Summary
An essential feature of mammary gland differentiation during pregnancy is the formation of alveoli composed of polarized epithelial cells, which, under the influence of lactogenic hormones, secrete vectorially and sequester milk proteins. Previous culture studies have described either organization of cells polarized towards lumina containing little or no demonstrable tissue-specific protein, or establishment of functional secretory cells exhibiting little or no glandular architecture. In this paper, we report that tissue-specific vectorial secretion coincides with the formation of functional alveoli-like structures by primary mammary epithelial cells cultured on a reconstituted basement membrane matrix (derived from Engelbreth-Holm-Swarm murine tumour). Morphogenesis of these unique three-dimensional structures was initiated by cell-directed remodelling of the exogenous matrix leading to reorganization of cells into matrix-ensheathed aggregates by 24 h after plating. The aggregates subsequently cavitated, so that by day 6 the cells were organized into hollow spheres in which apical cell surfaces faced lumina sealed by tight junctions and basal surfaces were surrounded by a distinct basal lamina.
The profiles of proteins secreted into the apical (luminal) and basal (medium) compartments indicated that these alveoli-like structures were capable of an appreciable amount of vectorial secretion. Immunoprecipitation with a broad spectrum milk antiserum showed that more than 80 % of caseins were secreted into the lumina, whereas iron-binding proteins (both lactoferrin and transferrin) were present in comparable amounts in each compartment. Thus, these mammary cells established protein targeting pathways directing milk-specific proteins to the luminal compartment. A time course monitoring secretory activity demonstrated that establishment of tissue-specific vectorial secretion and increased total and milk protein secretion coincided with functional alveolar-like multicellular architecture.
This culture system is unique among models of epithelial cell polarity in that it demonstrates several aspects of epithelial cell polarization: vectorial secretion, apical junctions, a sequestered compartment and formation of a basal lamina. These lumina-containing structures therefore reproduce the dual role of mammary epithelia to secrete vectorially and to sequester milk proteins. Thus, in addition to maintaining tissue-specific cytodifferentiation and function, a basement membrane promotes the expression of tissue-like morphogenesis.
Keywords: vectorial secretion, polarity, milk proteins, extracellular matrix, differentiation, epithelial cells
Introduction
Providing an extracellular matrix (ECM) to cultured primary epithelial cells has been shown to promote the retention of structural and functional characteristics typical of the tissue of origin. Studies of mouse mammary epithelial cells have demonstrated that attainment and maintenance of cytological and functional differentiation in culture are critically dependent on substratum, even when lactogenic hormones are present (Emerman & Pitelka, 1977; Emerman et al. 1977,1979; Lee et al. 1984, 1985; Chen & Bissell, 1987; Li et al. 1987; for review, see Bissell & Hall, 1987). This is also true for mammary cells from other species (Wicha et al. 1982; Foster et al. 1983; Suard et al. 1983; Blum et al. 1987). The increases in milk protein expression concomitant with cytological polarization observed in these studies in culture suggest that cell morphology and the expression of tissue-specific genes are both intimately related to the nature of the substrata.
Whereas some differentiated functions of epithelial cells can be maintained in two-dimensional sheets of cells, three-dimensional cellular organization represents an additional aspect of tissue specificity important in secretory epithelia. Formation of the mammary gland ductal tree during puberty and development of secretory alveoli during pregnancy represent two distinct phases of postnatal morphogenesis that are known to be dependent upon the interaction between epithelial cells and the surrounding stroma and ECM (Daniel & Silberstein, 1987). Mammary epithelial cells sandwiched between (Hall et al. 1982), embedded within (Yang et al. 1980; Flynn et al. 1982; Foster et al. 1983; Suard et al. 1983; Daniel et al. 1984) or on top of (Ormerod & Rudland, 1988) collagen gels often organize into duct-like structures that frequently have evidence of some milk protein production. Cocultures of mammary epithelial cells with adipocytes also induce ductal morphogenesis, regardless of whether milk proteins are produced (Wiens et al. 1987) indicating that the process of multicellular organization is not intrinsically linked to function. However, histiotypic organization has not been studied in relation to the quality or quantity of milk protein secretion, nor has the ability of epithelia to sequester protein been documented.
Recently, a reconstituted basement membrane derived from the Engelbreth-Holm-Swarm tumour (EHS) (Kleinman et al. 1986), has been shown to be particularly effective in eliciting tissue-specific cellular morphology and protein production in a variety of cultured epithelial cells (Hadley et al. 1985; Bissell et al. 1985; Medina et al. 1987; Li et al. 1987; Bissell et al. 1987; Scheutz et al. 1988). We have previously shown that culturing primary mammary epithelia or mammary cell strains on EHS matrix leads to dramatic increases in the levels of casein mRNAs relative to those on plastic or even floating collagen gels (Bissell et al. 1985; Medina et al. 1987; Li et al. 1987). We now describe in detail the development of three-dimensional multicellular structures that resemble secretory alveoli in vivo when cells are cultured on EHS matrix. We show that, during the first four days of culture, primary mammary epithelial cells aggregate, remodel the matrix and reorganize into structures composed of morphologically polarized cells facing an open lumen. Furthermore, tight junctions are established that partition these cavities from the medium. When we examined the composition of proteins secreted into the ‘luminal’ compartment, we found that not only are more proteins sequestered in this compartment in comparison to medium but a greater proportion are milk proteins. These alveolar-like structures remain stable and functional for several weeks in culture. This system provides a versatile and physiologically relevant model for studies on the relationship between tissue-specific morphogenesis and functional differentiation as well as epithelial cell polarity and vectorial secretion.
Materials and methods
Reagents
Medium 199, F12 medium, calcium-free Dulbecco’s modified minimum essential medium (CF-DME), gentamycin, and fetal bovine serum were obtained from Gibco Laboratories (Grand Island, NY). Hydrocortisone and insulin (bovine pancreas) were from Sigma Chemical Co. (St Louis, MO); aprotinin was obtained from Calbiochem–Behring Corp. (La Jolla, CA). Prolactin was obtained from the National Hormone and Pituitary Program (contracted to NIADDK, Baltimore, MD). Antiserum to mouse milk proteins was prepared as described by Lee et al. (1984). Rabbit anti-rat transferrin antibody was obtained from Sigma and rabbit anti-mouse lactoferrin antibody was the kind gift of Dr Mark LaForce (University of Colorado, Denver, CO). EHS-reconstituted basement membrane was obtained as Matrigel from Collaborative Research (Bedford, MA) or prepared in our laboratory according to published methods (Kleinman et al. 1986).
Animals
Cells for electron micrographic studies were isolated from 14- to 17-day pregnant Balb/c mice (Simonsen, Gilroy, CA). Midpregnant (11–14 day) CD–I mice (Charles River, Wilmington, MA), which provide more mammary gland tissue than the Balb/c mice, were used for all other experiments. The milk protein compositions of Balb/c and CD–I mice were essentially identical. Cultured CD–I cells responded similarly to previously reported substratum and hormone effects (Lee et al. 1984, 1985, 1987; Li et al. 1987).
Cell preparation and culture conditions
Cell isolation procedures optimized to obtain maximum cell yield were carried out as previously described (Lee et al. 1985). Cells were plated at 3×l05 cellscm−2 in F12 containing 50 μg ml−1 gentamycin, 10% fetal bovine serum and lactogenic hormones (insulin, 5 μg ml−; hydrocortisone, 1 μg ml−1; prolactin, 3μg ml−1), and incubated at 37°C in 95% air and 5% CO2. EHS extract was thawed on ice and coated dishes were prepared by carefully spreading EHS matrix (10μl cm−2) onto dishes kept on ice. The plates were then incubated for 3–4 h in a 37°C humidified incubator to allow the EHS matrix to gel. Cells were fed with fresh serum-free F12 plus hormones on the second day of culture and every day thereafter.
Growth determinations
At various times during 14 days of culture, cells plated in 25 mm tissue culture wells, with or without EHS matrix, were incubated for 1 h with 10 μm-bromodeoxyuridine and then removed using 0·05 % trypsin and 0·05 % EDTA. The bromodeoxyuridine labelling index was measured in cells fixed in cold 70% EtOH using the staining protocol of Dolbeare and colleagues (1985). DNA was fluorometrically quantified using the method of Burton (1956).
Microscopy
Living cells were observed by phase-contrast optics using a Nikon inverted microscope. For scanning electron microscopy, cells were fixed, dehydrated, dried at the critical point, gold-coated and observed in a Philips 500 scanning electron microscope as described (Glass et al. 1983). For transmission electron microscopy, the cells remaining in the culture well were dehydrated and embedded in situ in Epon/Araldite. Pieces of hardened Epon were reimbedded so that thin sections cut perpendicular to the dish surface could be prepared.
Demonstration of lumina and extraction of luminal proteins
On the basis of previous studies showing that EGTA disrupts intercellular junctions (Pitelka et al. 1983; Volberg et al. 1986), cells were exposed to this chelating agent for two purposes. First, EGTA was used in conjunction with tracer dye for visualization of cavities sequestered by tight junctions within multicellular structures. Second, it was used to release secreted proteins that were sequestered within these lumina.
To demonstrate lumen-containing structures, cultures were washed with CF-DME and incubated with 2·5 mm-EGTA/CF-DME plus 10mm-Hepes (pH7·4) for 10min at 37°C, followed by 1·2mm-EGTA/CF-DME containing 1% trypan blue. After 15min at room temperature, the EGTA/dye solution was replaced with F12 and the cultures were examined for blue staining using phase-contrast microscopy. Control cultures were treated identically except that EGTA was omitted from the incubation.
To release sequestered proteins, the medium from radio-labelled cultures was removed, the cells were washed twice with CF-DME and then incubated for 10min at 37°C in 2·5mm-EGTA/CF-DME plus 10mm-Hepes (pH7·4). The proteins from this EGTA-sensitive compartment are designated the ‘luminal’ fraction. Control studies were conducted to determine the optimal EGTA incubation time required to release sequestered proteins without affecting cellular integrity. The kinetics of EGTA-induced release of intraluminal proteins indicated that more than half of the total protein was released within the first 10min of EGTA treatment; the rate of release was greatest between 6 and 10min (determined in three experiments). Minimal counts (<1 % of total) were found in the CF-DME washes. To determine the effect of EGTA on cell integrity, we prelabelled cells with 14C-2-deoxy-d-glucose. Upon entering the cell, 2-deoxyglucose is converted to 2-deoxyglucose-6-phosphate, which is then retained intracellularly. In cultures on EHS matrix or plastic that were incubated with and without EGTA, less than 15 % of the label was released into the medium after 10min, indicating that a 10min EGTA treatment had minimal effect on the integrity of cell membranes.
Protein analysis
[35S]methionine labelling, trichloroacetic acid precipitation, immunoprecipitation and SDS/PAGE were carried out as described (Lee et al. 1985). Cellular and secreted proteins were labelled for 4h using 100/μCi ml−1 [35S]methionine (specific activity >1000Ci mM−1) in methionine-free medium 199 containing hormones under an atmosphere of air/CO2 (95:5). Aprotinin (2 %) was added to the samples, which were then stored at −70°C until analysed. Gels were enhanced for fluorography and dried gels were scanned using a Bio-Rad Laboratories (Richmond, CA) densitometer to quantify radioactivity incorporated into each band. This measurement, expressed in arbitrary units, was corrected for total secretion and normalized on the basis of DNA content per culture.
Results
Morphogenesis on EHS matrix
A striking reorganization in the morphology of primary mammary epithelial cells on EHS matrix was observed during the first 4 days of culture. When viewed by scanning electron microscopy, scattered single cells and small cell clusters were observed to be attached to the matrix 3h after plating (Fig. 1A). Note that the EHS matrix is present as a solid mat. By 24 h most cells were present in clumps that appeared to be ‘pulling’ on the fibrillar matrix leading to cleared areas of plastic and lines of stress in the matrix material (Fig. 1B). Larger islands of cells partially covered with EHS matrix had begun to form and few single cells were apparent. By the fourth or fifth day, the epithelial structures were larger (Fig. 1C) and completely enveloped in a fine, mesh-like material, which is presumed to be EHS matrix (Fig. 1D).
Fig. 1.
Development of lumen-containing structures by cells cultured on EHS matrix. Isolated primary mammary epithelial cells were cultured on EHS matrix in the presence of lactogenic hormones for 3h (A), 24h (B), or 5 days (C,D), fixed, critical point dried and observed with SEM. The isolated epithelial cells are seeded as small clumps and scattered single cells that adhere rapidly to the matrix substratum by 3h (A), but do not appear to spread out on it. After 24h in culture (B), the cell clumps have attached firmly to the matrix and have apparently pulled it in toward themselves, leaving large areas of the culture dish cleared. By 4–6 days in culture (C), most of the cells are present in spheroids, which are completely surrounded by matrix. At higher magnification (D), the fibrillar nature of the matrix meshwork surrounding the bracketed spheroid in C is clearly seen. (A-C) Bar, 100μm; (D) bar, 25μm.
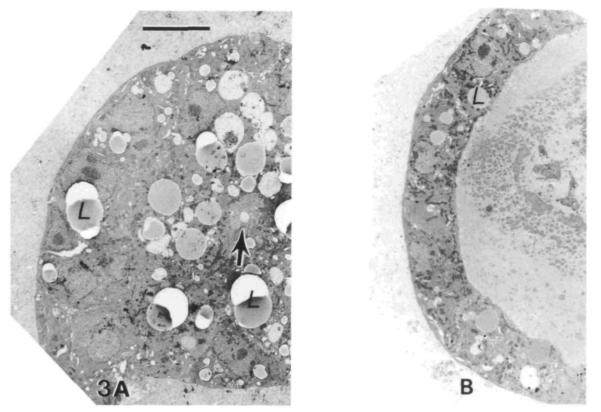
By viewing these structures in cross-section, we observed stages in the process of cavitation within cell aggregates that appeared to be a direct response to the basal orientation signal provided by the matrix. The cells originally plated were largely in heterogeneous aggregates that demonstrated little or no organization and contained significant numbers of pyknotic nuclei and dead cells (Fig. 2A). After 48 h in culture, cavities had formed within the aggregates, but the cells surrounding these spaces were not yet strongly polarized (Fig. 2B). By day 4, alveolar organization was indicated by junction formation around the ‘apical’ margins of cells, which were seen thereafter to be well organized into polarized epithelia facing a central lumen (Fig. 3). The alveolar morphology in fully established cultures (5–8 days) was heterogeneous. Some of the structures were composed of large, columnar cells with abundant secretory vesicles that enclosed small lumina (Fig. 3A), while others consisted of smaller cuboidal secretory cells lining lumina that were engorged with protein and cellular debris (Fig. 3B).
Fig. 2.
Early ultrastructural organization of cells cultured on EHS (e). (A) Cross-section of a clump of epithelial cells 3h after plating shows that they are not intact mammary gland ducts or alveoli. The cells are unorganized and dead cells and cell debris (d) are scattered throughout the clumps. After two days in culture (b) lumen (lu) formation has begun, apparently by a process of cavitation within clumps. Cells surrounding the nascent lumina are not yet well oriented. Bar, 5μm.
Fig. 3.
Cross-section views of secretory alveolar-like structures. After culture on EHS matrix for 8 days, cells are organized into a variety of alveolar-like structures ranging from 50-150 μm in diameter. The cells in these spheroids are polarized with their apices toward the lumen and their basal surfaces outward, contacting the basement membrane matrix. In some cases (A), the central lumen is quite small and filled with microvilli (arrow), while other (B) spheroids appear swollen with protein accumulated within their lumina. Likewise in A the cells are tall and columnar while in B the flattened cells are cuboidal. Individual cells in both of these structures show many morphological signs of secretory activity, including secretory granules, lipid droplets (L), and well-developed rough endoplasmic reticulum, although they are more prevalent in A. Bar, 10μm.
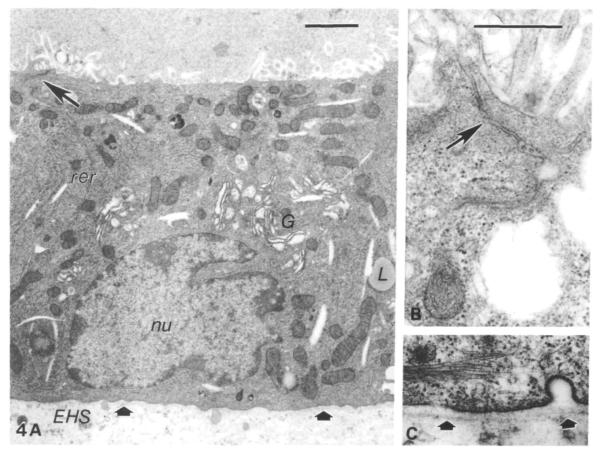
This dramatic three-dimensional morphogenesis was also accompanied by differentiation at the cellular level. The ultrastructure of cells cultured on EHS matrix (Fig. 4A) was highly reminiscent of secretory alveolar cells from lactating mammary gland itself. Individual cells showed apical junctional complexes between adjacent cells (Fig. 4B), supranuclear, distended Golgi complex, abundant rough endoplasmic reticulum and a basally located nucleus with a prominent nucleolus. Additional signs of secretory activity were the presence of fat droplets and various types of secretory vesicles. These high-magnification views also showed that the alveolar structures were completely surrounded by a thin, but distinct, basal lamina, which, both from its absence in earlier cultures (<4 days) and from its proximity to the basal plasma membrane, may have been assembled by the cultured cells (Fig. 4C). Thus, culturing primary mammary epithelial cells on EHS matrix provides the proper conditions for cells to undergo morphogenesis that results in structures typical of alveoli in vivo.
Fig. 4.
High-magnification view of secretory epithelial cell. In a section through an entire cell (A), many morphological indications of secretory activity are evident, including large indented basal nucleus (nu), multiple stacks of rough endoplasmic reticulum (rer), an elaborate Golgi complex (G), lipid droplets (L) and secretory vesicles in the apical cytoplasm, and numerous apical microvilli. These alveolar-like structures are characteristically sealed by tight and adherens junctions between cells near their apical borders (A,B, large arrows) and are completely surrounded by a distinct thin basal lamina (C, small arrows), apparently assembled by the cells during culture on the EHS matrix. (A) Bar, 1μm; (B,C) bar, 0·5 μm.
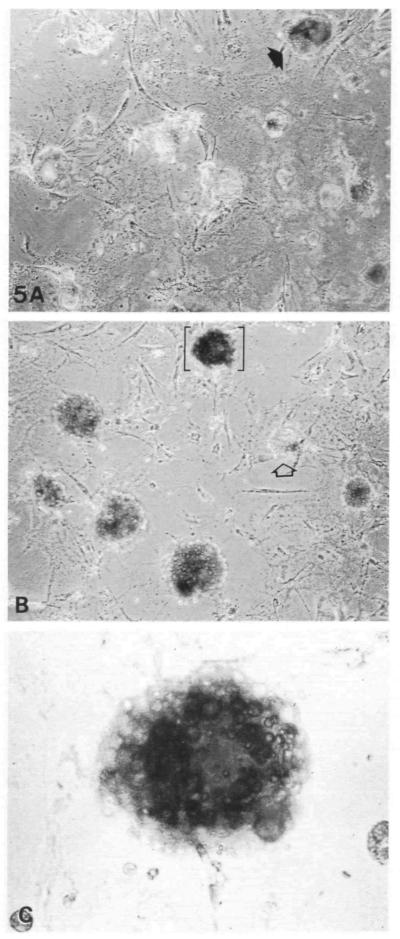
Evidence that the apical junctions observed in these alveoli-like structures were indeed tight was provided by determining whether EGTA-induced opening of junctions (Pitelka et al. 1983; Volberg et al. 1986) altered the ability of the multicellular structures to sequester dye over the course of time in culture. Between 24 and 48 h, small spots of dye were observed within aggregates, both with and without EGTA treatment; we concluded that they were nonviable cells that had taken up the trypan blue. In contrast, by day 4, very few single cells took up dye and large areas of dye indicative of lumina were localized within the multicellular structures. By day 6 and thereafter, 10–15 % of these structures took up dye in the absence of EGTA (Fig. 5A), while 50–85% had sequestered dye in EGTA-treated cultures (Fig. 5B,C). Cultures maintained on EHS matrix for 2 weeks still showed this alveolar-like multicellular organization. By comparison, cells on plastic exhibited some dye-retaining structures early in culture (day 2), but most of these multicellular structures had spread out by day 4, resulting in a monolayer that no longer retained dye (not shown).
Fig. 5.
Prevalence of lumen-containing structures in cultures on EHS matrix as demonstrated by phase-contrast microscopy after EGTA/trypan blue treatment. (A) Control culture exposed to trypan blue without EGTA; closed arrow designates dye-containing structure. (B) EGTA/trypan-blue-treated culture; open arrow designates dye-excluding structure; brackets designate the structure shown in C. (C) Higher magnification of bracketed structure in B containing a large trypan blue-containing lumen.
Vectorial secretion of proteins
Polarized secretion and compartmentalization of milk proteins are essential features of the lactating mammary gland. The morphological evidence of cell polarity and functional evidence of a tight epithelium suggested that cultured lumina-containing structures were capable of sequestering apically secreted proteins. The ability of the cells to compartmentalize secreted proteins was demonstrated using EGTA to extract proteins secreted apically into the lumina (as described in Materials and methods). Of newly labelled secreted proteins, 75% (6·3×106 cts min−1/8·5×106 ctsmin−1) were in the EGTA-sensitive compartment (Table 1), while in comparison negligible amounts were released in cultures on plastic. This experiment also clearly demonstrates the increased synthetic (twofold) and secretory (fivefold) function of cells cultured on EHS matrix as compared to cells cultured on plastic, the former of which was inferred from previous measurements of mRNA levels (Li et al. 1987), but was not clearly documented.
Table 1.
Synthetic and secretory activity of mammary epithelial cells on plastic and EHS matrix
| Substratum |
||
|---|---|---|
| Compartment | Plastic | EHS |
| Secreted | ||
| Medium | 1·4 ±0·4 | 2·2 ±0·5 |
| Luminal | 0·33 ±0·1 | 6·3 ±1·0 |
| Cell | 40·0 ±20 | 75·0 ±30 |
Cells cultured for 6 days on EHS matrix were labelled for 4 h with [35S]methionine and newly synthesized protein was fractionated and analysed as described in Materials and methods. Results are expressed as cts min−1 × 106; the mean ± the standard error of four experiments is shown.
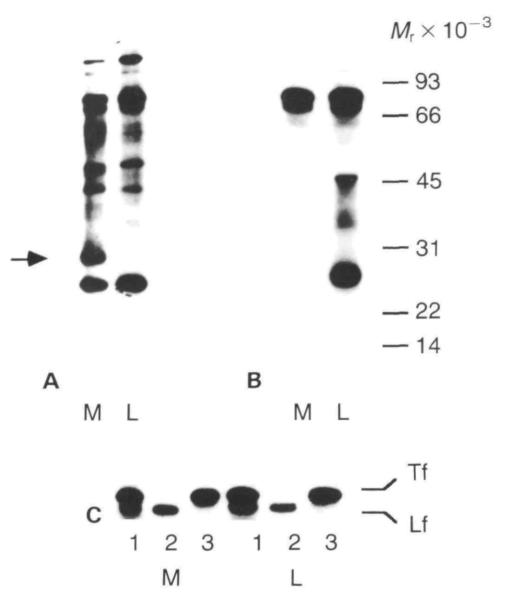
The proteins in the luminal and medium compartments on EHS matrix differed qualitatively as well as quantitatively (Fig. 6A). In particular, a 30×103 Mr protein (Fig. 6A, arrow) was found in the medium of the EHS matrix cultures but was absent from the luminal compartment. This protein was not immunoprecipitated by the broad spectrum milk antibody (see below), and its identity is not yet established.
Fig. 6.
Gel electrophoretic patterns of [35S]methionine-labelled proteins secreted into the medium and the luminal compartment by primary mammary epithelial cells cultured on plastic and EHS substrata for 6 days. (A) Unprecipitated proteins. Equal volumes of medium and EGTA extract were mixed 1:1 with sample buffer and run on 12·5% PAGE. The lanes in the fluorogram show medium (M) and lumina (L) compartments and position of molecular weight standards (Mr × 10−3). Note the protein band of approximately 30×l03 Mr (arrow) that is present only in the medium of the EHS cultures. (B) Immunoprecipitated milk proteins. Equal acid-precipitable counts from medium and luminal compartments were immunoprecipitated using a broad spectrum antibody to mouse skim milk proteins. (C) Specific immunoprecipitation of transferrin (Tf) and lactoferrin (Lf) by broad spectrum milk antibody (1), lactoferrin antibody (2), and transferrin antibody (3).
When the composition of milk proteins was analysed by immunoprecipitation, it was apparent that they were secreted differentially into the two compartments. In comparison with the medium, the luminal compartment of cells on EHS matrix contained a greater proportion of milk proteins (Fig. 6B). Although total secretion into the luminal compartment was threefold greater than into medium, there was an eightfold enrichment of milk protein in the luminal compartment. Additional evidence for the specificity of vectorial secretion was provided by comparisons of the relative amount of individual milk proteins in each compartment (Table 2). The milk-specific caseins were found preferentially in the luminal fraction. In comparison to the caseins, iron-binding proteins were found in comparable amounts in both compartments. Previously we identified transferrin as a major milk protein synthesized by mammary epithelial cells in culture (Lee et al. 1987; Chen & Bissell, 1987), but this is the first demonstration that lactoferrin is synthesized and secreted in these cultures (Fig. 6C). Note that the broad spectrum milk antiserum precipitates both proteins, but because of the similarity in their molecular weights and relative abundance, the bands are not well resolved. In summary, both total secretion and the proportion that was milk-specific were greater for cells on EHS matrix than on plastic; furthermore, both functions were greater in the luminal fraction than in the medium of cells on EHS matrix. This demonstrates that specific proteins can be correctly targeted to the luminal compartment in this culture system.
Table 2.
Percentage of individual milk proteins secreted apically and basally
| Medium | Luminal | |
|---|---|---|
| Transferrin | 49 | 51 |
| Lactoferrin | 44 | 56 |
| α-casein | 30 | 70 |
| β-casein | 11 | 89 |
Percentage of the secreted milk proteins found in the basal (medium) and apical (luminal) compartments as determined by densitometry of the immunoprecipitated protein bands shown in Fig. 6.
Functional stability of alveolar cultures
The physiologically relevant organization and function of this culture model should make it particularly useful for time-course studies. Therefore, we have documented the stability of cultures on EHS matrix as compared to those on plastic. At 24 h, approximately 75 % of cells on EHS matrix had attached compared to 45 % plated on plastic, indicating a substantial increase in plating efficiency. DNA determinations of cultures on EHS matrix showed an initial decline over the first 4 days; DNA levels continued to decline slightly over the next 3 days but then reached a plateau (Fig. 7A). The DNA levels on EHS matrix remained stable for an additional week, while cells on plastic deteriorated steadily over the entire 14-day culture period. That the cell population remains stable on EHS matrix was supported by the low labelling index (1–2%) throughout the duration of culture (data not shown). Thus, little or no growth occurred in these cultures, as would be expected from our culture conditions (removal of serum at day 2 and no growth factors).
Fig. 7.
Time course of DNA content and total protein secretion by cells cultured on EHS matrix. (A) DNA levels are expressed as a fraction of the amount of DNA plated at the initiation of culture (45 μg dish−1). Closed symbols represent the mean of duplicate cultures on EHS matrix of two separate experiments. Open symbols represent the mean of duplicate cultures on plastic. (B) Total secretion by cells cultured on EHS matrix, as indicated by acid-preciptable counts (corrected for DNA content); symbols represent the mean of duplicate cultures; closed symbols represent secretion into medium, open symbols represent secretion into the luminal compartment.
To determine when a functional luminal compartment was established, we studied the time course of milk protein secretion into the medium and the EGTA-sensitive compartments. In contrast to the decline in DNA content, secretion increased as a function of time in cultures on EHS matrix (Fig. 7B). Minimal, but equal, amounts of secreted proteins were found in the medium and EGTA-sensitive compartments during the first 4 days of culture. Between day 4 and 6, there was a tenfold increase in luminal secretion while there was only a twofold increase in the amount of protein secreted into the medium. Subsequently, most of the proteins were secreted into the luminal compartment.
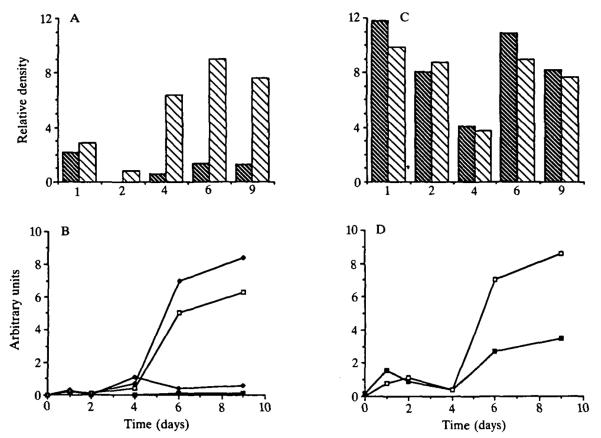
Noncoordinate regulation of the amount, proportion and direction of tissue-specific secretion was observed when the caseins were monitored and compared to the iron-binding proteins. After two days in culture, the proportion of secretion devoted to casein (Fig. 8B) was lower than on day 1, although the amount of total casein secretion was similar. On day 4, however, the proportion of luminal secretion devoted to casein increased by a factor of 6. Caseins first appeared in appreciable amounts in the EGTA-sensitive compartment on day 4 and in the medium at day 6, but the proportion of secretion as well as the total amount of casein increased at a greater rate in the luminal compartment, so that by day 9, there was seven times more casein being secreted than on day 1. In contrast, the proportion of secretion devoted to transferrin was equal in both compartments throughout culture (Fig. 8C) and, although the proportion of secretion devoted to transferrin varied somewhat during culture, it never exceeded the day-1 baseline. However, due to the increase in total secretory activity into the luminal compartment (Fig. 7B), the total amount of transferrin secreted into this compartment was greater than into the medium (Fig. 8D).
Fig. 8.
Time course of milk protein secretion into the medium and the EGTA-sensitive compartments by cells cultured on EHS matrix. (A) Proportion of β-casein in the medium (black bars) and lumina (open bars). The areas of the bands of interest were integrated by scanning densitometry and are expressed in arbitrary units; similar determinations for α-casein produced a comparable pattern (not shown). (B) Relative amounts of secreted α (squares) and β-casein (diamonds) in the medium (closed symbols) and lumina (open symbols) were determined by correcting the relative density of protein bands shown in A for total secretion and normalizing for DNA content. (C) Proportion of transferrin in the medium (black bars) and lumina (open bars) determined as in A. (D) Total amount of transferrin in the medium (closed symbols) and lumina (open symbols) determined as in B. These data were from the same experiment as represented in Fig. 7B.
In summary, these data indicate that during morphogenetic reorganization (up to day 4) there was little secretory activity associated with the EGTA-sensitive compartment, but that, once cell polarity and lumina were established, milk proteins were preferentially secreted into the lumina. Thus, biochemical evidence of tissue-specific vectorial secretion coincided with the earliest demonstration of cell polarity.
Discussion
The criteria by which ideal culture conditions for mammary epithelial cells are judged usually include individual cell morphology to determine cytological differentiation and milk protein synthesis and secretion to evaluate tissue-specific differentiation. An additional level of functional organization less frequently studied is multicellular architecture as a measure of the degree of histiotypic morphogenesis. Previous culture strategies have resulted in morphologically polarized cells capable of secreting milk proteins under the appropriate hormonal stimuli (Emerman et al. 1977,1979; Haeuptle et al. 1983; Lee et al. 1984, 1985, 1987; Blum et al. 1987; Parry et al. 1987). On the other hand, some culture conditions have been characterized as ductal in their multicellular organization (Daniel et al. 1984; Wiens et al. 1987; Ormerod & Rudland, 1988). In this paper, we have demonstrated that cells isolated from midpregnant mammary gland cultured on a reconstituted basement membrane satisfy all three criteria mentioned above and reproduce physiologically relevant alveolar organization and function.
Morphogenesis
Unlike the domes found in mammary epithelial cells cultured on plastic (‘blistering’, due to fluid accumulation under the epithelial pavement) (Pickett et al. 1975), the multicellular structures on EHS matrix appear to form by cells drawing up the flexible EHS matrix around them within the first 1–2 days of culture (Fig. 1). A process of cavitation then occurs within aggregates of cells; some internal cells deteriorate while other cells develop apical specializations, leading to the eventual formation of lumina. These structures are strikingly similar to alveoli in the pregnant and lactating mammary gland. Like alveoli, they contain morphologically polarized cells that rest on a basal lamina, are joined by apical junctions, and are capable of sequestering major secretory products. Furthermore, there is a precedent in vivo that may account for the heterogeneity, depicted in Fig. 3, of these lumina-containing structures. Pitelka and colleagues (1973) described dramatic differences in cell and alveolar shape in the lactating gland itself. The accumulation of milk within alveoli was seen to force cells to flatten and to increase the perimeters of inflated alveoli by 50%; this was interpreted as evidence that alveolar epithelia are ‘elastic’. Similarly, in our culture model some alveolar-like structures appear presecretory, as judged both by the size of the lumen and the abundance of cytoplasmic secretory vesicles (Fig. 3A) while others appear post-secretory, as characterized by swollen lumina and more cuboidal cells (Fig. 3B).
Functional polarity
The transjunctional permeability barrier in vivo exists to separate, and possibly filter, plasma and interstitial fluid from lumina that contain milk proteins. Based on the morphological differentiation observed in cells on EHS matrix, we reasoned that these alveolar structures might be compartmentalizing apically secreted proteins. Evidence that this compartmentalization exists and is maintained through functional intercellular junctions was provided by using EGTA to disrupt these complexes (Pitelka et al. 1983; Volberg et al. 1986). This treatment resulted in the release of more than 70 % of the total secreted proteins (acid-precipitable counts), while similar treatment of cells cultured on plastic released less than 20% of the secreted proteins. We conclude that the protein released by EGTA treatment was sequestered from the extracellular medium by tight junctions within structures containing lumina. Further evidence that the luminal compartment was distinct from the medium compartment was provided by the distinctive patterns of proteins secreted into them (Fig. 7A). Secretion of milk proteins into the sequestered luminal compartment explains the previously puzzling apparent lack of agreement between the increase in mRNA levels and the amount of milk protein in the medium of cells on EHS matrix, since only the medium was analysed in those experiments (Li et al. 1987).
We have shown that morphological polarity in EHS matrix cultures is accompanied by functional polarity in that milk proteins are preferentially secreted into the lumina of alveolar-like structures. This culture system is unique among models of epithelial cell polarity in that it reproduces several aspects of functional polarization: vectorial secretion, apical junctions, a sequestered compartment and formation of a basal lamina. Vectorial secretion is evident from the observation that milk proteins are not segregated equally. The caseins are found almost exclusively in the luminal compartment, while iron-binding proteins are secreted into both the medium and luminal compartments. This result is similar to that reported by Parry and colleagues (1987), who found that a mammary epithelial cell strain (COMMA-D) maintained in bicameral chambers also secreted caseins from the apical cell surface and transferrin from both the apical and basal surfaces. These data are consistent with earlier studies demonstrating that milk proteins are not coordinately regulated (Lee et al. 1984, 1985), and suggest that at least two different secretory pathways are utilized by mammary epithelial cells. A possible interpretation of our data is that the iron-binding protein transferrin is secreted through a ‘constitutive’ secretory pathway while caseins may be secreted through a regulated pathway (Burgess & Kelley, 1987). This aspect of the culture system offers further opportunities for study.
The directional secretion of proteins in vivo into the serosal or luminal compartments frequently suggests the physiological role that these proteins play. We previously demonstrated that transferrin is a major secretory product of mouse mammary epithelial cells from pregnant gland (Lee et al. 1987) and have now identified lactoferrin as a product of mammary epithelial cells in culture as well. Although these proteins have a common function of binding iron, their distributions in vivo are quite distinct. Transferrin is the most abundant iron-binding protein and is found in serum, while lactoferrin is predominantly found in secretions. They are both present in various proportions in the milk of different species (Masson & Heremans, 1971). Whether they have similar or distinct roles in mammary gland is not known. However, since transferrin (Lee et al. 1987) and its mRNA (Chen & Bissell, 1987) are more abundant during pregnancy than lactation, we have hypothesized that it may play a role in epithelial cell growth and differentiation. The apical secretion of transferrin is consistent with iron-delivery to offspring, whereas basal secretion would support the hypothesis that it may act in a paracrine or autocrine fashion during the complex differentiation of mammary gland in the virgin to lactating transition.
Mechanisms of EHS-induced morphogenesis and function
In addition to its effects on mammary epithelial cells (Bissell et al. 1985; Li et al. 1987; Medina et al. 1987), EHS-reconstituted basement membrane has been shown to promote histiotypic morphology and function in Sertoli cells (Hadley et al. 1985), hepatocytes (Bissell, D. M. et al. 1987; Scheutz et al. 1988), avian neural crest cultures (Maxwell & Forbes, 1987), exocrine acinar epithelial cells (Oliver et al. 1987), and even fibroblasts (Emonard et al. 1987). In each case, these effects on differentiation are contingent on the intrinsic properties of the tissue of origin. The mechanisms by which EHS matrix influences cytostructure, morphology and gene expression have yet to be elucidated, although composition and fluidity seem to be important factors.
In vivo, the differentiated mammary epithelium is in contact with a basal lamina composed of laminin, type IV collagen, entactin, heparan sulphate and proteoglycans (Silberstein & Daniel, 1982a; Warburton et al. 1982, 1984). The basement membrane matrix reconstituted from EHS tumour proteins has similar components (Kleinman et al. 1986). Laminin itself is regarded as being important for epithelial cell attachment (Kleinman et al. 1984; Hynes, 1987). However, although EHS matrix contains ~80% laminin, neither laminin nor any other individual basal lamina component can substitute completely for the effects of the complex reconstituted matrix in culture (Bissell et al. 1985; Li et al. 1987; Medina et al. 1987; Bissell, D. M. et al. 1987; Blum et al. 1987). Because basal lamina in vivo are heterogeneous three-dimensional arrays of proteins, spatial presentation of an individual component to the cell surface may be influenced by its interaction with other components of the ECM (Kleinman et al. 1983; Turley et al. 1985). In a similar vein, the reason that EHS matrix on top of collagen type I, a stromal ECM, elicits a greater response from mammary epithelial cells than EHS matrix on plastic (Li et al. 1987) may be that this configuration is similar to the stroma-parenchyma interface in the gland.
The mechanical properties of the EHS matrix gel may also influence the differentiation of epithelial cells by at least two mechanisms. First, like floating collagen gels, and unlike glutaraldehyde-fixed gels (Lee et al. 1984), EHS matrix is flexible and presumably allows cellular reorganization that alters cytostructure and membrane domains. Second, because EHS matrix is a thin gel in these experiments, its porosity allows basal access of nutrients, which may be important to polarized cell function (Emerman et al. 1979; Parry et al. 1987). However, the rapidity of the cell-directed remodelling of the EHS matrix belies a simplistic interpretation of this process; the dynamic character of the cell–ECM interaction suggests that it may be effected at many levels (Bissell et al. 1982). For example, both aspects of substratum flexibility permit cells to aggregate and form intercellular associations that foster multicellular structures. The resulting individual epithelial cell shape correlates with, and may even be a prerequisite for, the maintenance of differentiated function on many substrata (Watt, 1986; Bissell & Hall, 1987).
The demonstration of an organized basal lamina surrounding epithelial alveoli on EHS matrix is additional evidence of functional polarity in these cells and is another consequence of the cell–substratum interaction. Other culture configurations that produce differentiated function also elicit a distinct basal lamina (Emerman & Pitelka, 1977; Parry et al. 1985; Haeuptle et al. 1983; Bissell & Aggeler, 1987; David et al. 1987; Wiens et al. 1987). Functional changes in these cultures may be effected through the synthesis of basement membrane components (Bissell & Hall, 1987; Aggeler et al. 1988) or through assembly of these ECM components into the basal lamina (David & Bernfield, 1979; Parry et al. 1985; David et al. 1987). That this role for ECM may also be important in normal gland is indicated by reports that mammary gland development is disrupted when ECM deposition is inhibited (Wicha et al. 1980; Silberstein & Daniel, 1982b). Whether endogenous production of ECM is a primary event in the functional differentiation of mammary cells cultured on EHS matrix is currently under investigation in our laboratories.
Model of alveolar morphogenesis in culture
The mammary gland is composed of several distinct epithelial cell types, chiefly ductal, alveolar and myoepithelial. The ductal and alveolar epithelia differ in their morphology, cytostructure and function (Pitelka et al. 1973). Ductal epithelium is organized into inter-connecting tubes lined with low cuboidal cells with sparse cytoplasmic organelles and few apical specializations; it functions primarily as a conduit and is relatively non-secretory. The ductal tree is encased in a sheath of myoepithelial cells that separate the ductal cells from the basal lamina. Myoepithelial cells are thought to produce this basal lamina (Warburton et al. 1982, 1984; Silberstein & Daniel, 1982a). In contrast, alveolar epithelial cells directly contact, and may produce, their own basal lamina (Williams & Daniel, 1983). Mammary epithelial cell isolation protocols do not separate these two distinct epithelial populations. Unlike the tubular morphology obtained using collagen gel cultures (Yang et al. 1980; Daniel et al. 1984; Ormerod & Rudland, 1988) or adipocyte cocultures (Wiens et al. 1987), the culture conditions described here appear to drive the cells toward recapitulating the multicellular organization and secretory phenotype of alveolar epithelia. Therefore, the question of how EHS matrix induces alveolar morphogenesis and secretion is of interest.
We hypothesize that contact between the epithelial cells and EHS-reconstituted basement membrane is substituting for the contact of cells with their basal lamina, which itself influences alveolar morphogenesis in vivo. We thus envisage that the process of lumen formation is dependent upon directionality imparted to cells that are contacting EHS matrix. This basal anchorage, presumably through a specific receptor interaction with the ECM, promotes further membrane specialization, such as creation of tight junctions between the polarized cell and its neighbour, which, in turn, could give the latter cell directionality. Once polarity has been established in individual cells, they can proceed to refine their immediate environment by secreting their own basal lamina. This process continues in a reciprocal fashion, leading to a connected epithelium that encloses a space, which may be formed by cell death or possibly created by the distending forces of sequestered apical secretion. Thus, the formation of lumina in these cell aggregates is not fortuitous but is directly related to contact of the cell surface with an ECM.
Acknowledgments
These investigations were supported by the Health Effects Research Division, Office of Health and Environmental Research, US Department of Energy, Contract DE-AC-03-76SF00098 and a gift from Monsanto to MJB. MHB-H was a postdoctoral fellow under NIH 5T332 CA09272. JA was supported in part by a grant from the University of California Cancer Research Coordinating Committee and, in part, by grant DCB-87 19874 from the National Science Foundation.
We thank Dr Charles Streuli for critical reading of this manuscript, Dr Mark LaForce for providing the antibody to lactoferrin and Keith Seely for his technical assistance with the ultrastructural studies. A preliminary report of these results has appeared in abstract form (Barcellos-Hoff et al. 1987).
Abbreviations
- EHS
Engelbreth-Holm-Swarm tumour
- ECM
extracellular matrix
References
- Agoeler J, Park C, Bissell MJ. Regulation of milk protein and basement membrane gene expression: The influence of the extracellular matrix. J. Dairy Sci. 1988;71:2830–2842. doi: 10.3168/jds.S0022-0302(88)79879-3. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Neville PN, Aggeler J, Bissell MJ. Polarized secretion by mammary epithelial cell cultures on EHS-matrix. J. Cell Biol. 1987;105:220a. [Google Scholar]
- Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. J. Clin. Invest. 1987;79:801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Aggeler J. Dynamic reciprocity: How do extracellular matrix and hormones direct gene expression? In: Cabot MC, Mckeehan WL, editors. Mechanisms of Signal Transduction by Hormones and Growth Factors. Alan Liss; New York: 1987. pp. 251–262. [PubMed] [Google Scholar]
- Bissell MJ, Hall HG. Form and function in the mammary gland: The role of extracellular matrix. In: Neville M, Daniel C, editors. The Mammary Gland: Development, Regulation and Function. Plenum Press Publishing Corporation; New York: 1987. pp. 97–146. [Google Scholar]
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression. J. Theoret Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Lee EY-H, Li M-L, Chen L-H, Hall HG. Rogers CH, Coffey DC, Cunha GR, Grayhack JT, Hunman F, Horton R, editors. Role of extracellular matrix and hormones in modulation of tissue-specific functions in culture: mammary gland as a model for endocrine sensitive tissues. Benign Prostatic Hyperplasia. 1985;Vol. II:39–50. NIH Publication No. 87-2881. [Google Scholar]
- Blum JL, Zeigler ME, Wicha MS. Regulation of rat mammary gene expression by extracellular matrix components. Expl Cell Res. 1987;173:322–340. doi: 10.1016/0014-4827(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Kelley RB. Constitutive and regulated secretion of proteins. A. Rev. Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Burton K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 1956;62:315–322. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-H, Bissell MJ. Transferrin mRNA level in the mouse mammary gland is regulated by pregnancy and extracellular matrix. J. biol. Chem. 1987;262:17247–17250. [PubMed] [Google Scholar]
- Daniel CW, Berger JJ, Strickland P, Garcia R. Similar growth pattern of mouse mammary epithelium cultivated in collagen matrix in vivo and in vitro. Devi Biol. 1984;104:57–64. doi: 10.1016/0012-1606(84)90036-8. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB. Postnatal development of the rodent mammary gland. In: Neville M, Daniel C, editors. The Mammary Gland: Development, Regulation and Function. Plenum Press Publishing Corporation; New York: 1987. pp. 3–36. [Google Scholar]
- David G, Bernfield M. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: Possible mechanism for basal lamina formation. Proc. natn. Acad. Sci. U.S.A. 1979;76:786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Nusgens B, van der Schueren B, Cauwenberge DV, van der Berghe G, Lapiere C. Collagen metabolism and basement membrane formation in cultures of mouse mammary epithelial cells. Expl Cell Res. 1987;170:402–416. doi: 10.1016/0014-4827(87)90316-8. [DOI] [PubMed] [Google Scholar]
- Dolbeare F, Beisker W, Pallavicini MG, Vanderlaan M, Gray JW. Cytochemistry for bromodeoxyuridine/DNA analysis: stoichiometry and sensitivity. Cytometry. 1985;6:521–530. doi: 10.1002/cyto.990060606. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Burwen SJ, Pitelka DR. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue and Cell. 1979;11:109–119. doi: 10.1016/0040-8166(79)90011-9. [DOI] [PubMed] [Google Scholar]
- Emerman JT, Enami J, Pitelka DR, Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc. natn. Acad. Sci. U.S.A. 1977;74:4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonard H, Grimaud JA, Nusgens B, Lapiere CHM, Foidart JM. Reconstituted basement membrane matrix modulates fibroblast activities in vitro. J. cell. Physiol. 1987;133:95–102. doi: 10.1002/jcp.1041330112. [DOI] [PubMed] [Google Scholar]
- Flynn S, Yang J, Nandi S. Growth and differentiation of primary cultures of mouse mammary epithelium embedded in collagen gel. Different. 1982;22:191–195. doi: 10.1111/j.1432-0436.1982.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Foster CS, Smith CA, Dinsdale EA, Monagham P, Neville AM. Human mammary gland morphogenesis in vitro: The growth and differentiation of normal breast epithelium in collagen gel cultures defined by electron microscopy, monoclonal antibodies, and autoradiography. Devi Biol. 1983;96:197–216. doi: 10.1016/0012-1606(83)90323-8. [DOI] [PubMed] [Google Scholar]
- Glass RH, Aggeler J, Spindle A, Pedersen R, Werb Z. Degradation of the extracellular matrix by mouse trophoblast outgrowths: a model for implantation. J. Cell Biol. 1983;96:1108–1116. doi: 10.1083/jcb.96.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley MA, Byers SW, Suarez-Quian CA, Kleinman H, Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 1985;101:1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeuptle M-T, Suard YLM, Bogenmann E, Reggio H, Racine L, Kraehenbuhl J-P. Effect of cell shape change on the function and differentiation of rabbit mammary cells in culture. J. Cell Biol. 1983;96:1425–1434. doi: 10.1083/jcb.96.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall HG, Farson DA, Bissell MJ. Lumen formation by epithelial cell lines in response to collagen overlay: A morphogenetic model in culture. Proc. natn. Acad. Sci. U.S.A. 1982;79:4672–4676. doi: 10.1073/pnas.79.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: A family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Mcgarvey ML, Hassell JR, Martin GR. Formation of a supramolecular complex is involved in the reconstitution of basement membrane components. Biochem. 1983;22:4969–4974. doi: 10.1021/bi00290a014. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Mcgarvey ML, Hassell JR, Martin GR, van Evercooren AB, Dubois-Dalcqa M. The role of laminin in basement membranes and in the growth, adhesion, and differentiation of cells. In: Trelstad RL, editor. The Role of Extracellular Matrix in Development. Alan R. Liss, Inc.; New York: 1984. pp. 123–143. [Google Scholar]
- Kleinman HK, Mcgarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochem. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Lee EY-H, Barcellos-Hoff MH, Chen L-H, Parry G, Bissell MJ. Transferrin is a major mouse milk protein and is synthesized by mammary epithelial cells. In Vitro Cell, and Developmental Biol. 1987;23:221–226. doi: 10.1007/BF02623583. [DOI] [PubMed] [Google Scholar]
- Lee EY-H, Lee W-H, Kaetzel CS, Parry G, Bissell MJ. Interaction of mouse mammary epithelial cells with collagenous substrata: Regulation of casein gene expression and secretion. Proc. natn. Acad. Sci. U.S.A. 1985;82:1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY-H, Parry G, Bissell MJ. Modulation of secreted proteins of mouse mammary epithelial cells by the extracellular matrix. J. Cell Biol. 1984;98:146–155. doi: 10.1083/jcb.98.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M-L, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc. natn. Acad. Sci. U.S.A. 1987;84:136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Fink LM, Pierce GB. Removal of basement membrane in the involuting breast. Laboratory Investigation. 1976;34:455–462. [PubMed] [Google Scholar]
- Masson PL, Heremans JF. Lactoferrin in milk of different species. Comp. Biochem. Physiol. 1971;39B:119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- Maxwell GD, Forbes ME. Exogeneous basement-membrane-like matrix stimulates adrenergic development in avian neural crest cultures. Development. 1987;101:767–776. doi: 10.1242/dev.101.4.767. [DOI] [PubMed] [Google Scholar]
- Medina D, Li ML, Oborn CJ, Bissell MJ. Casein gene expression in mouse mammary epithelial cell lines: Dependence upon extracellular matrix and cell type. Expl Cell Res. 1987;172:192–203. doi: 10.1016/0014-4827(87)90105-4. [DOI] [PubMed] [Google Scholar]
- Oliver C, Waters JF, Tolbert CL, Kleinman HK. Growth of exocrine acinar cells on a reconstituted basement membrane gel. In Vitro cell, develop. Biol. 1987;23:465–473. doi: 10.1007/BF02628416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod EJ, Rudland PS. Mammary gland morphogenesis in vitro: Extracellular requirements for the formation of tubules in collagen gels by a cloned rat mammary epithelial cell line. In Vitro cell, develop. Biol. 1988;24:17–27. doi: 10.1007/BF02623811. [DOI] [PubMed] [Google Scholar]
- Parry G, Lee Y-H, Farson D, Koval M, Bissell MJ. Collagenous substrata regulate the nature and distribution of glycosaminoglycans produced by differentiated cultures of mouse mammary epithelial cells. Expl Cell Res. 1985;156:487–499. doi: 10.1016/0014-4827(85)90556-7. [DOI] [PubMed] [Google Scholar]
- Parry G, Cullen B, Kaetzel CS, Kramer R, Moss V. Regulation of differentiation and polarized secretion in mammary epithelial cells maintained in culture: Extracellular matrix and membrane polarity influences. J. Cell Biol. 1987;105:2043–2051. doi: 10.1083/jcb.105.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett PB, Pitelka DR, Hamamoto ST, Misfeldt DS. Occluding junctions and cell behavior in primary cultures of normal and neoplastic mammary gland cells. J. Cell Biol. 1975;66:316–332. doi: 10.1083/jcb.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitelka DR, Hamamoto ST, Duafala JG, Nemanic MK. Cell contacts in the mouse mammary gland. J. Cell Biol. 1973;56:797–818. doi: 10.1083/jcb.56.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitelka DR, Taggart BN, Hamamoto ST. Effects of extracellular calcium depletion on membrane topography and occluding junctions of mammary epithelial cells in culture. J. Cell Biol. 1983;96:613–624. doi: 10.1083/jcb.96.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheutz EG, Li K, Omiecinski CJ, Muller-Eberhad U, Kleinman HK, Elswick B, Guzelian PS. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J. cell. Physiol. 1988;134:309–323. doi: 10.1002/jcp.1041340302. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Devi Biol. 1982a;90:215–222. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Daniel CW. Elvax 40P implants, sustained local release of bioactive molecules influencing mammary ductal development. Devi Biol. 1982b;93:272–278. doi: 10.1016/0012-1606(82)90259-7. [DOI] [PubMed] [Google Scholar]
- Suard YML, Haeuptle M-T, Farinon E, Kraehenbuhl J-P. Cell proliferation and milk protein gene expression in rabbit mammary cell cultures. J. Cell Biol. 1983;96:1435–1442. doi: 10.1083/jcb.96.5.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley EA, Erickson CA, Tucker RP. The retention and ultrastructural appearance of various extracellular matrix molecules incorporated into three-dimensional hydrated collagen lattices. Devi Biol. 1985;109:347–369. doi: 10.1016/0012-1606(85)90461-0. [DOI] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Kartenbeck J, Franke WW. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. J. Cell Biol. 1986;102:1832–1842. doi: 10.1083/jcb.102.5.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton MJ, Mitchell D, Ormerod EJ, Rudland P. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J. Histochem. Cytochem. 1982;30:667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- Warburton MJ, Monoghan P, Ferns SA, Rudland PS, Perusinghe N, Chung AE. Distribution of entactin in the basement membrane of the rat mammary gland. Expl Cell Res. 1984;152:240–254. doi: 10.1016/0014-4827(84)90249-0. [DOI] [PubMed] [Google Scholar]
- Watt FM. The extracellular matrix and cell shape. TIBS. 1986;11:482–485. [Google Scholar]
- Wicha MS, Llotta LA, Vonderhaar BK, Kldwell WR. Effects of inhibition of basement membrane collagen deposition on rat mammary gland development. Devi Biol. 1980;80:253–266. doi: 10.1016/0012-1606(80)90402-9. [DOI] [PubMed] [Google Scholar]
- Wicha MS, Lowrie G, Klohn E, Bagavandoss P, Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc. natn. Acad. Sci. U.S.A. 1982;79:3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens D, Park CS, Stockdale FE. Milk protein expression and ductal morphogenesis in the mammary gland in vitro: Hormone-dependent and -independent phases of adipocyte-mammary epithelial cell interaction. Devi Biol. 1987;120:245–258. doi: 10.1016/0012-1606(87)90122-9. [DOI] [PubMed] [Google Scholar]
- Williams JM, Daniel CW. Mammary ductal elongation: Differentiation of myoepithelium and basal lamina during branching morphogenesis. Devi Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- Yang J, Richards J, Guzman R, Enami J, Imagawa W, Nandi S. Sustained growth and three-dimensional organizaton of primary mammary tumor epithelial cells embedded in collagen gels. Proc. natn. Acad. Sci. U.S.A. 1980;77:2088–2092. doi: 10.1073/pnas.77.4.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]