Summary
Hypoglycaemia is associated with poor prognosis in many severe childhood illnesses especially in sub-Saharan Africa where the prevalence of malaria, diarrhoea and malnutrition remains high. Uncertainty, however, still persists regarding the significance, definition and management of childhood hypoglycaemia. As a step towards defining optimal, evidence-based diagnostic and management criteria, we (i) reviewed the evidence underlying current recommendations for the management of hypoglycaemia, and (ii) analysed a large set of data on blood glucose levels and associated outcomes of paediatric admissions in a rural hospital over an 11-year period. Current definitions and treatment protocols for hypoglycaemia are based on observational data and expert opinion. Future large pragmatic randomized trials would help define optimal treatment thresholds. Emerging evidence suggests that sublingual sugar is a feasible and effective therapy for correction of hypoglycaemia, and should be considered where intravenous glucose is delayed or impossible.
Keywords: hypoglycaemia, glucose, dextrose, newborn, children
Introduction
Hypoglycaemia is common in the newborn and amongst seriously ill children [1]. In those ill enough to require hospital admission in rural Africa, prevalence in those aged <28 days is reported to be 23% and amongst infants and children prevalence is 7.3% [1, 2]. Disease-specific data similarly shows high prevalence (ranging from 6.6% to 30%) in children with severe malaria, anaemia, respiratory tract infections, diarrhoea and malnutrition [2]. In these same populations, the presence of hypoglycaemia is associated with increased mortality and/or serious neurological sequelae [odds ratio (OR) 3.7] [2]. Hypoglycaemia has also been identified as a prognostic indicator in children with severe malaria [OR for death = 3.3; 95% confidence interval (CI) 1.6–6.7; p < 0.001] [3]. Amongst the newborn, as well as evidence of clinical illness, known risk factors for hypoglycaemia include prematurity and/or low weight for gestational age, intrapartum asphyxia and maternal diabetes in pregnancy [4].
Definitions of hypoglycaemia are not completely standardized [5]. On the basis of observational data and expert opinion, the World Health Organization (WHO) currently provides different definitions for the different specific clinical categories indicated in Table 1. In 2005, and based on much of the same evidence as available to WHO, the Government of Kenya guidelines [6] were developed that define hypoglycaemia in sick infants/children to be a blood glucose <2.2 mmol l−1 or 40 mg dl −1 [2]. Other guidelines have definitions of hypoglycaemia as a blood glucose level more than 2SDs below the population mean, but this definition has been found to have limited physiological significance [4]. As hypoglycaemia in severe malnutrition probably deserves individual attention, for the remainder of this article we focus on those without this condition.
Table 1.
WHO definitions for hypoglycaemia
| Clinical category | BGC |
|---|---|
| Newborn/infant/child with ‘signs of illness’ | <2.5 mmol l−1 or 45 mgdl−1 |
| Healthy term/pre-term newborn—‘feeding well’ | <1.1 mmol l−1 or 19.8mg dl−1 |
| Infant/child with severe malnutrition | <3.0 mmol l−1 or 54 mg dl−1 |
WHO and Kenyan guidelines now recommend intravenous (IV) treatment of ‘symptomatic hypoglycemia’ using 10% glucose (5–10 ml kg−1), with continued monitoring and feeding or continued glucose infusion thereafter [7]. However, IV therapy is only feasible where trained personnel and essential supplies are available—raising the question of how most hypoglycaemic children in resource-constrained settings, who present to primary care, might be treated. There also remains uncertainty regarding the need to treat hypoglycaemia identified in an apparently ‘asymptomatic’ individual, particularly in the newborn. This article therefore aimed in general to identify recent evidence that might inform management of hypoglycaemia and specifically to contribute to revisions of Kenyan guidelines if required. In particular, we sought evidence on how best to define and treat hypoglycaemia in newborns and children without malnutrition.
Methodology
Articles were identified through electronic searches of The Cochrane Library and PubMed (January 2005–February 2009) without any language restrictions. PubMed was searched through the clinical queries therapy, broad sensitive filter using the following combination of search terms: hypoglyc* AND (glucose OR dextrose) AND (child* OR neonat* OR infant*). Limits were applied to exclude articles whose subjects of study were animals, and also those articles not published within the past 5 years as our aim was to identify new literature that might result in modification of guidelines published in 2006 [7]. The bibliographies of identified reviews and articles were searched for additional studies. The population of interest in this article was children aged 0–59 months with confirmed hypoglycaemia and managed with any dextrose formulation or any other management modality. Our outcome of interest was complete remission from hypoglycaemia or development of adverse events following dextrose therapy or any management modality. We excluded studies of hypoglycaemia in childhood diabetes or those where hypoglycaemia was as a result of treatment with insulin.
Retrospective analysis of hypoglycaemia dataset
In addition to the literature searches, and in an attempt to examine the consequences of alternative thresholds of blood glucose concentration (BGC) for diagnosis of hypoglycaemia in clinical practice, we undertook a retrospective analysis of a large dataset from Kilifi District Hospital (KDH) in rural Kenya. The dataset consisted of glucose measurements taken routinely from 23 805 children aged 1–59 months without a diagnosis of severe malnutrition admitted to KDH over an 11-year period (1998–2008). The association between BGC and pre-discharge mortality for children with an admission BGC ≤6.0 mmol l−1 was examined using ORs. Specifically, ORs were calculated by specifying (clinically relevant) cut-offs and comparing the odds of death for children with a BGC below the cut-off (‘exposed odds of death’) to the odds of death for all children (even those with a high BGC level) with a BGC above the cut-off level (‘non-exposed odds of death’). Successive ORs and their associated CIs were calculated in a stepwise manner by varying the threshold for ‘hypoglycaemia’ from a BGC level of 1.8–6.0 mmol l−1 in increments of 0.2 mmol l−1, a range spanning currently described recommendations [2, 7, 8]. We also calculated the proportion of admitted children identified as requiring parenteral glucose based on thresholds of <2.2 mmol l−1 and <2.5 mmol l−1. All analyses were conducted using STATA v 10.0 (Stata Corportation, TX, USA).
Results
In total, our literature search identified 72 studies: 67 from PubMed/The Cochrane Library and 5 studies from supplementary searches. Overall, four studies—one overview of a systematic and a narrative review and three randomized controlled trials (RCTs)—fulfilled our inclusion criteria. The study characteristics are summarized in Tables 2 and 3. In the KDH dataset, the mean age of admissions was 22 months with a mean weight of 8.5 kg.
Table 2.
Characteristics of included studies: summary of review articles
| Citation | Sample size | Participants | Interventions | Results |
|---|---|---|---|---|
| Joanna Briggs Institute 2006 |
Hewitt 2005 (one RCT, six non-RCTs) n=875 |
Healthy term (37–42 weeks gestation) neonates in the first 72 h Neonates with symptomatic hypoglycaemia, SGA new- borns,babies of diabetic mothers and LGA newborns were excluded |
All interventions that fell within the scope of a nurse/midwife:
|
Narrative summary:
|
| WHO 1997 Studies on screening for hypoglycaemia Four studies n=898 |
SGA, LGA, pre-term and term infants |
Screening neonates for hypoglycaemia using dextrostix Outcome measure: detection of hypoglycaemia |
|
SGA: small for gestation age; LGA: large for gestation age.
Table 3.
Characteristics of included studies: RCTs
| Citation/design | Setting | Participants | Interventions | Results |
|---|---|---|---|---|
| Graz et al. [12] Randomized open clinical trial |
Mali |
n=26 Children aged 6 months to 15 years— with WHO case definition of severe malaria, seizures, prostrated or in coma, and BGC<60mg dl−1 (<3.3 mmol l−1) |
The SLS group received a teaspoon of sugar (level teaspoon for those <15 kg, a heaped teaspoon for those above that weight, which approximates to 2.5 g and 3.5 g of sugar, respectively) moistened with water placed under the tongue The IV group received 5ml kg−1 10% glucose IV infusion |
|
| Nili et al. [11] Randomized open clinical trial |
Iran |
n=200 Pre-term infants (1500–2500 g) |
Group 1: 10% DW Group 2: 12.5% DW |
|
| Barennes et al. [13] Randomized open clinical trial |
Burkina Faso |
n=69 Moderately hypoglycaemic children (BGC between 0.5 g l−1 or 2.8 mmol l−1 and 0.8 gl−1 or 4.4 mmol l−1) No severe clinical symptoms of hypoglycaemia requiring immediate treatment |
Children were assigned to one of the four methods of administration:
|
|
RR: risk ratio; IVG: intravenous glucose.
Definition of hypoglycaemia
There remains considerable variation in the thresholds used to define hypoglycaemia in the different age groups in the identified studies. In common with current WHO recommendations, these definitions were based mainly on expert opinion. Recent research does not provide any new information to assist in establishing a consensus, evidence-based definition of hypoglycaemia.
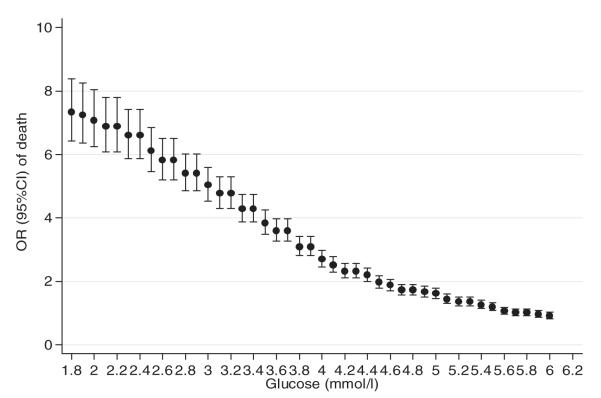
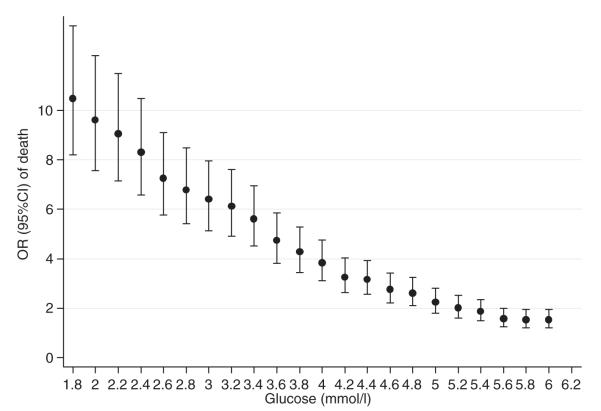
Results of the retrospective analysis of KDH data suggest that the current range of BGC cut-offs (2.0–3.0 mmol l−1) recommended by various consensus approaches are associated with considerably higher odds of death (Fig. 1). These analyses also indicate (i) an OR associating BGC levels <4.0 mmol l−1 with death with even the lower bound of the 95% CI of the OR >2.0 and (ii) absence of a clear inflection in the BGC/OR for death relationship that might be indicative of a clear and specific threshold for biological risk. The trend in odds of death did not change with analyses stratified by malaria diagnosis, sex or age (as shown in, e.g. Fig. 2).
FIG. 1.
OR of pre-discharge mortality at set BGC thresholds and their 95% CIs (ORs were calculated by dividing odds of death when BGC falls below the set-point with odds of death when BGC is above the set point).
FIG. 2.
ORs of pre-discharge mortality at set BGC and their 95% CIs in patients with a malaria diagnosis.
Management of hypoglycaemia
Outcomes following treatment of hypoglycaemia
No RCTs evaluating whether outcomes improve following treatment of hypoglycaemia were identified in neonates, infants or children. Available data from cross-sectional observational studies—summarized in a WHO 1997 report—indicate an association of low blood glucose levels with poor clinical outcomes in neonates [4]. In one study included in the WHO review [9], lower motor and neurodevelopmental scores were observed after long-term follow-up of sick hypoglycaemic neonates exposed to longer periods of low BGC. It is on the basis of these observational data, biological plausibility and the documented associations of hypoglycaemia with death that treatment is recommended.
Management of asymptomatic hypoglycemia in healthy term (37–42 weeks) infants
Is a low blood glucose measurement of any significance in asymptomatic, term infants? We identified one summary [8] of two existing reviews [4, 10] in this area. The overall incidence of hypoglycemia (defined as <1.1 mmol l−1 in term babies) was estimated at between 1 and 5 per 1000 of all live births. Although such biochemical ‘hypoglycaemia’ is therefore not uncommon, the expert authors of the 2006 review concluded that breastfeeding, when initiated early and associated with frequent sucking, provides adequate plasma glucose for the neonate in the first 48 h of life, with no need for supplemental feeds (to avert or treat biochemical hypoglycaemia) in a healthy neonate. Further they noted that the practice of supporting thermoregulation through skin-to-skin contact (‘kangaroo care’) was found to be effective in maintaining body temperature and safe blood glucose levels. Overall, the authors concluded that there is no evidence to show that low blood glucose levels (term babies: <1.1 mmol l−1 and pre-term babies: <2.0 mmol l−1) among healthy breast-fed infants who are feeding well are detrimental to outcome and therefore do not require pharmacological intervention. They further recommended that additional assessment is warranted only if hypoglycaemia persists beyond 48 h and is not resolved by additional feeds (as this could be suggestive of involvement of a metabolic or an endocrine disorder).
Alternative means of administering glucose
Alternative IV regimens
In sick premature infants weighing 1500–2500 g, a RCT [11] (n=200) compared the effect of IV 10% dextrose in water (DW) (Group 1) and IV 12.5% DW (Group 2) on the incidence of hypoglycaemia. Plasma glucose <36 mg dl−1 (2.0 mmol l−1) during the first 2–3 h of life and levels <45 mg dl−1 (2.5 mmol l−1) between 4 h and 24 h of life were defined as hypoglycaemia. When IV fluid therapy was started, the incidence of hypoglycaemia decreased especially in Group 2 (12.5% DW) with a greater risk of two consecutive low plasma glucose readings in the 10% DW group (risk ratio = 2.67; p=0.024). Although on the basis of these data the authors recommended 12.5% DW when initiating IV therapy in sick pre-term infants, this trial did not aim to investigate whether the 12.5% DW regimen improved other outcomes.
Studies comparing IV and other routes of administering glucose
One RCT [12] (n=23) assessed the efficacy of IV 10% glucose and sublingual sugar (SLS) in the treatment of hypoglycaemia defined as a BGC <60 mg dl−1 (<3.3 mmol l−1) in children with severe malaria. In the SLS group, a teaspoon of sugar, moistened with a few drops of water, was gently placed under the tongue every 20 min. The primary outcome measure was treatment response—defined as attainment of a blood glucose level of ≥60 mg dl−1 (3.3 mmol l−1) within 40 min after admission. Secondary outcome measures were early treatment response at 20 min, relapse (early and late) and maximal BGC gain (GCmax) and treatment delay. There was no significant difference between groups with regard to treatment response at 40 min (71% and 67% in the SLS and IV groups, respectively). Among the responders, relapses occurred in 30% on SLS at 40 min and in 17% on IVG at 20 min. One fatality was reported in each group. Treatment failures in the SLS group were attributed to clenched teeth, making administration of SLS impossible or due to swallowing the sugar; whereas in the IVG group, they were due to unavoidable delays in beginning an infusion [median time 17.5 min (range 3–40 min)]. The authors proposed the use of SLS as an immediate ‘first aid’ measure while awaiting IV glucose.
A second RCT [13] (n=69) compared outcomes of alternative treatments in children with moderate hypoglycaemia (BGC <80 mg dl−1 or 4.4 mmol l−1). Children were assigned to one of the four methods of administration: oral group (OG)—2.5 g of sugar; sublingual group (SG)—2.5 g of sugar under the tongue; IV group (IG)—8 ml of 30% dextrose in a single bolus; and water only group. Treatment failure was defined as the proportion of children not attaining BGCs ≥90 mg dl−1 (5.0 mmol l−1) during the study period and was not observed in the SG and IG groups, compared with eight (53%) and nine (81.8%) failures in the OG and water group, respectively. Children aged >7 years required repeated sublingual administrations to maintain normoglycaemia (BGC ≥90 mg dl−1 or 5.0 mmol l−1). The authors concluded that SLS administration was effective in moderately hypoglycaemic children.
Discussion
Interpretation of findings
The WHO currently defines hypoglycaemia as blood glucose <2.5 mmol l−1 in a sick infant/child without severe malnutrition [7]. This definition is based on observational studies linking low blood glucose measurements to poor outcomes and subsequent expert opinion on the need for treatment. There is an absence of evidence on whether treatment of hypoglycaemia improves clinical outcomes. Recent treatment studies continue to use different definitions of hypoglycaemia in inclusion and outcome criteria. The findings from the KDH data suggest that BGCs < 4.0 mmol l−1 are associated with ORs for death already >2.0 with a steady rise in the OR for death as BGC reduces further. No clear-cut threshold of BGC was observed resulting in a ‘step-change’ increase in OR for death in admitted infants and children could be identified. In this population, the WHO threshold (<2.5 mmol l−1) and the current Kenyan guideline threshold (<2.2 mmol l−1) would result in 8.1% and 6.5% of admissions warranting treatment, respectively. While there is current consensus that hypoglycaemia, however defined, warrants treatment, we lack evidence that treatment improves outcomes. The data we present indicate that a pragmatic RCT of treatment for children admitted with BGC between 2.2 mmol l−1 and 4.0 mmol l−1 might be warranted.
An influential expert review of evidence recommends that no action is required in healthy, term, newborn babies who are asymptomatic and feeding even if a measured blood glucose is <1.1 mmol l−1 [8]. This review encouraged early and exclusive breastfeeding as an adequate and safe means to prevent hypoglycaemia; and, additionally, thermal regulation—through skin-to-skin contact and ‘kangaroo care’—was found important in the prevention of hypoglycaemia in the neonatal period.
The findings from the two included RCTs also demonstrate that repeated doses of 0.2 g kg−1 of SLS is a child-friendly, feasible and effective alternative to IV glucose in raising blood glucose levels in critically sick children aged 6 months to 15 years. It is therefore recommended as a ‘first-aid’ measure, for example, as part of pre-referral care, while awaiting IV glucose and could potentially avert the need for IV bolus glucose.
Conclusions
Definitions of hypoglycaemia are based on observational data and expert opinion. Treatment of hypoglycaemia in sick newborns, infants and children is based on consensus opinion, but the treatment has not been proven to improve outcomes (absence of evidence). As risk of mortality in those aged 1–59 months increases significantly as BGC falls even <4 mmol l−1, large, pragmatic randomized trials might help define appropriate treatment thresholds in the future. Additional studies would be helpful to establish when and how best to treat hypoglycaemia in sick newborns. We further note that SLS appears to be a child-friendly, feasible and effective therapy in the correction of hypoglycaemia, particularly, in situations where administering IV dextrose is delayed or impossible.
Acknowledgements
The authors would like to thank Philip Ayieko for his assistance with data analysis. This manuscript is published with the permission of the Director of KEMRI.
Funding M.E. is funded by a Wellcome Trust Senior Fellowship (#076827) and N.O. is funded by a Wellcome Trust Strategic Award (#084538). General financial support for the KEMRI/Wellcome Trust Research Programme is provided through the Major Overseas Programme Grant (#077092).
References
- 1.Cornblath M, Hawdon JM, Williams AF, et al. Controversies regarding definition of neonatal hypoglycaemia: suggested operational thresholds. Pediatrics. 2000;105:1141–5. doi: 10.1542/peds.105.5.1141. [DOI] [PubMed] [Google Scholar]
- 2.Osier FH, Berkley JA, Ross A, et al. Abnormal blood glucose concentrations on admission to a rural Kenyan district hospital: prevalence and outcome. Arch Dis Child. 2003;88:621–5. doi: 10.1136/adc.88.7.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Hypoglycaemia of the Newborn: Review of the Literature in Hypoglycaemia. WHO; Geneva: 1997. [Google Scholar]
- 5.Koh TH, Eyre JA, Aynsley-Green A. Neonatal hypoglycaemia - the controversy regarding definition. Arch Dis Child. 1988;63:1386–8. doi: 10.1136/adc.63.11.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health, Republic of Kenya . Basic Paediatric Protocols for Paediatric Care. Ministry of Health; Republic of Kenya: 2007. [Google Scholar]
- 7.World Health Organization . Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. WHO; Geneva: 2005. [Google Scholar]
- 8.Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297:1304–8. doi: 10.1136/bmj.297.6659.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Management of asymptomatic hypoglycaemia in healthy term neonates for nurses and midwives. Aust Nurs J. 2006;13:32–5. [PubMed] [Google Scholar]
- 10.Hewitt V, Watts R, Robertson J, et al. Nursing and midwifery management of hypoglycaemia in healthy term neonates. Int J Evid Based Healthc. 2005;3:169–205. doi: 10.1111/j.1479-6988.2005.00025.x. [DOI] [PubMed] [Google Scholar]
- 11.Nili F, Ghafuri M. Hypoglycemia in sick preterm infants and the therapeutic effect of 12.5% dextrose in water compared with 10% dextrose in water. Acta Medica Iranica. 2005;43:182–6. [Google Scholar]
- 12.Graz B, Dicko M, Willcox ML, et al. Sublingual sugar for hypoglycaemia in children with severe malaria: a pilot clinical study. Malar J. 2008;7:242. doi: 10.1186/1475-2875-7-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barennes H, Valea I, Nagot N, et al. Sublingual sugar administration as an alternative to intravenous dextrose administration to correct hypoglycemia among children in the tropics. Pediatrics. 2005;116:e648–53. doi: 10.1542/peds.2004-2218. [DOI] [PubMed] [Google Scholar]
- 14.Sexson WR. Incidence of neonatal hypoglycemia: a matter of definition. J Pediatr. 1984;105:149–50. doi: 10.1016/s0022-3476(84)80382-0. [DOI] [PubMed] [Google Scholar]
- 15.Holtrop PC. The frequency of hypoglycemia in full-term large and small for gestational age newborns. Am J Perinatol. 1993;10:150–4. doi: 10.1055/s-2007-994649. [DOI] [PubMed] [Google Scholar]
- 16.Anderson S, Shakya KN, Shrestha LN, et al. Hypoglycaemia: a common problem among uncomplicated newborn infants in Nepal. J Trop Pediatr. 1993;39:273–7. doi: 10.1093/tropej/39.5.273. [DOI] [PubMed] [Google Scholar]