Abstract
Background
Olomoucine II, the most recent derivative of roscovitine, is an exceptionally potent pharmacological inhibitor of cyclin-dependent kinase activities. Here, we report that olomoucine II is also an effective antiviral agent.
Methods
Antiviral activities of olomoucine II were tested on a range of human viruses in in vitro assays that evaluated viral growth and replication.
Results
Olomoucine II inhibited replication of a broad range of wild-type human viruses, including herpes simplex virus, human adenovirus type-4 and human cytomegalovirus. Olomoucine II also inhibited replication of vaccinia virus and herpes simplex virus mutants resistant to conventional acyclovir treatment. This report is the first demonstration of a poxvirus being sensitive to a cyclin-dependent kinase inhibitor. The antiviral effects of olomoucine II could be observed at lower concentrations than with roscovitine, although both were short-term. A remarkable observation was that olomoucine II, when used in combination with the DNA polymerase inhibitor cidofovir, was able to almost completely eliminate the spread of infectious adenovirus type-4 progeny from infected cells.
Conclusions
Our results show that when targeting two complementary antiviral mechanisms, strongly additive effects could be observed.
Introduction
Many clinically useful antiviral drugs are nucleoside or nucleotide analogues that either directly or indirectly target viral DNA polymerases [1]. Despite their impressive safety record and efficacy, some nucleoside and nucleotide analogues exhibit significant toxicity and target active sites on virus-encoded kinases or DNA polymerases. Resistance to any antiviral agent that targets a specific virus-encoded function arises following virus mutation and can be rapidly selected in vivo. Because drug resistance and cross-resistance pose an increasing problem in disease management, the necessity to develop additional antiviral strategies is evident.
Cyclin-dependent kinases (CDKs) constitute a family of well conserved serine/threonine protein kinases that are active in complexes with their regulatory subunits, the cyclins [2]. The human genome encodes 13 CDKs, 48 CDK-related kinases and 25 cyclins [3,4]. Different members of the CDK family have been implicated in a range of key cellular processes: CDK1, CDK2, CDK3, CDK4, CDK6 and CDK7 regulate the cell cycle, CDK7, CDK8 and CDK9 interact directly with transcription factors, CDK5 and CDK11 control neuronal functions, CDK2, CDK5, CDK6 and CDK9 are involved in cell differentiation and CDK1, CDK2, CDK4, CDK5, CDK6 and CDK11 affect apoptosis [5]. CDK functions are regulated by cyclins, CDK inhibitory proteins and subcellular localization. Because many CDKs are crucial regulators of cell division, pharmaceutical firms have been targeting the discovery and development of pharmacological CDK inhibitors (CDK-Is) as potential anticancer drugs [6]. CDK-Is are a diverse and heterogeneous family of small, flat heterocyclic purines, pyrimidines, flavonoids or bis-indoles that bind to the ATP binding pocket of their target CDK, where they can compete with the ATP [7].
The earliest significant CDK-I was the CDK oligo-specific olomoucine [8] (Figure 1) and the CDK pan-specific flavopiridol [9]. Olomoucine belongs to C2-, N6- and N9-substituted adenines, which display high efficiency and selectivity towards some CDKs; olomoucine specifically inhibits CDK1, CDK2, CDK5 and, to a lesser extent, ERK1 [8]. With the specific objective of attaining enhanced inhibition of CDKs, olomoucine was subjected to structural modifications. A classical structure–activity relationship study directed at modifying olomoucine side chains generated two exceptionally potent CDK-Is: roscovitine (seliciclib; CYC202; Cyclacel Pharmaceuticals, Berkeley Heights, NJ, USA) and, more recently, olomoucine II [10,11] (Figure 1). The increased potency of roscovitine over olomoucine is caused by the introduction of a branched C2 side chain and a more bulky N9-isopropyl moiety. These changes markedly increased the complementarity of the inhibitor to the ATP binding site of CDK2, as demonstrated by X-ray crystallography [7]. Both structural changes did not alter the selectivity of roscovitine, but increased cellular potency of roscovitine 10-fold towards CDK1 and CDK2, and 20-fold towards CDK5 [7]. Olomoucine II differs from roscovitine in having an additional ortho-hydroxyl group on the benzyl ring, yet this single modification is associated with a 10-fold higher inhibitory activity for CDK9 [12]. This increased affinity of olomoucine II towards CDK9 is responsible for its enhanced effect on intracellular functional activities when compared with roscovitine. For example, olomoucine II induces the nuclear accumulation and transcriptional activation of the tumour suppressor protein p53 at two to threefold lower concentrations than roscovitine [12]. At higher concentrations, both inhibitors may inhibit RNA synthesis by attenuating C-terminal domain phosphorylation of RNA polymerase II [13,14].
Figure 1.
Three related cyclin-dependent kinase inhibitors showing progressive modifications to their structures
The realization that roscovitine possessed antiviral activity stimulated research aimed at targeting cellular functions, exemplified by CDKs, required for virus replication [15-17]. Roscovitine, and other CDK-Is, have the ability to inhibit replication of a broad range of viruses, even in non-dividing cells and including agents that do not require cell cycle progression [1]. Human cytomegalovirus (HCMV), herpes simplex type-1 (HSV-1) and -2 (HSV-2), varicella zoster virus, Epstein–Barr virus, human adenovirus (Ad), HIV and human T-cell leukaemia virus are all susceptible to roscovitine [1]. Antiviral activities of CDK-Is correlate with the extent of CDK inhibition, rather than the core structure of the inhibitor [18].
Although CDK-Is have been shown to inhibit multiple stages of viral replication, including splicing, DNA replication, reactivation from latency, activation of cellular or viral enzymes and intracellular trafficking, it is recognized that the antiviral activity of CDK-Is is primarily mediated by inhibiting virus-encoded transcription [18-24]. Interestingly, roscovitine can prevent initiation of viral transcription that is specific to viral genomes and independent of promoter elements [14,25]. Flavopiridol is CDK pan-specific and inhibits the transcription of most cellular and viral genes, whereas roscovitine is CDK oligo-specific and appears not to inhibit the transcription of cellular genes [14,26]. The selectivity of roscovitine in suppressing viral and plasmid-encoded gene expression resembles an activity described for interferon-α [27]. In this context, it is interesting to note that interferon-α also inhibits CDKs and promotes cell cycle arrest [28-30].
The mechanisms by which CDK-Is suppress virus replication have not been fully defined and can be expected to differ between agents. Consequently, it is extremely important to compare and contrast the potential antiviral properties of roscovitine and olomoucine II empirically. Our previous studies have determined that olomoucine II was a more potent inhibitor of CDK activities than roscovitine [12]. We now report that olomoucine II inhibits virus replication at substantially lower concentrations than roscovitine. In addition, olomoucine II exhibited antiviral activity across a wider range of human viruses, including an orthopoxvirus, human adenovirus type-4 (Ad4), HSV-1, HSV-2 and HCMV. Furthermore, we demonstrate that olomoucine II and cidofovir exerted a strongly additive effect on Ad4-infected cells when these two drugs were used in combination.
Methods
Chemistry
Antiviral agents
Where indicated, the following antivirals were used: acyclovir (9-[(2-hydroxyethoxy)methyl]guanine; Sigma–Aldrich, St Louis, MO, USA), cidofovir ((S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]-cytosine; Moravek, Biochemicals, Brea, CA, USA), iodo-deoxyuridine (1-(2-deoxy-β-d-ribofuranosyl)-5-iodouracil; Sigma–Aldrich), ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide; Sigma–Aldrich) and chloroquine (N′-(7-chloroquinolin-4-yl)-N,N-diethyl-pentane-1,4-diamine; Sigma–Aldrich). Olomoucine II (6-(2-hydroxybenzylamino)-2-{[(1R)-(hydroxymethyl)propyl]amino}-9-isopropylpurine) and roscovitine (2-(R)-(1-ethyl-2-hydroxyethylamino)-6-benzylamino-9-isopropylpurine) were prepared according to published methods [11,31]. The antivirals were used as indicated in text.
Virology
Cell culture
Human fetal foreskin fibroblast (HFFF) cells, human lung carcinoma A549 epithelial cells, human B95a B-cells and Madin–Darby canine kidney (MDCK) epithelial cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum (FBS), 105 IU/ml penicillin and 100 μg/ml streptomycin. MDCK cells were obtained from the European Collection of Cell Cultures (Porton Down, UK) and B95a from IZSBS–Istituto Zooprofilattico Sperimentale (Brescia, Italy).
Infection and viruses
HSV-1 strain 17, HSV-1 strain 17 containing green fluorescent protein (GFP) in the thymidine kinase (TK) locus (HSV-1-deltaTK) and HSV-2 strain HG52 were all kindly provided by the Medical Research Council Virology Unit (Glasgow, UK). Herpesviruses were all propagated and titrated in HFFF cells. Vaccinia virus Western Reserve strain encoding influenza PB2 in the TK locus (VV; kindly provided by Geoffrey Smith (Imperial College, London, UK) [32] were propagated and titrated in HFFF cells. Ad4 strain RI-6 [33] was propagated and titrated in A549 cells. HCMV strain Toledo encoding GFP in the β2.7 locus [34] was propagated and titrated in HFFF cells. Measles virus (MV) wild-type strain WTFb was a gift from Jurgen Schneider-Schaulies (Wuerzbug, Germany). MV was propagated and titrated in B95a cells. MV titres were determined as syncytium-forming units (SFU). Influenza virus (IV) H1N1 strain A/PR/8/34, obtained from the European Collection of Animal Cell Cultures (Porton Down, UK), was propagated in the allantoic cavity of embryonated chicken eggs (strain 0; Institute for Animal Health, Compton, UK) at 35°C. Eggs were infected on day 6 and allantoic fluid was collected on day 10. IV titres were determined as haemagglutination (HA) units (HAU; inverse of log2 end point dilution titre) by HA of chicken erythrocytes (Fiebig Naehrstofftechnik, Bad Kreuznach, Germany) and as infectious centre-forming units (ICFU) per ml of allantoic fluid. ICFU were determined in MDCK cells grown in 12-well plates (NUNC, Roskilde, Denmark) on 16 mm glass coverslips using a cocktail of directly fluorescein-isothiocyanate-labelled monoclonal antibodies against A-type influenza viruses (K6105 A reagent; DAKO, Glostrup, Denmark).
Plaque number reduction assays
The 50% inhibition concentrations (IC50) were calculated using standard plaque number reduction assays. The IC50 value was defined as the concentration of an antiviral that reduced the number of plaques by 50% relative to mock treatment without antiviral. Cells were grown in 25 cm2 tissue culture flasks (Corning, Amsterdam, the Netherlands) until confluent, then infected with 100 plaque-forming units (PFU) of appropriate virus (HSV-1, HSV-1-deltaTK, HSV-2, VV, HCMV on HFFF cells and Ad4 on A549 cells) for 1 h in a 37°C CO2 incubator on a rocking platform. Cells were then overlayed with medium containing 1% Avicel RC-591 (Camida Ltd, Tipperary, Ireland) [35] and the antiviral agent under test. Olomoucine II and roscovitine were typically tested at concentrations ranging from 0.04 μM to 20 μM. Where appropriate, acyclovir, cidofovir, iodo-deoxyuridine, chloroquine and ribavirin were used for comparison, and were applied over a range that included their reported peak IC50 value. Infected cell monolayers were stained with Giemsa (Sigma–Aldrich) after 3 days (HSV-1, HSV-1-deltaTK, HSV-2 and VV) or 5 days (Ad4) and plaques were counted. For HCMV, after 7 days of incubation, cell monolayers were washed with phosphate-buffered saline (PBS) and green fluorescent foci were counted using a Leica DMIRBE microscope (Leica Microsystems, Milton Keynes, UK). The plaque counts were plotted and analysed using Cricket Graph software (Computer Associates Inc., Smithfield, Ireland).
To perform an MV syncytium reduction assay, B95a cells were grown in 24-well plates (NUNC) until 80% confluent. Cells were pretreated with medium containing the appropriate amount of antiviral for 30 min prior to infection. Each well was infected with 100 SFU of MV and incubated in DMEM without FBS or antibiotics containing no antivirals or antivirals at a range of concentrations for 24 h. Syncytia formations were assessed by phase contrast microscopy. The IC50 values were defined as the concentration of antiviral that reduced the number of syncytia by 50% in comparison to cells infected in the absence of antiviral.
IV infectious centre reduction assays were performed in MDCK cells grown on 16 mm diameter glass cover slips in 12-well plates (NUNC) until 80% confluent. Cells were pretreated with medium containing the antiviral agent under test for 30 min prior to infection. Each well was infected with 100 ICFU of IV incubated in DMEM without FBS or antibiotics and containing no antivirals or with antivirals at a range of concentrations for 24 h. Cells were washed with PBS, fixed with 3% paraformaldehyde (Sigma–Aldrich), permeabilized with 1% Triton ×100 (Sigma–Aldrich) in PBS and stained with a cocktail of monoclonal antibodies against A-type influenza viruses (K6105 A reagent). ICFUs (green fluorescent cytoplasma and/or fluorescent nuclei) were quantitated using Axioplan epifluorescent microscope with Axiovision software (Carl Zeiss, Jena, Germany). IC50 values were defined as the concentration of antiviral that reduced the number of infectious centres by 50% in comparison to cells infected in the absence of antiviral. The final IC50 values for all virus/antiviral combinations are each a mean from four experiments.
The 50% cytotoxic concentrations (CC50) were calculated using a WST-1 kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. The CC50 value was defined as the concentration of an antiviral that reduced the absorbance at 450 nm readout by 50% relative to mock treatment without antiviral. Apoptosis was determined as caspase-3/7 activity using Caspase-Glo 3/7 kit (G8090; Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Ad4 yield reduction assays
Confluent monolayers of A549 cells in 6-well tissue culture dishes were infected with 2,000 PFU/well of Ad4 for 1 h in a 37°C CO2 incubator on a rocking platform. Cells were then rinsed and grown in medium containing 10 μM olomoucine II or 100 μM cidofovir or a cocktail of 10 μM olomoucine II and 100 μM cidofovir. The cells and media were separately harvested at 6, 12, 24, 48, 72 and 96 h after infection, then released virus and total virus (released and intracellular) yields in each sample were titrated using A549 cells.
Results
Inhibition of viral replication by olomoucine II and roscovitine
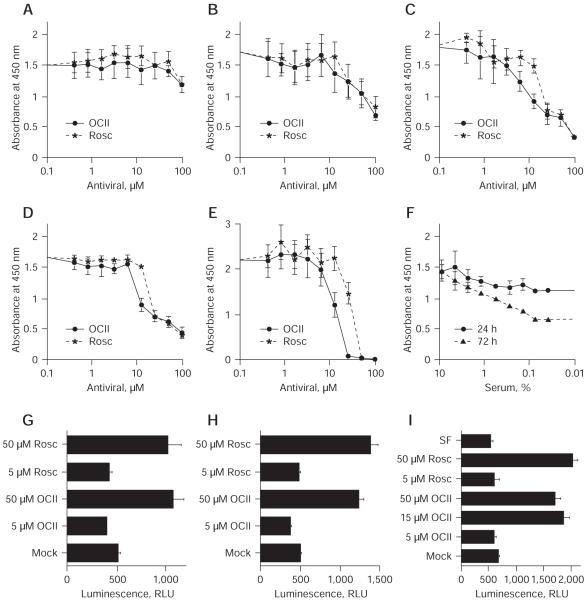
With the demonstration that olomoucine II was a more potent inhibitor of CDK activities than roscovitine [12], it was clearly important to evaluate the relative capacity of these two CDK-Is to impair virus replication. Using plaque reduction assays, we compared the effect of olomoucine II on the replication of a panel of DNA (HSV, VV, Ad4 and HCMV) and RNA (MV and IV) viruses against a range of reference antiviral agents (Table 1). Olomoucine II inhibited the replication of most of the tested DNA viruses more effectively than roscovitine. Roscovitine did not inhibit either HSV-1 or HSV-2, whereas olomoucine II did, albeit with a higher IC50 value than acyclovir. Acyclovir is a prodrug that becomes activated following phosphorylation by the virus-encoded TK. The HSV-1 TK deletion mutant remained sensitive to olomoucine II yet resistant to acyclovir (Table 1). Although the sensitivities of HSV-1 and HSV-2 to acyclovir differed, their IC50 values for olomoucine II were similar. Indeed, all the DNA viruses were inhibited at similar concentrations of olomoucine II, consistent with the drug targeting cellular (CDKs) rather than the specific virus-encoded functions. Vaccinia virus was inhibited equally with olomoucine II and iodo-deoxyuridine, whereas roscovitine exhibited no overt effect. Replication of VV had been previously thought not to require CDK functions, indeed it has been proposed that CDK-I may not be effective against such viruses [18]. To our knowledge, this is the first demonstration of a poxvirus being sensitive to a CDK-I. Both olomoucine II and roscovitine inhibited Ad4 replication and, interestingly, their IC50 values were substantially lower than for cidofovir. Likewise, HCMV was equally sensitive to olomoucine II and roscovitine, albeit at molar doses higher than for cidofovir. Both CDK-Is proved ineffective against both of the RNA viruses (MV and IV) tested. The WST-1 assay was used to establish CC50 values (Table 2). At the reported antiviral IC50 levels (2.4–5.3 μM olomoucine II), no significant cytotoxicity was detected (Figure 2). With further increase in olomoucine II concentration and prolonged exposure to the drug, cytotoxic effects became apparent in the fibroblasts (Figure 2). WST-1 assays measure the activity of mitochondrial dehydrogenases and do not discriminate between cytotoxic and cytostatic effects. This is illustrated in Figure 2, where serum-deprived cells (Figure 2F) display similar activity as cells treated with 16 μM olomoucine II (Figure 2C). We therefore performed more extensive analysis of this effect. With higher doses of olomoucine II (15–50 μM), we detected caspase activation, an increase in annexin V and propidium iodide staining, and registered cell cycle block (Figures 2G, 2H and 2I, and supplementary figures in Additional file 1), which indicated that olomoucine II at these concentrations had a cytotoxic effect on cells. In conclusion, we observed a clear antiviral effect of olomoucine II in the absence of any cytotoxicity (5 μM). The effect of increased doses of olomoucine II (15–50 μM) on cell viability/activity was complex but apparent; thus, limiting the selective index of the drug in fibroblasts in vitro.
Table 1.
Inhibition of virus replication
| Drug | HSV-1 | HSV-1-deltaTK | HSV-2 | VV | Ad4 | HCMV | MV | IV |
|---|---|---|---|---|---|---|---|---|
| Olomoucine II | 5.0 ±0.9 | 5.3 ±0.9 | 4.7 ±1.0 | 3.8 ±1.3 | 2.4 ±1.3 | 3.2 ±1.6 | >20 | >20 |
| Roscovitine | >20 | >20 | >20 | >20 | 3.1 ±1.6 | 4.9 ±2.2 | >20 | >20 |
| Iododeoxyuridine | - | - | - | 3.7 ±1.6 | - | - | - | - |
| Acyclovir | 0.5 ±0.2 | 73.0 ±18 | 2.2 ±0.5 | - | - | - | - | - |
| Cidofovir | - | - | - | - | 16.6 ±2.9 | 0.2 ±0.1 | - | - |
| Ribavirin | - | - | - | - | >40 | - | 12.0 ±1.1 | 10.5 ±1.8 |
| Chloroquine | - | - | - | - | - | - | - | 5.3 ±0.9 |
Values ar 50% inhibitory concentrations (IC50; μM) ±SD established by plaque reduction assays (n=4). Ad4, human adenovirus type-4; HCMV, human cytomegalovirus; HSV-1, herpes simplex virus type-1; HSV-2, herpes simplex virus type-2; HSV-1-deltaTK, HSV-1 strain 17 containing green fluorescent protein in the thymidine (TK) locus; IV, influenza virus; MV, measles virus; VV, vaccinia virus Western Reserve strain encoding influenza PB2 in the TK locus.
Table 2.
50% Cytotoxic concentrations of olomoucine II and roscovitine in HFFF and A549 cellsa
| HFFF cells |
A549 cells |
|||
|---|---|---|---|---|
| Treatment duration, h | Olomoucine II | Roscovitine | Olomoucine II | Roscovitine |
| 24 | >100 | >100 | ND | ND |
| 48 | 73 ±4 | 84 ±5 | >100 | >100 |
| 72 | 16 ±2 | 21 ±2 | ND | ND |
| 96 | 17 ±2 | 22 ±2 | >100 | >100 |
| 120 | ND | ND | >100 | >100 |
| 168 | 16 ±3 | 30 ±4 | ND | ND |
Values are 50% cytoxic concentrations (CC50; μM) ±SD.
n=2. HFFF, human fetal foreskin fibroblast; ND, not
Figure 2.
Cytotoxicity of olomoucine II and roscovitine in human fibroblasts
Confluent human fetal foreskin fibroblast cells were treated for (A) 24 h, (B) 48 h, (C) 72 h, (D) 96 h or (E) 168 h with indicated concentrations of olomoucine II (OCII) and roscovitine (Rosc) or were (F) serum-deprived (SF). The 50% cytotoxic concentration (CC50) values were determined using a WST-1 kit (Roche, Basel, Switzerland). A representative CC50 titration is shown for each time point. Plots show the absorbance at 450 nm (blank subtracted) ±SD. Similar samples were treated for (G) 24 h, (H) 48 h or (I) 72 h with indicated dilutions of OCII and Rosc, were mock-treated or were SF. Apoptosis was determined as caspase-3/7 activity, plotted as relative luminescence units (RLU; blank subtracted) ±SD.
Inhibition of adenovirus type-4 replication by olomoucine II and cidofovir
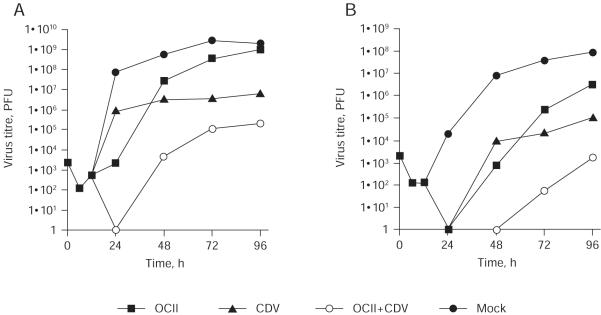
Olomoucine II had a profound effect on Ad4 replication, with a better IC50 value than even cidofovir in a plaque reduction assay (Table 1). Because olomoucine II and cidofovir act by distinct mechanisms, we wished to investigate whether the two antiviral agents would have an additive effect when used in combination. A growth curve of Ad4 was therefore performed in the presence of 10 μM olomoucine II, 100 μM cidofovir or both. As is conventional for Ad, the assay measured total virus yield from the cultures. At 24 h post-infection, both cidofovir and olomoucine II inhibited the production of infectious virus, reducing virus yields by 2 and 5 log (log10 PFU), respectively. No infectious virus was detected when both antivirals were used in combination. At 48 h post-infection, the relative efficacy of the antivirals was reversed: olomoucine II and cidofovir reduced virus yield by 1 and 2 log, respectively, whereas the combination of both produced a 5 log reduction. At this and later time points, the effect of the two drugs in combination was always additive; however, it was clear at later time points that the effect of olomoucine II diminished until at 96 h post-infection. Olomoucine II showed no overall effect on virus yield, whereas cidofovir inhibited the yield by 3 log. The combined effect of both drugs at 96 h post-infection showed a strong cumulative effect of 4 log. The titres of cell-associated (Figure 3A) and extracellular virus (Figure 3B) were also analysed independently. The treatment of infected cells with olomoucine II considerably delayed the appearance of extracellular virus, which in turn would delay the onset of each round of replication and thus the appearance of plaques. The effect of adding cidofovir and olomoucine II in combination on the yield of extracellular virus was substantially greater than either agent in isolation (Figure 3B). This combination of the rapid effect of olomoucine II with the delayed but sustained effect of cidofovir greatly enhanced their capacity to inhibit Ad4 replication. No infectious virus could be detected in the culture medium until 72 h post-infection. At 96 h post-infection, the virus yield was still lower than the initial virus input (0 h), approximately 5 log decrease relative to no drug treatment.
Figure 3.
The inhibition of human adenovirus type-4 replication
Multiple step growth curve (multiplicity of infection =0.01) of human adenovirus type-4 in the presence of 10 μM olomoucine II (OCII), 100 μM cidofovir (CDV) or both (OCII+CDV). Time point 0 h represents the virus inoculum. (A) Total virus yield from the culture (cell-associated plus extracellular). (B) Extracellular virus. PFU, plaque-forming units.
Discussion
We showed that olomoucine II is a potent inhibitor of virus replication and, when compared directly, has increased efficacy over roscovitine. Olomoucine II has a higher affinity for CDK9 than roscovitine, although CDK2 and CDK7 are affected approximately to the same extent. By contrast, roscovitine is a stronger inhibitor of CDK1, CDK4 and ERK2 [12]. Transcription from DNA virus genomes is recognized to be more sensitive to the level of CDK9 activity than endogenous cellular transcription [36,37]. CDK7 and CDK9 are general transcription factor subunits responsible for the transition from abortive to productive elongation, mediated through phosphorylation of the large subunit of RNA polymerase II. A study using step-by-step in vitro transcription in mammalian nuclear extracts has indicated that serine 5 is phosphorylated first in the initiation complex (likely by CDK7) and serine 2 is phosphorylated by CDK9 upon entry into elongation [38]. Both of the CDK-Is tested have been associated with the inhibition of CDK1/cyclin B, CDK2/cyclin E, CDK2/cyclin A and, to a lesser extent, CDK4/cyclin D [12], which are all involved directly not only in the regulation of the cell cycle, but also with the inhibition of the non-cycled kinases CDK7/cyclin H and CDK9/cyclin T [39,40].
Although antimicrobial agents have historically been targeted against specific microbial-encoded gene functions, most clinical drugs act on host functions. An obvious limitation of targeting a viral gene function is that resistance can emerge by virus mutation and can be rapidly selected for. Although a cellular target cannot readily develop resistance, a more specific concern with regards to developing olomoucine II as an antiviral agent is that most CDKs are essential in culture, and that cultured cells become irreversibly arrested when cell cycle progression is inhibited for prolonged periods. However, it has been shown that CDKs are functionally redundant in vivo [41] and that the application of CDK-Is in cancer trial studies has not been associated with toxicity [42]. The capacity of CDK-Is to selectively target expression from extrachromosomal genetic elements over endogenous functions encourages their therapeutic application as antiviral agents.
Olomoucine II efficiently inhibited replication of a range of DNA viruses (HSV-1, HSV-2, VV, Ad4 and HCMV), but exerted no obvious effect on the two RNA viruses (MV and IV) tested. To our knowledge, this is the first report of a CDK-I suppressing the replication of a poxvirus. As VV replicates in the cytoplasm, this result was unexpected; however, VV is believed both to require nuclear functions and, more specifically, host cell RNA polymerase II functions for efficient late-phase gene expression [43]. Furthermore, more recent studies imply that VV mediates the regulation of CDKs and promotes cell cycle progression [44]. Although the Adenoviridae family are primarily a common cause of mild, self-limiting upper respiratory tract infections, they can cause severe respiratory distress, keratoconjunctivitis and life-threatening systemic infections in immunocompromised individuals [45]. A prophylactic live Ad4 oral vaccine has been administered by the US military for over 40 years to prevent outbreaks of acute respiratory disease. The nucleotide analogue cidofovir has exhibited efficacy in studies of patients [46,47] and acts by inhibition of DNA replication and is thus effective during the late phase of infection. Although olomoucine II was effective in isolation, the combination of both olomoucine II and cidofovir proved strongly additive, and capable of a remarkably potent suppression of Ad4 replication and spread in culture. This result is consistent with the reported benefits of combining roscovitine and acyclovir to inhibit HSV-1 replication [18].
From the differential effects of roscovitine and flavopiridol on transcription of HSV-1 or cellular genes, Diwan et al. [14] showed that kinases that were inhibited by flavopiridol were required for transcription in general, whereas the kinases that were efficiently inhibited by roscovitine were required for pre-initiation or initiation of transcription and only from viral genomes. The CDK oligo-specific roscovitine is highly selective in that among 68 proteins tested to date, it inhibited only CDK1, CDK2, CDK5 and CDK7 with high potency and DYRK1a, ERK1 and ERK2 with lower potency [1]. Specific roscovitine-sensitive kinases required for transcription from the viral genome were not identified, although CDK9 was proposed to be responsible for flavopiridol’s effect on broad cellular and viral transcription [14]. It is now thought that because most of CDKs are redundant in their function, inhibition of any single one would be unlikely to have any major functional effect. This is reflected in a lack of enthusiasm towards mono-specific CDK-I, whereas the oligo-specific CDK-Is are being looked at most favourably because of their reasonably well defined range of targets [1]. As the human genome encodes 518 potential protein kinases [4] and numerous other ATP-binding proteins, the anticipated combination of kinases specifically inhibited by roscovitine and olomoucine II may prove too complex to establish.
Regardless of the specifics of a mechanism, viral genome-specific inhibition of transcription is clearly an interesting property for an antiviral. An antiviral with such specific activity would likely be effective against a wide variety of viruses and consequently could be promptly used against any new viral strain that becomes resistant to conventional therapy or against completely novel viral pathogens. As the primary target for olomoucine II is likely to be a cellular function, generation of resistance could be problematical for the virus. Indeed, it is possible that there may be no mechanism available for the virus to generate resistance by mutation of a single gene. Indeed, no CDK-I-resistant virus strain has been reported yet, despite extensive efforts to generate one [17,19]. The CDK-Is investigated here have both anti-tumour and antiviral activities. This could be beneficial for patients with nosocomial immunosupression, caused by oncotherapy, who suffer from exacerbated virus infections.
Supplementary Material
Acknowledgements
We are grateful to A Davison and R Everett for providing HSV-1 and HSV-2, to G Smith for providing VV and to J Schneider-Schaulies for providing MV. We are thankful to A Bulek and J Hobot for providing technical support. This work was supported by grants from MZ0MOU2005, MSMT LC06035, MSM 6198959216, GACR 301/08/1649 and the Wellcome Trust and Medical Research Council (UK).
Footnotes
Disclosure statement
The authors declare no competing interests.
Additional file
Additional file 1: An additional file containing a detailed study of olomoucine II cytotoxicity can be found at http://www.intmedpress.com/uploads/documents/AVCC-09-OA-0003_Holcakova_Add_file.pdf
References
- 1.Schang LM, St Vincent MR, Lacasse JJ. Five years of progress on cyclin-dependent kinases and other cellular proteins as potential targets for antiviral drugs. Antivir Chem Chemother. 2006;17:293–320. doi: 10.1177/095632020601700601. [DOI] [PubMed] [Google Scholar]
- 2.Meyerson M, Enders GH, Wu CL, et al. A family of human cdc2-related protein kinases. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray AW, Marks D. Can sequencing shed light on cell cycling? Nature. 2001;409:844–846. doi: 10.1038/35057033. [DOI] [PubMed] [Google Scholar]
- 4.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 5.Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol Sci. 2002;23:417–425. doi: 10.1016/s0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- 6.Arris CE, Boyle FT, Calvert AH, et al. Identification of novel purine and pyrimidine cyclin-dependent kinase inhibitors with distinct molecular interactions and tumor cell growth inhibition profiles. J Med Chem. 2000;43:2797–2804. doi: 10.1021/jm990628o. [DOI] [PubMed] [Google Scholar]
- 7.De Azevedo WF, Leclerc S, Meijer L, et al. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur J Biochem. 1997;243:518–526. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 8.Vesely J, Havlicek L, Strnad M, et al. Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem. 1994;224:771–786. doi: 10.1111/j.1432-1033.1994.00771.x. [DOI] [PubMed] [Google Scholar]
- 9.Losiewicz MD, Carlson BA, Kaur G, Sausville EA, Worland PJ. Potent inhibition of CDC2 kinase activity by the flavonoid L86-8275. Biochem Biophys Res Commun. 1994;201:589–595. doi: 10.1006/bbrc.1994.1742. [DOI] [PubMed] [Google Scholar]
- 10.Meijer L, Borgne A, Mulner O, et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 11.Kryštof V, Lenobel R, Havlicek L, Kuzma M, Strnad M. Synthesis and biological activity of olomoucine II. Bioorg Med Chem Lett. 2002;12:3283–3286. doi: 10.1016/s0960-894x(02)00693-5. [DOI] [PubMed] [Google Scholar]
- 12.Kryštof V, McNae IW, Walkinshaw MD, et al. Antiproliferative activity of olomoucine II, a novel 2,6,9-trisubstituted purine cyclin-dependent kinase inhibitor. Cell Mol Life Sci. 2005;62:1763–1771. doi: 10.1007/s00018-005-5185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ljungman M, Paulsen MT. The cyclin-dependent kinase inhibitor roscovitine inhibits RNA synthesis and triggers nuclear accumulation of p53 that is unmodified at Ser15 and Lys382. Mol Pharmacol. 2001;60:785–789. [PubMed] [Google Scholar]
- 14.Diwan P, Lacasse JJ, Schang LM. Roscovitine inhibits activation of promoters in herpes simplex virus type 1 genomes independently of promoter-specific factors. J Virol. 2004;78:9352–9365. doi: 10.1128/JVI.78.17.9352-9365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bresnahan WA, Boldogh I, Chi P, Thompson EA, Albrecht T. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology. 1997;231:239–247. doi: 10.1006/viro.1997.8489. [DOI] [PubMed] [Google Scholar]
- 16.Mancebo HS, Lee G, Flygare J, et al. P-TEFb kinase is required for HIV Tat transcriptional activation in vivo and in vitro. Genes Dev. 1997;11:2633–2644. doi: 10.1101/gad.11.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schang LM, Phillips J, Schaffer PA. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schang LM, Bantly A, Knockaert M, et al. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild-type and drug-resistant strains of herpes simplex virus and human immunodeficiency virus type 1 by targeting cellular, not viral, proteins. J Virol. 2002;76:7874–7882. doi: 10.1128/JVI.76.15.7874-7882.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, de la Fuente C, Deng L, et al. Inhibition of human immunodeficiency virus type 1 transcription by chemical cyclin-dependent kinase inhibitors. J Virol. 2001;75:7266–7279. doi: 10.1128/JVI.75.16.7266-7279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Deng L, Wu K, et al. Inhibition of HTLV-1 transcription by cyclin dependent kinase inhibitors. Mol Cell Biochem. 2002;237:137–153. doi: 10.1023/a:1016555821581. [DOI] [PubMed] [Google Scholar]
- 21.Kudoh A, Daikoku T, Sugaya Y, et al. Inhibition of S-phase cyclin-dependent kinase activity blocks expression of Epstein–Barr virus immediate-early and early genes, preventing viral lytic replication. J Virol. 2004;78:104–115. doi: 10.1128/JVI.78.1.104-115.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davido DJ, Leib DA, Schaffer PA. The cyclin-dependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J Virol. 2002;76:1077–1088. doi: 10.1128/JVI.76.3.1077-1088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapasi AJ, Spector DH. Inhibition of the cyclin-dependent kinases at the beginning of human cytomegalovirus infection specifically alters the levels and localization of the RNA polymerase II carboxyl-terminal domain kinases cdk9 and cdk7 at the viral transcriptosome. J Virol. 2008;82:394–407. doi: 10.1128/JVI.01681-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao SH, Fujinaga K, Marion JE, et al. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J Biol Chem. 2000;275:28345–28348. doi: 10.1074/jbc.C000446200. [DOI] [PubMed] [Google Scholar]
- 25.Schang LM, Rosenberg A, Schaffer PA. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J Virol. 1999;73:2161–2172. doi: 10.1128/jvi.73.3.2161-2172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senderowicz AM. Flavopiridol: the first cyclin-dependent kinase inhibitor in human clinical trials. Invest New Drugs. 1999;17:313–320. doi: 10.1023/a:1006353008903. [DOI] [PubMed] [Google Scholar]
- 27.Nicholl MJ, Preston CM. Inhibition of herpes simplex virus type 1 immediate-early gene expression by alpha interferon is not VP16 specific. J Virol. 1996;70:6336–6339. doi: 10.1128/jvi.70.9.6336-6339.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sangfelt O, Erickson S, Einhorn S, Grander D. Induction of Cip/Kip and Ink4 cyclin dependent kinase inhibitors by interferon-α in hematopoietic cell lines. Oncogene. 1997;14:415–423. doi: 10.1038/sj.onc.1200832. [DOI] [PubMed] [Google Scholar]
- 29.Satomoto K, Haisa M, Naomoto Y, Tanaka N, Orita K. Cyclin A and Cdk2 kinase activity are suppressed by combined treatment with tumor necrosis factor-α and interferon-α. Biochem Biophys Res Commun. 1995;213:1115–1121. doi: 10.1006/bbrc.1995.2242. [DOI] [PubMed] [Google Scholar]
- 30.Mandal M, Bandyopadhyay D, Goepfert TM, Kumar R. Interferon-induces expression of cyclin-dependent kinase-inhibitors p21WAF1 and p27Kip1 that prevent activation of cyclin-dependent kinase by CDK-activating kinase (CAK) Oncogene. 1998;16:217–225. doi: 10.1038/sj.onc.1201529. [DOI] [PubMed] [Google Scholar]
- 31.Havlicek L, Hanus J, Vesely J, et al. Cytokinin-derived cyclin-dependent kinase inhibitors: synthesis and cdc2 inhibitory activity of olomoucine and related compounds. J Med Chem. 1997;40:408–412. doi: 10.1021/jm960666x. [DOI] [PubMed] [Google Scholar]
- 32.Gotch F, McMichael A, Smith G, Moss B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J Exp Med. 1987;165:408–416. doi: 10.1084/jem.165.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs SC, Davison AJ, Carr S, et al. Characterization and manipulation of the human adenovirus 4 genome. J Gen Virol. 2004;85:3361–3366. doi: 10.1099/vir.0.80386-0. [DOI] [PubMed] [Google Scholar]
- 34.McSharry BP, Tomasec P, Neale ML, Wilkinson GW. The most abundantly transcribed human cytomegalovirus gene (beta 2.7) is non-essential for growth in vitro. J Gen Virol. 2003;84:2511–2516. doi: 10.1099/vir.0.19298-0. [DOI] [PubMed] [Google Scholar]
- 35.Matrosovich M, Matrosovich T, Garten W, Klenk HD. New low-viscosity overlay medium for viral plaque assays. Virol J. 2006;3:63. doi: 10.1186/1743-422X-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen VT, Kiss T, Michels AA, Bensaude O. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature. 2001;414:322–325. doi: 10.1038/35104581. [DOI] [PubMed] [Google Scholar]
- 37.Simone C, Bagella L, Bellan C, Giordano A. Physical interaction between pRb and cdk9/cyclinT2 complex. Oncogene. 2002;21:4158–4165. doi: 10.1038/sj.onc.1205511. [DOI] [PubMed] [Google Scholar]
- 38.Kim YK, Bourgeois CF, Isel C, Churcher MJ, Karn J. Phosphorylation of the RNA polymerase II carboxyl-terminal domain by CDK9 is directly responsible for human immunodeficiency virus type 1 Tat-activated transcriptional elongation. Mol Cell Biol. 2002;22:4622–4637. doi: 10.1128/MCB.22.13.4622-4637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer PM, Endicott J, Meijer L. Cyclin-dependent kinase inhibitors. Prog Cell Cycle Res. 2003;5:235–248. [PubMed] [Google Scholar]
- 40.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 41.Santamaria D, Ortega S. Cyclins and CDKS in development and cancer: lessons from genetically modified mice. Front Biosci. 2006;11:1164–1188. doi: 10.2741/1871. [DOI] [PubMed] [Google Scholar]
- 42.Buolamwini JK. Cell cycle molecular targets in novel anticancer drug discovery. Curr Pharm Des. 2000;6:379–392. doi: 10.2174/1381612003400948. [DOI] [PubMed] [Google Scholar]
- 43.Silver M, McFadden G, Wilton S, Dales S. Biogenesis of poxviruses: role for the DNA-dependent RNA polymerase II of the host during expression of late functions. Proc Natl Acad Sci U S A. 1979;76:4122–4125. doi: 10.1073/pnas.76.8.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo NK, Pyo CW, Kim Y, Ahn BY, Choi SY. Vaccinia virus-mediated cell cycle alteration involves inactivation of tumour suppressors associated with Brf1 and TBP. Cell Microbiol. 2008;10:583–592. doi: 10.1111/j.1462-5822.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 45.Walls T, Shankar AG, Shingadia D. Adenovirus: an increasingly important pathogen in paediatric bone marrow transplant patients. Lancet Infect Dis. 2003;3:79–86. doi: 10.1016/s1473-3099(03)00515-2. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman JA, Shah AJ, Ross LA, Kapoor N. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:388–394. doi: 10.1053/bbmt.2001.v7.pm11529489. [DOI] [PubMed] [Google Scholar]
- 47.Ljungman P, Ribaud P, Eyrich M, et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2003;31:481–486. doi: 10.1038/sj.bmt.1703798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.