Abstract
Background
Several studies suggest that herpes simplex virus type 2 (HSV-2) may enhance HIV-1 transmission and disease progression.
Methods
We conducted a randomised, double-blind, placebo-controlled trial of aciclovir 400mg BD for 3 months in 300 HSV-2/HIV-1 co-infected women not yet on HAART. Participants were evaluated pre-randomisation and at monthly visits for 3 months. Primary outcomes were the detection and quantity of genital HIV-1 RNA at the month 3 (M3) visit. Analyses were also undertaken using data from all visits. The treatment effects on plasma HIV-1 RNA, CD4+ count and genital HSV-2 DNA were also assessed.
Results
At M3 fewer women has detectable genital HIV in the aciclovir group compared to placebo, but this was not significant (61/132 [46%] vs.71/137 [52%], risk ratio [RR] 0.89, 95%CI 0.70 to 1.14, p=0.36). There was also little difference in quantity of HIV-1 RNA among shedders (+0.13 log10 copies/mL, 95%CI −0.14 to 0.39) at M3. However, aciclovir significantly decreased the frequency of HIV-1 shedding over all visits (adjusted odds-ratio 0.57, 95%CI 0.36 to 0.89). Significant reductions in M3 plasma HIV-1 RNA (−0.34 log10 copies/mL 95%CI 0.15 to 0.54), genital HSV-2 DNA (8% vs. 20%, RR 0.37, 95%CI 0.19 to 0.73) and genital ulceration (8% vs. 18%, RR 0.43, 95%CI 0.22 to 0.84) were observed in the aciclovir group.
Conclusion
HSV-2 suppressive therapy, by reducing HIV-1 plasma viral load and altering the pattern of genital HIV-1 shedding, may contribute to the reduction in sexual transmission of HIV-1 and may delay the requirement for HAART initiation.
Keywords: herpes simplex virus type-2 (HSV-2), HIV-1, aciclovir, suppressive therapy, randomised controlled trial, South Africa
Introduction
Herpes simplex virus type-2 (HSV-2) is one of the most common sexually transmitted infections (STIs) worldwide[1, 2], and 50-90% of HIV-infected persons are co-infected with HSV-2[3]. Evidence from clinical, epidemiological and biological studies underscores the synergistic bidirectional relationships between the two viruses. HIV-1 appears to alter the natural history of HSV-2 leading to more frequent reactivations of HSV-2. HSV-2 in turn appears to enhance HIV-1 transmissibility and disease progression through increased HIV-1 plasma levels and genital shedding [4, 5]. However, until recently, there has been no conclusive evidence of the causal role of HSV-2 on HIV-1 transmission. Two randomised trials, one conducted in 136 HIV-1 and HSV-2 seropositive women in Burkina Faso[6], and the other in 20 men who have sex with men in Peru[7] demonstrated that daily treatment with valaciclovir over a three-months period reduced both plasma and genital or rectal HIV-1 RNA. Further trials are needed to confirm these effects in larger populations, from settings where HIV-1 sub-types may also differ, and using aciclovir, the drug most commonly available to public health programmes.
We conducted a randomised, double-blind, placebo-controlled trial of oral aciclovir 400mg twice daily for three months in HIV-1 and HSV-2 seropositive women not taking highly active antiretroviral therapy (HAART) in Johannesburg, South Africa, in order to determine the impact of suppressive therapy on genital and plasma HIV-1 RNA, CD4 cell count and genital HSV-2 DNA.
Methods
Population and procedures
Women were recruited from the community or support groups for people living with HIV/AIDS in Johannesburg through a screening visit during which the following eligibility criteria were established: age ≥18 years; HIV-1 and HSV-2 seropositive; clinically asymptomatic for HIV according to WHO criteria [8]; a CD4 count ≥250 cells/mm3; negative urine pregnancy test; not breastfeeding; no contraindication to aciclovir; and not eligible for nor taking HSV-2 suppressive therapy;
Because genital HIV and HSV-2 shedding is not always a frequent event, enrolment procedures for eligible women were completed over two baseline visits (E1 and E2) separated by one week, in order to optimise detection of genital HIV-1 and HSV-2 at baseline amongst those that shed. Socio-demographic, sexual behaviour, and medical information was collected using an interviewer-administered questionnaire at E1. A physical and pelvic examination was performed at both visits, and blood and genital samples collected. Menstruating women had visits deferred until after menstruation had ceased.
At the E2 visit, participants were randomised 1:1 to aciclovir 400mg twice daily or matching placebo identical to aciclovir in all respects using random permuted blocks of varying size. Study drugs were pre-packaged and sequentially numbered by the drug company according to a randomisation list prepared by an independent statistician. Treatment packs were assigned consecutively. Both the investigators and participants were unaware of study-group assignments. Adherence counselling included instructions for study drug use, storage, and handling of missed doses.
Following randomisation, participants were seen at monthly clinic visits (visit interval 30-35 days) for a total of three visits (M1, M2 and M3), when drug packs were returned for pill count, a new supply of drugs dispensed, and adherence messages reiterated. Information on symptoms, adverse events and adherence was also obtained. Blood and genital specimens were collected as at enrolment. Urine pregnancy testing was performed. A study exit (F) visit was completed seven days after the M3 visit, following similar procedures. Study drug was only stopped after the F visit.
All participants received pre- and post-test STI/HIV counselling, regular risk reduction counselling, free condoms, treatment of laboratory-diagnosed STIs and STI syndromes, and referral to local HIV-1 treatment centres. Genital ulcer disease (GUD) episodes were treated presumptively as genital herpes with aciclovir 1200mg daily for 5 days, irrespective of study group.
Written informed consent was obtained from women at both screening and enrolment visits. The trial was approved by the Human Research Ethics Committees of the University of the Witwatersrand and of the London School of Hygiene and Tropical Medicine, and submitted to the South African Medicines Control Council. The trial was registered in the South African National Clinical Trials Register (DOH-27-0207-1376).
Specimen collection and laboratory procedures
HIV-1 serostatus was established using a rapid test on fingerprick blood with Determine HIV 1/2 (Abbott Diagnostics, Ill, USA) either alone or in combination with OraQuick® Rapid HIV-1 Antibody Test (Orasure Technologies Inc, Pennsylvania, USA). HSV-2 serostatus was determined using HerpeSelect® gG2 ELISA (Focus Diagnostics, Cypress, CA) at a higher cut-off of ≥3.5[9], with equivocal results resolved by Kalon® gG2 ELISA (Kalon Biologicals, Aldershot, UK)[10]. Syphilis serology was performed using standard treponemal and non-treponemal assays. Pregnancy was excluded using Quickvue One-Step hCG Urine test (Quidel, San Diego, CA).
Plasma samples were collected at all visits except F visit, for measurement of CD4+ cell count using the panLeucogating method (FlowCARE PLG CD4, Beckman Coulter)[11], and for plasma HIV-1 viral load, using the ultrasensitive Roche Amplicor Monitor version 1.5 (Roche Molecular Systems, Branchburg, NJ, USA).
A standardised cervico-vaginal lavage (CVL) was performed at all visits for the measurements of genital HIV-1 and HSV-2 shedding[12].
Nucleic acid extraction for detection and quantification of genital HIV-1 RNA was performed using the Roche Cobas Ampliprep/Cobas Amplicor system and the Roche Monitor HIV-1 version 1.5 assay (Roche Molecular Systems). The linear range of the standard assay is 400-750 000 RNA copies/mL. Samples with undetectable values using the standard assay were subsequently tested using the Roche Ultrasensitive assay, which has a lower limit of detection of 50 copies/mL.
For HSV-2 DNA detection and quantification, extracted nucleic acids were processed using an in-house HSV RT-PCR assay performed on the Roche LightCycler® 2.0 Real-Time PCR platform (Roche Applied Science, Mannheim, Germany), as described[13, 14]. For the trial, nucleic acid extracted from an Acrometrix HSV-2 QC sample by procedures described above were included with each batch of CVL samples analysed to allow calculations of HSV-2 viral loads using an external standard curve. The lower limit of quantification was 500 copies/mL, but the lower limit of detection was at least a log lower, at 50 copies/mL.
Cervical and vaginal swabs were tested for Neisseria gonorrhoeae and Chlamydia trachomatis (Roche Amplicor NG/CT, Roche Molecular Systems, Branchburg, NJ, USA), Trichomonas vaginalis (TV) (InPouch®TV, Biomed Diagnostics), and bacterial vaginosis (BV) using Nugent’s score of Gram-stained vaginal smears. Clinically-confirmed ulcers were swabbed and tested for ulcer aetiology (Multiplex PCR, Roche Molecular Systems).
Outcomes
The trial primary outcomes were the detection and quantity of genital HIV-1 RNA in CVL at the month 3 visit (M3). Secondary outcomes included quantity of plasma HIV-1 RNA (log10 copies/mL), CD4 count, detection and quantity of genital HSV-2 DNA in CVL, and occurrence of GUD episodes.
Statistical analysis
On the basis of published data[15, 16] and a pilot study, the proportion of women with detectable genital HIV-1 RNA in the placebo group at M3 was expected to be 40-60%. A sample of 300 women gave 80% power to demonstrate a 33% relative reduction in detection of genital HIV-1 RNA shedding after 3 months (5% type I error), assuming 10% loss to follow-up.
The primary analysis focused on the impact of treatment on genital HIV-1 RNA detection and quantity at the M3 visit. The effect of treatment was estimated as a risk ratio (RR) with 95% confidence intervals (CI). The impact of treatment on the mean quantity of genital HIV-1 RNA among those with detectable HIV-1 was assessed using linear regression, adjusting for the quantity of virus at baseline.
Values for plasma HIV-1 RNA and CD4+ count were also analysed using linear regression, adjusting for baseline values. The impact of treatment on genital HSV-2 DNA (detection and quantity) was assessed in a similar manner to that described for genital HIV-1 RNA. Additional secondary analyses were performed to explore the effect of treatment over time using data from all post-randomisation visits. Summary measures were used to combine the measurements for each woman into a single value. Impact of treatment on the frequency of genital HIV-1 RNA and HSV-2 DNA detection (proportion of visits during which the virus was detected, per woman) was estimated using ordered logistic regression, adjusting for baseline frequency of detection.
Analyses were also performed on a ‘per visit’ basis, using repeated measures analysis. Poisson regression models with robust standard errors were used to estimate the effect of the intervention on the presence of genital HIV-1 RNA or HSV-2 DNA[17]. The association between intervention and the quantity of genital or plasma HIV-1 RNA or genital HSV-2 DNA was assessed using random effects linear regression in those with detectable virus.
A limited number of predefined subgroup analyses were completed to assess whether any effects of treatment on genital HIV-1 RNA detection were dependent on (i) whether women had HIV-1 RNA at both baseline visits (“persistent HIV shedders”), and (ii) whether women had HIV-1 RNA at either baseline (E1/E2) visit and also HSV-2 DNA at either baseline visit (“HIV/HSV-2 shedders”). For these subgroup analyses, interaction tests were conducted.
Statistical analyses were performed using STATA version 9 (StataCorp, Texas), and all analyses used an intention-to-treat approach.
Results
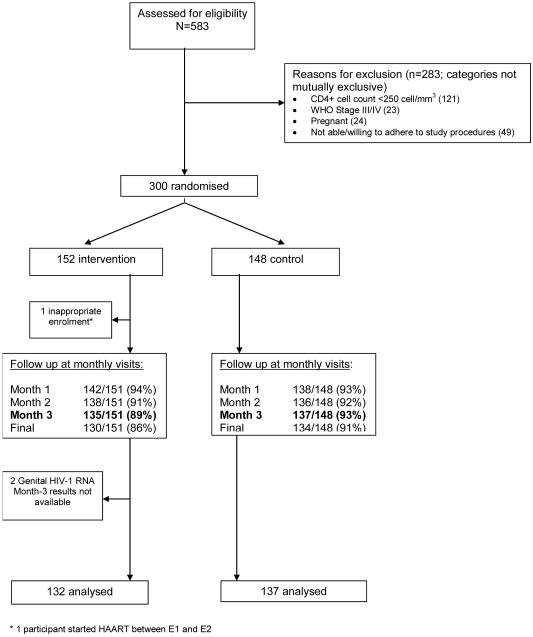
Between April 2005 and April 2006, 583 women were screened for eligibility, and 300 subsequently randomised to aciclovir (N=152) or placebo (N=148) (Fig 1). Results from 269 (90%) participants (132 aciclovir, 137 placebo) were available for inclusion in the primary analysis of outcomes at the M3 visit (Fig 1). Data were available for 288 (96%) participants (146 aciclovir, 142 placebo) for the summary and repeat measures analyses. Overall, 541/604 (90%) and 545/592 (92%) of the planned clinic visits were completed by participants in the aciclovir and placebo groups, respectively.. There was no association between treatment arm and missed visits. The average treatment adherence based on pill count over three months was 95% in both groups. There were 7 serious adverse events (3 in the aciclovir group), all due to hospitalisation for treatment of bacterial infections unrelated to treatment.
Figure 1. Enrolment and follow-up of participants during the trial.
The treatment groups were well balanced for most baseline characteristics (table 1). The mean age of participants was 32 years, with a median time of 1 year since their known HIV diagnosis. Across the two enrolment visits there were similar proportions of persistent HIV-1 shedders in the aciclovir and placebo groups (42% vs.41%). The proportion of HIV-1/HSV-2 shedders was similar in the two groups (30% vs. 28%).
Table 1. Baseline characteristics of trial participants.
| Aciclovir (N=151) |
Placebo (N=148) |
|
|---|---|---|
| Age group, years | ||
| ≤ 24 | 20 (13%) | 25 (17%) |
| 25-29 | 46 (31%) | 37 (25%) |
| 30-34 | 46 (31%) | 43 (29%) |
| ≥ 35 | 39 (26%) | 43 (29%) |
| Mean age (SD), years | 31 (6) | 32 (8) |
|
| ||
| Marital status | ||
| Never married | 109 (72%) | 112 (76%) |
| Married | 22 (15%) | 22 (15%) |
| Divorced/separated | 8 (5%) | 8 (5%) |
| Widowed | 12 (8%) | 6 (4%) |
|
| ||
| Age at first sex, years - mean (SD) | 17 (2) | 18 (2) |
|
| ||
| Lifetime sexual partners - median (IQR) | 5 (3-8) | 4 (3-7) |
|
| ||
| HIV status of most recent partner1 | ||
| Positive | 24 (19%) | 43 (33%) |
| Negative | 44 (35%) | 28 (22%) |
| Unknown | 57 (46%) | 59 (45%) |
|
| ||
| Condom use at last sex | 94 (63%) | 99 (67%) |
|
| ||
| Contraceptive use | ||
| None | 3 (2%) | 3 (2%) |
| Hormonal method only | 66 (44%) | 69 (47%) |
| Barrier method | 58 (39%) | 59 (40%) |
| Sterilisation | 8 (5%) | 5 (3%) |
| Abstinence | 14 (9%) | 12 (8%) |
|
| ||
| Practise vaginal cleansing | 70 (46%) | 70 (47%) |
|
| ||
| Years since HIV diagnosed – median (IQR) |
1 (0-3) | 1 (0-4) |
|
| ||
| CD4 count cells/mm3 - median (IQR) | 447 (330-615) | 500 (369-731) |
|
| ||
| Plasma HIV-1 RNA, log10 copies/mL – mean (SD) |
4.0 (1.0) | 3.9 (1.1) |
|
| ||
| Self-reported GUD in previous 3 months | 45 (30%) | 52 (35%) |
|
| ||
| Clinical GUD at enrolment | 19 (13%) | 17 (12%) |
|
| ||
| Serological syphilis | 3 (2%) | 3 (2%) |
|
| ||
| Chlamydia trachomatis | 8 (5%) | 10 (7%) |
|
| ||
| Neisseria gonorrhoeae | 7 (5%) | 4 (3%) |
|
| ||
| Trichomonas vaginalis | 18 (12%) | 22 (15%) |
|
| ||
| Bacterial vaginosis | 61 (41%) | 72 (50%) |
|
| ||
| Women with genital HIV-1 RNA | ||
| At no enrolment visits | 44 (29%) | 53 (36%) |
| At one enrolment visit | 43 (28%) | 34 (23%) |
| At both enrolment visits | 64 (42%) | 61 (41%) |
|
| ||
| Women with genital HSV-2 DNA | ||
| At no enrolment visits | 89 (59%) | 77 (52%) |
| At one enrolment visit | 45 (30%) | 54 (36%) |
| At both enrolment visits | 17 (11%) | 17 (11%) |
For those that reported having a sexual partner in the past 3 months (aciclovir, N=125, placebo N=130).
GUD, genital ulcer disease; IQR, interquartile range; SD, standard deviation.
Impact on genital HIV-1 RNA
At M3 visit, 61 (46%) women in the aciclovir group compared with 71 (52%) in the placebo group had detectable genital HIV-1 RNA (RR 0.89, 95%CI 0.70 to 1.14; p=0.36) (table 2). There was no evidence for an impact of aciclovir on the quantity of genital HIV-1 RNA among those with detectable genital HIV-1 RNA at the M3 visit (mean difference 0.13 log10 copies/mL, 95%CI −0.14 to 0.39).
Table 2. Impact of aciclovir on genital and plasma HIV-1 RNA, genital HSV-2 DNA, and CD4+ cell count at month 3 (M3) visit.
| Aciclovir (N=134*) |
Placebo (N=137*) |
Measure of effect** (95%CI) |
p-value | |
|---|---|---|---|---|
| HIV-1 RNA | ||||
| Women with detectable genital HIV-1 RNA | 61 (46%) | 71 (52%) | RR = 0.89 (0.70 to 1.14) | 0.36 |
| Mean genital HIV-1 RNA (SD) among women with detectable HIV-1 RNA, log10 copies/mL |
3.20 (0.87) | 3.12 (0.81) | 0.13 (−0.14 to 0.39) | 0.35 |
| Mean plasma HIV-1 RNA (SD), log10 copies/mL | 3.66 (1.16) | 3.90 (1.07) | −0.34 (−0.54 to −0.15) | 0.001 |
| Median CD4 cell count (IQR), cells/mm3 | 405 (300-522) | 435 (329-632) | 7 (−24 to 37) | 0.66 |
| HSV-2 DNA | ||||
| Women with detectable genital HSV-2 DNA | 10 (8%) | 28 (20%) | RR = 0.37 (0.19 to 0.73) | 0.002 |
| Mean genital HSV-2 DNA (SD) among women with detectable HSV-2 DNA, log10 copies/mL |
3.80 (1.72) | 3.62 (1.82) | 0.16 (−1.23 to 1.54) | 0.82 |
For aciclovir, there were 2 missing values for genital HIV-1 RNA and 1 missing value for genital HSV-2 DNA For placebo, there was 1 missing value for plasma HIV-1 RNA and 4 missing values for CD4 cell count
Risk ratios or mean differences. Mean difference outcomes adjusted for baseline values CI, confidence interval; RR, risk ratio; SD, standard deviation
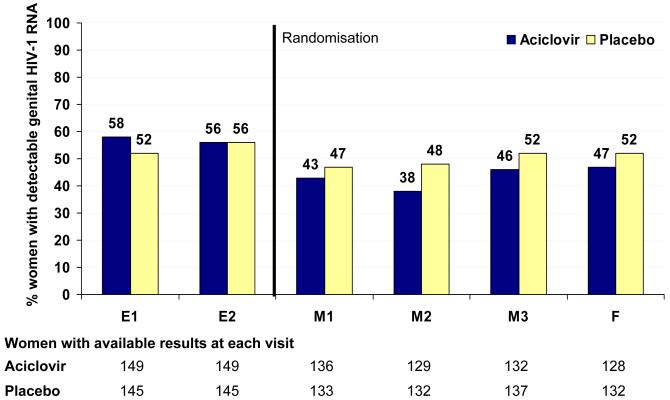
The proportion of women with detectable genital HIV-1 RNA was consistently lower in the aciclovir group at each post-randomisation visit (Fig 2A). Using summary measures analysis, there was evidence for a strong effect of aciclovir in reducing the frequency of HIV-1 shedding among women (adjusted odds-ratio 0.57, 95%CI 0.36 to 0.89; p=0.013) (table 3). Among women with detectable genital HIV-1, the mean quantity of HIV-1 RNA was lower in the aciclovir group (−0.13 log10 copies/mL, 95%CI −0.28 to 0.03), although the evidence for a difference was weak (p=0.12). The repeated measures analysis using all visits confirmed these results (table 3).
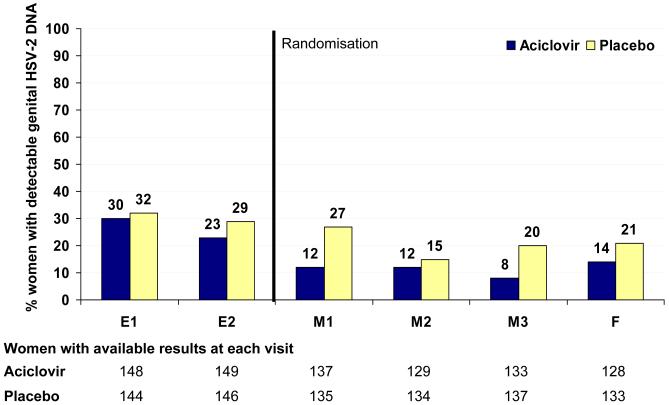
Figure 2. Impact of aciclovir on the frequency of detection of genital HIV-1 RNA (panel 2A) and HSV-2 DNA (panel 2B) over time.
Note: dashed line represents point of randomisation
Table 3. Impact of aciclovir on genital and plasma HIV-1 RNA, genital HSV-2 DNA, genital ulcer disease over follow-up visits (using Summary and Repeated measures analyses).
| Aciclovir (N=146) |
Placebo (N=142) |
Measure of effect* (95%CI) |
p-value | |
|---|---|---|---|---|
| Summary measures analysis (per woman) | ||||
|
| ||||
| HIV-1 RNA | ||||
|
| ||||
| Women with genital HIV-1 RNA detected at least once |
101 (69%) | 102 (72%) | RR = 0.96 (0.83 to 1.12) | 0.62 |
|
| ||||
| Women with genital HIV-1 RNA detected at: | ||||
| No visit | 45 (31%) | 40 (28%) | ||
| 0% < visits < 50% | 34 (23%) | 24 (17%) | OR = 0.57 (0.36-0.89) | 0.013 |
| 50% ≤ visits < 100% | 34 (23%) | 36 (25%) | ||
| All visits (100%) | 33 (23%) | 42 (30%) | ||
|
| ||||
| Mean genital HIV-1 RNA (SD) among women with detectable HIV-1 RNA, log10 copies/mL |
2.92 (0.63) | 3.04 (0.65) | −0.13 (−0.28 to 0.03) | 0.12 |
|
| ||||
| Mean plasma HIV-1 RNA (SD), log10 copies/mL |
3.67 (1.00) | 3.84 (0.97) | −0.27 (−0.41 to −0.13) | <0.001 |
|
| ||||
| HSV-2 DNA | ||||
|
| ||||
| Women with genital HSV-2 DNA detected at least once |
48 (33%) | 77 (54%) | RR = 0.61 (0.46 to 0.80) | <0.001 |
|
| ||||
| Women with genital HSV-2 DNA detected at: | ||||
| No visit | 98 (65%) | 65 (46%) | ||
| 0% < visits < 50% | 34 (23%) | 44 (31%) | OR = 0.40 (0.25 to 0.64) | <0.001 |
| 50% ≤ visits < 100% | 13 (9%) | 31 (22%) | ||
| All visits (100%) | 1 (1%) | 2 (1%) | ||
|
| ||||
| Mean genital HSV-2 DNA (SD) among women with detectable HSV-2 DNA, log10 copies/mL |
3.38 (1.44) | 3.81 (1.51) | −0.42 (−0.96 to 0.12) | 0.13 |
|
| ||||
| Proportion of women with at least one GUD episode |
11 (8%) | 25 (18%) | RR = 0.43 (0.22 to 0.84) | 0.01 |
|
| ||||
| Repeated measures analysis (per visit) | ||||
|
| ||||
| HIV-1 RNA | Visits = 525 | Visits = 535 | ||
|
| ||||
| Proportion of visits with detectable genital HIV- 1 RNA |
228 (43%) | 265 (50%) | RR = 0.88 (0.72 to 1.06) | 0.18 |
|
| ||||
| HSV-2 DNA | Visits = 527 | Visits = 539 | ||
|
| ||||
| Proportion of visits with detectable genital HSV-2 DNA |
60/527 (11%) | 113/539 (21%) | RR = 0.54 (0.39 to 0.74) | <0.001 |
Risk ratios, odds ratios or mean differences. Mean differences adjusted for baseline values and OR adjusted for baseline frequency of shedding
CI, confidence interval; GUD, genital ulcer disease; OR, odds-ratio; RR, risk ratio; SD, standard deviation.
We investigated the impact of aciclovir on predefined sub-groups of persistent genital HIV-1 shedders and on HIV-1/HSV-2 shedders at baseline. There was a suggestion of a greater impact of aciclovir at M3 in the subgroup of persistent HIV-1 shedders compared with intermittent or non-shedders. A similar pattern was seen at all follow-up visits. In the repeated measures analysis, genital HIV-1 RNA was detected at fewer visits overall in the aciclovir group among the persistent shedders (RR 0.78, 95%CI 0.67 to 0.91) compared to intermittent or non-shedders (RR 1.02, 95%CI 0.71 to 1.46) (interaction p-value=0.18). In contrast, there was little evidence that the effect of aciclovir depended on the presence of both HIV-1 and HSV-2 at baseline (interaction p-value=0.88).
Impact on plasma HIV-1 RNA and CD4+ cell count
Plasma HIV-1 RNA levels were substantially lower among women in the aciclovir group at M3 (mean difference −0.34 log10 copies/mL, 95%CI −0.54 to −0.15; p<0.001) (table 2). A similar result was observed when all four follow-up visits were included. There was a reduction of 0.27 log10 copies/mL (95%CI 0.13 to 0.41; p<0.001) across all visits, equivalent to a 46% reduction in mean HIV-1 plasma viral load.
This impact did not translate into an effect on CD4+ count after 3 months of treatment (mean difference 7 cells/mm3, 95%CI −24 to 37, p=0.66) (table 2).
Impact on genital HSV-2 DNA and GUD episodes
Aciclovir reduced the detection of genital HSV-2 DNA. At M3 visit, 10/133 (8%) women in the aciclovir group compared with 28/137 (20%) in the placebo group had detectable genital HSV-2 DNA (RR 0.37, 95%CI 0.19 to 0.73; p=0.002). However, there was little evidence of an impact on the quantity of HSV-2 DNA (mean difference 0.16 log10 copies/mL, 95%CI −1.23 to 1.54, p=0.82) (table 2).
The impact of treatment on the frequency of genital HSV-2 shedding was clearly observed in the summary measures analysis. Comparisons of monthly detection rates of genital HSV-2 DNA revealed consistently lower rates in the aciclovir group (Fig 2B). Over all visits, 33% of women in the aciclovir group had detectable genital HSV-2 DNA at least once compared to 54% in the placebo group (RR 0.61, 95%CI 0.46 to 0.80; p<0.001) (table 3). The impact of aciclovir was demonstrated in the reduced frequency of visits with detectable HSV-2 DNA (adjusted odds-ratio 0.40, 95%CI 0.25 to 0.64; p<0.001) (table 3). There was an observed reduction in the mean quantity of HSV-2 DNA (−0.42 log10 copies/mL (95%CI −0.96 to 0.12).
Aciclovir also had a substantial impact on the occurrence of GUD. The proportion of women with at least one clinically-confirmed ulcer during the three months of follow-up was significantly lower in the aciclovir group (8% vs. 18%, RR 0.43, 95%CI 0.22 to 0.84; p=0.01) (table 3)..
Discussion
Daily treatment with aciclovir to suppress HSV-2 reactivation reduced genital and plasma HIV-1 RNA levels in women co-infected with HIV-1 and HSV-2. In this proof-of-concept trial, women in the aciclovir arm had a lower rate of genital HIV-1 RNA detection after 3 months of treatment, although the evidence for impact at a single visit (M3) weak. Single measurements of genital HIV-1 RNA shedding are limited because HIV-1 genital shedding is subject to high within-person variability [18]. This variability is related in part to variability in both genital sample collection and the accuracy of measurement within mucosal samples. The impact of anti-HSV-2 suppressive therapy on genital HIV-1 RNA transmissibility therefore is best observed when multiple measurement points are included in the analysis. In this trial, anti- HSV-2 suppressive therapy with aciclovir was associated with a significant reduction in the frequency of genital HIV-1 RNA detection. The finding that these effects are even stronger in the sub-group of women who shed high levels of HIV-1 RNA and HSV-2 DNA simultaneously, provides support for earlier observations that HSV-2 reactivation can enhance HIV-1 transmissibility by increasing HIV-1 mucosal replication either through transactivation of HIV-1 expression by HSV-2 proteins[19-21]or stimulating the release of pro-inflammatory cytokines in addition to the recruitment of activated CD4+ cells to the genital mucosa and skin levels[22, 23]. Recent studies of the genital tract immune milieu have revealed that co-infection with HIV-1 results in depletion of the immune cells in the cervix responsible for the immune control of HSV-2 reactivation (DC-SIGN), confirming the biological synergy between these two viruses [24]. Our findings are consistent with the results from smaller trials conducted among women in Burkina Faso and Thailand, and among homosexual men in Peru, which have also shown reductions in plasma and genital or rectal HIV-1 viral loads using valaciclovir[6, 25].
It remains unclear, however, what reductions in the frequency of genital HIV-1 RNA shedding are required to reduce sexual transmission of HIV-1. A direct assessment of whether daily suppressive therapy with aciclovir can prevent the sexual transmission of HIV-1 is currently underway in a large, multi-centre, randomised controlled trial of HIV-1 serodiscordant couples[26]. While two recently published randomised controlled trials have shown that daily aciclovir does not reduce the risk of HIV-1 acquisition in HIV seronegative individuals, we need the results of ongoing trials to conclude about the value of daily aciclovir in reducing HIV infectiousness and transmission[27, 28]. Given that the biological mechanisms by which HSV-2 increases HIV-1 transmissibility are different, the results of our trial support the notion that that suppression of HSV-2 reactivation could prevent the sexual transmission of HIV-1.
A 0.34 log (46%) reduction in HIV-1 plasma viral load after three months of aciclovir was observed. Similar reductions of 0.33 and 0.53 log10 copies/mL in plasma HIV-1 RNA have been observed in the two smaller trials of valaciclovir suppression for 2 to 3 months in HSV-2 and HIV-1 co-infected men in Peru[7] and women in Burkina Faso[6]. Such impact may have important implications for HIV-1 transmission and disease progression. Plasma viral load remains one of the strongest predictors of HIV-1 transmission[29] and interventions that reduce plasma HIV-1 viral load are likely to influence HIV-1 transmission.
Reductions in plasma HIV-1 viral load of similar magnitude have previously been seen to be clinically significant[30]. Moreover, earlier studies showed a survival benefit for HIV-1 infected persons receiving aciclovir for treatment of cytomegalovirus (CMV)-associated conditions compared to untreated patients[31]. A meta-analysis of AIDS mortality in eight subsequent trials concluded that treatment with high-dose aciclovir offered a significant survival benefit for HIV-1 infected persons[32], despite the absence of a direct pharmacological activity of aciclovir against HIV-1[33]. Instead, the effects of aciclovir on HIV-1 plasma viral load are believed to be mediated through the suppression of clinical and sub-clinical reactivations of HSV-2, which have been shown to be associated with increases in systemic HIV-1 viral loads[34, 35]. In addition, an indirect effect mediated by impact on other Herpesviridae (HSV-1, Epstein-Barr virus, CMV, human herpesviruses −6 and −8) cannot be ruled out, although drugs and dosages used to control reactivation of these viruses might differ from the ones used to control HSV-2.
While we showed an impact of aciclovir on plasma HIV-1, we could not demonstrate any significant impact of aciclovir on CD4+ count. Trials of longer duration are required to confirm whether the observed reductions in HIV-1 plasma viral load would translate into immunological benefits in co-infected persons at high CD4+ count levels, potentially delaying the loss of CD4+ T-lymphocytes and prolonging the time before antiretroviral therapy initiation.
As anticipated, aciclovir significantly reduced both sub-clinical and clinical reactivations of HSV-2. Episodes of genital ulcerations were fairly frequent during the pre-randomisation visits of the trial (12%). While all episodes of GUD during the trial were treated with aciclovir irrespective of treatment arm, there was no evidence to suggest that this approach undermined our intent-to-treat analysis (data not shown). However, the most likely effect, if any, would have been to underestimate the impact of aciclovir on genital and plasma HIV-1 RNA.
In summary, this large proof-of-concept trial confirms the hypothesis that asymptomatic reactivation of HSV-2 leads to increased levels of genital and plasma HIV-1 RNA, and that HSV suppressive therapy can help reduce, but not eradicate, HIV-1 replication. The results from other ongoing or planned trials will provide further evidence of whether this translates into prevention of HIV-1 disease progression and transmission. Given the high prevalence of both HSV-2 and HIV-1 infections in Africa, HSV suppressive therapy in HIV-infected individuals offers a potential new HIV prevention tool which may also have individual clinical benefits for co-infected individuals.
Acknowledgement & funding
The authors wish to thank the volunteers for their participation in the study; the study staff; Jessica Dhookie for assistance with study drug management; Charlotte Ingram, Kirthi Hira and Ischelle Doddameade of Contract Laboratory Services for testing of routine laboratory specimens; colleagues at London School of Hygiene &Tropical Medicine who provided statistical or analytical advice (Helen Weiss, Richard Hayes, Simon Cousens, Mike Kenward, Nicolas Nagot); and the trial Data Safety &Monitoring Board (Chair: Andrew Nunn, Medical Research Council (UK), Rachel Jewkes, Medical Research Council, South Africa, and Adrian Puren, National Institute of Communicable Diseases, South Africa). The study was supported by grants from the Wellcome Trust (GR074151MA), the South African National Research Foundation (TTK2005071300016) and the UK’s DFID-funded Knowledge Programme on HIV/AIDS & STI of the London School of Hygiene & Tropical Medicine.
Footnotes
Conflicts of interest: None
Role of drug manufacturer: Drugs and placebo were purchased from Ranbaxy South Africa. The company had no involvement in the design or running of this trial.
Trial registered under no DOH-27-0207-1376 in the South African National Clinical Trials Register of the South African Department of Health.
REFERENCES
- 1.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11:24A–35A. [PubMed] [Google Scholar]
- 2.Malkin JE. Epidemiology of genital herpes simplex virus infection in developed countries. Herpes. 2004;11(Suppl 1):2A–23A. [PubMed] [Google Scholar]
- 3.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 4.Corey L, Wald A, Celum CL, Quinn TC. The Effects of Herpes Simplex Virus-2 on HIV-1 Acquisition and Transmission: A Review of Two Overlapping Epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 5.LeGoff J, Weiss HA, Gresenguet G, Nzambi K, Frost E, Hayes RJ, et al. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS. 2007;21:1569–1578. doi: 10.1097/QAD.0b013e32825a69bd. [DOI] [PubMed] [Google Scholar]
- 6.Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 7.Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Zuniga R, et al. Herpes Simplex Virus (HSV) Suppression with Valacyclovir Reduces Rectal and Blood Plasma HIV-1 Levels in HIV-1/HSV-2-Seropositive Men: A Randomized, Double-Blind, Placebo-Controlled Crossover Trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 8.WHO . Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance (African region) World Health Organisation; Geneva: 2005. [Google Scholar]
- 9.Hogrefe W, Su X, Song J, Ashley R, Kong L. Detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in African sera by using recombinant gG2, Western blotting, and gG2 inhibition. J Clin Microbiol. 2002;40:3635–3640. doi: 10.1128/JCM.40.10.3635-3640.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delany S, Stevens W, Weiss HA, Jentsh U, Moyes J, Ashley Morrow R, Mayaud P. Comparison of Focus HerpeSelect and Kalon glycoportein G-2 (G-G2) based ELISA serological tests to detect Herpes simplex virus type-2 (HSV-2) antibodies in South Africa. Abstract TP-011; 16th Biennial Meeting of the International Society for Sexually Transmitted Diseases Research (ISSTDR); Amsterdam, The Netherlands. 2005. [Google Scholar]
- 11.Glencross D, Scott LE, Jani IV, Barnett D, Janossy G. CD45-assisted PanLeucogating for accurate, cost-effective dual-platform CD4+ T-cell enumeration. Cytometry. 2002;50:69–77. doi: 10.1002/cyto.10068. [DOI] [PubMed] [Google Scholar]
- 12.Delany SRR, Mlaba N, Clayton T, Akpomiemie G, LeGoff J, Capovilla A, Belec L, Stevens W, Mayaud P. Comparison of cervico-vaginal lavage, cervico-vaginal lavage enriched with cervical swab and vaginal tampon for the detection of HIV-1 RNA and HSV-2 DNA in genital secretions. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/qai.0b013e31818c7f75. In press. [DOI] [PubMed] [Google Scholar]
- 13.Burrows J, Nitsche A, Bayly B, Walker E, Higgins G, Kok T. Detection and subtyping of Herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiol. 2002;2:12. doi: 10.1186/1471-2180-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Legoff J, Bouhlal H, Gresenguet G, Weiss H, Khonde N, Hocini H, et al. Real-time PCR quantification of genital shedding of herpes simplex virus (HSV) and human immunodeficiency virus (HIV) in women coinfected with HSV and HIV. J Clin Microbiol. 2006;44:423–432. doi: 10.1128/JCM.44.2.423-432.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mbopi-Keou FX, Gresenguet G, Mayaud P, Weiss HA, Gopal R, Matta M, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000;182:1090–1096. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 16.McClelland RS, Wang CC, Overbaugh J, Richardson BA, Corey L, Ashley RL, et al. Association between cervical shedding of herpes simplex virus and HIV-1. Aids. 2002;16:2425–2430. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 17.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 18.Coombs RW, Wright DJ, Reichelderfer PS, Burns DN, Cohn J, Cu-Uvin S, et al. Variation of human immunodeficiency virus type 1 viral RNA levels in the female genital tract: implications for applying measurements to individual women. J Infect Dis. 2001;184:1187–1191. doi: 10.1086/323660. [DOI] [PubMed] [Google Scholar]
- 19.Mosca JD, Bednarik DP, Raj NB, Rosen CA, Sodroski JG, Haseltine WA, Pitha PM. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987;325:67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- 20.Ostrove JM, Leonard J, Weck KE, Rabson AB, Gendelman HE. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J Virol. 1987;61:3726–3732. doi: 10.1128/jvi.61.12.3726-3732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rando RF, Pellett PE, Luciw PA, Bohan CA, Srinivasan A. Transactivation of human immunodeficiency virus by herpesviruses. Oncogene. 1987;1:13–18. [PubMed] [Google Scholar]
- 22.Clouse KAPD, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs JFA, Folks TM. Monokine regulation of human immune deficiency virus type 1 expression in a chronicall infected human t-cell line. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 23.Paludan SR. Requirements for the induction of interleukin-6 by herpes simplex virus-infected leukocytes. J Virol. 2001;75:8008–8015. doi: 10.1128/JVI.75.17.8008-8015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–598. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 25.Zuckerman RA, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Zuniga R, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–1508. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 26.Celum CL, Robinson NJ, Cohen MS. Potential effect of HIV type 1 antiretroviral and herpes simplex virus type 2 antiviral therapy on transmission and acquisition of HIV type 1 infection. J Infect Dis. 2005;191(Suppl 1):S107–114. doi: 10.1086/425272. [DOI] [PubMed] [Google Scholar]
- 27.Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–2119. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson-Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Rakai Project Study Group Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 30.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 31.Stein DS, Graham NM, Park LP, Hoover DR, Phair JP, Detels R, et al. The effect of the interaction of acyclovir with zidovudine on progression to AIDS and survival. Analysis of data in the Multicenter AIDS Cohort Study. Ann Intern Med. 1994;121:100–108. doi: 10.7326/0003-4819-121-2-199407150-00004. [DOI] [PubMed] [Google Scholar]
- 32.Ioannidis JP, Collier AC, Cooper DA, Corey L, Fiddian AP, Gazzard BG, et al. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998;178:349–359. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- 33.Ioannidis JP, Collier AC, Cooper DA, Corey L, Fiddian AP, Gazzard BG, et al. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998;178:349–359. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- 34.Duffus WA, Mermin J, Bunnell R, Byers RH, Odongo G, Ekwaru P, Downing R. Chronic herpes simplex virus type-2 infection and HIV viral load. Int J STD AIDS. 2005;16:733–735. doi: 10.1258/095646205774763298. [DOI] [PubMed] [Google Scholar]
- 35.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997;176:766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]