Abstract
Apoptosis and autophagy have been shown to be negatively regulated by prosurvival Bcl-2 proteins. We determined whether the anticancer agent celecoxib, alone or combined with a small molecule Bcl-2/Bcl-xL antagonist (ABT-737), can induce autophagy in colon cancer cells. Furthermore, we determined whether inhibition of autophagy can drive colon cancer cells into apoptosis. Celecoxib was shown to induce apoptosis that was attenuated by ectopic Bcl-2 or Bax knockout. ABT-737 synergistically enhanced celecoxib-induced cytotoxicity that was primarily due to apoptosis as shown by caspase cleavage and Annexin V labeling that was attenuated by a pan caspase inhibitor (z-VAD-fmk). Celecoxib triggered conversion of the autophagosome-associated protein light chain 3 (LC3) from a cytosolic (LC3I) to a membrane-bound (LC3II) form, as shown by immunoblotting and a punctate _uorescence pattern of an ectopic GFP-LC3 protein. Celecoxib-induced conversion of LC3 was due to autophagy induction, as supported using the lysosome inhibitor, ba_lomycin A1, which produced an accumulation of LC3II. ABT-737 enhanced celecoxib-induced LC3 conversion and p62/SQSTM1 degradation. Inhibition of autophagy was then studied in an e_ort to drive cells into apoptosis. 3-methyladenine (3-MA) blocked LC3 conversion, and 3-MA and wortmannin signi_cantly enhanced apoptotic signaling in cells treated with celecoxib plus ABT-737. Furthermore, knockdown of Atg8/LC3B or Vps34 using siRNA attenuated p62 degradation and enhanced apoptotic signaling; Vps34 siRNA potentiated annexin V+, PI− labeled cells induced by celecoxib + ABT-737. In conclusion, celecoxib induces apoptosis and autophagy that can both be potentiated by ABT-737. Inhibition of autophagy was shown to enhance apoptosis, suggesting a novel therapeutic strategy against colon cancer.
Keywords: celecoxib, NSAID, ABT-737, Bcl-2, apoptosis, autophagy
Introduction
Colorectal cancer is the second leading cause of cancer-related mortality in the United States1 which underscores the need for effective strategies to prevent and treat this malignancy. Celecoxib is an NSAID and selective cyclooxygenase-2 (COX-2) inhibitor that can regress colon cancer xenografts2,3 and enhance the efficacy of chemotherapy4 and/or radiation treatment.5 Celecoxib can also regress/reduce the recurrence of precancerous colon polyps in humans;6–8 however, its protracted use was associated with cardiovascular toxicities.6,8 The antitumor effect of celecoxib is associated with apoptosis induction,3,9 and this drug can engage both the death receptor (DR)10,11 and the mitochondria-mediated pathways.10,12 Ectopic Bcl-2 can attenuate apoptosis induction by the NSAID sulindac in human colon cancer cell lines;13 however, Bcl-2 overexpression was not sufficient to abrogate celecoxib-induced apoptosis in hematopoetic and other solid tumor cell types.12,14–16 Small molecule Bcl-2/Bcl-xL antagonists, including ABT-737, are a new class of anticancer drugs that mimic the function of endogenous BH3-only proteins (Bad, Bid, Bim, Puma, Noxa) that serve to neutralize prosurvival Bcl-2 proteins.17,18 ABT-737 binds with high affinity to Bcl-2, Bcl-xL and Bcl-w but not Mcl-1,18 and has shown single-agent activity in preclinical models of leukemia, lymphoma and small cell lung cancers where high levels of Bcl-2 and/or Bcl-xL and low/absent levels of Mcl-1 were found.17,19,20 ABT-737 can lower the apoptotic threshold for certain chemotherapeutic agents and demonstrated impressive antitumor activity against lymphoma in a murine model.18 Bcl-2 proteins are frequently expressed in human colon cancers21,22 and we have shown that ABT-737 can enhance chemotherapy-induced apoptosis in human colon23 and pancreatic cancer cells.24
Autophagy has been proposed as a mechanism of tumor suppression that may reverse or retard tumorigenesis.25,26 Several anticancer drugs have been shown to induce autophagy as well as apoptosis.27 Autophagy is a process of cell destruction whereby cytoplasmic proteins and organelles are sequestered in vacuoles and delivered to lysosomes for degradation and recycling.26,28 Autophagy is not always prodeath but can be prosurvival under conditions of cellular stress,26,29 including that induced by nutrient deprivation30 or chemotherapy.27 The adaptor protein p62, also known as sequestosome 1 (SQSTM1), can bind to LC3 and to ubiquitinated proteins to facilitate autophagic clearance.31 Evidence indicates that the level of p62 is regulated by autophagy and accumulates in autophagy-deficient cells.32 Since p62 accumulates when autophagy is inhibited, decreased levels can be observed when autophagy is induced and therefore, p62 can be used as a marker of autophagic flux. Recent studies suggest that autophagy inhibitors given in combination with pro-apoptotic agents may enhance chemosensitization in human cancer cells.26,27,33,34 The regulation of autophagy involves autophagy specific genes (ATGs) as well as the class III PI3 kinase complex containing human vacuolar protein sorting factor protein 34 (Vps34).35,36 Inhibition of autophagy can be accomplished by selective inhibition of Vps34 using RNAi or by targeting ATGs. LC3, the mammalian homology of yeast Atg8, is known to associate with the autophagosomal membrane,37 and, as such, is a common target for autophagy inhibition. Of the three LC3 isoforms (LC3A, LC3B, LC3C) in mammalian cells, an increase in LC3B-II was shown to correlate with the levels of autophagic vesicles.38 Alternatively, pharmacological inhibitors of autophagy such as the selective class III PI3K inhibitor, 3-methyladenine (3-MA)39 or the pan PI3K inhibitor, wortmannin40 can be utilized. Recent evidence indicates that pro-survival Bcl-2 proteins can inhibit autophagy whereas pro-apoptotic BH3-only proteins can induce autophagy by competitively disrupting the interaction between Bcl-2/Bcl-xL and the BH3 domain of Beclin 1.41,42 Beclin 1 is an essential autophagy protein that interacts with several cofactors (Ambra1, Bif-1, UVRAG) to activate the lipid kinase Vps34, thereby inducing autophagy.41 ABT-737 was shown to competitively dissociate Beclin 1 from prosurvival Bcl-2/Bcl-xL, thereby inducing autophagy which may limit the anti-tumor effect of this BH3 mimetic.42
In this study, we determined whether celecoxib-induced apoptosis and autophagy can be negatively regulated by prosurvival Bcl-2 proteins, and if the BH3 mimetic ABT-737 can potentiate these processes. Furthermore, we determined whether autophagy exerts a prosurvival effect in response to celecoxib and/or ABT-737, and whether inhibition of autophagy can potentiate apoptosis induction by these drugs.
Results
Prosurvival Bcl-2 proteins negatively regulate celecoxib-induced apoptosis
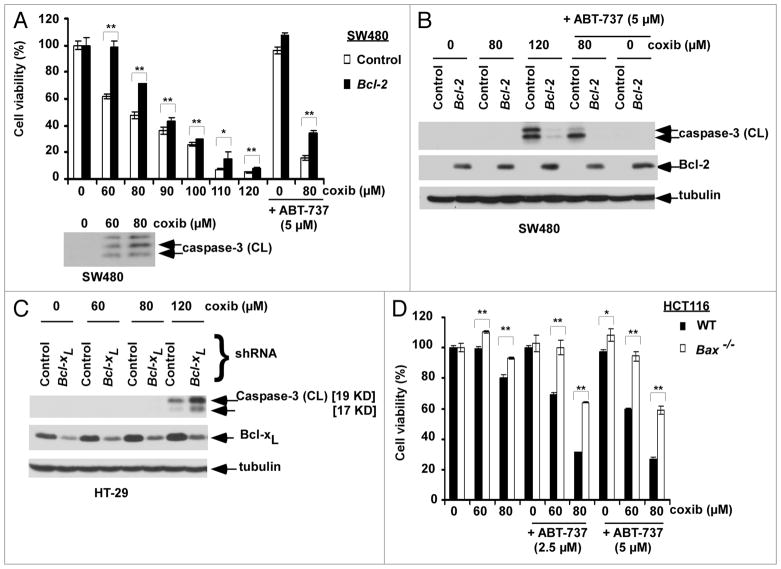
Controversy exists as to whether prosurvival Bcl-2 proteins can confer resistance to celecoxib-induced apoptosis. To address this issue, we utilized the SW480 colon cancer cell line that lacks endogenous Bcl-2 and was stably transfected with a Bcl-2 construct. Bcl-2 overexpression was shown to significantly (p < 0.05) attenuate celecoxib-induced cytotoxicity (Fig. 1A) and caspase-3 cleavage compared to parental cells (Fig. 1B). Celecoxib was shown to reduce cell viability coincident with caspase-3 cleavage and both were dose-dependent (Fig. 1A). Knockdown of Bcl-xL was shown to sensitize colon cancer cells to celecoxib-induced caspase-3 cleavage (Fig. 1C). We then determined the effect of ABT-737, a small molecule antagonist of Bcl-2/Bcl-xL, upon celecoxib-induced apoptosis in cells with/without ectopic Bcl-2 expression. The combination of celecoxib and ABT-737 cleaved caspase-3 to a greater extent than did either drug alone, and both cytotoxicity (p < 0.005; Fig. 1A) and caspase-3 cleavage (Fig. 1B) were attenuated in Bcl-2 overexpressing cells. Furthermore, the cytotoxic effects of celecoxib alone and combined with ABT-737 were attenuated (p < 0.005) in Bax knockout HCT116 cells (Fig. 1D). Together, these data indicate that celecoxib-induced apoptosis can be negatively regulated by Bcl-2/Bcl-xL proteins and is Bax-dependent.
Figure 1.
Prosurvival Bcl-2 proteins negatively regulate celecoxib (coxib)-induced apoptosis. (A) SW480 colon cancer cells with/without ectopic Bcl-2 expression were incubated with celecoxib and/or ABT-737 at indicated doses for 48 h at 37°C. Cytotoxicity was determined by the MTS assay with absorbance measured at 492 nm. Cells were treated with celecoxib at the given doses (48 h) and lysates were analyzed for caspase-3 cleavage. Columns, mean of triplicate experiments; bars, SD. *p < 0.05; **p < 0.005. (B) Caspase-3 cleavage in SW40 cells with/without ectopic Bcl-2 following treatment with celecoxib (0–120 μM) in the presence or absence of ABT-737 (5 μM) for 12 h. Caspase-3 activation is shown by immunoblotting in whole cell lysates. Tubulin serves as a loading control. (C) HT-29 cells with knockdown of Bcl-xL by shRNA (knockdown e_ciency ~60%) were treated with increasing doses of celecoxib for 12 h and caspase-3 activation was assayed by immnoblotting. (D) Wild-type (WT) and Bax knockout HCT116 cells were treated with celecoxib and/or ABT-737 for 48 h at 37°C. Drug-induced cytotoxicity was measured as described above. Columns, mean of triplicate experiments; bars, SD. *p < 0.05; **p < 0.005.
ABT-737 synergistically enhances celecoxib-induced apoptosis
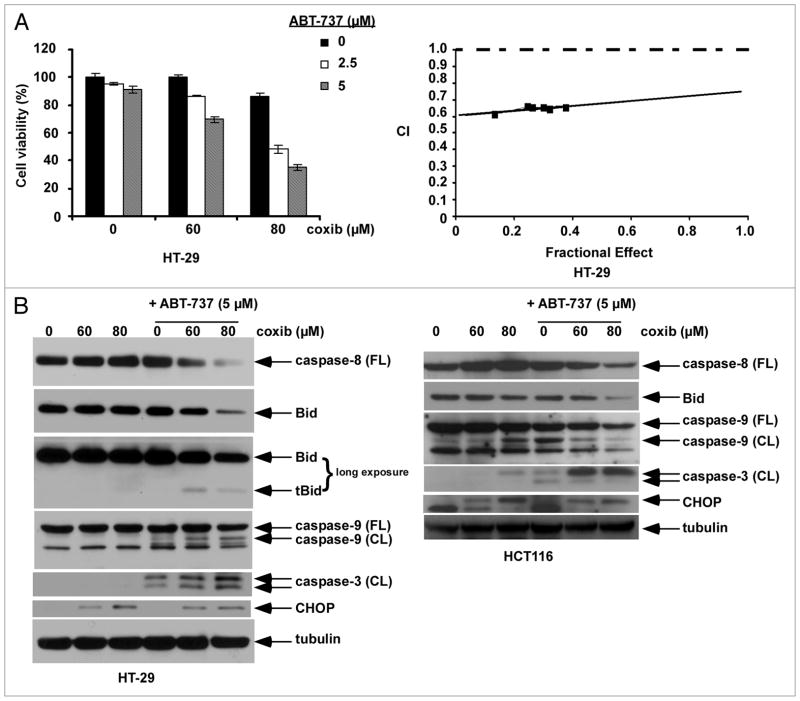
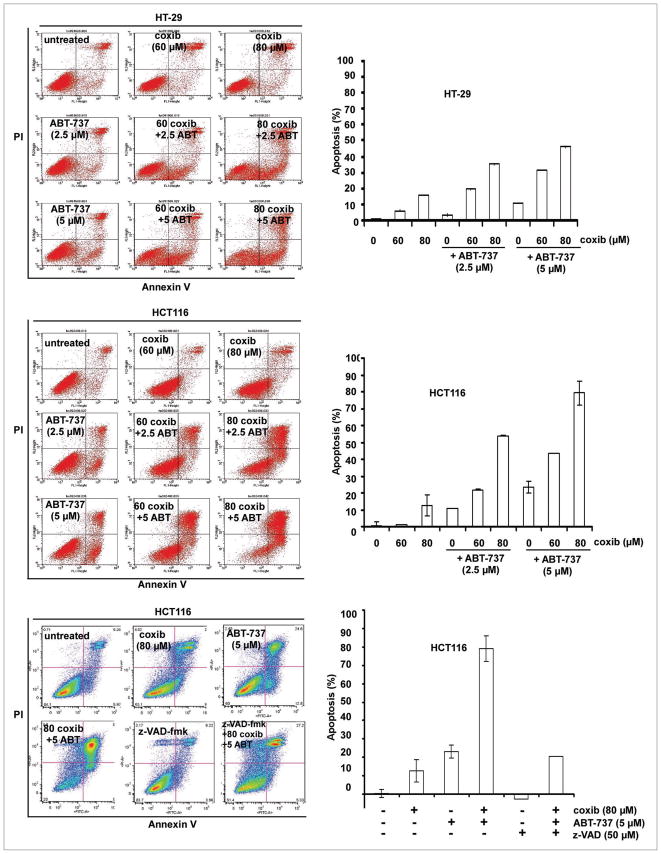
ABT-737 treatment was shown to significantly enhance celecoxib-induced cytotoxicity and caspase activation (Figs. 1A and D; 2A and B). To investigate the interaction between the study drugs, HT-29 cells were treated with celecoxib and ABT-737 at a fixed dose ratio and the combination index (CI) was determined using the median effect method.45 As shown in an isobologram, the CI values were <1 consistent with a synergistic interaction (Fig. 2A). The effect of celecoxib alone and combined with ABT-737 upon apoptotic signaling was then determined. At the doses of celecoxib utilized, no caspase activation was observed in HT-29 cells. However, the addition of ABT-737 resulted in enhanced activation of caspase-8, -9 and -3 as well as a reduction of full-length Bid in both cell lines although truncated Bid (tBid) was only observed in HT-29 cells (Fig. 2B). Furthermore, celecoxib was shown to induce expression of the ER stress chaperone, CHOP, that was not altered by ABT-737 treatment. Consistent with these observations, celecoxib has been shown to induce an ER stress response46–48 and to trigger both the DR-mediated and mitochondrial apoptotic pathways.10–12 We demonstrate that ABT-737 can significantly enhance celecoxib-induced externalization of phosphatidylserine, as shown by Annexin V labeling, in a dose-dependent manner in both cell lines tested. Specifically, ABT-737 treatment increased celecoxib-induced apoptosis in HT-29 and HCT116 cells by approximately three-fold and six-fold, respectively (Fig. 3). Use of the pan caspase inhibitor z-VAD-fmk was shown to inhibit ~80% of the Annexin V+ cells induced by celecoxib plus ABT-737, indicating that apoptosis accounts for the majority of cell death.
Figure 2.
ABT-737 synergistically augments cytotoxicity and enhances apoptotic signaling by celecoxib. (A) HT-29 cells were treated with celecoxib and/or ABT-737 for 48 h at 37°C and cytotoxicity was analyzed by the MTS assay. Cells were treated with drugs at a _xed dose ratio and the combination index (CI) was determined (see Materials and Methods). As shown in an isobologram, the CI < 1.0 indicates that the interaction between celecoxib and ABT-737 was synergistic. (B) HT-29 and HCT116 cells were treated with celecoxib and/or ABT-737 for 12 h, and whole cell lysates were then subjected to immunoblotting. Tubulin was utilized as a loading control.
Figure 3.
ABT-737 enhances celecoxib-induced apoptosis. HT-29 and HCT116 cells were treated with celecoxib and/or ABT-737 for 24 h and apoptosis was determined by Annexin V-FITC/PI labeling using _ow cytometry. Annexin V-positive cells were quanti_ed as described in Materials and Methods and are shown in bar graphs. Treatment with celecoxib and/or ABT-737 was also performed in the presence or absence of a pan caspase inhibitor, z-VAD-fmk, in HCT116 cells. Columns, mean of triplicate experiments; bars, SD.
Celecoxib induces autophagy in human colon cancer cells
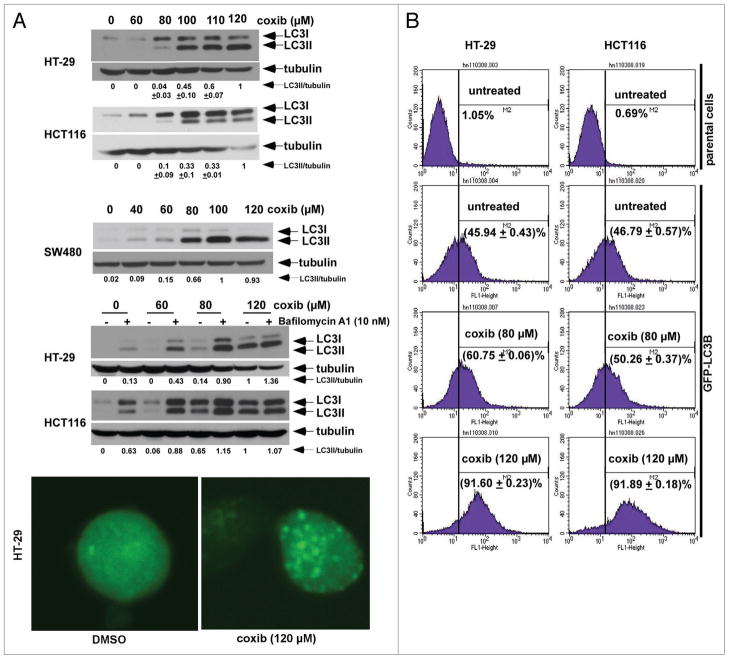
Several anticancer drugs have been shown to induce both apoptosis and autophagy.27 Autophagy is a mechanism of adaptation to cellular stress and may therefore, confer protection from drug-induced cell death.27,29 We determined whether celecoxib can induce autophagy, as detected by expression of the light chain 3 (LC3) protein that is associated with autophagosomal membranes.37 We found that celecoxib treatment induced a dose-dependent conversion of cytoplasmic LC3I to membrane-bound LC3II as detected by immunoblotting (Fig. 4A). To determine whether the increase in LC3 conversion was due to autophagy induction or from inhibition of completion, we utilized a lysosome inhibitor, bafilomycin A1, that inhibits vacuolar H+ ATPase.49 The addition of bafilomycin A1 was shown to stabilize LC3II induced by celecoxib (Fig. 4A), indicating that autophagy induction by celecoxib proceeds lysosomal degradation and is consistent with an increase of autophagic flux. In colon cancer cells stably transduced with GFP-LC3B, celecoxib treatment induced a characteristic punctate pattern of GFP-LC3B indicating autophagosome formation and produced an increase in fluorescence intensity as compared to control cells, as shown by fluorescence confocal microscopy (Fig. 4A). In support of these data, GFP-LCB stable transfectants showed a dose-dependent increase in fluorescence intensity after treatment with celecoxib compared to untreated cells, consistent with increased autophagy as shown by FACS analysis (Fig. 4B).
Figure 4.
Celecoxib induces autophagy in colon cancer cell lines. (A) Celecoxib induces conversion of cytosolic LC3I into membrane-bound L C3II in a dose-dependent manner in HT-29, HCT116 and SW480 cells, as shown by immunoblotting. The ratio of LC3II to tubulin, calculated by densitometry, is shown below. Autophagic _ux was determined by co-treatment with a lysosome inhibitor, Ba_lomycin A1 (10 nM), that produced accumulation of LC3II in HT-29 and HCT116 cells treated with celecoxib at the indicated doses for 12 h. HT-29 cells stably expressing a chimeric GFP-LC3B fusion protein were incubated with vehicle (DMSO) or celecoxib for 12 h. A punctate pattern of GFP-LC3B induced by celecoxib indicates autophagosome formation, as shown by confocal microscopy. (B) HT-29 and HCT116 cells containing a stable GFP-LC3B construct were treated with vehicle or celecoxib for 12 h and the fluorescent intensity was quanti_ed using _ow cytometry. Mean ± SD was obtained from triplicate experiments.
Celecoxib-induced autophagy is potentiated by ABT-737
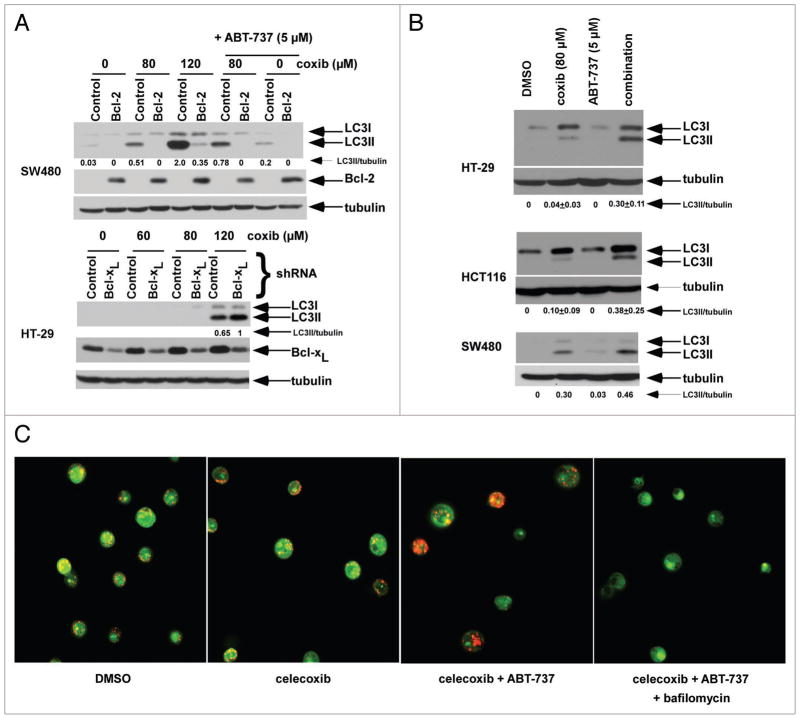
We found that ectopic Bcl-2 expression blocked the conversion of cytosolic LC3I to membrane-bound LC3II forms after treatment with celecoxib alone and combined with ABT-737 (Fig. 5A). Furthermore, knockdown of Bcl-xL modestly enhanced LC3I to LC3II conversion by celecoxib (Fig. 5A). We then determined whether ABT-737 can induce autophagy and studied its ability to enhance celecoxib-induced autophagy. In both cell lines tested, we found that the combination of ABT-737 and celecoxib induced a greater conversion of LC3I into LC3II than did either drug alone, consistent with an enhanced autophagic response (Fig. 5B). A mechanism for these effects is suggested by data demonstrating that ABT-737 can dissociate Beclin 1 (that contains a BH3 domain) from Bcl-2/Bcl-xL with the released Beclin 1 available to trigger autophagy.42 Autophagy begins with autophagosome formation that subsequently fuse with acidic lysosomes to form autolysosomes.50 Acridine orange staining was performed to visualize acidic autolysosomes in control and celecoxib ± ABT-737 treated HT-29 cells. Treatment with celecoxib and ABT-737 increased autolysosomes within the cells as shown by orange-red staining (Fig. 5C). Furthermore, the lysosome inhibitor bafilomycin A1 was shown to block acridine orange-positive vesicles and hence, autolysosome formation, providing further evidence that the autophagic process was being activated by drug treatment.
Figure 5.
Celecoxib-induced autophagy can be negatively regulated by Bcl-2/Bcl-xL and enhanced by the BH3 mimetic, ABT-737. (A) SW480 cells with/without ectopic Bcl-2 expression were treated with celecoxib and/or ABT-737, and LC3 expression was determined by immunoblotting and LC3II level was normalized to tubulin (top). HT-29 cells with stable expression of Bcl-xL shRNA were treated with increasing doses of celecoxib and LC3 expression was determined (bottom). (B) HT-29, HCT116 and SW480 cells were treated with celecoxib in the presence or absence of ABT-737 for 12 h and LC3 expression was measured by immunoblotting. (C) Acridine orange labeling of acidic vesicles in HT-29 cells treated with celecoxib (80 μM) ± ABT-737 (5 μM) in the presence or absence of ba_lomycin A1 (10 nM). Under acridine orange staining, the acidic vesicles showing orange-red _uorescence indicate autophagolysosomes whereas the cytoplasm shows green _uorescence. Ba_lomycin A1 suppressed acridine orange staining of acidic vesicles.
Autophagy inhibitors augment drug-induced apoptosis
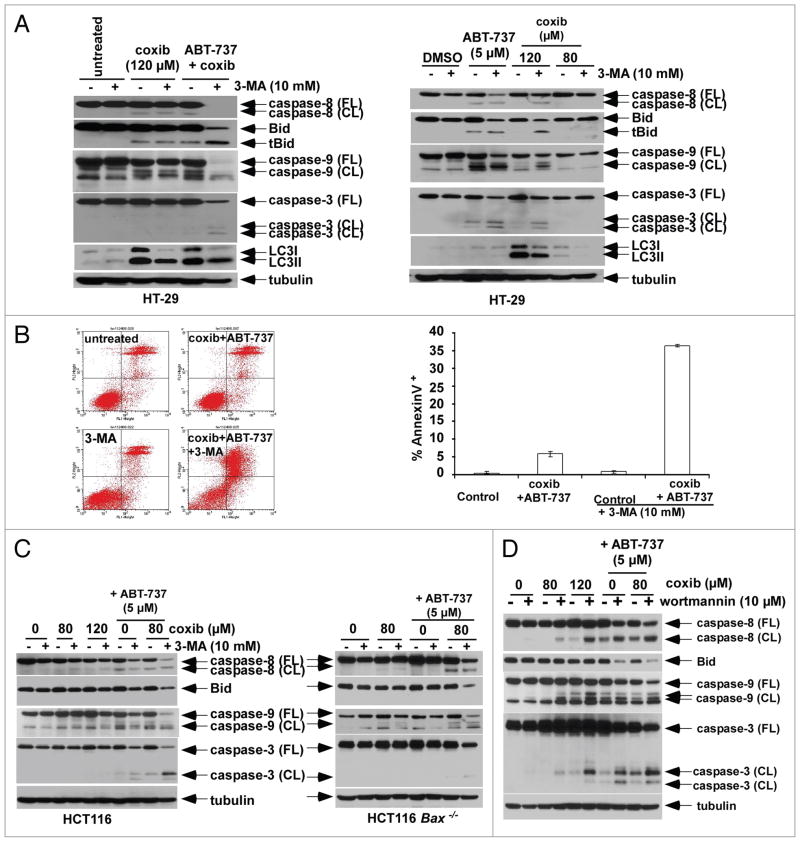
Recent data suggest that inhibitors of autophagy given in combination with pro-apoptotic drugs may enhance chemosensitization in human cancer cells.27,33 Therefore, we determined whether inhibition of autophagy, utilizing pharmacological or genetic means, can enhance celecoxib-induced apoptosis alone and in combination with ABT-737. To inhibit autophagy, we utilized the class III phosphatidylinositol 3-kinase (PI3K) inhibitor 3-methyladenine (3-MA) that has been shown to sensitize cancer cells to chemotherapy-induced apoptosis.39 Treatment with 3-MA attenuated the level of LC3II induced by celecoxib (Fig. 6A). In addition, 3-MA enhanced caspase cleavage induced by celecoxib or ABT-737 alone, or their combination (Fig. 6A). Moreover, 3-MA markedly increased apoptosis induction by the combination of celecoxib plus ABT-737, as measured by annexin V labeling (Fig. 6B). Whereas 3-MA alone caused minimal apoptosis (Fig. 6B), this agent produced a ~30% reduction in cell viability in our colon cancer cells (data not shown). We also observed that 3-MA can enhance caspase cleavage by celecoxib plus ABT-737 in apoptosis-resistant Bax knockout HCT116 cells, but to a lesser extent compared to wild-type cells (Fig. 6C). The ability of 3-MA to augment apoptotic signaling in apoptosis-deficient cells that populate most solid tumors suggests a novel strategy for chemosensitization. To confirm the finding that autophagy inhibition can enhance apoptosis induction, we used the nonselective PI3K inhibitor, wortmannin. Wortmannin similarly enhanced celecoxib-induced apoptotic signaling, as shown by caspase cleavage, alone or combined with ABT-737 (Fig. 6D).
Figure 6.
Autophagy inhibitors augment apoptosis induced by celecoxib and/or ABT-737. (A) HT-29 cells were treated with celecoxib plus ABT-737 in the presence or absence of the autophagy inhibitor, 3-methyladenine (3-MA). The addition of 3-MA was shown to inhibit LC3I to L C3II conversion, and to enhance caspase cleavage and Bid truncation by celecoxib and its combination with ABT-737 for 12 h (left). Co-administration of 3-MA with either celecoxib or ABT-737 alone for 24 h also enhanced apoptosis as indicated by caspase cleavage and Bid truncation compared to either drug alone (right). (B) Annexin V-positive cells were quanti_ed in HT-29 cells treated with celecoxib plus ABT-737 in the presence or absence of 3-MA for 12 h by FACS analysis. Columns, mean of triplicate experiments; bars, SD. (C) HCT116 wild-type (WT, left) and Bax−/− (right) cells were treated with celecoxib alone or combined with ABT-737 in the presence or absence of 3-MA for 12 h. Caspase cleavage and Bid expression were analyzed in whole cell lysates by immnublotting. (D) HCT116 cells were treated with celecoxib alone or combined with ABT-737 in the presence or absence of wortmannin for 16 h. Caspase cleavage and Bid expression were analyzed in whole cell lysates by immunoblotting.
Knockdown of LC3B or Vps34 increases p62 expression and potentiates apoptosis induction
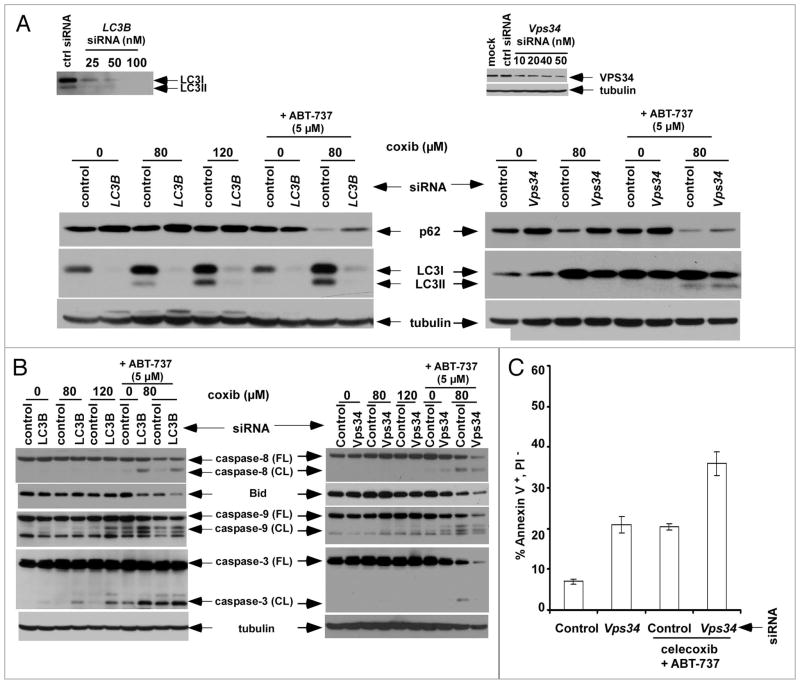
Autophagy-deficient cells have been shown to accumulate p62 and therefore, p62 is an indicator of autophagic flux.32 Treatment of HCT116 cells with celecoxib + ABT-737 reduced the level of p62 protein compared to either drug alone and increased LC3 conversion, consistent with enhancement of autophagy (Fig. 7A). Furthermore, knockdown of the autophagy-regulating gene Atg8/LC3B by siRNA was shown to produce an accumulation of p62 in drug treated cells indicating suppression of autophagic flux. Induction of autophagy requires Vps34 that forms a multiprotein complex with Beclin1, as well as Bif-1, and UVRAG, to initiate autophagosome formation.41 Similarly, knockdown of the class III PI3 kinase Vps34 by siRNA increased p62 expression, although LC3 conversion was not inhibited (Fig. 7A) as has been previously reported in HeLa cells stressed by nutrient deprivation.51
Figure 7.
Knockdown of Vps34 or Atg8/LC3 accumulates p62 and can enhance apoptosis induced by celecoxib plus ABT-737. (A) HCT116 cells were transfected with control siRNA or siRNA against LC3B (left) or Vps34 (right) and their knockdown e_ciencies were determined (insets). Cells transfected with control or siRNA against LC3B (25 nM) or Vps34 (50 nM) were then treated with celecoxib and/or ABT-737 (8 h), and expression of the autophagy degradation target protein (p62/SQSTM1) and LC3 were analyzed by immunoblotting. (B and C) E_ects of siRNA against LC3B or Vps34 upon apoptosis induction by celecoxib ± ABT-737 (8 h) was determined by analysis of caspase activation and Bid expression (B), and by annexin V+/PI− staining following drug treatment [celecoxib (80 μM) + ABT-737 (5 μM); 4 h] using FACS analysis (C).
In cells where LC3B or Vps34 are suppressed by siRNA, we demonstrate that caspase cleavage is enhanced by treatment with celecoxib plus ABT-737 (Fig. 7B). Furthermore, Vps34 siRNA was shown to significantly enhance annexin V+PI− staining by the drug combination indicating that inhibition of autophagy can enhance apoptosis induction (Fig. 7C). These results are consistent with findings observed for pharmacological inhibitors of autophagy (Fig. 6).
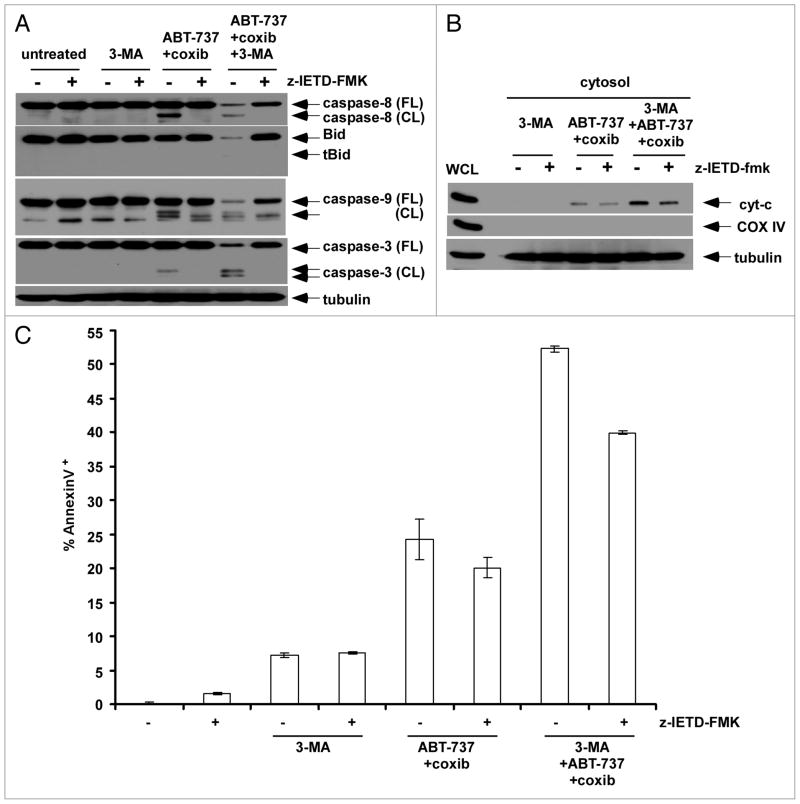
We determined the apoptotic signaling pathways triggered by celecoxib and ABT-737 upon autophagy inhibition. In the presence of 3-MA, we observed enhanced caspase-8-mediated signaling induced by celecoxib plus ABT-737 (Figs. 6A and C; 8A). Since caspase-8 is primarily activated via the death receptors (DR), we utilized a caspase-8 inhibitor (z-IETD-fmk) to determine the relative contribution of DR-mediated signaling. z-IETD-fmk was shown to block caspase-8 cleavage and to attenuate downstream caspase-9 and -3 cleavage induced by celecoxib plus ABT-737 in the presence or absence of 3-MA (Fig. 8A). Celecoxib plus ABT-737 triggered the release of mitochondrial cytochrome c that was enhanced by 3-MA. However, cytochrome c release triggered by celecoxib + ABT-737 + 3-MA was only slightly attenuated by z-IETD-fmk (Fig. 8B). Similarly, z-IETD-fmk was shown to modestly inhibit annexin V+ cells induced by celecoxib + ABT-737 + 3-MA (Fig. 8C) consistent with activation of both the DR-mediated and mitochondrial apoptotic signaling pathways when autophagy is inhibited.
Figure 8.
Celecoxib plus ABT-737 activates both the DR-mediated and mitochondrial apoptotic pathways upon inhibition of autophagy. (A) HT-29 cells were treated with 3-MA (10 mM), celecoxib (80 μM) plus ABT-737 (5 μM) or the three drug combination for 12 h in the presence or absence of a cas-pase-8 inhibitor, z-IETD-fmk. The e_ect of drug treatment upon caspase activation and Bid expression were determined by immunoblotting. (B) The release of mitochondrial cytochrome c into the cytosol was determined in cells treated with 3-MA, celecoxib plus ABT-737 or their combination for 4 h in the presence or absence of z-IETD-fmk. COX IV was utilized as a mitochondrial marker that indicates lack of contamination of the cytosolic fraction. (C) Annexin V staining was performed in HT-29 cells that were treated with 3-MA, celecoxib plus ABT-737 or the three drug combination (16 h) in the presence or absence of z-IETD-fmk. Columns, mean of triplicate experiments; bars, SD.
Discussion
Recent evidence indicates that cellular stress, including anticancer drugs, can trigger apoptosis and/or autophagy, both of which can regulated by the Bcl-2 protein family.27,41 We studied the effect of celecoxib alone and combined with the small molecule Bcl-2/Bcl-xL antagonist, ABT-737, upon apoptosis and autophagy in human colon cancer cell lines and their modulation by Bcl-2 proteins. We found that celecoxib-induced apoptosis is negatively regulated by Bcl-2/Bcl-xL and is Bax dependent. Treatment of cells with ABT-737 combined with celecoxib produced a synergistic cytotoxic effect that was due primarily to a caspase-dependent apoptosis. Celecoxib was also shown to induce autophagy, as evidenced by conversion of the autophagosomal marker LC3 from the cytosol to the membrane and an alteration in the pattern of GFP-LC3 fluorescence. The observed increase in LC3 conversion by celecoxib was shown to result from autophagy induction rather than from inhibition of completion, since the lysosome inhibitor bafilomycin A1 was able to retard LC3 degradation as indicated by its accumulation. Induction of both apoptosis and autophagy by celecoxib may be related to its known ability to trigger endoplasmic reticulum (ER) stress,52,53 as shown here by CHOP expression that occurs secondary to celecoxib-induced leakage of calcium into the cytosol.46–48 The ER stress response is known to be involved in both apoptosis and autophagy.40,54 Accumulating evidence suggests that apoptosis and autophagy are regulated by the Bcl-2 protein family.41,42 Cells with ectopically expressed Bcl-2 and treated with celecoxib showed attenuated autophagy, indicated by a reduced conversion of LC3 from cytosolic to membrane-bound forms compared to parental cells, whereas knock-down of Bcl-xL enhanced LC3 conversion. ABT-737 was shown to potentiate celecoxib-induced autophagy as shown by LC3 conversion, accumulation of acridine orange-labeled acidic vesicles consistent with autophagolysosomes, and decreased p62 protein levels. p62 is known to be degraded by autophagy and can be used as a marker of autophagic flux.32 Conversely, p62 is known to accumulate in autophagy-deficient cells32 and we demonstrate that p62 accumulation occurs when autophagy is inhibited by knockdown of LC3B or Vps34 using siRNA. The mechanism by which ABT-737 can potentiate autophagy may be related to its ability to competitively disrupt the binding of Bcl-2/Bcl-xL to the autophagic protein Beclin 1 [the mammalian homologue of the autophagy-related gene 6 (Atg6)], whose autophagic function was shown to be inhibited by Bcl-2 proteins.41,42 Taken together, these data demonstrate a dual role of Bcl-2 family proteins in the regulation of both apoptosis and autophagy.
Autophagy can be prodeath or prosurvival depending upon the cellular context. Autophagy can be induced by treatment with certain anticancer drugs27 and demonstrates tumor selectivity in that autophagosome formation was seen only in tumor cells but not in the adjacent noncancerous epithelial cells of colorectal cancer specimens.30 Autophagy may also serve as a cell survival mechanism that occurs in response to cellular stress induced by nutrient deprivation30 or chemotherapy.27 In this regard, recent evidence suggests that autophagy may attenuate a drug-induced apoptotic response.27,29 To date, however, the molecular mechanisms that govern the interplay between autophagy and apoptosis are poorly understood. In an effort to determine whether autophagy serves a prosurvival or prodeath role in response to treatment with celecoxib plus ABT-737, we evaluated pharmacological and genetic approaches to inhibit autophagy. Since autophagosome formation is regulated by a class III phosphoinositide-3-kinase (PI3K)-complex,41 we utilized the class III PI3K inhibitor 3-methyladenine (3-MA)39 and the nonspecific PI3K inhibitor, wortmannin40 that have been shown to inhibit autophagy. We found that 3-MA treatment can suppress autophagy, and that both 3-MA and wortmannin can significantly enhance apoptotic signaling by celecoxib alone and in combination with ABT-737. Moreover, the addition of 3-MA to the combination of celecoxib and ABT-737 produced a 5-fold increase in apoptosis, as shown by annexin V labeling. To confirm that inhibition of autophagy was responsible for enhanced apoptosis, we performed knockdown of Atg8/LC3, the mammalian homology of yeast Atg8, that was shown to accumulate the autophagy target p62 and to enhance apoptotic signaling by celecoxib plus ABT-737. We also targeted Vps34 by siRNA, as it has been shown to form a multiprotein complex with the pro-autophagic tumor suppressors Beclin1, Bif-1 and UVRAG to initiate autophagosome formation.35,36,41 Suppression of Vps34 was shown to similarly accumulate p62 and to enhance apoptosis induction by celecoxib combined with ABT-737. Together, these data indicate that autophagy is serving a prosurvival function in our drug-treated colon cancer cells. Consistent with these results are studies demonstrating that autophagy inhibition can enhance the anti-cancer effects of arsenic trioxide,34 hyperthermia,34 sulforaphane55 and alkylating agents.27 Therefore, autophagy may represent a common prosurvival mechanism utilized by cancer cells to protect against cellular stress and thus, represents a potential therapeutic target.
We determined the effect of autophagy inhibition by 3-MA on apoptotic signaling via the DR-mediated and mitochondrial apoptotic pathways that have been shown to be utilized by celecoxib.10–12 We found that a caspase-8 inhibitor can attenuate apoptotic signaling by celecoxib plus ABT-737 in the presence of 3-MA, indicating the involvement of the DR-FADD-caspase-8 axis. The caspase-8 inhibitor only minimally attenuated mitochondrial cytochrome c release by celecoxib plus ABT-737 in the presence of 3-MA. These data support the contribution of both DR-mediated and mitochondrial signaling to enhancement of apoptosis by autophagy inhibition. In HCT116 Bax knockout cells, autophagy inhibition by 3-MA was able to enhance apoptotic signaling by celecoxib plus ABT-737. An explanation for this observation was shown in a recent study where inhibition of autophagy increased TRAIL-mediated apoptosis in Bax knockout HCT116 cells that was Bak-dependent.56 Activation of caspase-8 and Bak-dependent mitochondrial permeabilization may therefore, explain the shift to apoptosis in Bax-deficient cells. Inhibiting autophagy in apoptosis-defective cells has important implications for the treatment of human cancer given the intrinsic apoptosis resistance of colorectal and many other solid tumors.
In summary, our novel findings demonstrate that celecoxib can induce both apoptosis and autophagy in human colorectal cancer cells, and that both processes can be negatively regulated by Bcl-2/Bcl-xL. ABT-737 was shown to potentiate both celecoxib-mediated apoptosis and autophagy and exerted a synergistic cytotoxic effect. Moreover, inhibition of autophagy by pharmacologic or genetic means was shown to drive colon cancer cells into apoptosis, indicating that autophagy serves a prosurvival role in these colon cancer cells subjected to cellular stress. Together, these data indicate that Bcl-2/Bcl-xL antagonism and/or autophagy inhibition may represent novel therapeutic strategies against human colorectal cancer.
Materials and Methods
Cell culture, chemical compounds and biological reagents
Human colorectal cell lines [HT-29, SW480, HCT116 wild-type or Bax−/− (gift from B. Vogelstein, Johns Hopkins Univ.)] were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 100 μg/mL penicillin and 100 μg/mL streptomycin (Invitrogen, 15140163). SW480 cells with stable Bcl-2 expression were utilized, as previously described by our laboratory.43 ABT-737 (Abbott Laboratories, A-779024.0) was dissolved in DMSO at a stock concentration of 20 mmol/L that was aliquoted and stored at −20°C. Celecoxib (LKT Laboratories, C1644), was dissolved in DMSO, aliquoted and used within a one month period. Cells were treated in the presence or absence of a caspase-8 inhibitor (z-IETD-fmk) [R&D Systems, FMK007], 3-methyladenine (3-MA), bafilomycin A1 [both from Sigma Chemical Co., M9281 and B1793], or wortmannin (Calbiochem, 681675). Antibodies used for immunoblot analysis included mouse anti-caspase-8 (BD Biosciences, 51-8084N), mouse anti-p62 (MBL International, 162-3), and rabbit anti-Bid, anti-caspase-9, anti-caspase-3, anti-cleaved caspase-3 and anti-LC3 (all from Cell Signaling Technology, 2002, 9502, 9665, 9661, 2775). Additionally, we utilized the anti-rabbit Vps34 and mouse anti-Bcl-xL (both from EMD Chemicals, ab5451 and AM05). An anti-rabbit antibody against CHOP (Sigma, 6916) was also utilized.
Bcl-xL knockdown using lentiviral shRNA
The targeting sequence for Bcl-xL was CAG GGA CAG CAT ATC AGA G. Cloning of shRNA and generation of lentivirus in the producer cells and transduction of lentivirus into colon cancer cell lines were performed as previously described.44
Knockdown using siRNA
Atg8/LC3B siRNA was synthesized and the targeting sequence was GAA GGC GCT TAC AGC TCA A (Qiagen, 1027020). Vps34 siRNA was obtained as siGENOME SMARTpool reagents that consisted of four different oligoduplexes (Dharmacon, M-005250-00-0005). The control siRNA used was the siCONTROL non-targeting siRNA pool 2 (Dharmacon, D-001206-14-05), which also contains four nontargeting siRNAs. HCT116 cells (2.5 × 105) were plated in RPMI supplemented with 10% FBS in a 6-well plate. After 16 h and at ~30% confluence, the cells were transfected with siRNA in Opti-MEM medium (Invitrogen, 31985) using Lipofectamine RNAi MAX reagent (Invitrogen, 13778), according to the manufacturer’s protocol. After 12 h, normal growth medium was added and at the end of the siRNA treatment period, the cells were treated with drug and assayed.
Cell viability assay
Cell viability was analyzed by the MTS assay per the manufacturer’s protocol (Promega), as previously described.24 Each experimental condition was performed in triplicate and the SD was calculated.
Annexin V labeling
After drug treatment, floating cells were collected and combined with adherent cells that were detached from culture dishes by treating with trypsin (Invitrogen, 12604) for 3 to 5 min. Annexin V labeling was performed as previously described.23 The extent of apoptosis was quantified as a percentage of Annexin V+ cells, and the extent of drug-specific apoptosis was assessed by using a formula: % specific apoptosis = (test - control) × 100/(100 - control).44
Construction and stable expression of GFP-LC3B vector
A lentiviral GFP-LC3B fusion protein expression vector was constructed by sequential cloning steps. First, the GFP coding sequence without a stop codon was PCR-amplified using pEGFP-C1 (Clontech, 6084-1) as the template. The PCR product was flanked by restriction enzyme recognition sites and digested and ligated into pCDH1-MCS1-EF1-puro vector (System Biosciences, CD510A-1). Second, an LC3B coding sequence was PCR amplified using a true clone cDNA (Origene, sc322139) as a template and inserted into the vector containing the GFP coding sequence. The generation and transduction of lentivirus (e.g., GFP-LC3B) was performed as previously described.24 HT-29 cells were transduced with lentiviral GFP-LC3B vector and then selected in the presence of 2 μg/ml puromycin. The puromycin-resistant pool of HT-29 cells were then treated with the study drugs and analyzed by confocal microscopy.
Confocal microscopy for GFP-LC3B fluorescence
Cells transduced with the lentiviral GFP-LC3B construct were fixed with 3% paraformaldehyde. Fluorescent signals were visualized and captured by a LSM 5 Pascal Laser Scanning Microscope (Carl Zeiss) with appropriate filter and detector combinations according to the spectrum of the fluorochrome used.
Acridine orange staining for autophagy detection
After drug treatment, acridine orange (Invitrogen, A1301) was added to the culture medium and cells were incubated at 37°C for 15–30 min. Cells were then trypsinized and washed with cold PBS × 2 and observed under a confocal microscope. Fluorescence was excited with a 490-nm band-pass blue filter and the fluorescence of the green and red channel were recorded and merged. A shift from green to red fluorescence indicates acidic vesicles consistent with autolysosomes. In the presence of bafilomycin A1, a lysosome inhibitor that blocks the fusion of autophagosome with lysosome, only green but not red fluorescence was observed, and this treatment served as a negative control for staining.
Western blotting
Protein samples were prepared in a lysis buffer [5 mmol/L MgCl2, 137 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% CHAPS, 10 mmol/L HEPES (pH 7.5)], normalized using nanodrop measurement (Thermo Scientific), and boiled in LDS sample buffer (Invitrogen, NP0008). Samples were then loaded onto 14% SDS-PAGE gels (8% for detection of Vps34 proteins) with electrophoretic transfer onto a polyvinylidene difluoride membrane (Bio-Rad, 162-0177). Western blotting was performed as previously described,44 and blots was quantified using Image J software (National Institutes of Health). All experiments were repeated at least twice and mean values and SDs were derived from triplicate experiments.
Release of mitochondrial cytochrome c
Following drug treatment, the release of mitochondrial cytochrome c was analyzed using a selective digitonin lysis method, as previously described.44 An antibody against cytochrome oxidase subunit IV (COX IV, Molecular Probes, A-21348) was used as a marker of the cytosolic fraction.
Calculation of combination index
The interaction between celecoxib and ABT-737 was analyzed using Calcusyn software (Biosoft) as previously reported.44
Statistical analysis
The statistical significance of the differences between experimental variables and their reference group was determined using the Student’s t test. p < 0.05 was considered statistically significant. The values shown represent the mean ± SD for triplicate experiments.
Acknowledgments
We express our gratitude to Dr. S. Kaufmann for his assistance with calculation of the combination index.
Grant support: Supported in part by a Fraternal Order of Eagles Foundation Award and a National Cancer Institute R01 grant CA113681 [both to F.A.S.].
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/11124
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Johnsen JI, Lindskog M, Ponthan F, Pettersen I, Elfman L, Orrego A, et al. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Res. 2004;64:7210–5. doi: 10.1158/0008-5472.CAN-04-1795. [DOI] [PubMed] [Google Scholar]
- 3.Williams CS, Watson AJ, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–51. [PubMed] [Google Scholar]
- 4.Hida T, Kozaki K, Muramatsu H, Masuda A, Shimizu S, Mitsudomi T, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6:2006–11. [PubMed] [Google Scholar]
- 5.Nakata E, Mason KA, Hunter N, Husain A, Raju U, Liao Z, et al. Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int J Radiat Oncol Biol Phys. 2004;58:369–75. doi: 10.1016/j.ijrobp.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 6.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 7.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 8.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 9.Grosch S, Tegeder I, Niederberger E, Brautigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. Faseb J. 2001;15:2742–4. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 10.Kern MA, Haugg AM, Koch AF, Schilling T, Breuhahn K, Walczak H, et al. Cyclooxygenase-2 inhibition induces apoptosis signaling via death receptors and mitochondria in hepatocellular carcinoma. Cancer Res. 2006;66:7059–66. doi: 10.1158/0008-5472.CAN-06-0325. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96:1769–80. doi: 10.1093/jnci/djh322. [DOI] [PubMed] [Google Scholar]
- 12.Jendrossek V, Handrick R, Belka C. Celecoxib activates a novel mitochondrial apoptosis signaling pathway. Faseb J. 2003;17:1547–9. doi: 10.1096/fj.02-0947fje. [DOI] [PubMed] [Google Scholar]
- 13.Sinicrope FA, Penington RC. Sulindac sulfide-induced apoptosis is enhanced by a small-molecule Bcl-2 inhibitor and by TRAIL in human colon cancer cells overexpressing Bcl-2. Mol Cancer Ther. 2005;4:1475–83. doi: 10.1158/1535-7163.MCT-05-0137. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AJ, Smith LL, Zhu J, Heerema NA, Jefferson S, Mone A, et al. A novel celecoxib derivative, OSU03012, induces cytotoxicity in primary CLL cells and transformed B-cell lymphoma cell line via a caspase- and Bcl-2-independent mechanism. Blood. 2005;105:2504–9. doi: 10.1182/blood-2004-05-1957. [DOI] [PubMed] [Google Scholar]
- 15.Ding H, Han C, Zhu J, Chen CS, D’Ambrosio SM. Celecoxib derivatives induce apoptosis via the disruption of mitochondrial membrane potential and activation of caspase 9. Int J Cancer. 2005;113:803–10. doi: 10.1002/ijc.20639. [DOI] [PubMed] [Google Scholar]
- 16.Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275:11397–403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 17.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 19.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Tahir SK, Yang X, Anderson MG, Morgan-Lappe SE, Sarthy AV, Chen J, et al. Influence of Bcl-2 family members on the cellular response of small-cell lung cancer cell lines to ABT-737. Cancer Res. 2007;67:1176–83. doi: 10.1158/0008-5472.CAN-06-2203. [DOI] [PubMed] [Google Scholar]
- 21.Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–41. [PubMed] [Google Scholar]
- 22.Krajewska M, Kim H, Kim C, Kang H, Welsh K, Matsuzawa S, et al. Analysis of apoptosis protein expression in early-stage colorectal cancer suggests opportunities for new prognostic biomarkers. Clin Cancer Res. 2005;11:5451–61. doi: 10.1158/1078-0432.CCR-05-0094. [DOI] [PubMed] [Google Scholar]
- 23.Okumura K, Huang S, Sinicrope FA. Induction of Noxa sensitizes human colorectal cancer cells expressing Mcl-1 to the small-molecule Bcl-2/Bcl-xL inhibitor, ABT-737. Clin Cancer Res. 2008;14:8132–42. doi: 10.1158/1078-0432.CCR-08-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S, Sinicrope FA. BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res. 2008;68:2944–51. doi: 10.1158/0008-5472.CAN-07-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin S, White E. Tumor suppression by autophagy through the management of metabolic stress. Autophagy. 2008;4:563–6. [PMC free article] [PubMed] [Google Scholar]
- 26.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amaravadi RK, Thompson CB. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin Cancer Res. 2007;13:7271–9. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 28.Levine B, Kroemer G. Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell Death Differ. 2009;16:1–2. doi: 10.1038/cdd.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 30.Sato K, Tsuchihara K, Fujii S, Sugiyama M, Goya T, Atomi Y, et al. Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res. 2007;67:9677–84. doi: 10.1158/0008-5472.CAN-07-1462. [DOI] [PubMed] [Google Scholar]
- 31.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–45. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 32.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–75. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–36. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–34. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 35.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–5. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophago-some membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Nat Acad Sci USA. 1982;79:1889–92. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abedin MJ, Wang D, McDonnell MA, Lehmann U, Kelekar A. Autophagy delays apoptotic death in breast cancer cells following DNA damage. Cell Death Differ. 2007;14:500–10. doi: 10.1038/sj.cdd.4402039. [DOI] [PubMed] [Google Scholar]
- 41.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–6. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, et al. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–6. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 43.Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14–1, in human colon cancer cells. Clin Cancer Res. 2004;10:8284–92. doi: 10.1158/1078-0432.CCR-04-1289. [DOI] [PubMed] [Google Scholar]
- 44.Huang S, Okumura K, Sinicrope FA. BH3 mimetic obatoclax enhances TRAIL-mediated apoptosis in human pancreatic cancer cells. Clin Cancer Res. 2009;15:150–9. doi: 10.1158/1078-0432.CCR-08-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 46.Pyrko P, Kardosh A, Liu YT, Soriano N, Xiong W, Chow RH, et al. Calcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Mol Cancer Ther. 2007;6:1262–75. doi: 10.1158/1535-7163.MCT-06-0629. [DOI] [PubMed] [Google Scholar]
- 47.Johnson AJ, Hsu AL, Lin HP, Song X, Chen CS. The cyclo-oxygenase-2 inhibitor celecoxib perturbs intracellular calcium by inhibiting endoplasmic reticulum Ca2+-ATPases: a plausible link with its anti-tumour effect and cardiovascular risks. Biochem J. 2002;366:831–7. doi: 10.1042/BJ20020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka K, Tomisato W, Hoshino T, Ishihara T, Namba T, Aburaya M, et al. Involvement of intracellular Ca2+ levels in nonsteroidal anti-inflammatory drug-induced apoptosis. J Biol Chem. 2005;280:31059–67. doi: 10.1074/jbc.M502956200. [DOI] [PubMed] [Google Scholar]
- 49.Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem. 2006;281:36303–16. doi: 10.1074/jbc.M607031200. [DOI] [PubMed] [Google Scholar]
- 50.Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad or both? Cancer Res. 2006;66:9349–51. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 51.Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–72. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, et al. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ. 2004;11:1009–16. doi: 10.1038/sj.cdd.4401436. [DOI] [PubMed] [Google Scholar]
- 53.Tsutsumi S, Namba T, Tanaka KI, Arai Y, Ishihara T, Aburaya M, et al. Celecoxib upregulates endoplasmic reticulum chaperones that inhibit celecoxib-induced apoptosis in human gastric cells. Oncogene. 2006;25:1018–29. doi: 10.1038/sj.onc.1209139. [DOI] [PubMed] [Google Scholar]
- 54.Heath-Engel HM, Chang NC, Shore GC. The endoplasmic reticulum in apoptosis and autophagy: role of the BCL-2 protein family. Oncogene. 2008;27:6419–33. doi: 10.1038/onc.2008.309. [DOI] [PubMed] [Google Scholar]
- 55.Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–35. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 56.Han J, Hou W, Goldstein LA, Lu C, Stolz DB, Yin XM, Rabinowich H. Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J Biol Chem. 2008;283:19665–77. doi: 10.1074/jbc.M710169200. [DOI] [PMC free article] [PubMed] [Google Scholar]