Abstract
Purpose
Obesity is associated with an increased risk of colon cancer. However, the influence of body mass index (BMI) on the prognosis of colon cancer survivors and its relationship to gender remains unknown.
Experimental Design
BMI (kg/m2) was categorized in patients with tumor-node-metastasis stage II and III colon carcinomas (n = 4,381) enrolled in seven randomized trials of 5-fluorouracil–based adjuvant chemotherapy. Cox proportional hazards models were used to determine the association of BMI with disease-free survival (DFS) and overall survival (OS).
Results
Among colon cancer patients, 868 (20%) were obese (BMI, ≥30 kg/m2), of which 606 were class 1 (BMI, 30–34 kg/m2) and 262 were class 2,3 (BMI, ≥35 kg/m2). Obese versus normal-weight patients were more likely to be younger, have distal tumors, show intact DNA mismatch repair, and have more lymph node metastases (P < 0.017). In a multivariate analysis, BMI was significantly associated with both DFS (P = 0.030) and OS (P = 0.0017). Men with class 2,3 obesity showed reduced OS compared with normal-weight men [hazard ratio, 1.35; 95% confidence interval, 1.02–1.79; P = 0.039]. Women with class I obesity had reduced OS [hazard ratio, 1.24; 95% confidence interval, 1.01–1.53; P = 0.045] compared with normal-weight women. Overweight status was associated with improved OS in men (P = 0.006), and underweight women had significantly worse OS (P = 0.019). BMI was not predictive of therapeutic benefit.
Conclusions
Obesity is an independent prognostic variable in colon cancer survivors and shows gender-related differences. These data suggest that obesity-related biological factors can influence clinical outcome.
The prevalence of obesity, as determined by body mass index (BMI), continues to steadily increase in the United States (1). Large prospective cohort studies have shown that obesity and a sedentary lifestyle confer an increased risk of developing colorectal cancer (CRC) relative to normal-weight individuals (2–8). The Cancer Prevention Study II prospectively followed >900,000 U.S. adults and found a 1.5- to 1.8-fold increased risk of incident colon cancers for obese individuals (9). Studies indicate that the observed associations between obesity and CRC risk are generally stronger in men than in women (8–11). To date, fewer studies have examined the association between BMI and risk of cancer recurrence and mortality in patients with established colon cancer (12–14). In a study of 1,053 patients with surgically resected stage III colon cancer treated in an adjuvant chemotherapy trial conducted by the Cancer and Leukemia Group B (CALGB), obesity was not significantly associated with a higher risk of colon cancer recurrence or death compared with normal-weight patients (14). Among participants in two colon cancer adjuvant trials conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP), very obese patients (class 2,3: BMI, ≥35 kg/m2) were shown to have an increased risk of recurrence and death compared with normal-weight patients (13). Whereas the association of BMI and risk of CRC seems stronger in men, conflicting data exist about obesity and gender in relation to prognosis in colon cancer survivors (12, 13). Although obesity was reported to be associated with a significant increase in mortality among women with stage II and III colon carcinomas (12), no evidence for a differential association of BMI and outcome by gender was reported in the NSABP studies (13).
The mechanism by which obesity influences colon cancer prognosis remains poorly understood. Evidence suggests that hyperinsulinemia and perturbations in the insulin-like growth factor (IGF) axis may be important mechanisms by which obesity can confer an increased risk of colon cancer, and such factors may also influence recurrence and death (15–18). We determined the association of obesity, as measured by BMI, with colon cancer recurrence and death in patients with curatively resected stage II and III colon cancers who participated in National Cancer Institute–sponsored randomized clinical trials of 5-fluorouracil–based adjuvant chemotherapy. We also determined whether an association of BMI and prognosis was related to patient gender. Because sporadic colon cancers with defective DNA mismatch repair (MMR) are more frequent in older women (19), we studied the relationship between obesity, MMR status, and patient gender. Lastly, we explored whether BMI was related to the therapeutic response to adjuvant chemotherapy.
Materials and Methods
Study population
The study population (n = 4,381) consisted of participants in seven colon cancer adjuvant therapy trials sponsored by the U.S. National Cancer Institute and conducted by Mayo Clinic/North Central Cancer Treatment Group (NCCTG) and the Southwest Oncology Group. The details and findings of the individual studies have been previously reported (20–23). All patients had tumor-node-metastasis (TNM) stage II or III colon carcinomas (24) that had been surgically resected with curative intent. Of these 4,381 patients, 3,226 received effective 5-fluorouracil–based adjuvant chemotherapy and the remaining 1,155 patients received either ineffective treatment (n = 516) or no treatment (n = 639). Demographic variables were obtained at study entry, and patients were required to have a baseline Eastern Cooperative Oncology Group performance score of 0 to 2 (25). Institutional Review Boards at the study sites had approved these trials, and all participants provided written informed consent. Furthermore, this study was conducted under an active Institutional Review Board–approved protocol to examine and correlate prognostic marker data from adjuvant colon cancer trials.
Determination of height, weight, and BMI
At study enrollment, patient height and weight were measured and recorded by trained staff. These measurements were also used to calculate chemotherapy dosages. In none of the trials was the computed body surface area “capped” for chemotherapy dosing. We calculated BMI (kg/m2) by dividing the weight (kilograms) by the patient’s squared height (meters). BMI categories were created on the basis of WHO classifications and prior reports (12, 26). For this analysis, BMI was categorized as underweight (BMI, <20 kg/m2), normal weight (BMI, 20–24.9 kg/m2), overweight (BMI, 25–29.9 kg/m2), class 1 obesity (BMI, 30–34.9 kg/m2), and class 2,3 obesity (BMI, ≥35 kg/m2).
Analysis of DNA MMR
MMR status was determined in primary colon carcinomas from NCCTG adjuvant studies, where tumor blocks and sufficient tissue were available. Tumors were analyzed for MMR protein expression using immunohistochemistry (n = 893), as previously described (27). Alternatively, tumors had been analyzed for microsatellite instability (MSI; n = 217) or for both MSI and MMR proteins (n = 198), as previously reported (28, 29). MSI was classified as MSI-high, MSI-low, or microsatellite stable, with the latter two groups being combined for the analysis (29, 30). Tumors were regarded as showing defective MMR if there was absence of nuclear staining for any MMR protein or if they were MSI-high.
Statistical analyses
χ2 and Wilcoxon rank-sum tests were used to test for an association between the categorical BMI and other clinicopathologic variables. The Co-chran-Armitage test was used to test for a trend across the BMI ordered categories for the two-level clinicopathologic variables. Overall survival (OS; censored at 8 y) was calculated as the number of years from random assignment to the date of death or last contact. Disease-free survival (DFS; censored at 5 y) was calculated as the number of years from random assignment to the first of either disease recurrence or death. The distributions of OS and DFS were estimated using Kaplan-Meier methodology. Univariate and multivariate Cox proportional hazards models (31) were used to explore the association of BMI with other clinicopathologic variables and with DFS and OS. The score and likelihood ratio test P values were used to test the significance of each covariate in the univariate and multivariate models, respectively, after stratifying by the patient’s original treatment study. Interaction effects between BMI and other variables of interest were tested in multivariate Cox models with the use of the likelihood ratio test. Statistical tests were two-sided, with P ≤ 0.05 considered significant. P values were not adjusted for multiple comparisons. Statistical analyses were done using SAS software (SAS Institute).
Results
BMI baseline characteristics
The study population included 4,381 participants from seven randomized trials of 5-fluorouracil–based adjuvant chemotherapy for tumor-node-metastasis stage II and III colon cancer. Using our predetermined BMI categories (see Materials and Methods), 868 (20%) were obese, 1,605 (37%) patients were overweight, 281 (6%) were underweight, and 1,627 (37%) were of normal weight (Table 1). Obese patients were further classified into class 1 (BMI 30–34.9 kg/m2) and class 2,3 (BMI ≥ 35 kg/m2)obesity, where 606 (70%) of the 868 obese patients were class 1 and the remaining 262 (30%) were class 2,3. Median BMI in the entire cohort was 25.7 (range, 14.0–70.3). Obese and normal-weight patients both received a median of six cycles of adjuvant chemotherapy from our NCCTG cohort (P = 0.9436).
Table 1.
Clinical characteristics by BMI categories
| Underweight (n = 281) | Normal (n = 1,627) | Overweight (n = 1,605) | Obese (n = 868) | Total (n = 4381) | Overall P* | Obese vs normal P† | |
|---|---|---|---|---|---|---|---|
| Histologic grade‡ | 0.1370 | 0.6825 | |||||
| 1/2 | 124 (68.9%) | 763 (73.5%) | 786 (76.1%) | 388 (74.5%) | 2,061 (74.4%) | ||
| 3/4 | 56 (31.1%) | 275 (26.5%) | 247 (23.9%) | 133 (25.5%) | 711 (25.6%) | ||
| Stage | 0.0129 | 0.0516 | |||||
| III | 200 (71.7%) | 1,235 (76%) | 1,219 (76.1%) | 684 (79.4%) | 3,338 (76.5%) | ||
| II | 79 (28.3%) | 390 (24%) | 382 (23.9%) | 177 (20.6%) | 1,028 (23.5%) | ||
| Tumor site | 0.0144 | 0.0575 | |||||
| Distal | 78 (43.3%) | 497 (47.9%) | 512 (49.7%) | 275 (53%) | 1,362 (49.2%) | ||
| Proximal | 102 (56.7%) | 541 (52.1%) | 518 (50.3%) | 244 (47%) | 1,405 (50.8%) | ||
| MMR | 0.0082 | 0.0347 | |||||
| Intact | 40 (75.5%) | 339 (84.5%) | 370 (85.1%) | 201 (90.5%) | 950 (85.5%) | ||
| Defective | 13 (24.5%) | 62 (15.5%) | 65 (14.9%) | 21 (9.5%) | 161 (14.5%) | ||
| Gender | <0.0001 | 0.6163 | |||||
| Women | 205 (73%) | 840 (51.6%) | 623 (38.8%) | 439 (50.6%) | 2,107 (48.1%) | ||
| Men | 76 (27%) | 787 (48.4%) | 982 (61.2%) | 429 (49.4%) | 2,274 (51.9%) | ||
| Age | 0.0005 | 0.0382 | |||||
| Mean (SD) | 57.8 (14.25) | 61.0 (12.11) | 61.7 (10.50) | 60.6 (10.24) | 61.0 (11.38) | ||
| Median | 60.5 | 63.0 | 63.0 | 61.3 | 62.1 | ||
| Range | 18.0–84.2 | 18.3–89.0 | 22.0–89.7 | 23.7–83.3 | 18.0–89.7 | ||
| Treatment status | 0.0349 | 0.0293 | |||||
| Ineffective | 81 (28.8%) | 438 (26.9%) | 437 (27.2%) | 199 (22.9%) | 1,155 (26.4%) | ||
| Effective | 200 (71.2%) | 1,189 (73.1%) | 1,168 (72.8%) | 669 (77.1%) | 3,226 (73.6%) | ||
| Lymph nodes | 0.0714 | 0.0171 | |||||
| 0 | 66 (28.2%) | 333 (25.9%) | 342 (27.4%) | 148 (23.8%) | 889 (26.2%) | ||
| 1–3 | 113 (48.3%) | 670 (52.1%) | 632 (50.6%) | 299 (48.1%) | 1,714 (50.5%) | ||
| >3 | 55 (23.5%) | 284 (22.1%) | 275 (22%) | 174 (28%) | 788 (23.2%) | ||
| T stage | 0.3508 | 0.8280 | |||||
| T1,2 | 19 (8.4%) | 141 (10.9%) | 145 (11.5%) | 71 (11.3%) | 376 (11%) | ||
| T3,4 | 206 (91.6%) | 1,148 (89.1%) | 1,115 (88.5%) | 559 (88.7%) | 3,028 (89%) |
Kruskal-Wallis test for continuous data; Χ2 test for variables with 3+ categories; Cochran-Armitage test for trend P value for variables with only 2 categories.
Χ2 test for categorical data and Wilcoxon rank-sum test for continuous data.
Grade1/2: well/moderate differentiation; grade 3/4: poor/undifferentiated.
Association of BMI with clinicopathologic variables
We examined the association of the categorical BMI with demographic and clinicopathologic variables (Table 1). Across BMI categories, we found significant associations between BMI and tumor stage and site, where obese patients had the highest rate of stage III tumors and were more likely to have distal colon cancers (Table 1). Distal tumors were defined relative to the splenic flexure with those at the flexure included in the distal category. Obesity was associated with a higher number of metastatic lymph nodes in that obese patients were more likely to have greater than three metastatic lymph nodes (N2 disease; ref. 24) compared with normal-weight patients (28% versus 22%; P = 0.017; Table 1). Age was also associated with BMI (P = 0.0005) in that obese and underweight patients were younger than were normal and overweight patients (Table 1). Similarly, gender was associated with BMI (P < 0.0001), where underweight patients were more likely female, overweight patients were more likely male, and normal-weight and obese patients were evenly split by gender. No relationship between BMI and patient performance status was found.
The prevalence of defective MMR colon cancers was 14.5% (Table 1), and the frequency was higher in women than in men (18% versus 11%; P = 0.001). Defective MMR was associated with older age in women (P = 0.0005) but not in men (P = 0.5818; Pinteraction = 0.0009). Obese patients had a lower rate of defective MMR compared with normal-weight patients (9.5% versus 15.5%; P = 0.0347; Table 1), and this association was stronger in patients younger (P = 0.0037) compared with those older than 60 years of age (Pinteraction = 0.0310; adjusted for covariates).
Impact of BMI on cancer recurrences or death
After a median follow-up of 8 years, 1,585 (36%) of the 4,381 eligible patients experienced cancer recurrence, and 1,833 (42%) had died. In a univariate analysis, the categorical BMI was significantly associated with both DFS and OS (Table 2). Specifically, patients who were obese (BMI, ≥30 kg/m2) had worse DFS and OS rates compared with normal-weight patients that were of borderline statistical significance. When obesity was further categorized, those with class 2,3 obesity (BMI, ≥35 kg/m2) had worse DFS [hazard ratio (HR), 1.23; 95% confidence interval (95% CI), 1.01–1.49); P = 0.0370] and OS (HR, 1.18; 95% CI, 0.98–1.44; P = 0.0879) rates compared with normal-weight patients (Table 2). The prognosis of underweight or overweight patients did not differ univariately compared with normal-weight patients (Table 2). The prognostic effect of other clinicopathologic variables is shown in Table 2. We found that patients with higher tumor stage (stage III versus II), poor differentiation, intact MMR status, increased number of metastatic lymph nodes, and none or ineffective treatment had worse rates of DFS and OS by univariate analysis. In addition, male gender and increased age were also associated with worse OS. The relationship between BMI and survival was not significantly modified by age or stage.
Table 2.
Univariate analysis in all patients (n = 4,381)
| Variable | DFS, HR (95% CI) | P* | OS, HR (95% CI) | P* |
|---|---|---|---|---|
| Categorical BMI (overall) | 0.0477 | 0.0218 | ||
| Underweight vs normal | 1.08 (0.89–1.31) | 0.4336† | 1.13 (0.93–1.36) | 0.2209† |
| Overweight vs normal | 0.94 (0.84–1.05) | 0.2880† | 0.93 (0.83–1.03) | 0.1623† |
| Obese vs normal | 1.13 (0.99–1.28) | 0.0670† | 1.13 (0.99–1.28) | 0.0622† |
| Class 1 obese | 1.08 (0.94–1.25) | 0.2768† | 1.10 (0.96–1.27) | 0.1785† |
| Class 2,3 obese | 1.23 (1.01–1.49) | 0.0370† | 1.18 (0.98–1.44) | 0.0879† |
| Stage | ||||
| II vs III | 0.45 (0.39–0.52) | <0.0001 | 0.47 (0.41–0.53) | <0.0001 |
| Gender | ||||
| Men vs women | 1.05 (0.95–1.15) | 0.3348 | 1.10 (1.00–1.20) | 0.0490 |
| Histologic grade‡ | ||||
| Grade 3/4 vs grade 1/2 | 1.41 (1.24–1.61) | <0.0001 | 1.43 (1.26–1.63) | <0.0001 |
| Tumor site | ||||
| Proximal vs distal | 0.90 (0.80–1.01) | 0.0836 | 1.02 (0.91–1.15) | 0.6984 |
| MMR status | ||||
| Defective vs intact | 0.68 (0.49–0.94) | 0.0174 | 0.71 (0.53–0.96) | 0.0252 |
| Age (1-unit increase) | 1.00 (0.996–1.004) | 0.9007 | 1.01 (1.005–1.014) | <0.0001 |
| Number of lymph nodes | <0.0001 | <0.0001 | ||
| 1–3 vs 0 | 1.75 (1.50–2.04) | (1.51–2.04) | ||
| >3 vs 0 | 3.57 (3.04–4.20) | 3.58 (3.05–4.19) | ||
| Treatment status | ||||
| Effective vs ineffective/none | 0.61 (0.52–0.72) | <0.0001 | 0.69 (0.59–0.81) | <0.0001 |
Score test P value from a Cox regression model after stratifying by study.
Wald Χ2 P value for the individual categories shown.
Grade1/2: well/moderate differentiation; grade 3/4: poor/undifferentiated.
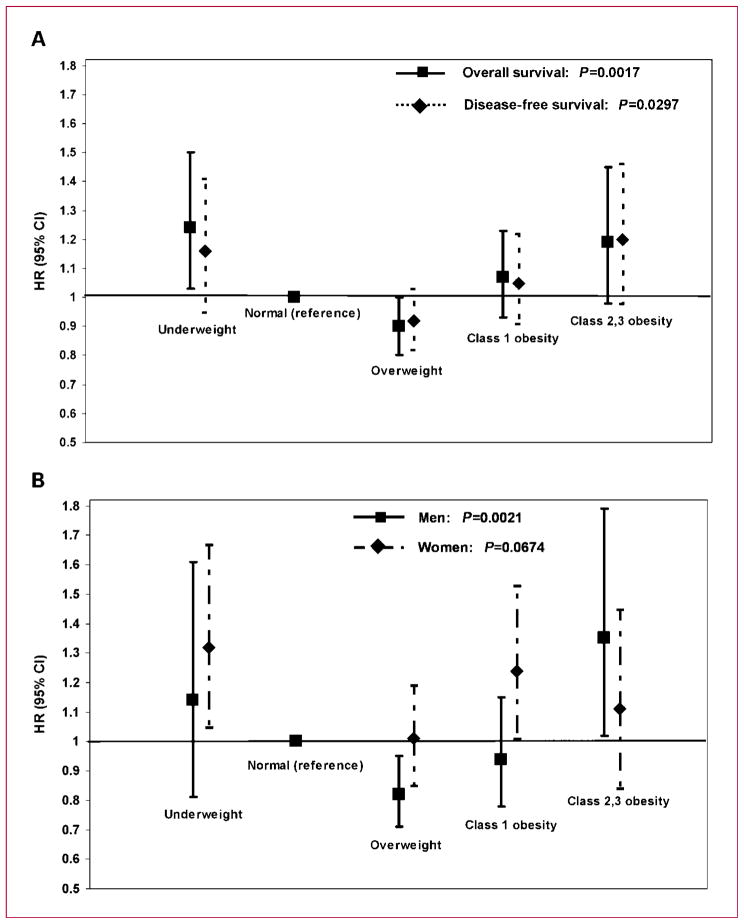
In a multivariate analysis, the categorical BMI remained significantly associated with both DFS (overall P = 0.0297) and OS (overall P = 0.0017; Table 3) after adjusting for age, stage, adjuvant treatment status, and gender. Class 2,3 obese patients showed a trend toward worse DFS (P = 0.0725) and OS (P = 0.0805; Table 3). Underweight patients had a significantly worse OS (P = 0.0258), whereas overweight patients had better OS (P = 0.0500) after adjusting for these same covariates (Table 3). The HRs and 95% CIs for both DFS and OS by the individual BMI categories are shown in Fig. 2A. As shown by the HRs, an adverse prognostic effect of BMI is seen for both underweight and class 2,3 obese patients. When the data were analyzed by patient gender (Table 3), we found that BMI was significantly associated with OS in men (overall P = 0.0021) and was of borderline significance in women (OS; overall P = 0.0674; adjusted Pinteraction = 0.081 for categorical BMI and gender for OS). Specifically, men with class 2,3 obesity had a significantly worse OS (HR, 1.35; 95% CI, 1.02–1.79; P = 0.0391) compared with normal-weight men (Table 3; Fig. 1A). In women, class 1 obesity was associated with worse OS compared with normal-weight women (HR, 1.24; 95% CI, 1.01–1.53; P = 0.0447; Fig. 1B), whereas this same effect was not observed in men (Table 3; Fig. 1A). Overweight men had a significantly improved OS (P = 0.0063) compared with normal-weight men after adjustment for covariates (Table 3). The poor prognosis of underweight patients was more evident in women (OS; P = 0.019; Table 3). A comparison of the OS HRs (95% CI) by BMI category and gender is shown graphically in Fig. 2B to illustrate the gender-related differences. Age, tumor stage, and treatment were at least of borderline significance for OS among all patients and in both genders (Table 3).
Table 3.
Multivariate analysis of BMI and OS
| All patients (n = 4,366) | ||
|---|---|---|
| Variable | HR (95% CI) | P* |
| Categorical BMI (overall) | 0.0017 | |
| Underweight vs normal | 1.24 (1.03–1.50) | 0.0258† |
| Overweight vs normal | 0.90 (0.80–1.00) | 0.0500† |
| Class 1 obese vs normal | 1.07 (0.93–1.23) | 0.3717† |
| Class 2,3 obese vs normal | 1.19 (0.98–1.45) | 0.0805† |
| Age | 1.01 (1.01–1.02) | <0.0001 |
| Stage (II vs III) | 0.47 (0.41–0.53) | <0.0001 |
| Treatment (effective vs control/ineffective) | 0.72 (0.62–0.85) | <0.0001 |
| Gender (men vs women) | 1.15 (1.04–1.26) | 0.0045 |
| Men (n = 2,268) | ||
| Variable | HR (95% CI) | P* |
| Categorical BMI (overall) | 0.0021 | |
| Underweight vs normal | 1.14 (0.81–1.61) | 0.4474† |
| Overweight vs normal | 0.82 (0.71–0.95) | 0.0063† |
| Class 1 obese vs normal | 0.94 (0.78–1.15) | 0.5599† |
| Class 2,3 obese vs normal | 1.35 (1.02–1.79) | 0.0391† |
| Age | 1.01 (1.01–1.02) | <0.0001 |
| Stage (II vs III) | 0.51 (0.43–0.61) | <0.0001 |
| Treatment (effective vs control/ineffective) | 0.64 (0.51–0.80) | <0.0001 |
| Women (n = 2,098) | ||
| Variable | HR (95% CI) | P* |
| Categorical BMI (overall) | 0.0674 | |
| Underweight vs normal | 1.32 (1.05–1.67) | 0.0194† |
| Overweight vs normal | 1.01 (0.85–1.19) | 0.9366† |
| Class 1 obese vs normal | 1.24 (1.01–1.53) | 0.0447† |
| Class 2,3 obese vs normal | 1.11 (0.84–1.45) | 0.4651† |
| Age (1-y increase) | 1.01 (1.00–1.02) | 0.0052 |
| Stage (II vs III) | 0.41 (0.34–0.51) | <0.0001 |
| Treatment (effective vs control/ineffective) | 0.83 (0.66–1.04) | 0.0948 |
Likelihood ratio P value after stratifying by study.
Wald Χ2 P value.
Fig. 2.
A, HRs and 95% CIs for DFS and OS in relation to BMI category in all patients. B, HRs and 95% CIs for OS by BMI category in men versus women. HRs were adjusted for age, stage, treatment, and gender.
Fig. 1.
Relationship between obesity and clinical outcome for stage II and III colon cancer patients participating in adjuvant chemotherapy trials. A, OS in men only. B, OS in women only.
Because normal-weight and overweight patients had the best prognoses, we combined these groups and conducted a secondary analysis. Using these combined groups as the reference (BMI, 20–30), we found that obesity (class 1–3; BMI, >30) was associated with a significantly worse DFS (P = 0.0254) and OS (P = 0.0093) after adjusting for age, stage, treatment, and gender. Given the findings for individual BMI categories and patient outcome, we determined whether a curvilinear or quadratic relationship could also describe the results observed. We found that continuous BMI displayed a significant curvilinear relationship with OS (P = 0.0071) after adjustment for age, stage, treatment, and gender.
Predictive analysis
We determined whether BMI was predictive for 5-fluorouracil–based chemotherapy outcome among all patients and within stage III patients. In the multivariate models, there was no significant relationship between BMI and treatment efficacy (adjusted P > 0.15) for any of the BMI categories tested.
Discussion
Although obesity and a sedentary lifestyle are known to increase the risk of developing colon cancer (2–7), only recently have studies examined whether obesity can influence the outcome of survivors of this disease. To determine whether obesity is associated with differences in colon cancer recurrence and/or survival, we studied stage II and III colon cancer patients from completed adjuvant chemotherapy trials. Using study baseline BMI measurements, we observed that obesity (BMI, ≥30 kg/m2) was significantly associated with an increased number of metastatic regional lymph nodes compared with normal-weight patients. The number of lymph node metastases is an accepted adverse prognostic variable in colon cancer patients (24). Obese patients were more likely to have distal versus proximal colon cancers, and distal tumors have been shown to have higher rates of chromosomal instability, p53 mutation, and DNA aneuploidy that may confer a worse prognosis (32, 33).
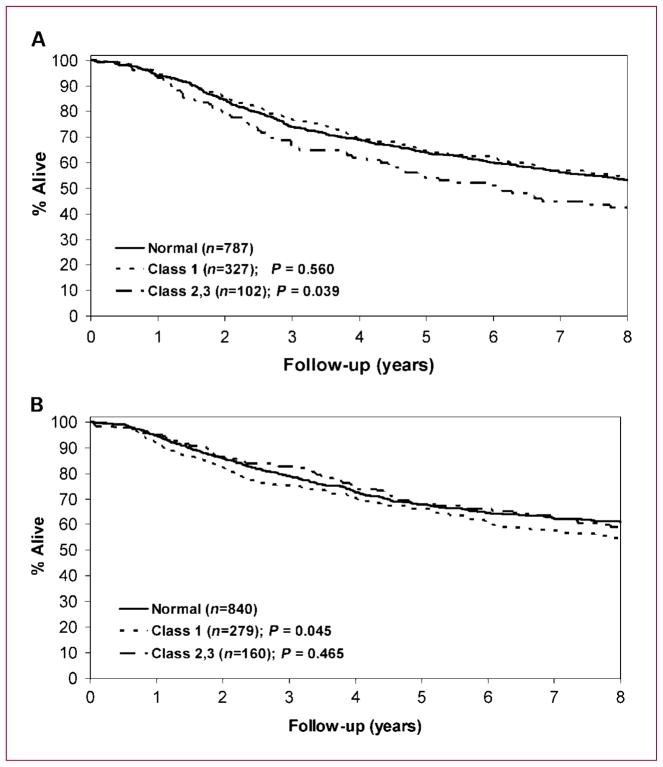
We found that obese patients showed higher rates of cancer recurrence and mortality compared with normal-weight patients that was most evident overall for class 2,3 (BMI, ≥35 kg/m2) obesity. In a multivariate analysis, class 2,3 obesity was associated with a 19% increase in the risk of death (i.e., OS) compared with normal-weight patients. It is important to note that unlike the NSABP colon cancer adjuvant trials (13), our studies did not limit chemotherapy dosing based on body surface area, and therefore, the less favorable outcomes among obese patients are not related to differences in the amount of chemotherapy received. In support of the prognostic effect of obesity, we found that chemotherapy dosing and the number of cycles of adjuvant chemotherapy received were similar in obese versus normal-weight subjects. Although we did not have data on the total number of lymph nodes removed at surgical resection, we further addressed the adequacy of surgical resection by examining local recurrence rates in patients where such data were available (n = 243). Across all BMI categories studied, local recurrence rates were similar (P = 0.67) and did not differ significantly among obese versus normal-weight patients (n = 130; P = 0.20). Therefore, our data suggest that obesity did not adversely affect the adequacy of surgical resection when local recurrence is used as a surrogate. Regrettably, we did not have colon cancer–specific survival data; however, DFS is a useful surrogate for such information and results for DFS and OS were similar.
We determined whether the prognostic effect of obesity was related to patient gender. Data about BMI, gender, and prognosis have been conflicting in patients with established colon cancer. In an adjuvant chemotherapy trial, obese women (BMI, ≥30.0 kg/m2) but not men experienced significantly worse overall mortality (12). In an analysis of pooled adjuvant colon cancer studies conducted by a single cooperative group (NSABP), no differences between obesity and colon cancer outcome by gender were found (13). However, we found a stronger relationship between BMI and clinical outcome in men compared with women. Men who were very obese (BMI, ≥35 kg/m2) had a 35% increased risk of death that was statistically significant, whereas very obese women had only an 11% increased risk of death that was not significant compared with normal-weight patients after adjustment for covariates. Women with class 1 (BMI, 30–34 kg/m2) obesity, however, had worse OS (P = 0.045) compared with normal-weight women, whereas this same effect was not observed in men. These data are consistent with the finding that the association of BMI and colon cancer incidence and mortality is stronger and more linear in men than in women (9, 10, 34–39). Stronger associations for BMI and prognosis in men compared with women may be explained, in part, by the observation that BMI is more closely related to the amount of abdominal or central adiposity in men than in women (40, 41). In this regard, a 20% increase in the risk of colon cancer recurrence or death was observed for every 10-cm increase in waist circumference (42). Another potential explanation for gender differences in colon cancer prognosis is an effect modification by menopausal status and/or hormone replacement therapy in that a protective effect of hormone replacement therapy has been associated with a significant reduction in colon cancer mortality (3, 43, 44).
Patient gender was related to the molecular pathway of colon tumorigenesis in that colon cancers with defective MMR were more prevalent in older women but not in men (19, 45). In this regard, we found that defective MMR was associated with older age in women but not in men (Pinteraction = 0.0009). An excess of colon cancers with defective MMR found in older women as shown here may be related to a withdrawal of estrogens (45, 46). Interestingly, we found that obese patients had significantly fewer colon cancers with defective MMR compared with normal-weight patients. Similarly, obesity and lack of physical activity were associated with a lower prevalence of colon cancers with defective MMR in women but not in men in a population-based, case-controlled study (45). Obesity is associated with increased levels of circulating estrogen (47), and estrogen may protect against the development of colon cancers with defective MMR in women (45).
We found that colon cancer mortality was increased among underweight (BMI, <20.0 kg/m2) patients in a multivariate analysis. A worse outcome for underweight patients has been a consistent finding among studies, and underweight may reflect underlying comorbidities that increase mortality risk (48). Mortality in underweight CRC patients was more often due to noncan-cerrelated causes than was mortality in normal-weight patients (13). We also observed that overweight patients had significantly better OS rates in a multivariate analysis. This effect was limited to men, whereby overweight men showed significantly improved OS (P = 0.006) after adjusting for age, stage, and treatment. Some longitudinal studies have shown that overweight subjects without a cancer history have the lowest mortality among BMI categories when examining BMI and risk of death (49, 50). This finding may reflect a limitation of BMI in that it makes no distinction between body weight from muscle versus fat, and therefore, muscular individuals can be categorized as overweight or obese when they are not. Together, our data show the complex relationship of BMI and clinical outcome whereby the prognostic effect of BMI varies by category. Based on WHO guidelines and prior studies (13, 26), we analyzed BMI as a categorical variable. The results of this analysis suggested that BMI has a curvilinear or quadratic relationship with OS. We also modeled BMI as a continuous curvilinear variable and found that it was associated with OS (P = 0.0071). Therefore, our data show consistent results when modeling BMI as a categorical variable or as a continuous curvilinear variable.
The mechanism underlying the effect of obesity on the clinical behavior of colon cancers remains poorly understood but may involve interactions among insulin, IGFs, and IGF-binding proteins (16, 17, 51–54) that have all been implicated in colon cancer development. Increased circulating levels of insulin and free IGF-I have been associated with obesity and physical inactivity (18, 55, 56). Furthermore, both insulin and IGF-I promote cell proliferation and inhibit apoptosis in colon cancer cells (57, 58), suggesting that they may promote the growth of micrometastases. In a prospective case-control study nested within the Physicians’ Health Study, men in the highest quintile for IGF-I had 2.5 times the risk of CRC as did men in the lowest quintile (51). An analysis of the Nurses’ Health Study cohort found a comparable risk increase among women, with a relative risk of 2.17 for those in the highest quartile of IGF-I compared with those in the lowest (15). Studies have also shown (16, 52) an increase in CRC risk with increasing levels of C-peptide, a marker of insulin production, and in men with the high levels of the hormone leptin (59, 60). Another mechanism that may contribute to differences in colon cancer survival based on BMI is suggested by data for fatty acid synthase (FASN) expression (61). Among normal-weight and minimally overweight patients (BMI, <27.5 kg/m2), FASN positivity was associated with a reduction in mortality, whereas among moderately overweight and obese patients (BMI, ≥27.5 kg/m2), FASN overexpression conferred a significant increase in mortality (61). FASN plays an important role in de novo lipogenesis and is physiologically regulated by energy balance in that exercise and energy restriction downregulate FASN (62).
Important strengths of our study include its large size, accuracy of BMI measurements done at study entry by trained staff rather than self-reporting of height and weight, and rigorous collection of recurrence and survival data during an extended follow-up period. Limitations include the retrospective design and measurement of obesity by BMI versus measures of central obesity such as waist-to-hip ratio or waist circumference that may be more predictive of the risk of developing colon cancer than BMI (3, 42, 63). We were also unable to analyze factors such as diet, physical activity, or menopausal status and hormone replacement therapy use that may have independent associations with colon cancer outcomes as well as risk of death from other causes. Importantly, Meyerhardt et. al. (14) reported that changes in weight (either gain or loss) during the period of adjuvant chemotherapy in colon cancer patients were not associated with clinical outcome.
Our findings extend the effect of obesity beyond its known association with colon cancer risk by showing that obesity is an independent prognostic variable in colon cancer survivors that shows differences by gender. Obesity was a poor prognostic factor despite adjuvant chemotherapy. Such information has the potential to influence patient management decisions and surveillance strategies. Further study is needed to determine the mechanism of the adverse effect of obesity on survivors of colon cancer. Lastly, our findings suggest the potential for evaluating interventions among obese survivors of colon cancer with the goal of improving patient outcomes.
Translational Relevance.
Multiple studies have shown that obesity is associated with an increased risk of developing colon cancer; however, the influence of obesity on the prognosis of colon cancer survivors is poorly understood. Furthermore, the link between obesity and colon cancer risk seems stronger in men, yet conflicting data exist about colon cancer outcomes based on gender. We determined the association of body mass index with clinical outcome in colon carcinoma patients who participated in seven randomized trials of 5-fluorouracil–based adjuvant chemotherapy. Twenty percent of patients were obese (body mass index, ≥30 kg/m2) and were more likely to have distal tumors, show intact DNA mismatch repair, and have increased lymph node metastases compared with normal-weight patients. Moreover, obesity was an independent and adverse prognostic variable in colon cancer survivors with distinct gender-related differences. These data suggest that interventions to reduce rates of obesity may improve colon cancer outcomes.
Acknowledgments
Grant Support
Supported in part by National Cancer Institute grant CA 104683 (F.A. Sinicrope).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Larsson SC, Rutegard J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer. 2006;42:2590–7. doi: 10.1016/j.ejca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:920–31. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Le Marchand L, Wilkens LR, Mi MP. Obesity in youth and middle age and risk of colorectal cancer in men. Cancer Causes Control. 1992;3:349–54. doi: 10.1007/BF00146888. [DOI] [PubMed] [Google Scholar]
- 6.Martinez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses’ Health Study Research Group. J Natl Cancer Inst. 1997;89:948–55. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 7.Ford ES. Body mass index and colon cancer in a national sample of adult US men and women. Am J Epidemiol. 1999;150:390–8. doi: 10.1093/oxfordjournals.aje.a010018. [DOI] [PubMed] [Google Scholar]
- 8.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 9.Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol. 2000;152:847–54. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 10.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–47. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 11.Potter J, Hunter D. Colorectal cancer. Oxford University Press; New York, NY: 2001. [Google Scholar]
- 12.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–95. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 13.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–54. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 14.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–15. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005;14:850–5. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Giovannucci E, Pollak M, et al. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004;96:546–53. doi: 10.1093/jnci/djh082. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 18.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–80. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 19.Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208–15. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poplin EA, Benedetti JK, Estes NC, et al. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol. 2005;23:1819–25. doi: 10.1200/JCO.2005.04.169. [DOI] [PubMed] [Google Scholar]
- 21.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–8. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 22.Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122:321–6. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 23.O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16:295–300. doi: 10.1200/JCO.1998.16.1.295. [DOI] [PubMed] [Google Scholar]
- 24.Greene FL, Stewart AK, Norton HJ. A new TNM staging strategy for node-positive (stage III) colon cancer: an analysis of 50,042 patients. Ann Surg. 2002;236:416–21. doi: 10.1097/00000658-200210000-00003. discussion 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zubrod C, Scheiderman M, Frei E. Appraisal of methods for the study of chemotherapy in man: comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. J Chronic Dis. 1960;11:7–33. [Google Scholar]
- 26.WHO. World Health Organization technical report series. Geneva: WHO; 1995. Physical status: the use and interpretation of anthropometry; pp. 1–47. [PubMed] [Google Scholar]
- 27.Parc YR, Halling KC, Wang L, et al. HMSH6 alterations in patients with microsatellite instability-low colorectal cancer. Cancer Res. 2000;60:2225–31. [PubMed] [Google Scholar]
- 28.O’Connell MJ, Sargent DJ, Windschitl HE, et al. Randomized clinical trial of high-dose levamisole combined with 5-fluorouracil and leucovorin as surgical adjuvant therapy for high-risk colon cancer. Clin Colorectal Cancer. 2006;6:133–9. doi: 10.3816/ccc.2006.n.030. [DOI] [PubMed] [Google Scholar]
- 29.Halling KC, French AJ, McDonnell SK, et al. Microsatellite instability and 8p allelic imbalance in stage B2 and C colorectal cancers. J Natl Cancer Inst. 1999;91:1295–303. doi: 10.1093/jnci/91.15.1295. [DOI] [PubMed] [Google Scholar]
- 30.Thibodeau SN, French AJ, Cunningham JM, et al. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998;58:1713–8. [PubMed] [Google Scholar]
- 31.Cox D. Regression models and life tables. JR Stat Soc. 1972;34:187–200. [Google Scholar]
- 32.Offerhaus GJ, De Feyter EP, Cornelisse CJ, et al. The relationship of DNA aneuploidy to molecular genetic alterations in colorectal carcinoma. Gastroenterology. 1992;102:1612–9. doi: 10.1016/0016-5085(92)91721-f. [DOI] [PubMed] [Google Scholar]
- 33.Bell SM, Scott N, Cross D, et al. Prognostic value of p53 over-expression and c-Ki-ras gene mutations in colorectal cancer. Gastroenterology. 1993;104:57–64. doi: 10.1016/0016-5085(93)90835-z. [DOI] [PubMed] [Google Scholar]
- 34.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2007;13:4199–206. doi: 10.3748/wjg.v13.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–65. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 36.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 37.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–5. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 38.Russo A, Franceschi S, La Vecchia C, et al. Body size and colorectal-cancer risk. Int J Cancer. 1998;78:161–5. doi: 10.1002/(sici)1097-0215(19981005)78:2<161::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 39.Engeland A, Tretli S, Austad G, Bjorge T. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control. 2005;16:987–96. doi: 10.1007/s10552-005-3638-3. [DOI] [PubMed] [Google Scholar]
- 40.Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99:931–40. doi: 10.1017/S0007114507853347. [DOI] [PubMed] [Google Scholar]
- 41.Krotkiewski M, Bjorntorp P, Sjostrom L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest. 1983;72:1150–62. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–7. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan JA, Meyerhardt JA, Chan AT, Giovannucci EL, Colditz GA, Fuchs CS. Hormone replacement therapy and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:5680–6. doi: 10.1200/JCO.2006.08.0580. [DOI] [PubMed] [Google Scholar]
- 44.Rennert G, Rennert HS, Pinchev M, Lavie O, Gruber SB. Use of hormone replacement therapy and the risk of colorectal cancer. J Clin Oncol. 2009;27:4542–7. doi: 10.1200/JCO.2009.22.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slattery ML, Potter JD, Curtin K, et al. Estrogens reduce and withdrawal of estrogens increase risk of microsatellite instability-positive colon cancer. Cancer Res. 2001;61:126–30. [PubMed] [Google Scholar]
- 46.McCourt CK, Mutch DG, Gibb RK, et al. Body mass index: relationship to clinical, pathologic and features of microsatellite instability in endometrial cancer. Gynecol Oncol. 2007;104:535–9. doi: 10.1016/j.ygyno.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 47.McTiernan A, Wu L, Chen C, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity. 2006;14:1662–77. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- 48.Katzmarzyk PT, Janssen I, Ardern CI. Physical inactivity, excess adiposity and premature mortality. Obes Rev. 2003;4:257–90. doi: 10.1046/j.1467-789x.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 49.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 50.Orpana HM, Berthelot JM, Kaplan MS, Feeny DH, McFarland B, Ross NA. BMI and mortality: results from a National Longitudinal Study of Canadian Adults. Obesity. 2010;18:214–8. doi: 10.1038/oby.2009.191. [DOI] [PubMed] [Google Scholar]
- 51.Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–5. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 52.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 53.Giovannucci E, Pollak MN, Platz EA, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000;9:345–9. [PubMed] [Google Scholar]
- 54.Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–54. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 55.Wolpin BM, Meyerhardt JA, Chan AT, et al. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27:176–85. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 57.Guo YS, Narayan S, Yallampalli C, Singh P. Characterization of insulinlike growth factor I receptors in human colon cancer. Gastroenterology. 1992;102:1101–8. [PubMed] [Google Scholar]
- 58.Koenuma M, Yamori T, Tsuruo T. Insulin and insulin-like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res. 1989;80:51–8. doi: 10.1111/j.1349-7006.1989.tb02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wauters M, Mertens I, Considine R, De Leeuw I, Van Gaal L. Are leptin levels dependent on body fat distribution in obese men and women? Eat Weight Disord. 1998;3:124–30. doi: 10.1007/BF03339999. [DOI] [PubMed] [Google Scholar]
- 60.Stattin P, Palmqvist R, Soderberg S, et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep. 2003;10:2015–21. [PubMed] [Google Scholar]
- 61.Ogino S, Nosho K, Meyerhardt JA, et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26:5713–20. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev. 2007;7:763–77. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 63.Moore LL, Bradlee ML, Singer MR, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obes Relat Metab Disord. 2004;28:559–67. doi: 10.1038/sj.ijo.0802606. [DOI] [PubMed] [Google Scholar]