Abstract
Purpose
Prosurvival Bcl-2 proteins inhibit the mitochondrial and death receptor-mediated apoptotic pathways. Obatoclax is a small-molecule antagonist of the BH3-binding groove of Bcl-2 proteins that may enhance tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sensitivity and efficacy.
Experimental Design
Human pancreatic cancer cell lines were incubated with obatoclax and/or TRAIL and cell viability, Annexin V labeling, caspase cleavage, and cytochrome c release were measured. In drug-treated cell lines, protein-protein interactions were studied by immunoprecipitation. Bax/Bak activation was analyzed using conformation-specific antibodies. Lentiviral short hairpin RNA was used to knockdown Bim, Bid, and apoptosis-inducing factor (AIF) expression.
Results
Obatoclax reduced the viability of PANC-1 and BxPC-3 cell lines and synergistically enhanced TRAIL-mediated cytotoxicity. Obatoclax enhanced TRAIL-mediated apoptosis, as shown by Annexin V labeling, which was accompanied by caspase activation (caspase-8, -9, and -3) and cleavage of Bid. Obatoclax potentiated TRAIL-mediated Bax/Bak activation and the release of mitochondrial cytochrome c, Smac, and AIF. Mechanisms underlying the apoptotic effect of obatoclax include displacement of Bak from its sequestration by Bcl-xL or Mcl-1 and release of Bim from Bcl-2 or Mcl-1. Bid knockdown by short hairpin RNA attenuated caspase cleavage and cytotoxicity of obatoclax plusTRAIL. Bim knockdown failed to inhibit the cytotoxic effect of obatoclax alone or combined with TRAIL yet attenuated TRAIL-mediated cytotoxicity. AIF knockdown attenuated cytotoxicity of the drug combination.
Conclusions
Obatoclax potentiates TRAIL-mediated apoptosis by unsequestering Bak and Bim from Bcl-2/Bcl-xL or Mcl-1 proteins. This drug combination enhances Bid-mediated cross-talk between the mitochondrial and death receptor-mediated apoptotic pathways and may represent a novel therapeutic strategy against pancreatic cancer.
Pancreatic cancer carries a poor prognosis and is the fourth leading cause of cancer-related mortality in the United States. These tumors show refractoriness to cytotoxic chemotherapy due, in part, to their frequent expression of prosurvival Bcl-2 proteins (Bcl-2, Bcl-xL, Mcl-1, and Bcl-w) that are potent inhibitors of the intrinsic mitochondrial apoptotic pathway used by most anticancer drugs and radiation (1). Mitochondrial permeabilization results in the release of apoptogenic molecules including cytochrome c that, on release, activate caspases to execute the cell death pathway (2). The Bcl-2 family consists of prosurvival proteins and two factions of proapoptotic proteins that include multidomain Bak and Bax and the BH3-only proteins (Bad, Bim, Bid, Puma, and Noxa). BH3 proteins are induced by cellular stress and bind to the prosurvival Bcl-2 family proteins to neutralize them, allowing apoptosis to occur (3). Specifically, protein-protein interactions occur with the insertion of the amphophilic BH3 domain of proapoptotic members into a hydrophobic cleft at the surface of prosurvival members (4). BH3-only proteins either directly or indirectly activate Bak and/or Bax proteins that involve conformational changes at the mitochondrial membrane whose permeabilization they regulate (5, 6). An alternative, extrinsic apoptotic pathway is engaged by members of the tumor necrosis factor family, including the death receptor ligand, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). TRAIL is a promising anticancer drug that binds its membrane death receptors resulting in DISC formation, caspase-8 and Bid cleavage, and subsequent effector caspase-3 and -7 activation (7). Most human cancer cells are referred to as type II in that they require a mitochondrial amplification step (intrinsic pathway) after a death receptor stimulus to induce apoptosis (7, 8). Cross-talk between the extrinsic and the intrinsic apoptotic pathways is mediated by caspase-8-induced Bid cleavage (9–11) with translocation of truncated Bid to mitochondria to activate Bax and to stimulate the release of cytochrome c (12, 13).
TRAIL-induced apoptosis has been shown to be inhibited by prosurvival Bcl-2 proteins (14, 15). Therefore, strategies to circumvent Bcl-2-mediated resistance are needed to increase the efficacy of TRAIL for treatment of human cancers. Moreover, therapeutic modulation of the Bcl-2 pathway may represent an important therapeutic target in human cancers (16, 17). A novel class of small-molecule Bcl-2 antagonists [obatoclax (GeminX Pharmaceuticals) and ABT-737 (Abbott)] were identified by chemical library screening to bind the hydrophobic groove of prosurvival Bcl-2 proteins to antagonize their function (18, 19). These agents mimic endogenous proapoptotic BH3-only proteins (Bad, Bid, Bim, Noxa, and Puma) that are activated by cellular stressors including anticancer drugs (3). Obatoclax, also known as GX015-070, induces apoptosis that is dependent on Bax and Bak. In contrast to ABT-737, obatoclax can neutralize Mcl-1, whereas ABT-737 disables Bcl-2 and Bcl-xL but binds to Mcl-1 with low affinity (20). Obatoclax was shown to potently interfere with the direct interaction between Mcl-1 and Bak in the mitochondrial outer membrane and inhibited their association in intact cells (21). Therefore, the ability of obatoclax to target Mcl-1 suggests a broad clinical utility for this agent to include tumors that overexpress Mcl-1 (22). In a recent study, we found that knockdown of Mcl-1 sensitized human pancreatic cancer cells to ABT-737-induced apoptosis (23), indicating that Mcl-1 is a relevant therapeutic target in this malignancy. Obatoclax has been shown to induce apoptosis in lymphoma and melanoma cells and to enhance the cytotoxicity of bortezomib against mantle cell lymphoma (24, 25). Obatoclax is currently undergoing evaluation in multiple single-agent and combination phase I and II clinical trials directed at leukemia, lymphoma, and selected solid tumor malignancies.
Translational Relevance.
Pancreatic cancers display broad resistance to anticancer drug-induced apoptosis that is related to the expression of prosurvival Bcl-2 family proteins. The recent development of small-molecule antagonists of prosurvival Bcl-2 family proteins holds promise for the therapy of pancreatic and other malignancies. These novel compounds, also known as BH3 mimetics, bind to prosurvival Bcl-2 proteins to neutralize them. BH3-only proteins are induced by cellular stress including chemotherapy, and gene knockout or suppression of their expression confers apoptosis resistance. Obatoclax is a BH3 mimetic and pan-Bcl-2 inhibitor, whereas another BH3 mimetic, ABT-737, selectively inhibits Bcl-2 and Bcl-xL butnot Mcl-1. In this report, we examine its ability to enhance TRAIL-mediated apoptotic signaling and efficacy. TRAIL is a promising anticancer cytokine that has shown selectivity for cancer cells. TRAIL engages the membrane death receptor-mediated or extrinsic apoptotic pathway. Most human cancer cells require a mitochondrial amplification step after a death receptor-mediated stimulus, and prosurvival Bcl-2 proteins have been shown to inhibit TRAIL-mediated apo ptosis. Therefore, removal of the mitochondrial “block” im posed by Bcl-2 proteins using a BH3 mimetic may enhance TRAIL-induced apoptosis and efficacy. Examining the mechanisms by which BH3 mimetics promote apoptosis, including their effects on protein-protein interactions, may guide the use and further development of this novel class of compounds.
In this report, we determined the ability of a small-molecule inhibitor of prosurvival Bcl-2 proteins to synergistically enhance the cytotoxic effects of the death receptor agonist TRAIL, thereby enabling cross-talk between the death receptor and mitochondrial apoptotic pathways. We used human pancreatic cancer cell lines that express prosurvival Bcl-2 proteins, including Mcl-1, and display variable resistance to TRAIL. We found that obatoclax can antagonize Bcl-2/Bcl-xL/Mcl-1 to release proapoptotic Bak and the BH3-only protein Bim. Furthermore, obatoclax augmented Bak/Bax activation by TRAIL and triggered a caspase-dependent apoptosis. Dependence on Bid was shown in knockdown experiments, whereby Bid short hairpin RNA (shRNA) attenuated caspase activation and cytotoxicity induced by obatoclax plus TRAIL. Bim knockdown attenuated the cytotoxic effect of TRAIL but not its combination with obatoclax. Together, these data provide a mechanistic basis for the enhanced apoptotic effect of the drug combination in the treatment of pancreatic cancer cells.
Materials and Methods
Cell culture, chemical compounds, and biological reagents
Human pancreatic cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 100 µg/mL penicillin, 100 µg/mL streptomycin (Invitrogen), 10 mmol/L HEPES, and 1% sodium pyruvate. Obatoclax was obtained from GeminX Pharmaceuticals and dissolved in DMSO at a stock concentration of 5 mmol/L that was aliquoted and stored at −20°C. Human recombinant TRAIL was obtained from R&D Systems. Antibodies used for immunoblot analysis included mouse anti-Bcl-2, mouse anti-Mcl-1, and mouse anti-caspase-8 (BD Biosciences); rabbit anti-Bim (Santa Cruz Biotechnology); rabbit anti-Bid, anti-caspase-9, anti-Bax, and anti-cleaved caspase-3 (Cell Signaling Technology); and mouse anti-Bcl-xL and mouse anti-Bak (EMD Chemicals). Antibodies used for immunoprecipitation included rabbit anti-Bak (Millipore), rabbit anti-Bim (Santa Cruz Biotechnology), mouse anti-Bax 6A7 (Sigma), and mouse anti-Bak Ab-1 or Ab-2 (EMD Chemicals).
Knockdown using lentiviral shRNA
Target sequences for Bak, Bax, and Bim (23) were selected and were synthesized by the Mayo Clinic Molecular Biology Core Facility. The targeting sequence for Bid was AAGAAGACATCATCCGGAATA. The targeting sequence was CCCATTCACTACAGGTGAA for Bak (26), was GCTCTGAGCAGATCATGAA for Bax (26), and was GATCCTCCCCGAATACCTC for apoptosis-inducing factor (AIF; ref. 27). After annealing, the template forms a double-stranded DNA flanked by EcoRI and BamHI sites and can be ligated into the lentiviral shRNA cloning and expression vector pSIH-H1 (System Biosciences). Insertion at the intended site was confirmed by DNA sequencing. For pseudovirus production, 2 µg endotoxin-free lentivector expression construct was mixed with lentivector packaging plasmid mix (System Biosciences) and diluted in serum reduction Opti-MEM containing Plus reagent (Invitrogen). After incubation at room temperature for 15 min, the Lipofectamine reagent containing Opti-MEM was added dropwise into the above DNA/Plus complex and incubated for another 15 min. Lentivirus producer cell line 293T was transfected with the DNA/Lipofectamine/Plus complex in Opti-MEM overnight in the 5% CO2 incubator at 37°C. The next day, the medium was replaced with fresh DMEM containing 2% heat-activated fetal bovine serum and the incubation at 37°C was continued. At 48 h post-transfection, the supernatants were collected, clarified, and filtered through Millex-HV 0.45 µm polyvinylidene difluoride filters (Millipore). The supernatants were then concentrated by adding 10% (final concentration) of PEG-8000 (Sigma), incubated at 4°C overnight for no less than 12 h, and centrifuged at 1,500 × g for 10 min at 4°C. The pseudovirus pellet was resuspended in a small volume of Opti-MEM and stored at −80 °C. For transduction of the lentivector expression construct (packaged in pseudo-type viral particles) into target cells, appropriate amounts of virus in Opti-MEM were directly added to target cells grown in Opti-MEM containing 8 µg/mL polybrene (Sigma) and incubated overnight at 37°C. Target gene knockdown was then tested 72 h post-transduction.
Cell viability assay
Cell viability was analyzed by the MTS assay per the manufacturer’s protocol (Promega) and as previously described (23). Absorbance was recorded at 492 nm using a Versamax microplate reader (Molecular Devices). Each experimental condition was done in triplicate and the SD was calculated.
Annexin V labeling
After drug treatment, adherent cells were detached from culture dishes by treating with trypsin (Invitrogen) for 3 to 5 min and combined with floating cells. Total cells were then washed with cold PBSs and resuspended in 1× Annexin V binding buffer (BD Biosciences) at a concentration of 1 × 106 to 1 × 107 cells/mL. A 100 µL single-cell suspension was labeled with 10 µL Annexin V-APC (BD Biosciences) for 15 min at room temperature in the dark. Then, 1 × Annexin V binding buffer (390 µL) was added to each tube. Flow cytometry (fluorescence-activated cell sorting) was done on a FACScan (Becton Dickinson). A minimum of 20,000 cells per sample were analyzed. The extent of apoptosis was quantified as percentage of Annexin V-positive cells, and the extent of drug-specific apoptosis was assessed by this formula: % specific apoptosis = (test - control) × 100 / (100 - control) (28).
Immunoprecipitation
Cells were lysed in CHAPS buffer [5 mmol/L MgCl2, 137 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% CHAPS, 10 mmol/L HEPES (pH 7.5)] for 30 min on ice and clarified by centrifugation at 14,000 rpm for 10 min at 4°C. The protein concentration of the supernatant was measured by nanodrop (Nano-Drop Technologies) and was normalized. Lysates were then incubated with an antibody/protein A/G bead complex, made by incubating antibody with protein A/G beads in 0.5 mL PBS at room temperature for 2 h, at 4°C for 3 h or overnight. Unbound proteins were washed three times with 1 mL CHAPS buffer without protease inhibitors. Bound proteins on the beads were eluted by boiling the sample in LDS sample buffer (Invitrogen) and then loaded for Western blotting.
Detection of Bak/Bax conformational change
Bak or Bax proteins were immunoprecipitated with mouse anti-Bak Ab-1 or Ab-2 (EMD Chemicals) or mouse anti-Bax 6A7 (Sigma) conformation-specific antibodies, respectively (26, 29). Proteins were subjected to SDS-PAGE fractionation and Western blotting. Bak and Bax bands were probed with rabbit anti-Bak (Millipore) or rabbit anti-Bax (Cell Signaling) antibodies. To minimize the interference of denatured mouse IgG light chains that originated from the mouse anti-Bak antibody used for immunoprecipitation and ran at the similar size as Bak, a longer time (70 min) of electrophoresis on 14% SDS-PAGE mini-gel was done to differentiate protein bands and a native IgG detection kit (Pierce Biotechnology) was used as per the manufacturer’s protocol.
Release of mitochondrial apoptogenic proteins
Following drug treatment, the release of Smac, cytochrome c, and AIF was analyzed using a selective digitonin lysis method. For these assays, 4 × 106 cells per condition were resuspended in 50 µL permeabilization buffer containing 75 mmol/L NaCl, 10 mmol/L HEPES (pH 7.5), 250 mmol/L sucrose (added fresh before use), 1 mmol/L EDTA, and 700 µg/mL digitonin (final concentration of digitonin, 35 µg/4 × 106 cells). After incubation at room temperature for 5 min, cells were pelleted by centrifugation for 3 min at 13,000 × g and supernatants containing cytochrome c, Smac, and AIF proteins were obtained and subjected to Western blotting. An antibody against cytochrome oxidase subunit IV (Molecular Probes) was used as a marker of the cytosolic fraction.
Western blotting
Protein samples were prepared in a lysis buffer, as above, normalized using nanodrop measurement (NanoDrop Technologies), boiled in LDS sample buffer (Invitrogen), and loaded onto 14% SDS-PAGE gels with electrophoretic transfer onto a polyvinylidene difluoride membrane (Bio-Rad). The membrane was blocked with 0.2% I-Block (Applied Biosystems) in PBS-T (PBS containing 0.1% Tween 20) and incubated with the primary antibodies in PBS-T containing 0.2% I-Block at room temperature for 3 h or overnight at 4°C. The membrane was then incubated with a secondary antibody conjugated to alkaline phosphatase in PBS-T containing 0.2% I-Block. The specific protein bands were then developed with CDP-star substrate (Applied Biosystems).
Calculation of combination index
The effect of combination between obatoclax and TRAIL was analyzed using Calcusyn software (Biosoft) as reported previously (30).
Statistical analysis
The values represent the mean ± SD for triplicate or duplicate experiments. The statistical significance of the differences between experimental variables was determined using the Student’s t test. P < 0.05 was considered statistically significant.
Results
Obatoclax synergistically enhances TRAIL-mediated cytotoxicity
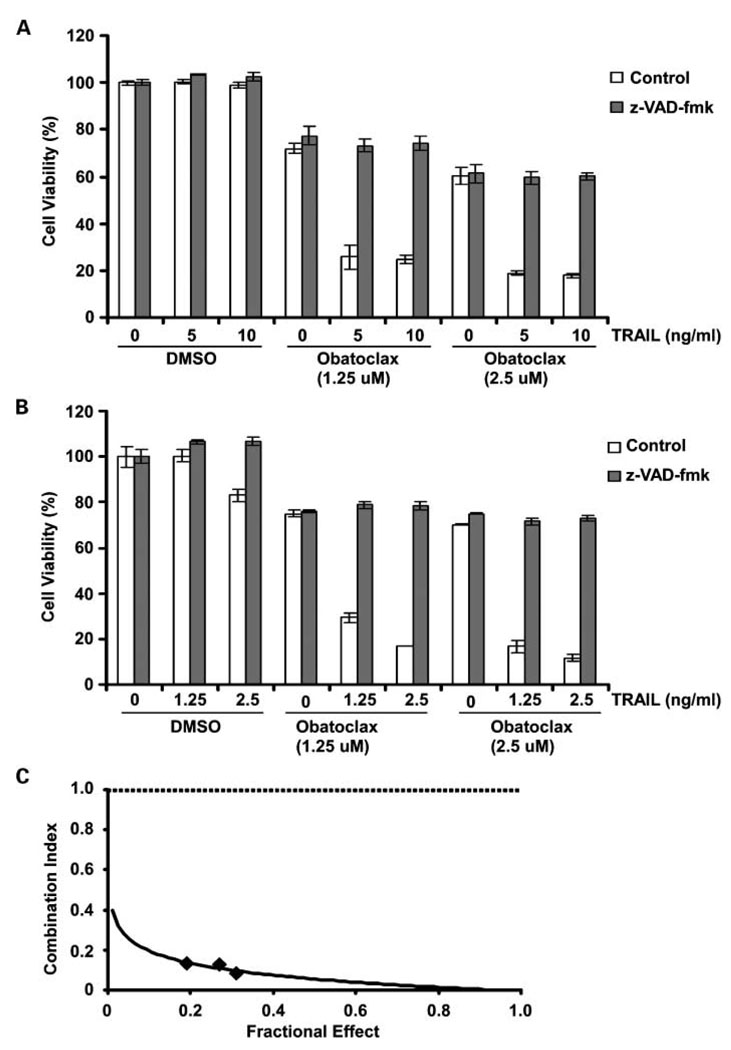
We determined the effect of obatoclax monotherapy on cell viability in human pancreatic cancer cell lines (PANC-1 and BxPC-3). BxPC-3 show relatively higher endogenous levels of Bcl-2, Bcl-xL, and Mcl-1 compared with PANC-1 cells (23). PANC-1 cells are relatively TRAIL-resistant compared with BxPC-3 cells (23, 31); therefore, these cells were treated with relatively higher doses of TRAIL. Single-agent obatoclax treatment was associated with significant reductions in cell viability (Fig. 1A and B). Obatoclax was shown to significantly enhance TRAIL-mediated cytotoxicity in both cell lines (P < 0.05; Fig. 1A and B) and also in CFPAC-1 cells (data not shown), indicating its ability to sensitize cells to TRAIL. Furthermore, the cytotoxic effect of the drug combination was caspase-dependent as shown by the ability of z-VAD-fmk to significantly attenuate drug-induced cytotoxicity in PANC-1 and BxPC-3 cells (Fig. 1A and B). To determine whether the cytotoxic effect of the obatoclax plus TRAIL was synergistic or additive, we performed an analysis using the median effect method. Different concentrations of obatoclax and/or TRAIL at a fixed ratio were chosen for analysis, and the combination index values were calculated per the method of Chou and Talalay (32). As shown in an isobologram, the combination index values were <1, indicating a synergistic interaction (Fig. 1C).
Fig. 1.
Obatoclax synergistically enhances TRAIL-mediated cytotoxicity in a caspase-dependent manner in human pancreatic cancer cell lines. PANC-1 (A) and BxPC-3 (B) cells were incubated with obatoclax alone and in combination with TRAIL at indicated doses in the presence or absence of the pan-caspase inhibitor, z-VAD-fmk (50 µmol/L). Drug treatments were done for 48 h at 37 °C, and cytotoxicity was assessed by the MTS assay with absorbance measured at 492 nm. Columns, mean of triplicate experiments; bars, SD. C, BxPC-3 cells were treated with a range of concentrations of obatoclax (1.25–5 µmol/L) and TRAIL (1.25–5 ng/mL) at a fixed ratio for 48 h. Cell viability was measured using the MTS assay. A combinational index was then calculated as described in Materials and Methods and plotted against the fractional effect as shown in an isobologram. Synergism between obatoclax and TRAIL is indicated by a combination index < 1.0.
Obatoclax enhances TRAIL-mediated apoptosis
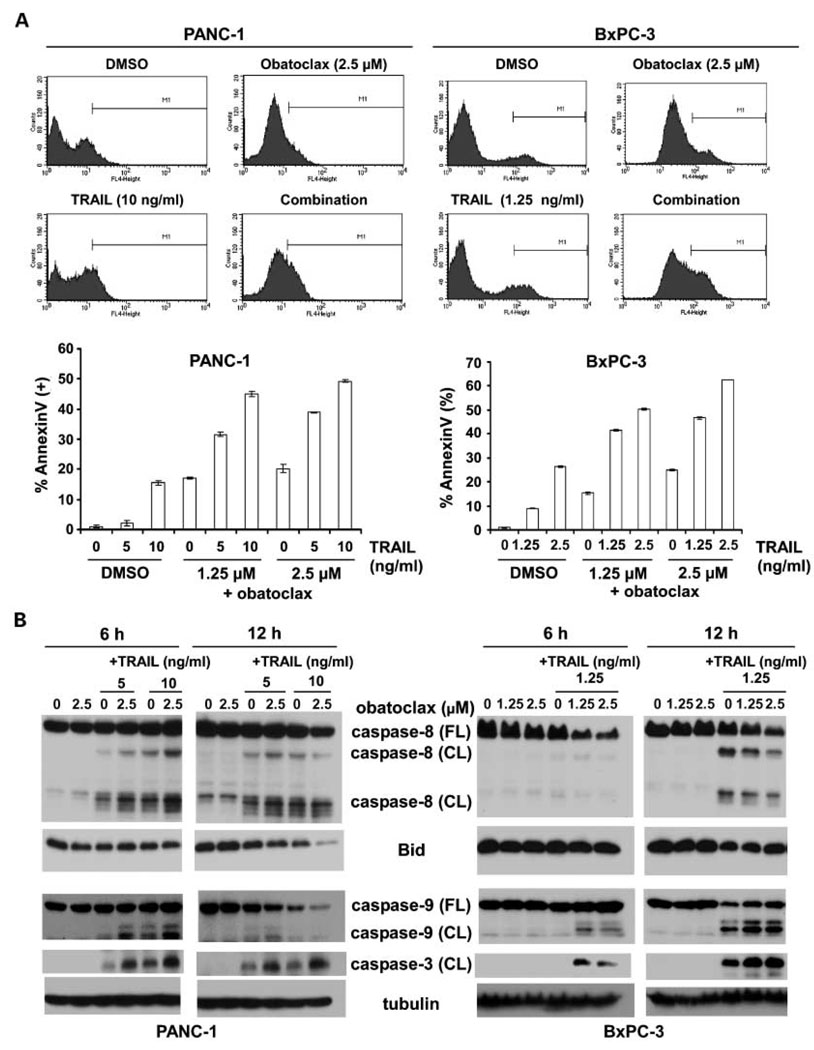
We determined the ability of obatoclax alone and in combination with TRAIL to induce apoptosis using Annexin V labeling. Single agent obatoclax (0–2.5 µmol/L) induced apoptosis in both cell lines (Fig. 2A). Obatoclax was found to significantly enhance TRAIL-mediated apoptosis in both cell lines and to a greater extent compared with either drug alone (Fig. 2A and B). Enhancement of apoptosis by the drug combination was associated with increased activation of caspase-8, Bid, caspase-9, and caspase-3 (Fig. 2B). A feedback amplification loop mediated by caspase-3 may contribute to activation of caspase-8 and Bid (33). The extent of apoptosis, as detected by Annexin V labeling, approximates the reduction in cell viability produced by obatoclax, TRAIL, or their combination, suggesting that apoptosis accounts for most of the observed cytotoxicity (Figs. 1A and B and 2A).
Fig. 2.
Obatoclax augments TRAIL-mediated apoptotic signaling. A, PANC-1 and BxPC-3 cells were treated with obatoclax and/or TRAIL for 24 h and apoptosis was determined by phosphatidylserine externalization as detected by Annexin V-APC binding using flow cytometry. Annexin V-positive cells were quantitated as described in Materials and Methods. Results of triplicate determinations are shown. B, combination of obatoclax and TRAIL enhances the cleavage of caspase-8, -9, and -3 and reduces full-length Bid in PANC-1 and BxPC-3 cell lines. Cells were treated with drugs for 6 or 12 h and whole-cell lysates were prepared and then subjected to immunoblot analysis for determination of protein expression. β-Tubulin served as a loading control.
Obatoclax plus TRAIL induces Bak/Bax activation and cytochrome c release
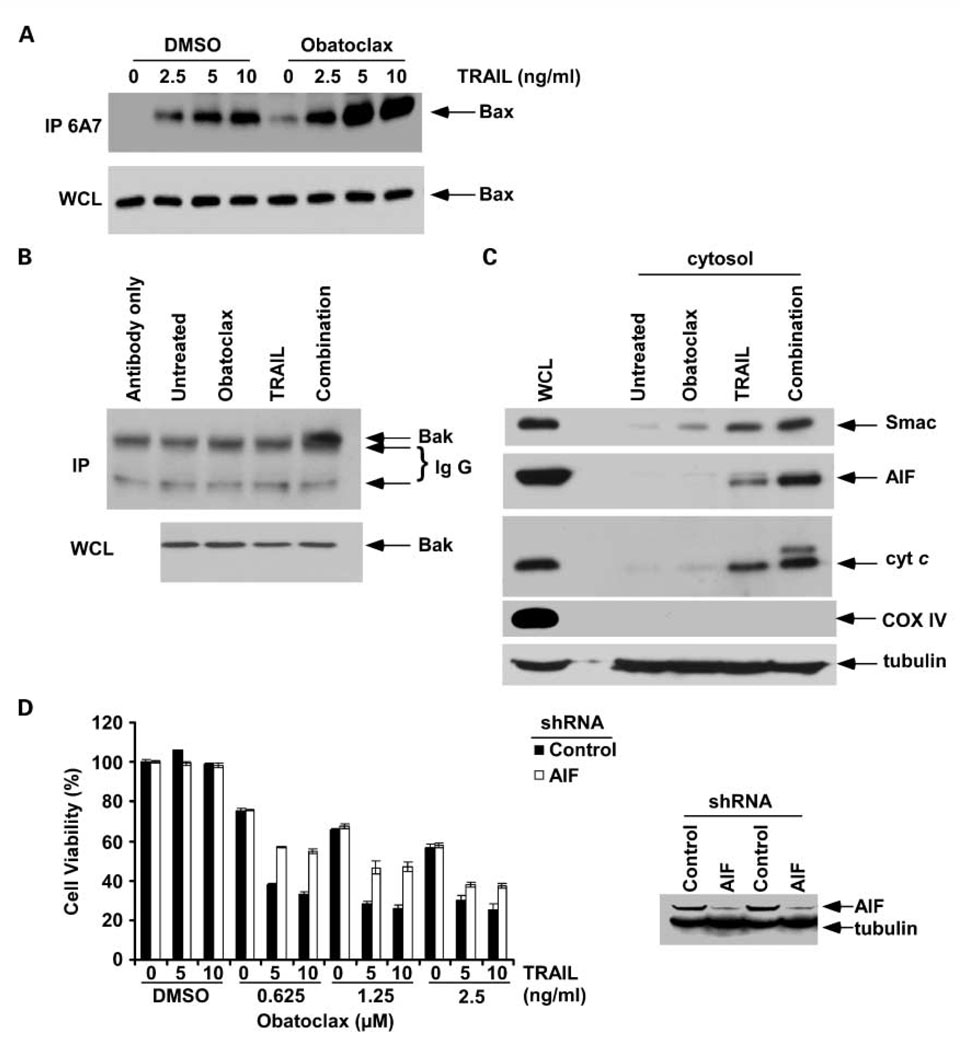
We studied the effect of obatoclax, TRAIL, and their combination on proapoptotic Bak and Bax activation as shown using antibodies recognizing the active protein conformers (29). Following an apoptotic stimulus, Bak and Bax undergo a conformational change, and Bax translocates to the mitochondrial outer membrane to induce permeabilization, indicating a commitment to cell death (29). TRAIL, and to a lesser extent, obatoclax monotherapy induced Bax activation. Coadministration of these drugs enhanced Bax activation to a greater extent compared with TRAIL alone in PANC-1 cells (Fig. 3A). Furthermore, coadministration of obatoclax and TRAIL induced a marked activation of Bak, whereas either drug alone failed to do so at the selected time point (6 h; Fig. 3B). To show that treatment of cells with the obatoclax and TRAIL can permeabilize the mitochondria, we analyzed the release of mitochondrial apoptogenic proteins. We found that obatoclax or TRAIL induced the release of cytochrome, c and Smac/DIABLO into the cytosol (Fig. 3C). TRAIL was also shown to release minimal AIF from the mitochondria. Furthermore, the combination of obatoclax plus TRAIL released cytochrome c, Smac, and AIF to a greater extent than did either drug alone in PANC-1 cells (Fig. 3C). Cytochrome oxidase subunit IV, a mitochondrial marker, is seen in the whole-cell lysate but not the cytosol, indicating the lack of mitochondrial contamination of the cytosolic fraction. Given the inability of 2-VAD-fmk to completely inhibit the cytotoxicity of obatoclax + TRAIL (Fig. 1B), we determined whether AIF contributes to apoptosis induction since AIF can mediate caspase-independent cell death (34). We generated AIF knockdown PANC-1 cells using shRNA. Knockdown of AIF was shown to protect cells from the cytotoxic effects induced by obatoclax plus TRAIL but not obatoclax alone compared with control shRNA cells (Fig. 3D). These results for AIF suggest that obatoclax plus TRAIL can also produce a caspase-independent cell death (34).
Fig. 3.
Obatoclax treatment enhances TRAIL-mediated activation of proapoptotic Bak/Bax proteins and induces cytochrome c release in PANC-1 cells. A, cells were treated with obatoclax (2.5 µmol/L),TRAIL (2.5, 5, 10 ng/mL), or their combination for 6 h. Immunoprecipitation was done using a conformation-specific antibody to Bax (mouse anti-Bax 6A7). Precipitants were then probed for Bax by immunoblotting using a rabbit polyclonal antibody. Whole-cell lysates show equal protein loading for Bax. B, cells were treated with obatoclax (2.5 µmol/L),TRAIL (10 ng/mL), or their combination for 6 h, and a Bak conformation-specific antibody was used to recognize and immunoprecipitate the activated Bak proteins. A rabbit anti-Bak polyclonal antibody and native IgG detection kit were used as primary and secondary antibodies in immunoblotting, respectively. C, immunoblot analysis of mitochondrial cytochrome c, Smac, and AIF release into the cytosol after treatment of cells with obatoclax (2.5 µmol/L),TRAIL (10 ng/mL), or their combination for 12 h. Cytochrome oxidase subunit IV was used as a mitochondrial marker. β-Tubulin was reprobed and served as a loading control. D, knockdown of AIF by lentiviral shRNA attenuates the cytotoxic effect of obatoclax combined withTRAIL, but not obatoclax alone, compared with shRNA control PANC-1 cells. Columns, mean of triplicate experiments; bars, SD.
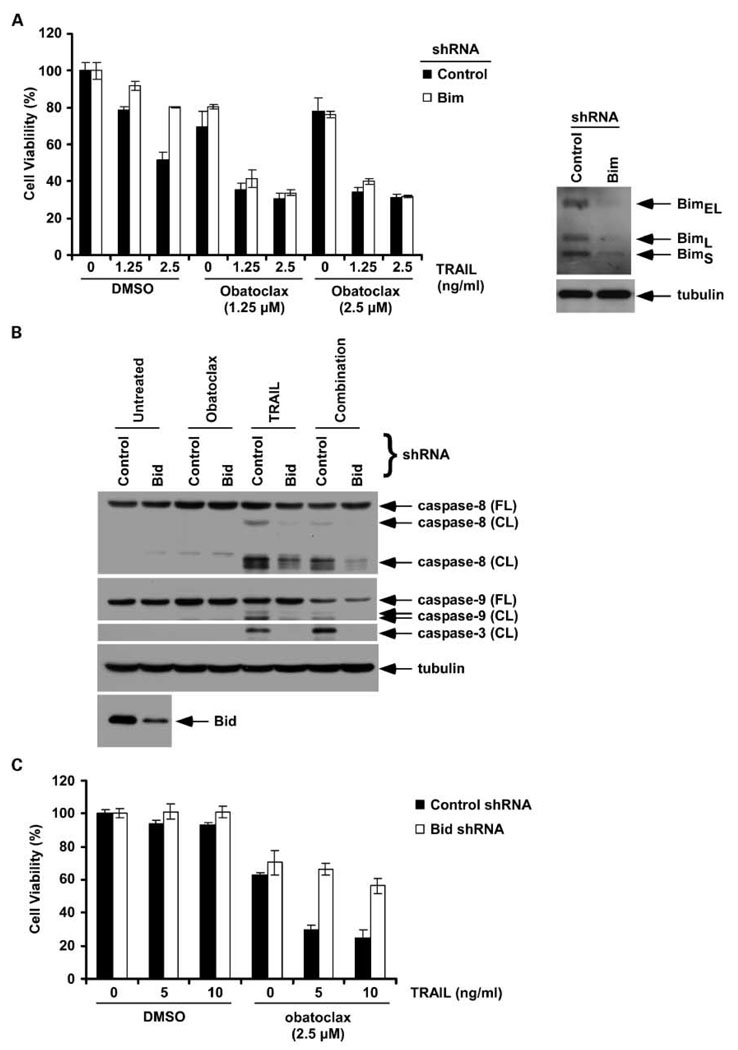
Effect of knockdown of Bim and Bid on drug-induced cytotoxicity and caspase activation
Bim has been shown to bind to all prosurvival Bcl-2 proteins and is therefore a potent proapoptotic BH3-only protein (17). To determine the role of Bim in the cytotoxic effect of obatoclax and/or TRAIL, we generated Bim knockdown BxPC-3 cells using shRNA. Bim knockdown was shown to reduce TRAIL-mediated cytotoxicity but did not alter the cytotoxic effect of obatoclax alone or combined with TRAIL (Fig. 4A). Given the ability of obatoclax to enhance cross-talk between the death receptor-mediated (induced by TRAIL) and the mitochondrial apoptotic pathways (Figs. 2A–C and 3A–C), we sought to confirm the role of Bid in mediating this cross-talk. Using Bid knockdown PANC-1 cells generated by lentiviral shRNA, we found that Bid knockdown markedly attenuated caspase cleavage (caspase-8, -9, and -3; Fig. 4B) and cytotoxicity induced by obatoclax plus TRAIL (Fig. 4C). We also determined whether obatoclax and/or TRAIL depend on Bak/Bax by using cells with knockdown of these proteins by shRNA. Lentiviral shRNA- transduced PANC-1 and BxPC-3 cell lines were treated with obatoclax, TRAIL, or their combination and caspase cleavage was analyzed. Suppression of Bak or Bax expression in both cell lines was shown to attenuate caspase-3 cleavage by the combination of obatoclax and TRAIL (data not shown).
Fig. 4.
Effect of knockdown of Bim and Bid expression on drug-induced cytotoxicity or caspase activation. A, knockdown of Bim by lentiviral shRNA in BxPC-3 cells attenuates the cytotoxic effect induced by TRAIL alone but not obatoclax or its combination with TRAIL. Columns, mean of triplicate experiments; bars, SD. B, knockdown of Bid by lentiviral shRNA (versus control) in PANC-1 cells attenuates the cleavage of caspase-8, -9, and -3 induced by coadministration of obatoclax and TRAIL. C, Bid knockdown in PANC-1 cells attenuates the cytotoxic effect of the combination of obatoclax plus TRAIL compared with treated control shRNA cells.
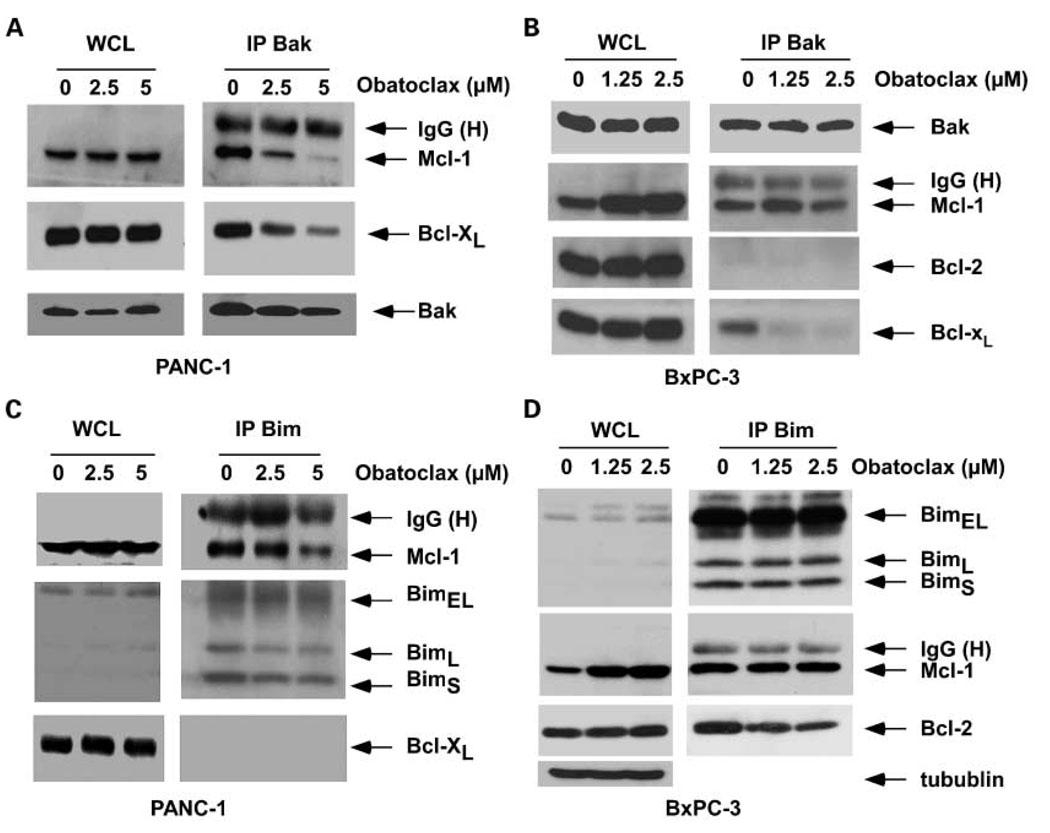
Obatoclax targets Bcl-2/Bcl-xL and Mcl-1 to unsequester proapoptotic binding partners
Recent studies have shown the importance of unsequestering Bak from Bcl-2/Bcl-xL in determining the lethality of obatoclax (24, 25). To examine the mechanism by which obatoclax can enhance TRAIL-mediated apoptosis, we studied interactions between proapoptotic multidomain or BH3-only proteins with prosurvival Bcl-2 proteins. Immunoprecipitation of Bak yielded abundant Mcl-1 and Bcl-xL proteins in vehicle-treated PANC-1 (Fig. 5A) and BxPC-3 cell lines (Fig. 5B), consistent with heterodimerization of these proteins in the control state (35). Treatment with obatoclax released Bak from its sequestration by Mcl-1 or Bcl-xL in both cell lines. The effect of obatoclax treatment on the association of the BH3-only Bim protein with prosurvival Bcl-2 proteins was then determined. Bim binds to and disables all the prosurvival Bcl-2 proteins and is therefore a potent proapoptotic molecule (17). Treatment with obatoclax (5 µmol/L) partially disrupted the association between Bim and its binding partner Mcl-1 in PANC-1 cells (Fig. 5C). Although obatoclax (1.25 and 2.5 µmol/L) failed to disrupt the interaction between Bim and Mcl-1 in BxPC-3 cells, it partially unsequestered Bim from its complex with Bcl-2 (Fig. 5D). Of note, PANC-1 cells lack Bcl-2 proteins (23). Treatment with obatoclax was associated with an increased level of Mcl-1 protein in BxPC-3 cells (Fig. 5D). Bim has been shown to bind to and stabilize Mcl-1, thereby preventing its degradation (36). The ability of obatoclax to release Bim from Bcl-2 may potentially contribute to stabilization of Mcl-1. In a separate study, we found that ABT-737 treatment can increase Mcl-1 expression in PANC-1 cells (23).
Fig. 5.
Obatoclax displaces proapoptotic Bak and Bim from their sequestration by prosurvival Mcl-1, Bcl-xL, or Bcl-2 proteins. PANC-1 (A) and BxPC-3 (B) cells were treated with obatoclax or diluent (DMSO) for 24 h and Bak proteins were immunoprecipitated. Precipitated proteins were then subjected to immunoblot analysis for the designated proteins. Whole-cell lysates and IgG heavy chain indicate similar protein loading. PANC-1 (C) and BxPC-3 (D) cells were treated with obatoclax or diluent (DMSO) for 24 h and Bim splice variants were immunoprecipitated. Precipitated proteins were then subjected to immunoblot analysis for the designated proteins. Whole-cell lysates and IgG heavy chain were also shown for similar loading for each condition. β-Tubulin served as a loading control.
Discussion
Pancreatic cancer cells frequently express prosurvival Bcl-2 proteins that contribute to their intrinsic drug resistance (22). Obatoclax is a novel proapoptotic agent that binds to and disables prosurvival Bcl-2 proteins (Mcl-1, Bcl-2, Bcl-xL, and Bcl-w; refs. 24, 25). Obatoclax also targets Mcl-1, whereas Mcl-1 has been shown to confer resistance to another BH3 mimetic, ABT-737 (37). We determined whether obatoclax can enhance TRAIL-mediated apoptosis by disabling Bcl-2 prosurvival proteins to enhance cross-talk between the extrinsic and intrinsic apoptotic pathways. We show for the first time that obatoclax can synergistically enhance TRAIL-mediated cytotoxicity in human pancreatic cancer cell lines. Cytotoxicity was observed at low concentrations of both obatoclax and TRAIL that are clinically relevant. Obatoclax enhanced TRAIL-mediated Bax/Bak activation as shown by their conformational change. The cytotoxicity of this drug combination involves a caspase-dependent apoptosis associated with mitochondrial permeabilization. Specifically, single-agent obatoclax or TRAIL treatment induced the release of mitochondrial cytochrome c, Smac, or AIF into the cytosol that was enhanced by their combination. Mechanisms underlying the synergy between obatoclax and TRAIL include the ability of obatoclax to displace Bak from its interaction with Bcl-xL or Mcl-1. Evidence suggests that Mcl-1 constitutively interacts with and regulates Bak within the mitochondrial outer membrane (35). Disruption of the Mcl-1/Bak complex, as shown here, may be required for Bak activation. We found that obatoclax can enhance TRAIL-mediated activation of both Bak and Bax proteins. In human lymphoma cells, single-agent obatoclax was shown to activate Bak/Bax and to release cytochrome c from mitochondria upstream of caspase activation (25). We found that obatoclax can unsequester Bim from its complex with Bcl-2 or Mcl-1. The ability of obatoclax to dissociate both Bak and Bim from their sequestration by Mcl-1 confirms that obatoclax can target Mcl-1. However, Bim knockdown failed to inhibit the cytotoxic effect of obatoclax alone or combined with TRAIL yet attenuated TRAIL-mediated cytotoxicity. Lack of dependence on Bim was shown in another report where Bim wild-type and knockout murine embryonic fibroblast cells showed similar sensitivity to obatoclax (21). Together, these findings show that obatoclax can circumvent the mitochondrial blockade conferred by prosurvival Bcl-2 proteins to enhance TRAIL-mediated apoptosis.
Knockdown of Bid was shown to markedly attenuate cytotoxicity and caspase cleavage induced by the combination of obatoclax and TRAIL, indicating that obatoclax can enhance cross-talk between the death receptor-mediated pathway and the mitochondrial apoptotic pathway that is negatively regulated by prosurvival Bcl-2 proteins. This effect occurs through antagonism of both Mcl-1 and Bcl-2/Bcl-xL proteins and activation of the mitochondrial regulators Bak and Bax that commit cells to a mitochondria-mediated death (38) as shown here by release of cytochrome c, Smac, and AIF proteins. Because obatoclax-induced cell death was partially independent of caspase activation, we determined the dependence of cell death on AIF. Using PANC-1 cells with AIF knockdown, we found that obatoclax-induced cytotoxicity is independent of AIF. However, the cytotoxic effect of obatoclax plus TRAIL was significantly reduced in AIF knock-down cells compared with control shRNA-transduced cells (Fig. 3D). In addition to our findings, obatoclax was shown to antagonize Mcl-1 and to overcome Mcl-1-mediated apoptosis resistance in human melanoma cells (24). Together, these observations indicate the ability of obatoclax to disrupt complexes between Mcl-1 and its high affinity binding partners to enhance apoptosis. These findings suggest the broad therapeutic utility of this agent to include pancreatic cancers and other tumors that overexpress Mcl-1 proteins (21, 22). In addition to the ability of obatoclax or ABT-737 (23) to enhance TRAIL-mediated cytotoxicity, obatoclax was also shown to enhance the efficacy of the proteasome inhibitor bortezomib in mantle cell lymphoma (25).
In conclusion, we show that obatoclax can exert a synergistic apoptotic effect when combined with TRAIL in human pancreatic cancer cells. The mechanism underlying the ability of obatoclax to enhance TRAIL-mediated apoptosis involves unsequestering Bak and Bim from Bcl-2/Bcl-xL or Mcl-1 proteins to lower the mitochondrial apoptotic threshold and to enhance Bid-mediated cross-talk between the death receptor-induced and mitochondrial apoptotic pathways. These data suggest that this drug combination may represent a novel therapeutic strategy against pancreatic cancer.
Acknowledgments
We thank Scott Kaufmann for assistance with calculation of the combination index and Jonelle Morales for capable secretarial assistance.
Grant support: Hirschberg Foundation for Pancreatic Cancer Research and National Cancer Institute grant CA 104683.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol. 2006;3:388–398. doi: 10.1038/ncponc0538. [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Wang X. Cytochrome c-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- 3.Labi V, Erlacher M, Kiessling S, Villunger A. BH3-only proteins in cell death initiation, malignant disease and anticancer therapy. Cell Death Differ. 2006;13:1325–1338. doi: 10.1038/sj.cdd.4401940. [DOI] [PubMed] [Google Scholar]
- 4.Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Annis MG, Soucie EL, Dlugosz PJ, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dlugosz PJ, Billen LP, Annis MG, et al. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006;25:2287–2296. doi: 10.1038/sj.emboj.7601126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, El-Deiry WS. TRAIL and apoptosis induction byTNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 8.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broaddus VC, Dansen TB, Abayasiriwardana KS, et al. Bid mediates apoptotic synergy between tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and DNA damage. J Biol Chem. 2005;280:12486–12493. doi: 10.1074/jbc.M408190200. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 11.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activa tion of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 12.Desagher S, Osen-Sand A, Nichols A, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res. 2004;10:8284–8292. doi: 10.1158/1078-0432.CCR-04-1289. [DOI] [PubMed] [Google Scholar]
- 15.Sun SY, Yue P, Zhou JY, et al. Overexpression of BCL2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. Biochem Biophys Res Commun. 2001;280:788–797. doi: 10.1006/bbrc.2000.4218. [DOI] [PubMed] [Google Scholar]
- 16.Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 17.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 19.Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006;13:1419–1421. doi: 10.1038/sj.cdd.4401937. [DOI] [PubMed] [Google Scholar]
- 20.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen M, Marcellus RC, Roulston A, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto Y, Hosotani R, Wada M, et al. Immunohistochemical analysis of Bcl-2, Bax, Bcl-X, Mcl-1 expression in pancreatic cancers. Oncology. 1999;56:73–82. doi: 10.1159/000011933. [DOI] [PubMed] [Google Scholar]
- 23.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiatesTRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–2951. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–4449. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
- 26.Kepp O, Rajalingam K, Kimmig S, Rudel T. Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. EMBO J. 2007;26:825–834. doi: 10.1038/sj.emboj.7601533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vahsen N, Cande C, Briere JJ, et al. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23:4679–4689. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konopleva M, Watt J, Contractor R, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi Y, Karbowski M, Yamaguchi H, et al. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol. 2005;25:9369–9382. doi: 10.1128/MCB.25.21.9369-9382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adjei AA, Davis JN, Bruzek LM, Erlichman C, Kaufmann SH. Synergy of the protein farnesyltransferase inhibitor SCH 66 33 6 and cisplatin in human cancer eel I lines. Clin Cancer Res. 2001;7:1438–1445. [PubMed] [Google Scholar]
- 31.Wang P, Zhang J, Bellail A, et al. Inhibition of RIP and c-FLIP enhances TRAIL-induced apoptosis in pancreatic cancer cells. Cell Signal. 2007;19:2237–2246. doi: 10.1016/j.cellsig.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 33.Slee EA, Keogh SA, Martin SJ. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 2000;7:556–565. doi: 10.1038/sj.cdd.4400689. [DOI] [PubMed] [Google Scholar]
- 34.Cande C, Vahsen N, Garrido C, Kroemer G. Apoptosis-inducing factor (AIF): caspase-independent after all. Cell Death Differ. 2004;11:591–595. doi: 10.1038/sj.cdd.4401400. [DOI] [PubMed] [Google Scholar]
- 35.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czabotar PE, Lee EF, van Delft MF, et al. Structur al insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sei U S A. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahir SK, Yang X, Anderson MG, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–1183. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 38.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]