Abstract
The central nervous system (CNS) consists of trillions of interconnected neurons. The specialised regions of intercellular contact between neurons where information, usually in chemical form, is transmitted are called synapses. The last decade has seen an unprecedented advance in our understanding of the molecular nature, formation and maintenance of synapses. A major question that remains is how synaptic identity is established to ensure the coordinated recruitment of the correct synaptic components on both sides of the synapse so that the neurotransmitter accumulating on the presynaptic side is matched with its cognate receptor on the postsynaptic membrane. Until recently, Fibroblast Growth Factors (FGFs) have been thought of as general regulators of synaptic aptitude through their ability to increase the expression of synaptic proteins or promote neurite branching. A recent study shows that the decision to form an excitatory vs. inhibitory synapse may to a large extent be determined by the identity of the FGF ligand present at the postsynaptic membrane. This observation establishes FGFs as key target-derived cues that are involved in determining synaptic identity.
Introduction
It has been estimated that approximately 1014-1015 synapses are formed between the 1011 neurons in the human brain. As such, human intelligence, and in fact, any kind of complex behaviour in a multicellular organism, can be though of as an emergent property of the complex interconnectedness of neurons. Neurological disorders such as schizophrenia, autism spectrum disorders, Tourette's syndrome and epilepsy may have a basis in defective connectivity (reviewed by Mohler, 2006; Rubenstein and Merzenich, 2003; Singer and Minzer, 2003; Wassef et al., 2003). Thus, elucidating the molecular basis of synapse formation, maintenance and activity are essential to understand the basis of behaviour and neurological disorders.
Several factors involved in synaptic organisation have been identified by testing candidate proteins for their ability to induce the formation of varicosities and the clustering of synaptic vesicles in cultured neurons. Recently, unbiased approaches such as biochemical purification methods have been employed to identify organising molecules. These experimental strategies together have identified a range of molecules which include cell-cell adhesion molecules such as neuroligins and SynCAM; lectins; signalling molecules like EphB/ephrin-B; basement membrane associated molecules like laminin and secreted factors of the Wnt, TGFβ and FGF families (reviewed in Dalva et al., 2007; Fox and Umemori, 2006; Salinas, 2005; Waites et al., 2005). In addition, factors produced by glia such as thrombospondin and cholesterol, have been found to induce synaptic differentiation in the CNS (reviewed by Fox and Umemori, 2006).
FGF ligands are best known for their diverse roles in embryonic development. However, it is becoming clear that the functional variability in the FGF signalling system has been employed to coordinate reciprocal cell-cell signalling in many other contexts. A recent paper by Terauchi et al. now shows that this functional variability is also used to provide specific signals from postsynaptic to presynaptic specialisations during synapse formation (Terauchi et al., 2010).
The structure and molecular composition of a synapse
The ultrastructure of a variety of synapses has been investigated. Excitatory synapses are characterised by an electron-dense region at the presynaptic terminal, called the active zone (AZ), underneath which clusters of neurotransmitter-filled synaptic vesicles accumulate. The postsynaptic terminal also contains an electron-dense region, called the postsynaptic density (PSD). The PSD is formed by clusters of receptor proteins and cell adhesion molecules and is directly apposed to the AZ (Rollenhagen and Lubke, 2006).
Depolarisation of the axonal membrane opens voltage-gated calcium channels (VGCCs) and Ca2+ influx initiates the fusion of neurotransmitter-filled vesicles with the AZ, resulting in the release of neurotransmitter into the synaptic cleft. A postsynaptic response is elicited upon neurotransmitter binding to receptors in the postsynaptic terminal in the PSD, which may depolarise (excitatory) or hyperpolarise (inhibitory) the postsynaptic membrane. Neurotransmitter receptors in the PSD are tethered to several scaffold proteins, which can modulate the functional response (as reviewed by Kim and Sheng, 2004).
There is great variation in pre- and postsynaptic specialisations in different organisms and neuronal types, representing significant functional diversity. Two main functional synaptic types form between neurons in the CNS: excitatory and inhibitory synapses.
Synapses of the CNS
The majority of synapses in the nervous system occur between presynaptic axons and postsynaptic dendrites (axo-dendritic) using glutamate as the excitatory neurotransmitter. Synaptic vesicles containing glutamine (marked by vesicular glutamate transporter, VGLUT) localise underneath the AZ. Upon depolarisation of the presynaptic membrane, glutamate-filled vesicles fuse with the membrane at AZs and release neurotransmitter into the synaptic cleft. Glutamate then diffuses across the cleft and acts upon glutamate receptor channels in the postsynaptic terminal. The main glutamate receptors are AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) receptors (AMPARs), and NMDA (N-methyl-D-aspartic acid) receptors (NMDARs). Ligand binding to these receptors allows for the influx of Na+ ions (and Ca2+ ions for NMDARs) leading to depolarisation of the postsynaptic membrane and propagation of an electrical current.
GABAergic synapses make up the majority of inhibitory transmission in the CNS. These synapses can be identified by the presence of the vesicular GABA transporter (VGAT), which is responsible for the selective transport of GABA into synaptic vesicles under the AZ. GABA (γ-aminobutyric acid) is released from synaptic vesicles into the synaptic cleft upon depolarisation of the presynaptic membrane. The neurotransmitter then diffuses across the synaptic cleft and binds GABA receptors on the postsynaptic membrane to elicit a response.
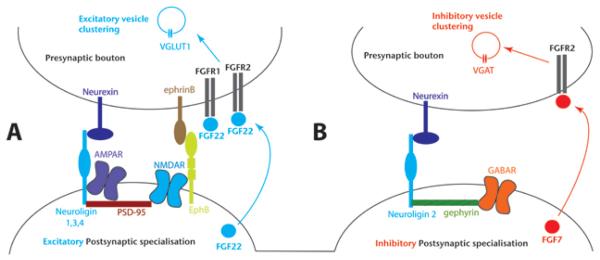
The structure of the pre- and postsynaptic specialisations of excitatory and inhibitory synapses has been uncovered by electron microscopy. Excitatory synapses contain presynaptic AZs and prominent PSD thickenings, and as such, they are termed asymmetric synapses (Figure 1). By contrast, Inhibitory GABAergic synapses lack a prominent PSD thickening, and hence inhibitory synapses are termed symmetric. In addition to the obvious differences in presynaptic vesicles in excitatory vs. inhibitory synapses, many postsynaptic proteins are present in one, but not the other type. Gephyrin, present in inhibitory synapses, may be the equivalent of PSD-95 in excitatory synapses as it is responsible for clustering receptors in the postsynaptic membrane (Essrich et al., 1998; Levi et al., 2004; Yu et al., 2007; Figure 1).
Figure 1. Diagrammatic representation of some of the factors implicated in the formation of excitatory and inhibitory synapses in the CNS.
Molecules expressed on, or secreted from pre- or postsynaptic membranes are indicated. Putative or confirmed interactions are indicated by contacts between different molecules. For example, interactions between Neuroligin, AMPAR, PSD-95, NMDAR and EphB have been reported which may result in the formation of a multimolecular complex consisting of clusters of all or some of these molecules on the postsynaptic membrane during postsynaptic specialisation. A) Typical composition of excitatory synaptic specialisations. Postsynaptic cell adhesion (neuroligin 1,3,4) and signalling molecules (EphB) bound to presynaptic partners (Neurexin, ephrinB), interact with scaffold proteins (PSD-95) to cluster neurotransmitters (AMPAR, NMDAR) in complexes with other molecules (not shown). FGF22 is secreted from the postsynaptic terminal and is proposed to signal through FGFR1b and FGFR2b on the presynaptic membrane to promote VGLUT1 vesicle clustering. B) Molecular components of an inhibitory synapse. Postsynaptic adhesion molecules (Neuroligin 2) interact with presynaptic partners (Neurexin) to help cluster GABAR in complexes with scaffold molecules (gephyrin). FGF7 is secreted and signals through FGFR2b in the presynaptic terminal to promote VGAT vesicle clustering.
Central synapses differ from peripheral synapses like the neuromuscular junction (NMJ), in that the synaptic cleft of excitatory and inhibitory synapses lacks a basal lamina. Molecules such as β-laminin and Agrin that are associated with the basal lamina have key roles in NMJ formation and function (reviewed by Sanes and Lichtman, 1999). Instead, cell adhesion molecules such as N-cadherin and neuroligin/neurexin interactions appears to function in the formation and/or stability of central synapses (Togashi et al., 2002; Varoqueaux et al., 2006; Figure 1).
As in the NMJ, glial cell processes also contribute significantly to the cellular composition of central synapses. These processes appear to isolate synapses from the environment and have been suggested to regulate neurotransmitter concentration in the synaptic cleft by acting as physical barriers to diffusion and/or active removal of neurotransmitter by specific transporters (reviewed by Oliet et al., 2004; Rollenhagen and Lubke, 2006). Glial cells also produce factors that can influence synaptogenesis (Allen and Barres, 2005; Christopherson et al., 2005; Feng et al., 2005; Mauch et al., 2001; Ullian et al., 2004; Xu et al., 2010).
Fibroblast growth factors as presynaptic organisers in the CNS
Fibroblast growth factors (FGFs) are a large family of proteins with diverse roles in embryonic development, homeostasis and metabolism. The FGF family consists of twenty-two members in mammals, with 15 canonical heparin-binding, secreted polypeptides and 7 non-canonical FGFs: 3 being secreted ligands with low affinity for heparan sulfate (FGF15/18, FGF21, 23) and 4 being intracellular FGFs (FGF11-14) (reviewed by Itoh and Ornitz, 2008). Several FGFs have roles in regulating neural cell proliferation, migration and differentiation in the developing CNS (reviewed by Mason, 2007; Reuss and von Bohlen und Halbach, 2003). FGF1-10 and FGF15-23 elicit their effect by binding to FGF receptors (FGFRs), and activating their receptor tyrosine kinase activity. So far, four genes encoding FGF receptors with signalling activity have been identified (Fgfr1 - 4). The Fgfr1-3 genes can generate two different receptor isoforms by the selection of one of two alternative exons (denoted ‘b’ or ‘c’) that encode the 3rd extracellular Ig loop. The different splice isoforms have entirely different spectra of ligand specificities, for example FGF7, 10 and 22 preferentially activate the FGFR2b isoform with negligible affinity for the FGFR2c isoform (Zhang et al., 2006). FGFRs consist of three domains: an extracellular ligand binding domain; a transmembrane domain; and an intracellular tyrosine kinase domain. The binding of an FGF ligand-heparan sulphate proteoglycan (HSPG) complex to FGFRs (the exact stoichiometry of this complex is still under debate), results in receptor dimerisation and tyrosine phosphorylation on its intracellular domain (Mohammadi et al., 2005). Receptor phosphorylation creates docking sites for the recruitment of intracellular adaptor proteins to the receptor, which themselves recruit a host of effectors that mediate the activation of downstream ras/MAPK, PI3K/AKT and PLCγ/PKC signalling pathways (Lemmon and Schlessinger, 2010). The nature and strength of pathway activation is a function of many different variables. These include the expression levels of ligands and cognate receptors, HSPGs modifications (Ford-Perriss et al., 2002), as well as positive and negative feedback mechanisms (Lemmon and Schlessinger, 2010; Mason et al., 2006).
The observation that some FGF ligands were expressed during late embryogenesis and early postnatal life, when synaptogenesis is at its peak, led to the suggestion that FGFs may play a role during this process (Caday et al., 1990). It was shown several years later that exogenously provided FGF2 could increase the number of excitatory synapses in cultured hippocampal neurons (Li et al., 2002). This observation was mainly attributed to the ability of FGF to increase neurite branching and the expression of synaptic proteins such as GluR1. Whether FGF2 also has the ability to promote the formation of inhibitory synapses was not investigated in this study.
Our understanding of the mechanism whereby an FGF molecule could promote synaptogenesis made a significant leap in 2004, when Hisashi Umemori and co-workers identified FGF22 as the active component present in biochemically purified fractions from mouse brain that had activity in an in vitro assay for synaptic vesicle clustering activity (Umemori et al., 2004). They showed that neutralising FGF22 activity using an FGFR2b-AP soluble decoy inhibited presynaptic differentiation in cerebellar granule cells in vivo, and that the deletion of FGFR2 from the postnatal brain had the same effect. This study was unable to discriminate between the activities of FGF7, FGF10 and FGF22 in vivo as FGFR2b-AP is also capable of neutralising FGF7 and FGF10. However, this study clearly established the FGF7/10/22 family as a novel presynaptic organising molecules. Furthermore, it appeared that this activity of FGF22 was mediated through its canonical receptor, FGFR2b, expressed on the presynaptic axon terminal. However, as FGF7 and FGF10 are also expressed in cerebellar granule cells analysed in this study, the relative contribution of different FGF ligands in this context was not clear. Furthermore, as mossy fibre synapses on cerebellar granule cells studied in these expriments are excitatory (glutamatergic), the effect of FGF7/10/22 on inhibitory synapses was not investigated.
The most recent study by the Umemori group analysed synaptogenesis in the CA3 region of the hippocampus in FGF7- and FGF22-deficient mice (Terauchi et al., 2010). In agreement with their previous studies, they found that presynaptic vesicle clustering was also diminished in CA3 in both these mutants. By contrast, clustering of postsynaptic molecules PSD-95 (excitatory) and gephyrin (inhibitory) was unaffected, confirming a specific effect of FGF7 and FGF22 on presynaptic differentiation. Furthermore, the formation of the active zone on the presynaptic membrane as marked by clustering of bassoon, was unaffected, implying a role for FGF signalling in mechanisms that control vesicle clustering but not AZ formation. Intriguingly, the clustering of vesicles containing excitatory glutamate (VGLUT1) was only affected in FGF22-deficient mice, whereas the clustering of inhibitory, GABA-containing vesicles (VGAT) was only reduced in FGF7-deficient mice. The authors further confirmed that FGF7 was sufficient to induce VGAT clustering and FGF22 sufficient to induce VGLUT1 clustering in culture. Based on these findings, it can be concluded that there is a requirement for FGF7 during the formation of inhibitory GABAergic synapses and a requirement for FGF22 during excitatory glutamatargic synaptogenesis.
Perhaps the most intriguing finding was that tagged FGF7 protein expressed in hippocampal neurons largely co-localised with gephyrin in dendrites whilst tagged FGF22 co-localised with PSD-95 (Figure 1). This was true, even in the same dendrite, suggesting that these proteins specifically localised to distinct subdomains on a dendritic membrane. Thus, the ability of these factors to cluster a particular type of vesicle correlated with the localisation of each factor to the corresponding postsynaptic specialisation. However, when exogenous protein was added to hippocampal cultures, the same functional specificity was observed i.e. FGF7 selectively promoted the formation of VGAT-containing synapses. Thus, the specificity of these factors cannot wholly be explained by their subcellular localisation, unless the mechanisms whereby these factors are recruited to specific presynaptic domains are also accessible to exogenous protein. Investigating the mechanisms controlling selective recruitment of different FGF ligands to different subdomains at the postsynaptic membrane is an important area of future investigation. The authors suggest that the ability of FGF22 to signal through FGFR1b in addition to FGFR2b may help explain this finding. Two obvious mechanisms can be envisaged that might explain the different activities of FGF7 and FGF22: 1) FGFR1b is preferentially recruited to the glutamatergic presynaptic membrane or 2) both receptor isoforms are equally represented at inhibitory and excitatory presynaptic specialisations but there are qualitative or quantitative differences in the signalling evoked by FGF7 and FGF22 due to the latter being able to activate both receptor types. By showing that FGFR2 deletion affected GABAergic synapse formation more drastically than glutamatergic synapses, the authors has some evidence in support of the second possibility. Indeed, Zucchini et al. reported a year and a half ago that the overexpression of FGF2, which can activate a number of FGF receptors, including FGFR1b and FGFR2b, was associated with increased density of glutamatergic synaptic vesicles in the hippocampus and an associated increase susceptibility to kainite (a glutamate mimic)-induced seizures (Zucchini et al., 2008).
Future directions
As with most seminal studies of this kind, the observations reported by Terauchi et al. will stimulate the formulation of numerous new hypotheses and research questions. What mechanism allows for the selective targeting of specific FGF ligands to particular domains on the postsynaptic cell? Once recruited, do different FGFs have different intrinsic activities or is the presence of any FGF sufficient to promote synapse formation if presented at the correct time and place? Developing means to mis-direct FGF7 to excitatory and FGF22 to inhibitory presynaptic regions may help address this question. If, as the experiments by Terauchi et al. suggest, the difference in intensity or quality of signalling evoked by different FGF ligands is key to understand functional specificity, experimental manipulation of in the intracellular signalling pathways should affect the type of synapse that forms. It is likely that several surprising findings are in store. For example, Umemori and Sanes showed that blocking MEK activity, a key mediator of the ras/ERK signalling pathway downstream of FGF receptors, failed to prevent the presynaptic organising activity of FGF22 on motoneurons in vitro, whereas it did prevent the action of another presynaptic organising molecule, SIRP-α (Umemori and Sanes, 2008). Is it possible that FGF22 preferentially signals through a different intracellular pathway to mediate its effects on the presynaptic cell?
At what stage of synapse assembly do FGFs function? Synaptic membranes appear specialised, active zone formation is unaffected and synapses are morphologically visible in FGF mutant hippocampi. The main defects associated with the loss of FGF7 or FGF22 appear to be in the density and number of synaptic vesicles (Terauchi et al., 2010). Studies on the NMJ suggest that FGF functions early to initiate synapse formation. It is clear that the exact sequence of events during synaptic assembly remains to be elucidated. This is a Herculaneum task given the large number of molecules that are already known to be involved. However, the ability to image synapse formation in real time and follow the movement of individually tagged molecules will no doubt be instrumental in addressing these questions (Barrow et al., 2009; Niell et al., 2004).
To what extent are the roles of FGF signalling in synaptogenesis evolutionary conserved? It is intriguing to note that the Drosophila orthologue of the neural cell adhesion molecule (NCAM), called fasciclin II, has a role in synaptic maturation (Kristiansen and Hortsch, 2008). Given the well-established interaction between FGF receptors and NCAM proteins (Kiselyov, 2008), it is tempting to speculate that this conserved interaction may be important for similar functions in mammals.
What is the relevance of these findings to understanding the origin of neurological disorders? The ability to study synaptogenesis in experimental models such as the mouse, and combine it with increasingly more sophisticated behavioural tests will no doubt result in an improved understanding of how defects in synaptogenesis affect behaviour. Fibroblast growth factor signalling has been linked to a number of neurological diseases, including schizophrenia (Terwisscha van Scheltinga et al., 2009), and it will be interesting to see how these conditions are linked to imbalances in synapse formation. Studies in mouse models have shown that the number of and balance between excitatory and inhibitory synapses has to be tightly regulated in the brain to ensure normal function. Terauchi et al. showed that this type of imbalance could affect the sensitivity of an animal to epileptic attacks. Using an established protocol for inducing epilepsy in rodents, which involves the injection of a GABA receptor antagonist, FGF7-deficient mice showed an increased susceptibility to epileptic seizures. FGF22-deficient animals on the other hand showed some resistance to seizures, compared to wild-type controls (Terauchi et al., 2010). These results of course correlated with a reduction in GABAergic synapses in FGF7-deficient animals and vice versa for FGF22-deficient animals.
Concluding remarks
It has become clear from the study of synaptic formation and maturation that a full understanding of synaptogenesis will require a detailed molecular picture of how the large multimolecular complexes are assembled at the pre- and postsynaptic membrane. Despite a huge recent explosion in our knowledge, of which this commentary merely scratches the surface, much remain to be learnt. An in-depth understanding of these mechanisms will not only represent a major advance in cell biology, but may eventually result in novel treatments of many debilitating and distressing neurological conditions.
Acknowledgements
We wish to thank Martin Meyer, Jeff Huang and Mohi Ahmed for comments on the manuscript. Research in our group is supported by the Wellcome Trust (WT080470) and Medical Research Council (G0601104). Kieran Jones is a recipient of a BBSRC Doctoral Training Award.
References
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–8. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Barrow SL, Constable JR, Clark E, El-Sabeawy F, McAllister AK, Washbourne P. Neuroligin1: a cell adhesion molecule that recruits PSD-95 and NMDA receptors by distinct mechanisms during synaptogenesis. Neural Dev. 2009;4:17. doi: 10.1186/1749-8104-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caday CG, Klagsbrun M, Fanning PJ, Mirzabegian A, Finklestein SP. Fibroblast growth factor (FGF) levels in the developing rat brain. Brain Res Dev Brain Res. 1990;52:241–6. doi: 10.1016/0165-3806(90)90240-y. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–33. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–20. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–71. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Feng Z, Koirala S, Ko CP. Synapse-glia interactions at the vertebrate neuromuscular junction. Neuroscientist. 2005;11:503–13. doi: 10.1177/1073858405277409. [DOI] [PubMed] [Google Scholar]
- Ford-Perriss M, Guimond SE, Greferath U, Kita M, Grobe K, Habuchi H, Kimata K, Esko JD, Murphy M, Turnbull JE. Variant heparan sulfates synthesized in developing mouse brain differentially regulate FGF signaling. Glycobiology. 2002;12:721–7. doi: 10.1093/glycob/cwf072. [DOI] [PubMed] [Google Scholar]
- Fox MA, Umemori H. Seeking long-term relationship: axon and target communicate to organize synaptic differentiation. J Neurochem. 2006;97:1215–31. doi: 10.1111/j.1471-4159.2006.03834.x. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Kiselyov VV. NCAM and the FGF-Receptor. Neurochem Res. 2008 doi: 10.1007/s11064-008-9666-0. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Hortsch M. Fasciclin II: The NCAM Ortholog in Drosophila melanogaster. Neurochem Res. 2008 doi: 10.1007/978-1-4419-1170-4_24. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signalling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Logan SM, Tovar KR, Craig AM. Gephyrin is critical for glycine receptor clustering but not for the formation of functional GABAergic synapses in hippocampal neurons. J Neurosci. 2004;24:207–17. doi: 10.1523/JNEUROSCI.1661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Suzuki S, Suzuki M, Mizukoshi E, Imamura T. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur J Neurosci. 2002;16:1313–24. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8:583–96. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–7. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–37. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABAA receptors in central nervous system disease: anxiety, epilepsy, and insomnia. J Recept Signal Transduct Res. 2006;26:731–40. doi: 10.1080/10799890600920035. [DOI] [PubMed] [Google Scholar]
- Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–60. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA, Theodosis DT. Glial modulation of synaptic transmission: Insights from the supraoptic nucleus of the hypothalamus. Glia. 2004;47:258–67. doi: 10.1002/glia.20032. [DOI] [PubMed] [Google Scholar]
- Reuss B, von Bohlen und Halbach O. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–57. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- Rollenhagen A, Lubke JH. The morphology of excitatory central synapses: from structure to function. Cell Tissue Res. 2006;326:221–37. doi: 10.1007/s00441-006-0288-z. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–67. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC. Signaling at the vertebrate synapse: new roles for embryonic morphogens? J Neurobiol. 2005;64:435–45. doi: 10.1002/neu.20159. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Singer HS, Minzer K. Neurobiology of Tourette's syndrome: concepts of neuroanatomic localization and neurochemical abnormalities. Brain Dev. 2003;25(Suppl 1):S70–84. doi: 10.1016/s0387-7604(03)90012-x. [DOI] [PubMed] [Google Scholar]
- Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–7. doi: 10.1038/nature09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwisscha van Scheltinga AF, Bakker SC, Kahn RS. Fibroblast Growth Factors in Schizophrenia. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–16. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–70. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Umemori H, Sanes JR. Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules. J Biol Chem. 2008;283:34053–61. doi: 10.1074/jbc.M805729200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–54. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–74. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–40. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Xu J, Xiao N, Xia J. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci. 2010;13:22–4. doi: 10.1038/nn.2459. [DOI] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles CP, Li RW, Chen G, de Blas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchini S, Buzzi A, Barbieri M, Rodi D, Paradiso B, Binaschi A, Coffin JD, Marzola A, Cifelli P, Belluzzi O, et al. Fgf-2 overexpression increases excitability and seizure susceptibility but decreases seizure-induced cell loss. J Neurosci. 2008;28:13112–24. doi: 10.1523/JNEUROSCI.1472-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]