio (1:1) and cell viability was determined. The combination index (CI) was then calculated per the method of Chou and Talalay (22). As shown in an isobologram, the CI values in HCT116 cells were <1, indicating a synergistic interaction (Fig. 1C). In HT-29 cells, calculation of the CI revealed that the combination of ABT-737 and CPT-11 was additive (Fig. 1D).
Fig. 1.
ABT-737 and CPT-11 cooperatively enhance cytotoxicity. Cultured HCT116 (A) and HT-29 (B) human colorectal cancer cell lines were incubated with ABT-737 and/or CPT-11for 48 h at indicated doses. Cell viability was determined using the MTS reduction assay. Experiments were conducted in triplicate. Mean ± SD. HCT116 (C) and HT-29 (D) cells were treated with increasing concentrations of ABT-737 or CPT-11for 48 h and the cell viability was determined using the MTS assay. IC50 values forABT-737 or CPT-11were calculated. Cells were treated with ABT-737 and CPT-11at a fixed ratio (1:1), cell viability was measured, and the CI was calculated (see Materials and Methods). Isobologram showing CI < 1indicates synergy.
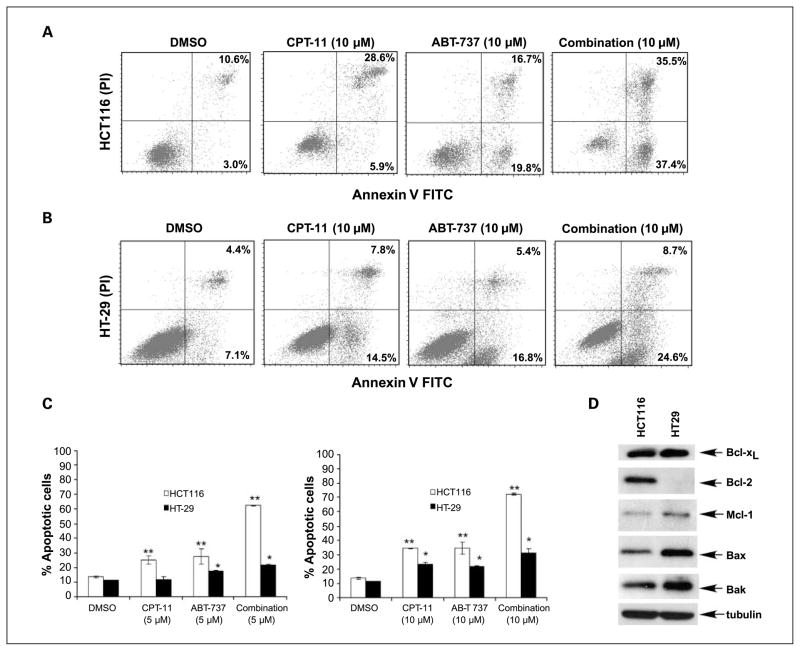
We analyzed and quantified the apoptotic effect of ABT-737 or CPT-11 alone and in combination by Annexin V staining. ABT-737 plus CPT-11 induced apoptosis to a greater extent than did either drug alone in both cell lines (Fig. 2A-C). ABT-737 induced apoptosis to a greater extent in HCT116 versus HT-29 cells, which may be explained by lower levels of endogenous Mcl-1 and higher levels of Bcl-2 in HCT116 cells (Fig. 2D). Furthermore, the drug combination produced a 1.7-fold increase in cell death compared with ABT-737 (5 μmol/L) alone in HT-29 cells versus a 3.5-fold increase in HCT116 cells.
Fig. 2.
ABT-737 and CPT-11 cooperatively enhance apoptosis induction. Flow cytometric analysis of Annexin V and propidium iodide staining in HCT116 (A) and HT-29 (B) cells treated with vehicle (DMSO), CPT-11, ABT-737, or their combination (24 h) at the indicated doses. C, extent of drug-induced cell death (percentage) in HCT116 (A) and HT-29 (B) cell lines are shown in histograms. Mean ± SD of triplicate experiments. *, P < 0.005; **, P < 0.0005, relative to DMSO. D, endogenous expression of prosurvival (Bcl-2, Bcl-xL, and Mcl-1) and proapoptotic (Bax and Bak) proteins was determined by immunoblotting in whole-cell lysates of HCT116 and HT-29 cells. β-Tubulin was used as a control for protein loading.
ABT-737 and CPT-11 cooperatively enhance apoptotic signaling
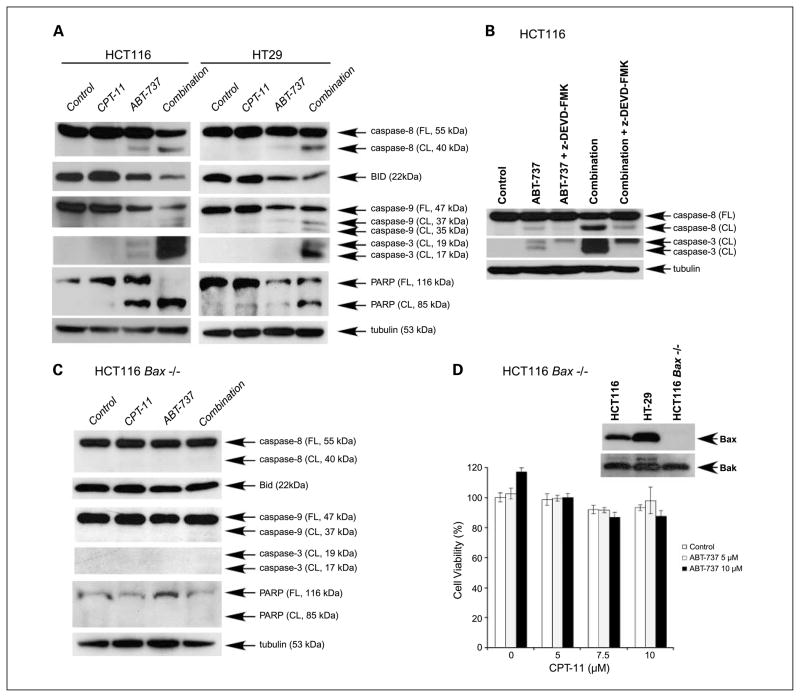
Exposure of HCT116 and HT-29 cell lines to the combination of ABT-737 plus CPT-11 resulted in enhanced activation of caspase-8, caspase-9, and caspase-3 and cleavage of Bid and poly(ADP-ribose) polymerase (PARP) compared with treatment with either drug alone (Fig. 3A). To determine whether drug-induced caspase-8 activation is due to a feedback amplification loop mediated by caspase-3 (23), we used a caspase-3 inhibitor (z-DEVD-FMK). z-DEVD-FMK was shown to attenuate caspase-8 cleavage by ABT-737 and its combination with CPT-11 in HCT116 cells (Fig. 3B), consistent with a feedback amplification loop mediated by caspase-3. To confirm that cell death occurred by an apoptotic mechanism, Bax knockout HCT116 cells were used. Treatment of these cells with ABT-737, CPT-11, or their combination failed to activate caspases or to cleave PARP (Fig. 3C), and their cytotoxic effects were completely abrogated (Fig. 3D).
Fig. 3.
ABT-737 and CPT-11enhance Bax-dependent apoptotic signaling. A, human colon carcinoma cell lines HCT116 or HT-29 were incubated with vehicle (DMSO), ABT-737 (10 μmol/L), CPT-11 (10 μmol/L), or their combination for 24 h. The effects of drug treatment on cleavage of caspases, Bid, and poly(ADP-ribose) polymerase (PARP) were determined in whole-cell lysates subjected to immunoblotting. β-Tubulin served as a control for protein loading. B, HCT116 cells were incubated with vehicle (DMSO) orABT-737 (10 μmol/L) alone or in combination with CPT-11 (10 μmol/L) for 24 h and caspase-8 and caspase-3 cleavage were analyzed in the presence or absence of a caspase-3 inhibitor (z-DEVD-FMK, 20 μmol/L). Experiments were done in whole-cell lysates subjected to immunoblotting. C, HCT116 Bax−/− cells were incubated with vehicle (DMSO), ABT-737 (10 μmol/L), CPT-11 (10 μmol/L), or their combination for 24 h. Caspase-8, caspase-9, and caspase-3 cleavage and Bid and PARP cleavage were determined. D, effect of ABT-737 and/or CPT-11at the indicated doses on cell viability (MTS assay; 48 h) was also determined in HCT116 Bax−/− cells. Experiments were conducted in triplicate. Mean ± SD.
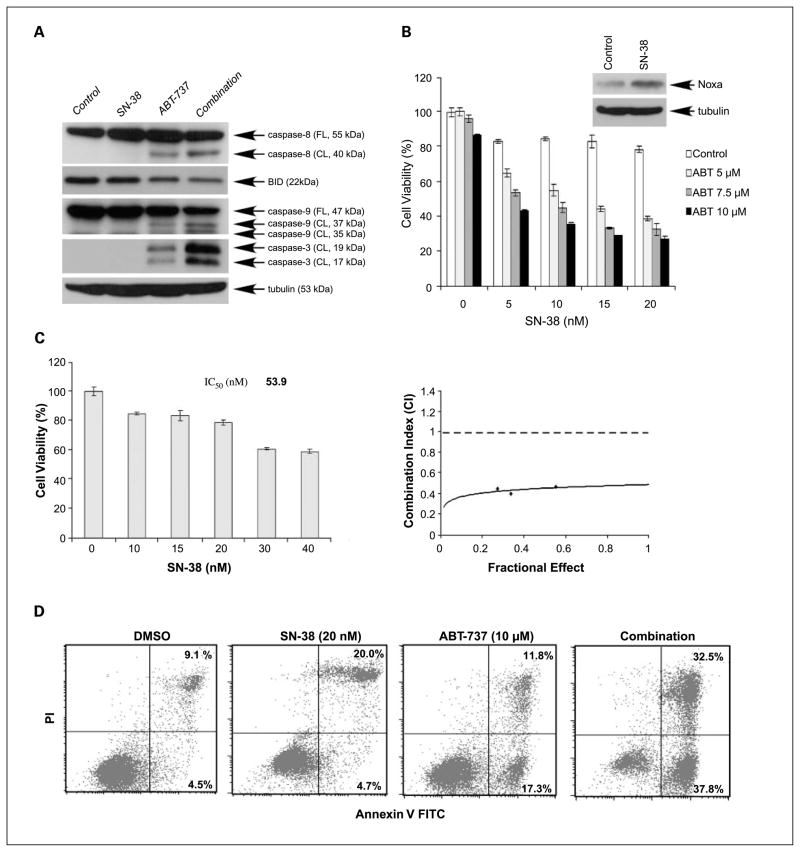
Given that SN-38 is the active metabolite of CPT-11, we evaluated the effects of the combination of SN-38 and ABT-737 on caspase activation and cytotoxicity. SN-38 was used at nanomolar dosages because it has been shown to be ~1,000-fold more potent than CPT-11 (15, 24, 25). The combination with SN-38 and ABT-737 enhanced caspase cleavage (Fig. 4A) and cooperatively reduced cell viability (Fig. 4B) to a greater extent than did either drug alone in HCT116 cells. We determined the IC50 value for SN-38 (53.9 nmol/L) and evaluated the combination of SN-38 and ABT-737 at fixed ratios. Calculation of the CI of SN-38 and ABT-737 yielded CI < 1, indicating a synergistic interaction, as shown in an isobologram (Fig. 4C). We also measured apoptosis induction by SN-38, ABT-737, or their combination by Annexin V staining. The drug combination induced apoptosis to a greater extent than did either drug alone (Fig. 4D).
Fig. 4.
A, HCT116 cells were incubated with ABT-737 (10 μmol/L), SN-38 (10 nmol/L), or their combination for 24 h. DMSO was used as a vehicle control. The effects of drug treatment on caspase cleavage and Bid were analyzed by immunoblotting. B, cytotoxic effects of ABT-737 and/or SN-38 treatment for 48 h at indicated doses were determined using the MTS assay. Experiments were conducted in triplicate. Mean ± SD. Inset, treatment of HCT116 cells with SN-38 (10 nmol/L) was shown to induce Noxa expression. C, HCT116 cells were treated with increasing concentrations of SN-38 for 48 h and the cell viability was determined using the MTS assay. The IC50 value for SN-38 was calculated. Cells were treated with ABT-737 and SN-38 at a fixed ratio (500:1) and the CI was calculated (see Materials and Methods). An isobologram showing CI < 1indicates synergy. D, apoptosis was determined using Annexin V and propidium iodide staining in HCT116 cells treated with vehicle (DMSO), SN-38, ABT-737, or their combination for 24 h at the indicated doses by FACS analysis.
ABT-737 displaces Bim and Bak from its complex with Bcl-xL/Bcl-2 or Bcl-xL
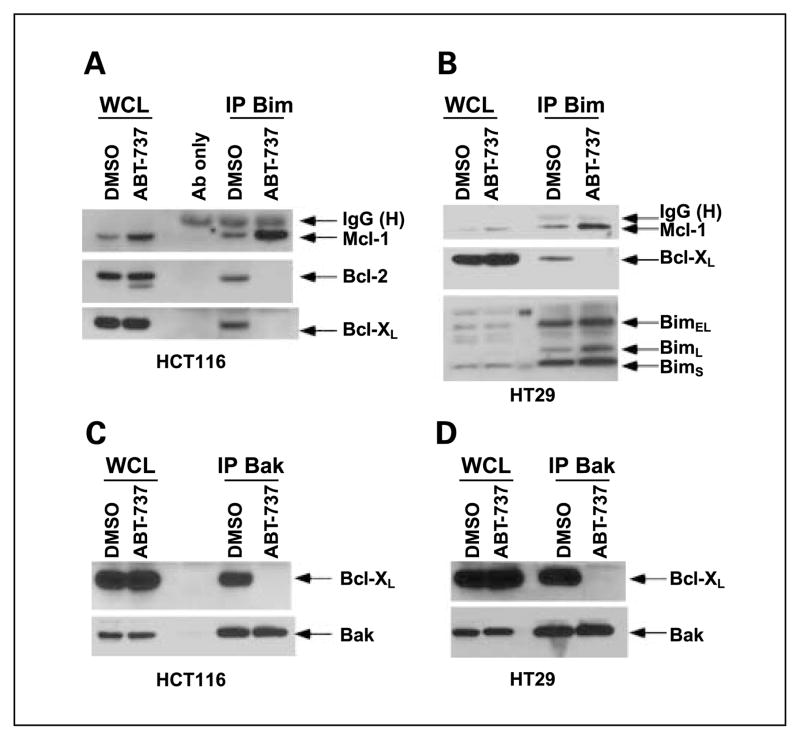
We studied the mechanism by which ABT-737 can potentiate CPT-11-induced apoptosis. As a BH3 mimetic, ABT-737 binds to and neutralizes Bcl-2/Bcl-xL and can thereby disrupt important protein-protein interactions. Bim can bind to all prosurvival Bcl-2 proteins and is therefore a potent proapoptotic molecule (3). We determined the effect of ABT-737 treatment on the interaction between Bim and Bcl-2 proteins by immunoprecipitation of Bim and then probing for Bcl-xL, Bcl-2, and Mcl-1. ABT-737 treatment was shown to unsequester Bim from its complex with Bcl-xL or Bcl-2 in HCT116 cells compared with control-treated cells (Fig. 5A). ABT-737 also displaced Bim from its complex with Bcl-xL in HT-29 cells that lack endogenous Bcl-2 (Fig. 5B). We also observed that ABT-737 can induce Mcl-1 expression and Bim/Mcl-1 complexes in both cell lines (Fig. 5A and B).
Fig. 5.
ABT-737 displaces Bim or Bak from its complex with Bcl-xL/Bcl-2 or Bcl-xL. HCT116 (A) and HT-29 (B) cells were treated with ABT-737 (10 μmol/L) or vehicle (DMSO) for 24 h and immunoprecipitation was done for Bim proteins. Whole-cell lysates (WCL) from beads and eluted proteins from beads (IP) were separated by SDS-PAGE and then probed for Bim, Bcl-xL, Bcl-2, or Mcl-1proteins. HCT116 (C) and HT-29 (D) cells were incubated with vehicle (DMSO) or ABT-737 (10 μmol/L) for 24 h and immunoprecipitation was done for Bak proteins. Whole-cell lysates were then probed for Bak or Bcl-xL proteins.
Recent studies have shown the importance of untethering Bak from Bcl-xL in determining the lethality of ABT-737 (26). Accordingly, we studied the effect of ABT-737 on the interaction between Bak and Bcl-xL in HCT116 and HT-29 cells. By immunoprecipitation of Bak and probing for Bcl-xL, we found that ABT-737 can disrupt the binding of Bak to Bcl-xL in both cell lines (Fig. 5C and D). Taken together, our data show that ABT-737 can activate Bax and unsequester Bak, which function to permeabilize the outer mitochondrial membrane resulting in cellular commitment to apoptosis.
CPT-11 up-regulates Noxa to sequester Mcl-1 and to disrupt Mcl-1/Bak complexes
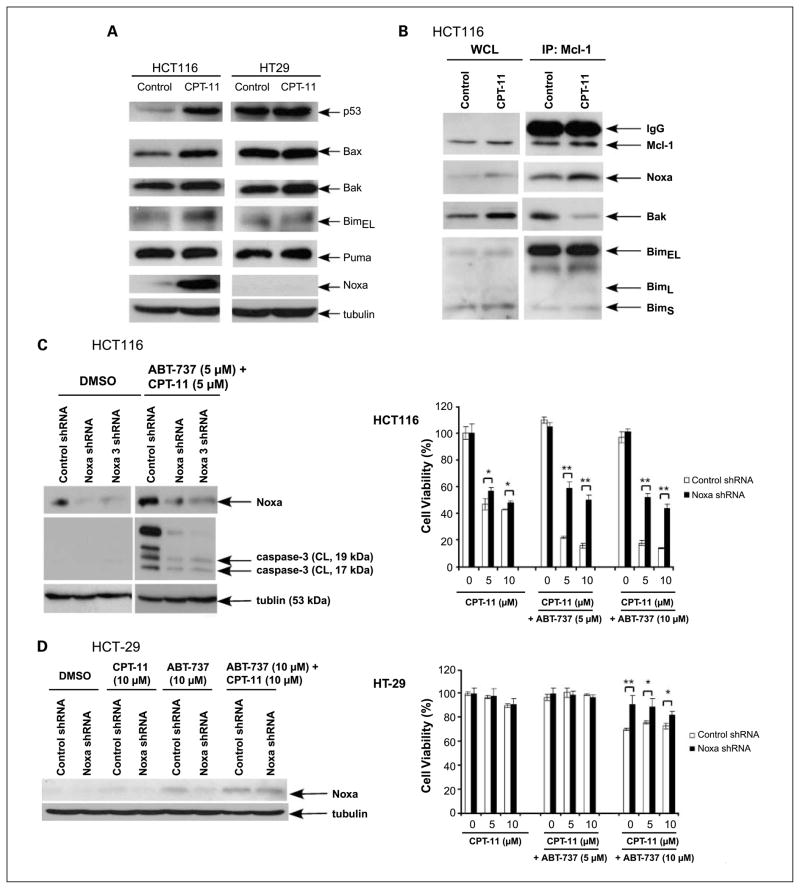
Noxa is a BH3-only protein whose expression can be induced by p53-dependent or p53-independent apoptotic stimuli (27). In HCT116 (wild-type p53) but not HT-29 (mutated p53) cells, CPT-11 treatment markedly induced expression of Noxa and also up-regulated Bax compared with vehicle-only treated cells (Fig. 6A). We also found that SN-38 significantly up-regulated Noxa expression in HCT116 cells (Fig. 4B). Given the induction of Noxa by CPT-11 in HCT116 cells, we determined the effect of drug treatment on the interaction between Noxa and its high-affinity partner Mcl-1 that result in Noxa/Mcl-1 complexes (3, 19). To address this issue, we immunoprecipitated Mcl-1 and probed for Noxa in HCT116 cells treated with CPT-11 or vehicle. CPT-11 treatment was shown to enhance the interaction of Mcl-1 and Noxa (Fig. 6B). It has been proposed that up-regulation of Noxa can activate Bak by displacing it from Mcl-1 (26). Consistent with this model, treatment with CPT-11 was shown to release Bak from its interaction with Mcl-1 (Fig. 6B). Although a prior study suggested that Mcl-1 binding of endogenously induced Noxa can interfere with the ability of Mcl-1 to sequester Bim (28), CPT-11 treatment failed to show an appreciable effect on Mcl-1 and Bim complexes (Fig. 6B). Taken together, induction of Noxa by CPT-11 can sequester Mcl-1 and also release Bak from Mcl-1, which may contribute to the enhanced apoptotic effect of the CPT-11 and ABT-737 combination.
Fig. 6.
CPT-11up-regulates Noxa to sequester Mcl-1and to disrupt Mcl-1/Bak complexes. Knockdown of Noxa attenuates the cytotoxicity of CPT-11plus ABT-737. The effect of CPT-11treatment on the expression of BH3-only and proapoptotic proteins was determined. A, HCT116 and HT-29 cells were incubated with CPT-11 (10 μmol/L) for 48 h and whole-cell lysates were subjected to immunoblot analysis for the expression of indicated proteins. B, HCT116 cells were treated with vehicle (DMSO) or CPT-11 (10 μmol/L) for 24 h and immunoprecipitation was done for Mcl-1 proteins. Whole-cell lysates from beads and eluted proteins from beads were separated by SDS-PAGE and then probed for Mcl-1, Bim, Noxa, or Bak proteins. C and D, knockdown of Noxa was achieved using lentiviral-delivered shRNA in HCT116 (C, left) or HT-29 (D, left) cells and the level of Noxa expression was determined in untreated (vehicle) and drug-treated cells by immunoblotting. HCT116 cells (C, left) were treated with vehicle (DMSO) or the combination of ABT-737 and CPT-11at the indicated doses for 24 h and caspase-3 cleavage was analyzed in whole-cell lysates subjected to immunoblotting. β-Tubulin served as a control for protein loading. We then determined the effect of Noxa knockdown versus control shRNA on cell viability in drug-treated HCT116 (C, right) and HT-29 (D, right) cell lines. Cell viability was determined using two Noxa shRNA constructs in HCT116 cells, and similar results for the Noxa and Noxa 3 (Supplementary Fig. S1) constructs were found. Cells were incubated with ABT-737 and/or CPT-11for 48 h at the indicated doses and cell viability was determined using the MTS assay. Experiments were conducted in triplicate. Mean ± SD. *, P < 0.005; **, P < 0.0005.
Knockdown of Noxa attenuates the cytotoxicity of CPT-11 plus ABT-737
To further show the importance of Noxa up-regulation as a potential mechanism for the synergistic cytotoxic effect of CPT-11 and ABT-737, we generated Noxa knockdown HCT116 cells using lentiviral-delivered shRNA. Knockdown of Noxa was found to significantly reduce sensitivity to CPT-11 plus ABT-737 as shown using two shRNA constructs (Fig. 6C; Supplementary Fig. S1). Furthermore, Noxa knockdown blocked the activation of caspase-3 induced by the drug combination (Fig. 6C). Because CPT-11 did not induce Noxa in HT-29 cells, we generated Noxa knockdown HT-29 cells. Knockdown of Noxa had no effect on the sensitivity of HT-29 cells to the combination of ABT-737 and CPT-11, although a slight protection was seen at an ABT-737 dose of 10 μmol/L (Fig. 6D). At a dose of 10 μmol/L, ABT-737 was shown to weakly induce Noxa (Fig. 6D). Together, these data show that Noxa is a key regulator of apoptotic susceptibility to this drug combination.
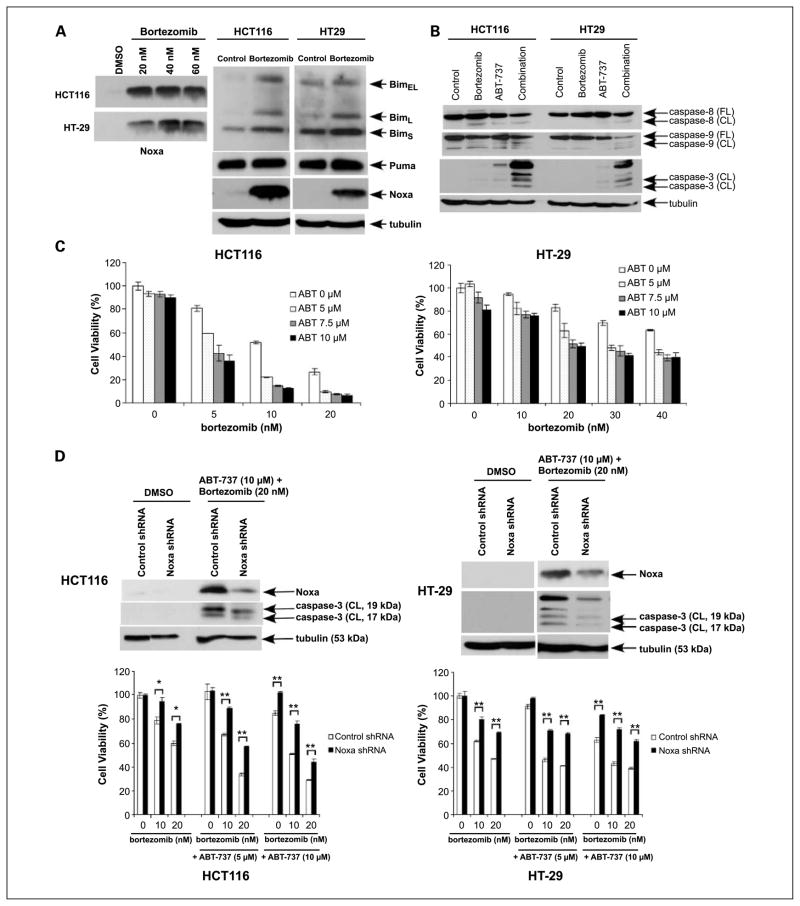
To show that induction of Noxa represents an important mechanism to increase apoptotic susceptibility, we used the proteasome inhibitor bortezomib that has been shown to induce Noxa in human myeloma cells (19), mantle-cell lymphoma cells (29), and non-small cell lung cancer cells (30). Treatment of HCT116 and HT-29 cell lines with bortezomib (20–60 nmol/L) was shown to markedly induce Noxa in both cell lines (Fig. 7A). Coadministration of ABT-737 and bortezomib was shown to enhance caspase cleavage (Fig. 7B) and to enhance cytotoxicity in both cell lines to a greater extent compared with either drug alone (Fig. 7C). Using Noxa knockdown HCT116 and HT-29 cells, we observed that Noxa shRNA protected cells from caspase-3 cleavage and cytotoxicity induced by bortezomib plus ABT-737 (Fig. 7D). We also found that CPT-11 can induce Noxa in another colon cancer cell line, RKO, which expresses high levels of Mcl-1. In these cells, ABT-737 was again shown to enhance CPT-11-mediated cytotoxicity and caspase cleavage (Supplementary Fig. S2). These data suggest that our findings can be generalized to other colon cancer cell lines.
Fig. 7.
Bortezomib up-regulates Noxa to cooperatively enhance the effects of ABT-737 on cytotoxicity and apoptosis. A, HCT116 and HT-29 cells were treated with bortezomib (20–60 nmol/L) and Noxa expression was analyzed by immunoblotting. Cells were treated with bortezomib (20 nmol/L) or vehicle (DMSO) for 24 h and Bim, Puma, and Noxa expression was determined in whole-cell lysates by immunoblot analysis. B, HCT116 and HT-29 cells were incubated with vehicle (DMSO), ABT-737 (10 μmol/L), and/or bortezomib (20 nmol/L) for 24 h and their effects on caspase cleavage were analyzed in whole-cell lysates subjected to immunoblotting. β-Tubulin served as a control for protein loading. C, cytotoxic effects of ABT-737 and/or bortezomib treatment for 48 h at indicated doses were determined in HCT116 and HT-29 cells using the MTS assay. Experiments were conducted in triplicate. Mean ± SD. D, knockdown of Noxa was achieved using lentiviral-delivered shRNA in HCT116 (left) and HT-29 (right) cells. Cells were incubated with ABT-737 (10 μmol/L) and bortezomib (20 nmol/L) or vehicle (DMSO) for 24 h. Noxa expression and caspase-3 cleavage were then analyzed by immunoblotting. β-Tubulin served as a control for protein loading. The effects of ABT-737 and/or bortezomib treatment (48 h) at indicated doses on cell viability were determined using the MTS assay in the presence of Noxa shRNA or control shRNA-transduced HCT116 (left) and HT-29 (right) cell lines. Experiments were conducted in triplicate. Mean ± SD. *, P < 0.005; **, P < 0.0005.
Discussion
ABT-737 is a potent small-molecule antagonist of prosurvival Bcl-2 proteins (8). Although sparse data exist as to the activity of ABT-737 in solid tumor cell lines, this drug has been shown to lower the apoptotic threshold for certain chemotherapeutic agents (8, 12). Colorectal cancers display intrinsic apoptosis resistance related, in part, to overexpression of prosurvival Bcl-2 proteins (2). Therefore, we determined whether the combination of ABT-737 and CPT-11 exerts an enhanced apoptotic effect in human colon cancer cells. We found that ABT-737 mono-therapy induced a dose-dependent apoptosis in HCT116 and HT-29 colon cancer cells. Moreover, we show that coadministration of ABT-737 with CPT-11 results in a synergistic (HCT116) or additive (HT-29) cytotoxic effect that is caspase-dependent and requires Bax as shown using Bax knockout HCT116 cells where caspase cleavage and apoptosis induction were completely abrogated. We also show that nanomolar doses of SN-38, the active metabolite of CPT-11, in combination with ABT-737 exerts a synergistic cytotoxic effect that is due to apoptosis. Highly concordant results with SN-38 and CPT-11 support the biological relevance of our observations.
The ability of ABT-737 to enhance apoptosis induction was due to its ability to disrupt the interaction of Bcl-xL with Bak and to displace Bim from its sequestration by Bcl-xL or Bcl-2 in both cell lines. Recent studies have shown the importance of unsequestering Bak from Bcl-xL in determining the lethality of ABT-737 (26). We also found that CPT-11 releases Bak from Mcl-1; together, these data for ABT-737 and CPT-11 are consistent with the indirect activation model (3). Recently, we (17) reported that ABT-737 can release Bim from Bcl-2 or Bcl-xL and that Bim shRNA can attenuate the cytotoxic effects of ABT-737 and TRAIL in human pancreatic cancer cell lines. Potentially, the ABT-737-induced dissociation of Bim from Bcl-2 or Bcl-xL may contribute to Bax activation. In this regard, Bim may act directly on Bax/Bak as shown by the observation that Bim, but not a Puma BH3 peptide, was sufficient to induce oligomerization and activation of Bax and Bak to permeabilize the outer mitochondrial membrane (5). Further evidence for the direct activation model is that activation of Bax or Bak by Bim has been shown in thymocytes from Bim/Bax or Bim/Bak double-knockout mice (31). Bim is a potent inducer of apoptosis because Bim, Puma, and tBid can neutralize all prosurvival Bcl-2 proteins, whereas Bad and Noxa show selectivity (6).
The ability of ABT-737 to release Bim from Bcl-xL or Bcl-2 may enable its complex formation with Mcl-1, as shown in both cell lines, and this Bim: Mcl-1 complex may attenuate the apoptotic effect of ABT-737. Unsequestered Bim has been shown to stabilize Mcl-1 (32) and this may account for the increase in Mcl-1 expression seen in ABT-737-treated cells. We found that CPT-11 or SN-38 treatment up-regulated Noxa expression in HCT116 cells, consistent with the role of BH3-only proteins, which act as molecular sensors of cellular stress or damage (6, 26, 33). Furthermore, CPT-11 was shown to increase Noxa/Mcl-1 complexes and also to disrupt the interaction of Mcl-1 with Bak. This finding is consistent with the observation that up-regulated Noxa promotes Bak activation by displacing it from Mcl-1 (26, 29). Noxa can bind specifically to Mcl-1 and A-1 but not to Bcl-2 or Bcl-xL (6). Higher Noxa levels have been observed in cell lines sensitive to ABT-737, and ectopic expression of Noxa in a resistant cell line increased its sensitivity to ABT-737 (12). The converse has been shown for Mcl-1 in that ABT-737 binds to Mcl-1 with low affinity; thus, Mcl-1 reduces responsiveness to ABT-737 (12, 13). To confirm the importance of Noxa in the synergistic cytotoxicity of CPT-11 plus ABT-737, suppression of Noxa by shRNA was shown to markedly attenuate the cytotoxic effect of this drug combination and blocked caspase-3 cleavage in HCT116 cells. In HT-29 cells where CPT-11 did not induce Noxa, knockdown of Noxa failed to confer resistance to CPT-11 plus ABT-737. To confirm the role of Noxa in sensitizing cells to apoptosis, we used bortezomib that is known to induce Noxa in myeloma cells (19) and did so in our colon cancer cell lines. The combination of bortezomib and ABT-737 enhanced apoptosis induction in HCT116 and HT-29 cells compared with either drug alone, and this effect was attenuated using Noxa shRNA constructs.
In summary, we show that ABT-737 and CPT-11 induce a cooperative cytotoxic effect against human colorectal carcinomas cell lines. ABT-737 antagonizes Bcl-2/Bcl-xL to release Bim and Bak. Although ABT-737 does not target Mcl-1 (10, 11), our data indicate that CPT-11-induced up-regulation of Noxa can sequester Mcl-1 and thereby disrupt Mcl-1/Bak complexes to enhance apoptotic susceptibility. Together, these findings suggest that Noxa induction can sensitize tumors expressing Mcl-1 to ABT-737 and thereby suggest a strategy to improve the therapeutic efficacy of ABT-737 against human colorectal cancers.
Translational Relevance.
Colorectal cancers display intrinsic drug resistance due to overexpression of prosurvival Bcl-2 family proteins. Recently, small-molecule antagonists of Bcl-2 proteins, also known as BH3 mimetics, have been developed and represent a promising therapeutic strategy. These novel compounds bind to the BH3 domain of prosurvival Bcl-2 proteins to neutralize them and thus enable Bax and Bak activation. BH3-only proteins, including Noxa, are induced by cellular stress including chemotherapy, and gene knockout or suppression of BH3 protein expression confers apoptosis resistance. ABT-737 is a BH3 mimetic that selectively inhibits Bcl-2 and Bcl-xL but not Mcl-1. Therefore, strategies to disable Mcl-1 may increase the efficacy of ABT-737. Noxa is a high-affinity binding partner of Mcl-1, and in human colon carcinoma cells, we show that CPT-11 or bortezomib can induce Noxa expression and thereby sequester Mcl-1to enhance apoptosis induction. Therefore, induction of Noxa in cancer cells expressing Mcl-1 represents a promising strategy to increase the therapeutic efficacy of ABT-737.
Supplementary Material
Acknowledgments
We thanks Dr. Scott Kaufmann (Mayo Clinic) for assistance in the calculation of IC50 values and the CI.
Grant support: National Cancer Institute R01 grant CA104683-02 (F.A. Sinicrope), Japan-North America Medical Exchange Foundation (K. Okumura), and Mayo Clinic Cancer Center National Cancer Institute core grant CA15083.
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Violette S, Poulain L, Dussaulx E, et al. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int J Cancer. 2002;98:498–504. doi: 10.1002/ijc.10146. [DOI] [PubMed] [Google Scholar]
- 3.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 2007;67:2908–11. doi: 10.1158/0008-5472.CAN-07-0082. [DOI] [PubMed] [Google Scholar]
- 5.Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 8.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan D, Velankar M, Brahmandam M, et al. A novel Bcl-2/Bcl-XL/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2007;26:2374–80. doi: 10.1038/sj.onc.1210028. [DOI] [PubMed] [Google Scholar]
- 10.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 11.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahir S, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 14.Houghton PJ, Cheshire PJ, Hallman JC, Bissery MC, Mathieu-Boue A, Houghton JA. Therapeutic efficacy of the topoisomerase I inhibitor 7-ethyl-10-(4-[1-piperidino]-1-piperidino)-carbonyloxy-camptothecin against human tumor xenografts: lack of cross-resistance in vivo in tumors with acquired resistance to the topoisomerase I inhibitor 9-dimethylaminomethyl-10-hydroxycamptothecin. Cancer Res. 1993;53:2823–9. [PubMed] [Google Scholar]
- 15.Hayward RL, Macpherson JS, Cummings J, Monia BP, Smyth JF, Jodrell DI. Antisense Bcl-xl down-regulation switches the response to topoisomerase I inhibition from senescence to apoptosis in colorectal cancer cells, enhancing global cytotoxicity. Clin Cancer Res. 2003;9:2856–65. [PubMed] [Google Scholar]
- 16.Ravi R, Jain AJ, Schulick RD, et al. Elimination of hepatic metastases of colon cancer cells via p53-independent cross-talk between irinotecan and Apo2 ligand/TRAIL. Cancer Res. 2004;64:9105–14. doi: 10.1158/0008-5472.CAN-04-2488. [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Sinicrope FA. The BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–51. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Bougie P, Wuillème-Toumi S, Ménoret E, et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007;67:5418–24. doi: 10.1158/0008-5472.CAN-06-4322. [DOI] [PubMed] [Google Scholar]
- 20.Alves NL, Derks IA, Berk E, Spijker R, van Lier RA, Eldering E. The Noxa/Mcl-1axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24:703–16. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Adjei AA, Davis JN, Bruzek LM, Erlichman C, Kaufmann SH. Synergy of the protein farnesyl-transferase inhibitor SCH66336 and cisplatin in human cancer cell lines. Clin Cancer Res. 2001;7:1438–45. [PubMed] [Google Scholar]
- 22.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Slee EA, Keogh SA, Martin SJ. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 2000;7:556–65. doi: 10.1038/sj.cdd.4400689. [DOI] [PubMed] [Google Scholar]
- 24.Boyer J, McLean EG, Aroori S, et al. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Cancer Res. 2004;10:2158–67. doi: 10.1158/1078-0432.ccr-03-0362. [DOI] [PubMed] [Google Scholar]
- 25.Jansen WJM, Zwart B, Hulscher STM, Giaccone G, Pinedo HM, Boven E. CPT-11 in human colon-cancer cell lines and xenografts: characterization of cellular sensitivity determinants. Int J Cancer. 1997;70:335–40. doi: 10.1002/(sici)1097-0215(19970127)70:3<335::aid-ijc15>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei Y, Xie C, Xie W, Tian X, Li M, Wu M. Noxa/Mcl-1 balance regulates susceptibility of cells to camptothecin-induced apoptosis. Neoplasia. 2007;9:871–81. doi: 10.1593/neo.07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Goldstein LA, Hou W, Rabinowich H. Functional linkage between NOXA and Bim in mitochondrial apoptotic events. J Biol Chem. 2007;282:16223–31. doi: 10.1074/jbc.M611186200. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Galán P, Roué G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 30.Voortman J, Checinska A, Giaccone G, Rodriguez JA, Kruyt FA. Bortezomib, but not cisplatin, induces mitochondria-dependent apoptosis accompanied by up-regulation of Noxa in the non-small cell lung cancer cell line NCI-H460. Mol Cancer Ther. 2007;6:1046–53. doi: 10.1158/1535-7163.MCT-06-0577. [DOI] [PubMed] [Google Scholar]
- 31.Hutcheson J, Scatizzi JC, Bickel E, et al. Combined loss of proapoptotic genes Bak or Bax with Bim synergizes to cause defects in hematopoiesis and in thymocyte apoptosis. J Exp Med. 2005;201:1949–60. doi: 10.1084/jem.20041484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czabotar PE, Lee EF, van Delft MF, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci U S A. 2007;104:6217–22. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.