Abstract
LL-37 is a human cationic host defense peptide that is present in the specific granules of neutrophils, produced by epithelial cells from a variety of tissues, and is upregulated during inflammation, infection, and injury. It has been proposed to have a variety of antimicrobial functions, including both direct antimicrobial activity and immunomodulatory functions. Using the TUNEL assay it was demonstrated that LL-37 induced apoptosis in vitro in the A549 human lung and 16HBE4o- human airway epithelial cell lines, and in vivo in the murine airway. Peptide-induced apoptosis in vitro involved the activation of caspase pathways and was substantially inhibited by an inhibitor of caspase 3. Apoptosis was also inhibited by human serum, but not fetal bovine serum. Similarly, human but not fetal bovine serum inhibited the cellular internalization of LL-37 and the production of IL-8 in response to LL-37 treatment of epithelial cells. The protective effects of human serum were also observed with high-density lipoproteins but not by the core peptide apolipoprotein A1, providing one possible mechanism of human serum inhibition of apoptosis. We propose that LL-37–induced apoptosis of epithelial cells at low serum tissue sites may have a protective role against bacterial infection.
Keywords: apoptosis, cathelicidin, epithelial cells, host defense peptide, LL-37
Cationic host defense (also termed “antimicrobial”) peptides have been shown to be involved in many aspects of the innate immune response to infection, including in some instances direct antimicrobial activity (1, 2). In addition, many of these peptides can interact directly with monocytic and epithelial cells to selectively stimulate specific aspects of the host innate immune response. These peptides have been demonstrated to have a number of immunomodulatory properties in vitro, and there is now a growing list of these properties that have been demonstrated in vivo, including chemokine production, angiogenesis, anti-endotoxin activity, and chemotaxis (3-7). Host defense peptides do not, however, mediate these effects through typical proinflammatory pathways and indeed often have anti-inflammatory properties, with an ability to neutralize the biological effects of bacterial inflammatory mediators such as lipopolysaccharide and lipoteichoic acid (3).
The human cathelicidin hCAP18 was first isolated from human bone marrow (8) and is found at high concentrations in the specific granules of human neutrophils (9). LL-37 is a proteolytically processed form of hCAP18 that is released upon stimulation of cells and cleaved extracellularly by proteinase-3 (10). LL-37 is also produced by epithelial cells, including those of the lung (11, 12), and by the epidermis, and is upregulated in response to inflammatory stimuli (13, 14). It can be found in a number of bodily fluids, including gastric juices, saliva, semen, sweat, plasma, airway surface liquid, and breast milk (15, 16). LL-37 can be detected at mucosal surfaces of healthy individuals at concentrations of around 2–5 μg/ml, is upregulated to approximately 20 μg/ml in bronchoalveolar lavage fluid from children with lung infections (14), and is present in psoriatic skin plaques at concentrations exceeding 1 mg/ml (17). In addition, plasma has been reported to contain hCAP18 bound to lipoproteins at a concentration of 1.2 μg/ml (18).
LL-37 stimulates the expression, in monocytes/macrophages, of a wide variety of genes involved in the innate immune response, including those encoding chemokines (e.g., IL-8 and MCP-1), differentiation factors, and anti-inflammatory cytokines (3). LL-37 has also been reported to be directly chemotactic for human neutrophils, monocytes, and T cells through formyl peptide receptor like-1 (FPRL-1, a Gi protein–coupled receptor) (19), and for human mast cells through two unknown other receptors (20). Independent of the FPRL-1 receptor, LL-37 induces IL-8 production through phosphorylation and activation of the mitogen-activated protein kinases (MAPK) ERK1/2 and p38 in human peripheral blood–derived monocytes and human bronchial epithelial cell lines, but not in B or T lymphocytes (21, 22). Recently, LL-37 has been shown to promote processing of the proinflammatory cytokine, IL-1β, via activation of P2X7 receptors (23). Thus, LL-37 appears to be an important component of both the phagocyte and epithelial defense systems in humans, using several different receptors. LL-37 has a variety of other functions in immunity, including the promotion of mast cell histamine release (24), stimulation of wound healing (25) and angiogenesis (5), and the modulation of dendritic cell differentiation (26).
Although cationic peptides can have rapid microbicidal activity, most natural peptides are relatively less toxic to eukaryotic cells, by virtue of differences in membrane composition. However, there have been a number of reports regarding the induction of mammalian cell death by particular cationic peptides. Two bovine cathelicidins, BMAP-27 and BMAP-28, were found to induce cell death in cell lines and fresh haematopoietic tumor cells, but not in resting lymphocytes (27, 28). This was associated with cationic peptide–induced membrane permeabilization, as assessed by propidium iodide uptake, and was followed by programmed cell death. Recently BMAP-28 was shown to affect mitochondrial membrane permeability, causing mitochondrial depolarization and cytochrome c release, leading to cell death (29). In addition, LL-37–induced eukaryotic cell death has been documented (30). In a carcinoma cell line, using derivatives of LL-37, this was attributed to caspase-independent apoptosis (31); however, it is not clear whether the induction of apoptosis is generally involved in LL-37–mediated cell death in other mammalian cell types.
The control of cell death is a critical biological function, in both homeostasis and the resolution of inflammation. Programmed cell death, also known as apoptosis, is required for normal development and maintenance of tissue physiology, in addition to having a role as a defense mechanism to remove damaged and infected cells. There are many prominent morphologic features that accompany apoptosis, including membrane blebbing, cell shrinkage, and nuclear fragmentation. These events take place over a prolonged period of time while the cell retains the integrity of its plasma membrane and organelles, thus reducing the induction of proinflammatory responses in the surrounding tissues (32). It is important to note that apoptosis is a relatively immunologically silent process, whereby immunosuppressive cytokines such as IL-10 and TGF-β can be produced, and phagocytes rapidly engulf the apoptotic bodies to prevent further immunologic responses (33). However, apoptotic cells may become necrotic when the apoptotic cells cannot be cleared in diseases such as cystic fibrosis (34).
Necrosis is a form of cell death, distinct from apoptosis, and has characteristically different morphologic features, including cytoplasmic swelling, organelle breakdown, and rapid plasma membrane rupture leading to an inflammatory response (32). Historically, necrotic cell death was considered to be an accidental type of death caused by gross cell injury resulting in the death of groups of cells within tissues. However, it has been recently shown that signaling pathways, involving such components as death receptors, the caspase cascade, and the mitochondrial pathway, participate in both necrosis and apoptosis, and as a result allow cells to switch between the two pathways. The decision points between the two pathways include short or prolonged opening of the mitochondrial pore, the extent of oxidative stress, the concentration of cellular ATP content, and the levels of Bcl-2 present (35-37). Therefore, necrosis, along with apoptosis, may be considered to be interrelated with programmed cell death and the disturbance of the balance between these two processes may lead to the development of disease.
The effect of cationic host defense peptides on eukaryotic cell death is of significant interest with regards to the function of these peptides in immunity to infection. The continuing development of analogous synthetic peptides as novel antimicrobial therapeutics further adds to the importance of such investigations. The aim of this study, therefore, was to characterize the potential for, and determinants of, LL-37–induced mammalian cell death. Our data indicates that LL-37 was able to induce eukaryotic cell apoptosis in a dose-dependent manner, involved the activation of caspase pathways, and that LL-37–induced cell death was inhibited by human serum components, including high-density lipoproteins.
MATERIALS AND METHODS
Cell Maintenance and Growth
The human lung epithelial cell line A549 was obtained from the American Type Culture Collection (ATCC, Manassas, VA). It was maintained in complete DMEM (Dulbecco's Modified Eagle Medium; Invitrogen, Burlington, ON, Canada), consisting of DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT), and grown in cell culture flasks (Costar, Cambridge, MA) at 37°C in a 5% CO2 atmosphere. The cells were passaged at least twice a week by treating the monolayer with Typsin-EDTA (Invitrogen) at 37°C for 5 min to dissociate the cells from the flask. The detached cells were transferred to a 50-ml centrifuge tube containing 20 ml complete DMEM medium and then centrifuged for 5 min at 135 × g. The supernatant was discarded and the cells were resuspended in DMEM complete medium. A175cm2 flask was seeded with 106 viable cells in 40 ml complete DMEM and incubated at 37°C in 5% CO2. The SV40-transformed, immortalized 16HBE4o- cell line was a gift from Dr. D. Gruenert (University of California, San Francisco, CA). Cells were passaged as above in MEM with Earle's salts (Invitrogen) containing 10% FBS and 2 mM L-glutamine.
For experiments, A549 epithelial cells were seeded in 24-well plates at a density of 105 cells/well in DMEM supplemented with 10% FBS and incubated at 37°C in 5% CO2 overnight. The medium was removed from the cells and replaced with either fresh DMEM supplemented with 10% FBS, DMEM supplemented with 10% pooled AB human serum (Sigma, Oakville, ON, Canada), or DMEM without serum supplementation. 16HBE4o- cells were seeded in 48-well plates at a density of 2 × 104 cells / well in MEM with Earle's salts, supplemented with 10% FBS and incubated at 37°C in 5% CO2 until confluence (2–3 d). The medium was removed from the cells and replaced with either fresh MEM supplemented with 10% pooled AB human serum, or DMEM without serum supplementation.
Peptide Synthesis and Reagents
LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVP-RTES) and LL-37C (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTESC) were synthesized at the Nucleic Acid/Protein Synthesis (NAPS) Unit at UBC by Fmoc [(N-(9-fluorenyl) methoxycarbonyl)] chemistry using an Applied Biosystems Model 431 peptide synthesizer. Peptide concentration was determined by using amino acid analysis. LL-37C was biotinylated at the C-terminal cysteine side chain with N-α-(3-maleimidyl-propionyl) biocytin (Molecular Probes, Eugene, OR), as previously described (38) to generate LL-37B. Peptide purity (> 90%) was confirmed by HPLC and MALDI-TOF mass spectrometry. Pertussis toxin was supplied by List Biological Laboratories Inc. (Campbell, CA). The epidermal growth factor (EGFR) receptor inhibitors, PD1683, AG1478, were purchased from Calbiochem Biosciences Inc. (La Jolla, CA). The caspase 3 antibody was purchased from ALEXIS Biochemicals (San Diego, CA). The caspase 3 inhibitor (Ac-DEVD-CHO) was purchased from Sigma.
LDH Assay
A549 and 16HBE4o- cells were treated with LL-37 at a range of concentrations (5–300 μg/ml) at 37°Cin 5% CO2. The level of LDH in the cell supernatant was assayed in duplicate using a colorimetric Cytotoxicity Detection Kit (Roche, Mannheim, Germany). As a positive control for maximum LDH release, cells were treated with 1% Triton X-100 (Sigma), resulting in complete cell lysis, while medium-alone wells were used to assess background (0%) LDH release. For inhibition studies, A549 cells were incubated with Pertussis toxin (100 ng/ml), PD1683 (2 μM), or AG1478 (1 μM) for 1 h before stimulation with LL-37 for 24 h at 37°C in DMEM in the absence of serum.
Immunofluorescence
Immunofluorescence experiments were performed as described previously (38). Briefly, A549 cells were seeded onto 12-mm-diameter coverslips (VWR, Edmonton, AB, Canada) at a density of 5 × 104 cells per coverslip in DMEM supplemented with 10% FBS and incubated overnight at 37°C, 5% CO2. The following day, media was removed from the cells and replaced with either fresh DMEM supplemented with 10% FBS, 10% pooled AB human serum, or without serum supplementation. Biocytin was used as a negative control for all conditions. Biotinylated LL-37 (LL-37B, see above) was incubated with the cells for 4 h at 37°C, 5% CO2 and then coverslips processed by fixing the cells with 4% paraformaldehyde, permeabilizing with 0.1% Triton X-100 in PBS, and probing LL-37B with streptavidin/biotin-Oregon green. Actin was detected using Alexa 568–conjugated phalloidin (300 U; Molecular Probes), while cellular DNA was stained with 4′,6′-diamidino-2-phenylinodole (DAPI, 1.5 μg/ml; Vector Laboratories, Burlington, ON, Canada). Coverslips were viewed using a Bio-Rad radiance confocal microscope (Bio-Rad Laboratories, Mississauga, ON, Canada).
Binding Assay
Binding of LL-37B to A549 cells was performed exactly as described previously (38) for 2 h at 4°C. After incubation, the cells were washed gently with PBS and incubated with streptavidin–horseradish peroxidase (1/200 dilution; R&D Systems, Minneapolis, MN) at room temperature for 1 h and bound LL-37B detected using 3,3′,5,5′-tetramethylbenzidine liquid substrate (TMB; Sigma). The Bmax and Kd were obtained by nonlinear regression analysis using GraphPad (GraphPad Software Inc., San Diego, CA).
RNA Isolation
Total RNA was isolated from A549 cells and treated with or without LL-37 for 2 h, using RNAqueous (Ambion, Austin, TX) as per the manufacturer's directions. RNAse inhibitor (Ambion) was added to the RNA samples to prevent RNA degradation. To remove contaminating genomic DNA, the RNA samples were incubated with DNAse I (Ambion) for 30 min at 37°C. DNase I was subsequently inactivated using DNase inactivation reagent (Ambion). RNA quality and quantity was assessed using spectrophotometer at absorbance of 260 and 280 nm. Visualization of RNA was performed by electrophoresis on 2% agarose gel.
Semiquantitative RT-PCR
RNA was isolated as described above. Total RNA (500 ng) was reverse transcribed into cDNA using SuperScript II (Invitrogen). One microliter of the resulting cDNA was used as a template in PCR for IL-8 using as primers 5′-GTG CAG AGG GTT GTG GAG AAG-3′ and 5′-TTC TCC CGT GCA ATA TCT AGG-3′. Results were analyzed in the linear phase of amplification and normalized to the housekeeping control, GAPDH (5′-GAA ACT GTG GCG TGA TGG-3′, 5′-GTC GCT GTT GAA GTC AGA GG-3′). A lack of genomic DNA contamination was verified by including controls without reverse transcriptase.
Quantification of IL-8
Samples from the LDH experiments described above were also used to quantify the amount of IL-8 produced and secreted by A549 cells. Human IL-8 from the supernatants of A549 epithelial cells was measured using a commercially available ELISA kit (BioSource International, Montreal, PQ, Canada) as per the manufacturer's directions.
TUNEL Assay
A549 cells were seeded onto 12-mm-diameter coverslips at a density of 1 × 105 cells/coverslip in DMEM supplemented with 10% FBS and incubated overnight at 37°C, 5% CO2. The following day, medium was removed and A549 cells were washed with PBS before the addition of fresh DMEM supplemented with 10% FBS, 10% pooled AB human serum, or no serum. LL-37 was added to the media at final concentrations ranging from 10–100 μg/ml and incubated for 24 h. 16HBE4o- cells were seeded into 8-chamber Tissue culture slides (BD Falcon, Franklin Lakes, NJ) at a density of 2 × 104 cells/chamber in MEM with Earle's salts supplemented with 10% FBS and cultured to confluence at 37°C, 5% CO2. Cells were then serum starved for 4 h before addition of fresh MEM with Earle's salts supplemented with 10% pooled AB human serum or no serum. LL-37 was added to the media at final concentrations ranging from 0–50 μg/ml and incubated for 20 h. When specified, caspase 3 inhibitor was added at the same time as LL-37. A TUNEL assay assessing the generation of 3′OH ends as a result of the action of caspase-activated deoxribonuclease was performed using an In Situ Cell Death Detection Kit, Fluorescein (Roche) as per manufacturer's directions. Coverslips were mounted onto slides using Vecta-shield containing DAPI (Vector Laboratories). Coverslips were viewed using a Carl Zeiss fluorescence microscope (Thornwood, NY). TUNEL-positive cells were quantified by counting the ratio of TUNEL-positive cells to total cells in three fields of view in three independent experiments.
Immunoblot Analysis
After overnight growth, the medium bathing A549 cells was replaced with fresh DMEM supplemented with 10% FBS, 10% pooled AB human serum, or no serum. Cells were then incubated with LL-37 ranging from 10–100 μg/ml for 24 h. After stimulation, supernatants were removed and cells were washed with PBS. Cells were solubilized with preheated (95°C) 5× sample buffer (300 mM Tris-HCl pH 6.8, 0.5 M DTT, 12% SDS, 10% glycerol). For 16HBE4o-, cells were seeded into 6-well tissue culture plates (BD Falcon) at a density of 2 × 105 cells/well in MEM with Earle's salts supplemented with 10% FBS and cultured to confluence at 37°C, 5% CO2. Cells were then serum starved for 4 h before addition of fresh MEM with Earle's salts without serum. LL-37 was added to the media at final concentrations ranging from 0–50 μg/ml and incubated for 20 h. After stimulation, supernatants were removed and cells were washed with PBS. Cells were solubilized with RIPA buffer on ice for 10 min. Cell lysates (same procedure for A549 and 16HBE4o-) were then incubated at 95°C for 10 min before loading onto 14% SDS-PAGE. After electrophoretic separation, proteins were transferred to a nitrocellulose membrane. The membrane was blocked with 5% skim milk powder for 1 h and then incubated overnight at 4°C with primary antibody in TBS + 0.05% Tween 20 (TBST). Membranes were rinsed with TBST and then incubated for 2 h at room temperature with secondary antibody conjugated to horseradish peroxidase. After washing membranes for 30 min with TBST, immunoreactive bands were visualized by enhanced chemiluminescence (ECL) detection (Amersham, Piscataway, NJ). As a loading control, membranes were re-probed with anti-actin antibody. Quanititation of bands was performed using ImageJ (NIH, Bethesda, MD).
Murine Pulmonary Exposure to LL-37
Animal studies were performed with the approval of the UBC Animal Care Committee (UBC ACC # A01–0008). BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in standard animal facilities. Age-, sex-, and weight-matched adult mice were anesthetised with an intraperitoneal injection of Avertin (4.4 mM 2–2-2-tribromoethanol, 2.5% 2-methyl-2-butanol, in distilled water), using 200 μl per 10 g body weight. The intratracheal instillation was performed using a nonsurgical method, as previously described (3). Mice were given either (1) no intratracheal instillation, (2) 25 μl of sterile water, or (3) 25 μl of 2 mg/ml LL-37 solution in sterile water, gently instilled into the trachea with 200 μl of air and allowed to drain into the respiratory tree, n = 2 per condition. After 4 h the mice were killed by intraperitoneal injection of 300 mg/kg of pentobarbital. The lungs were removed, inflated with fixative, and processed to paraffin as previously described (39), but fixed with 1:1 acetone:methanol solution.
Lung Immunohistochemisty and TUNEL Analysis In Situ
Representative 5-μm lung sections were prepared as previously detailed (39). Immunohistochemical staining was performed as previously described (40), using a 50 μg/ml solution of mouse monoclonal antibody raised against LL-37 (clone 3D11, a kind gift from Pieter Hiemstra and Sandra Tjabringa, Dept. of Pulmonology, Leiden University Medical Center, The Netherlands), and 1 in 100 dilution of secondary FITC-labeled goat anti-mouse IgG (Sigma). Specimens were counterstained with Mayer's haematoxylin for 5 min, rinsed three times in PBS, and mounted in Vectashield (Vector Laboratories). TUNEL analyses were performed using an In Situ Cell Death Detection kit (Roche Diagnostics, Laval, PQ, Canada), according to the manufacturer's instructions, with detection of fluorescent enzymatic in situ labeling of apoptosis DNA strand breaks, but using acetone:methanol fixed samples. Specimens were counterstained with Mayer's haematoxylin as described above. Three representative sections were examined, blinded for treatment, for each animal in each form of analysis. Images were prepared using an Axioplan 2 fluorescent microscope (Carl Zeiss), DXC-390P digital camera (Sony, Tokyo, Japan), and Northern Eclipse, version 6.0, software (Empix Imaging Inc., Mississauga, ON, Canada).
HDL/Apolipoprotein A-1 Isolation from Whole Blood
Blood was taken from healthy individuals according to UBC Clinical Research Ethics Board protocol C02–0091 and collected in sodium heparin Vacutainer collection tubes (Becton Dickinson, Mississauga, ON, Canada). Blood samples were centrifuged for 25 min at 1,740 rpm. Plasma was transferred into 50-ml conical tubes, adjusted to 25 ml volumes with PBS, and the following was added: 0.04% ethylene diamine tetraacetic acid, 0.005% gentamicidin sulphate, 0.05% sodium azide, and 0.015% phenylmethanesulfonyl fluoride. Density of the resulting solution was adjusted to 1.063 g/ml with potassium bromide and centrifuged for 24 h at 165,000 × g, 4°C. Low density lipoproteins layer were removed, the volume of the resulting solution was re-adjusted to 25 ml with PBS and density to 1.21 g/ml with KBr and centrifuged for 24 h at 165,000 × g, 4°C. HDL layer was removed and HDL were delipidated with 2:1 chloroform:methanol to obtain apolipoprotein A-1. Each fraction was run on an 8% SDS-PAGE gel to confirm lipoprotein/protein isolation. A549 cells were simultaneously exposed to LL-37 and HDL or apolipoprotein A-1, and incubated for 24 h at 37°C.
Statistical Analysis
Results are expressed as the mean ± SEM. The paired Student's t test was used, with significance indicated by P < 0.05.
RESULTS
Serum Dependence of LL-37–Mediated Alteration in Cellular Membrane Permeability
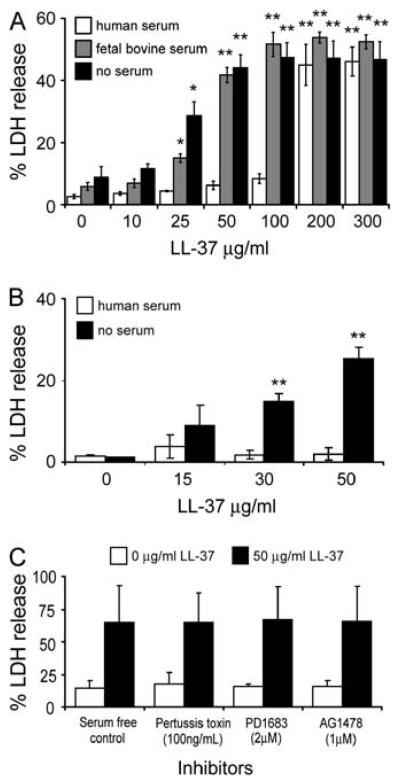
Cathelicidins have been shown to be capable of inducing eukaryotic cell membrane permeabilization, and the induction of apoptosis in specific cell types (23, 28, 31, 38). To investigate the ability of LL-37 to impact of the integrity of human pulmonary cells, A549 epithelial cells were incubated with a range of 10–300 μg/ml LL-37 for 24 h at 37°C in DMEM in the absence of serum, with 10% FBS, or with 10% pooled AB human serum. The level of lactate dehydrogenase (LDH) released into the supernatant was quantified as a marker of impaired cell membrane integrity (Figure 1A). With no serum supplementation, or in the presence of 10% FBS, LL-37 caused significant LDH release by A549 cells at concentrations as low as 25 μg/ml (P < 0.05). However, in human serum, no significant LDH release was observed at concentrations < 200 μg/ml LL-37 (P < 0.01). Similar results were achieved with 16HBE14o- cells (Figure 1B), a bronchial epithelial cell line that is not tumor-derived, but rather is transformed with SV40 and maintains many of the characteristics of primary cells including the ability to form tight junctions and differentiate to produce microvilli and cilia (41). These findings indicated that LL-37 induced perturbation of mammalian cell membrane integrity. This effect was not confined to tumor-derived cells, and could be antagonised by one or more serum components in human serum. To demonstrate specificity, we assessed the ability of the mouse homolog CRAMP (60% identity to LL-37) to induce LDH release. In contrast to LL-37, no statistically significant LDH release was observed at concentrations <100 μg/ml of CRAMP, although interestingly, the effects observed at >100 μg/ml CRAMP were similarly suppressed in the presence of 10% human serum (data not shown).
Figure 1.
LL-37–mediated alteration in membrane permeability in different serum conditions. (A) A549 epithelial cells were stimulated with 10–300 μg/ml of LL-37 for 24 h in DMEM supplemented with 10% pooled AB human serum, 10% FBS, or no serum, and the levels of lactate dehydrogenase (LDH) in the supernatant were determined. (B) 16HBE14o- epithelial cells were stimulated with 0–50 μg/ml of LL-37 for 20 h in MEM supplemented with 10% pooled AB human serum, or no serum, and the levels of LDH in the supernatant were determined. (C) A549 epithelial cells were pretreated with inhibitors for either Gi protein–coupled receptors (pertussis toxin, 100 ng/ml), or epidermal growth factor receptor (PD1683, 2 μM or AG1478, 1 μM) or untreated, for 1 h before stimulation with 50 μg/ml of LL-37 or a vehicle control for 24 h, and the levels of LDH in the supernatant determined. 100% LDH release was the amount of LDH released into the supernatant when 1% Triton X-100 was incubated with cells. 0% LDH release was taken as the amount of LDH in media alone. Results are expressed as mean ± SE of three independent experiments. Student's two-tailed t test was performed. **P < 0.01; *P < 0.05.
LL-37–Induced LDH Release Is Not Dependent upon Signaling via Known Receptors
To date there have been two receptors proposed for LL-37 on epithelial cells: the Gi protein–coupled receptor FPRL-1 (19) and EGFR (22). To investigate whether the LL-37–mediated LDH release was linked to either of these receptors, A549 epithelial cells were pre-treated with inhibitors specific for GPCR (pertussis toxin, 100 ng/ml), or for EGFR (PD1683 2 μM or AG1478 1 μM) for 1 h and then stimulated with a 50 μg/ml LL-37 for 24 h at 37°C in DMEM in the absence of serum. No decrease in LDH release was observed in the presence of these inhibitors (Figure 1C), indicating that this effect of LL-37 was not mediated through either FPRL-1 or EGFR.
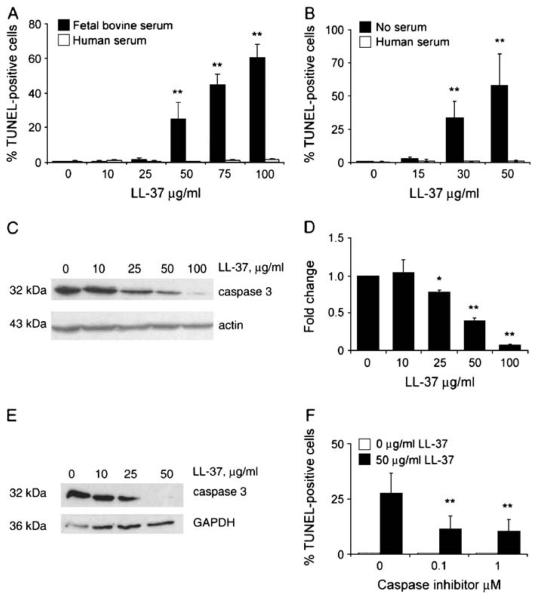
LL-37 Induces Apoptosis in A549 and 16HBE14o- Epithelial Cells
Certain cathelicidins have been shown to induce apoptosis in specific eukaryotic cell types (28, 29, 31). Given the effects of serum components upon perturbation of membrane integrity, LL-37 was tested for its ability to induce apoptosis in A549 epithelial cells under different serum conditions. One of the trademarks of apoptosis is the cleavage of genomic DNA, which can be assessed using the TUNEL assay. Free 3′-OH DNA ends are created when caspase-activated DNAse generates 180-bp DNA fragments from high molecular weight DNA fragments. Fluorescent labels can then be incorporated by terminal deoxynucleotidyl transferase (TdT), an enzyme that catalyzes the repetitive addition of mononucleotides from dNTP to the terminal 3′-OH of a DNA strand. Similar to the effects on membrane permeabilization, LL-37 exposure induced a dose-dependent increase in TUNEL-positive cells (indicative of caspase-activated DNAse activity) in the presence of 10% FBS, but not human serum, with significant apoptosis observed at concentrations ≥ 50 μg/ml (Figure 2A). At a concentration of 50 μg/ml of LL-37, ~ 30% of the cells were TUNEL-positive after 24 h, increasing to ~ 60% at a concentration of 100 μg/ml of LL-37. In contrast, in the presence of 10% pooled AB human serum, LL-37 did not induce A549 cells to become TUNEL-positive, even at peptide concentrations as high as 100 μg/ml. Similar data was obtained for the transformed bronchial epithelial cell line 16HBE14o- (Figure 2B), with a concentration-dependent induction of apoptotic cells in the absence of serum, inhibited by human serum.
Figure 2.
Induction of epithelial cell apoptosis by LL-37 in A549 and 16HBE14o- cells. (A) A549 epithelial cells were incubated with 0–100 μg/ml of LL-37 in the presence of 10% FBS or 10% pooled AB human serum for 24 h. The percentage of TUNEL-positive cells was quantified by counting three fields of view. Results are expressed as mean ± SE for three separate experiments. Student's two tailed t test was performed. **P < 0.01. (B) 16HBE14o- epithelial cells were stimulated with 0–50 μg/ml LL-37 for 20 h in MEM supplemented with 10% pooled AB human serum, or no serum, and quantified as above. (C) Representative Western immunoblot of total protein lysates from A549 epithelial cells incubated in DMEM with 10% FBS, over a range of concentrations of LL-37, probed for immunoreactivity with anti–caspase 3 antibody, and an anti-actin antibody as a loading control. (D) Ratio of caspase 3 protein detected in LL-37–stimulated and unstimulated A549 epithelial cells. A ratio of one indicates no change versus control. The intensities of three independent Western blots were measured, normalized to the intensity of actin, to correct for inconsistencies in loading. The LL-37–stimulated protein levels relative to unstimulated levels were calculated as the mean ± SE for three separate experiments. Student's two tailed t test was performed. **P < 0.01; *P < 0.05. (E) Representative Western immunoblot of total protein lysates from 16HBE14o- epithelial cells incubated in MEM without serum, over a range of concentrations of LL-37, probed for immunoreactivity with anti–caspase 3 antibody, and an anti-GAPDH antibody as a loading control. (F) A549 cells were grown overnight in DMEM media supplemented with 10% FBS, and incubated for 20 h with LL-37 (50 μg/ml) or untreated, with or without caspase 3 inhibitor (0.1 or 1 μM Ac-DEVD-CHO). Cells were then washed in PBS and fixed for subsequent TUNEL staining.
LL-37–induced apoptosis was confirmed by Western blot analysis, by examining the activation of caspase 3 (Figures 2C-2E), a common effector for all apoptotic pathways. The native pro–caspase 3 is 32 kD, and in the process of inducing apoptosis through the extrinsic or intrinsic pathways, it is cleaved at Asp-175 to form two fragments, 17 and 19 kD. Thus, a decrease in the amount of the pro-caspase 3 is indicative of activation. LL-37 induced a significant decrease in the levels of pro-caspase 3 starting at concentrations of 25–30 μg/ml in A549 and 16HBE14o- cells, demonstrating greater sensitivity than the TUNEL assay, and correlating with the dose dependence of LDH release. A caspase 3 inhibitor reduced the LL-37–mediated induction of TUNEL-positive A549 cells by approximately two thirds (Figure 2F), indicating the significance of caspase pathway induction in LL-37–induced cell death.
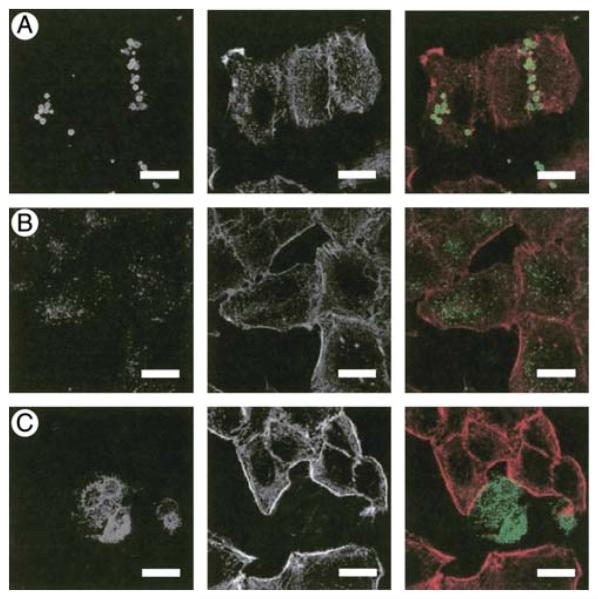
Serum Dependence of LL-37B Binding to and Localization in Lung Epithelial Cells
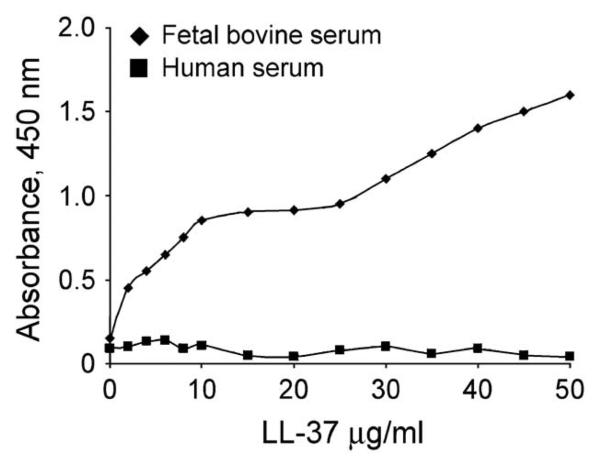
We previously demonstrated that LL-37B, in the presence of FBS, binds to two receptors on the surface of A549 epithelial cells, with Kd values of 0.76 and 2.46 μM, and is subsequently taken up via an atypical tubulin-dependent endocytic pathway and localizes to a perinuclear location (38). To determine the effects of serum on the localization of LL-37B, A549 epithelial cells were incubated with 10 μg/ml LL-37B in the presence of DMEM together with 10% pooled AB human serum or in the absence of serum (Figure 3). In contrast to the perinuclear distribution observed with FBS, LL-37B was not observed to be present within cells in the presence of human serum. In the absence of serum, LL-37B became localized to the nucleus in approximately 20% of the cells. On the contrary, in the presence of 10% FBS, a much higher concentration of LL-37B (50 μg/ml) was required to observe equivalent intense nuclear localization of peptides within a similar proportion of cells (38). The ability of LL-37B to translocate to the nucleus or to the perinuclear region may thus correlate with or be responsible for cell death under these conditions. The binding of LL-37B to A549 epithelial cells in the presence of human serum was negligible when compared to LL-37B in the presence of FBS (Figure 4), and was equivalent to the binding of the biocytin control (data not shown).
Figure 3.
Localization of LL-37B in the presence of different serum sources. A549 epithelial cells were incubated at 37°C with 10 μg/ml (2.2 μM) of LL-37B for 4 h in DMEM with (A) 10% pooled AB human serum, (B) 10% FBS, or (C) no serum. Cells were prepared for immunofluorescence assessment as described in Materials and Methods. Right panels represent a merge of LL-37B (left panels, green) and actin (middle panels, red). Bars represent 20 μm.
Figure 4.
Inhibition of LL-37B binding to A549 epithelial cells by human serum. A549 cells were preincubated at 4°C for 20 min before the addition of 2–50 μg/ml of LL-37B in the presence of 10% FBS, or 10% human serum, then incubated for 2 h at 4°C. Streptavidin–horseradish peroxidase and TMB was added sequentially to visualize binding. Each point represents the means from three individual experiments, each of which was performed in duplicate.
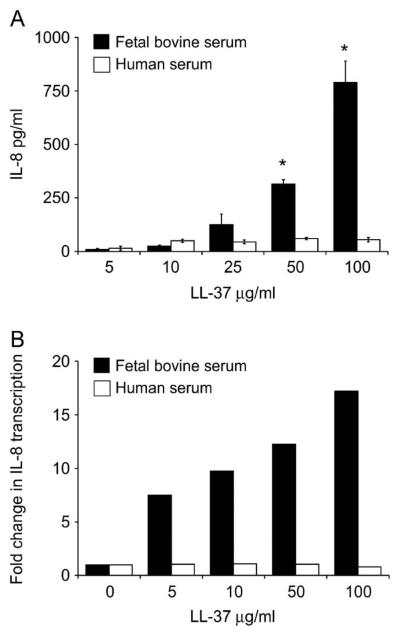
The Presence of Human Serum Inhibits LL-37–Induced IL-8 Gene Expression and Secretion
LL-37 is known to induce production of IL-8, a neutrophil-specific chemokine, in A549 cells (3, 38). We have previously shown this to be dependent upon active endocytosis in these cells and correlated with uptake of LL-37 (38, 42). Since the presence of human serum altered the cellular localization of LL-37 and peptide-induced cell death, we determined whether the presence of human serum would also alter the expression and production of IL-8 from A549 cells. A549 cells were stimulated over a range of LL-37 concentrations, in the presence of 10% FBS, or 10% pooled AB human serum, and the supernatants were tested for IL-8. As anticipated, in the presence of FBS, there was a dose-dependent increase in IL-8 production (Figure 5A). In contrast, in the presence of human serum, no significant increase in IL-8 secretion was observed in response to increasing concentrations of LL-37 (Figure 5A). The transcriptional expression of the IL-8 gene was also examined. LL-37 induced IL-8 expression in a dose-dependent manner in FBS, whereas in the presence of human serum there was no increase in IL-8 expression in response to LL-37 stimulation (Figure 5B). Therefore, human serum inhibits IL-8 expression and secretion in response to LL-37.
Figure 5.
Inhibition of LL-37–induced IL-8 gene expression and secretion by human serum. A549 cells were stimulated with LL-37 in the presence of FBS or human serum. (A) IL-8 protein secretion determined by ELISA; means ± SD of three independent assays are shown, *P < 0.01. (B) Relative IL-8 gene expression determined by semiquantitative RT-PCR corrected for RNA concentration using GAPDH expression levels. Fold change over expression in untreated control cells is demonstrated and represents the mean results for three independent assays.
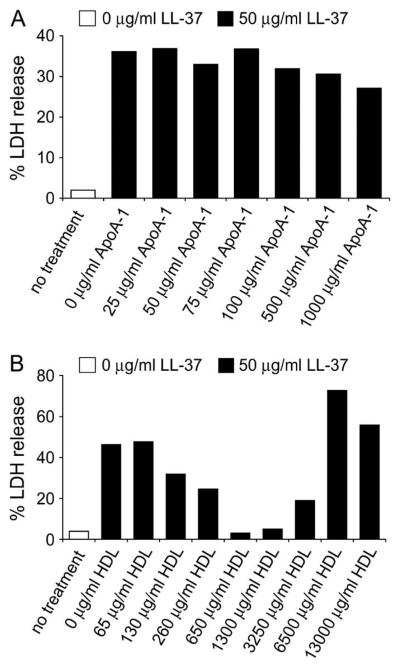
Suppression of LL-37–Induced Membrane Permeabilization by HDL
It has been demonstrated that apolipoprotein A-1 binds LL-37 in human plasma, potentially working as a scavenger of LL-37 and inhibiting antimicrobial function (18, 43). We hypothesized that it might also affect peptide-induced membrane permeabilization and cell death. To determine whether apolipoprotein A-1 or HDL are involved in reducing the cytotoxic effects of LL-37, A549 epithelial cells were incubated for 24 h with either apolipoprotein A-1 or HDL in the presence or absence of 10% FBS. The levels of LDH released into the supernatant in response to LL-37 exposure were measured. Apolipoprotein A-1 was unable to block the effects of 50 μg/ml LL-37, in the presence of 10% FBS (Figure 6). However, a dose-dependent inhibition was observed in the presence of HDL. At a concentration of 650 μg/ml, HDL completely blocked the effect of 50 μg/ml LL-37, although concentrations of HDL > 3,250 μg/ml appeared themselves to induce LDH release (Figure 6). The same trend was observed in the absence of serum whereby HDL, but not apolipoprotein A-1, was able to reduce the induction of cell death by LL-37 (data not shown).
Figure 6.
Protection by HDL against LL-37–induced LDH release. A549 epithelial cells were incubated with 50 μg/ml of LL-37 alone or with increasing concentrations of (A) Apolipoprotein A-1 or (B) HDL in the presence of 10% FBS for 24 h and the levels of LDH in the supernatant assessed. 100% LDH release was the amount of LDH released into the supernatant when 1% Triton X-100 was incubated with cells. 0% LDH release was taken as the amount of LDH in media alone. Results are expressed as the mean of two separate experiments.
Murine Pulmonary Exposure to High-Dose LL-37 Induces Airway Epithelial Cell Apoptosis In Vivo
To examine peptide-induced cell death in vivo, mice were exposed to a high dose of LL-37, or distilled water as a carrier control, by nonsurgical intratracheal instillation. Lung sections from these mice were compared to each other and to controls from animals not exposed to any manipulations. We previously observed that this method of intratracheal instillation results in a focal distribution pattern in the alveoli, but more homogeneous distribution through the conducting airways, with approximately 10% of the dose localized to the larger airways (data not shown). Given a mean total conducting airway surface area in an adult mouse of 10 cm2 (44) with an approximate airway surface liquid depth of between 50 μm in the trachea (45), and 5 μm in smaller bronchioles, we estimated that the peptide load delivered would distribute within the conducting airways and would be subject to a 1 in 10 dilution in airway surface liquid. Thus, 25 μl of a 2 mg/ml solution (i.e., 50 μg) of LL-37 was delivered intratracheally to generate an estimated exposure concentration of approximately 200 μg/ml to the airway epithelial cells, although we would anticipate that the concentration would be much more dilute, and quite variable over the entire lung due to the large surface area of the alveoli, and effects of liquid bolus delivery.
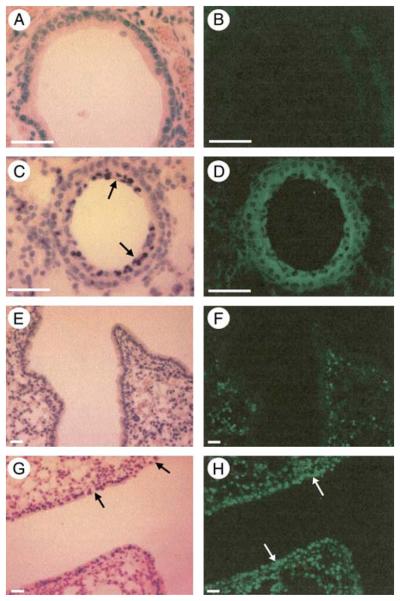
The lungs were collected for analysis 4 h after intratracheal instillation. Immunohistochemical staining demonstrated no signal in the carrier control treated animals (Figure 7B). In contrast there was widespread distribution of LL-37 in the airways of mice treated with peptide (Figure 7D). Nuclear localization of LL-37 in epithelial cells, observed in vitro in the absence of serum, at high peptide concentrations and longer time points (38) was not observed in vivo. This could be due either to leakage of serum or serum components into the lungs of LL-37–infused mice, to lower than predicted concentrations of LL-37 in contact with epithelial cells, or other as yet undefined native constituents of murine airway surface liquid that impact on the effects of LL-37 in this model. Histochemical analysis of the peptide-treated animals revealed evidence of cell death in LL-37–treated animals, with irregular epithelial cell organization, substantial loss of integrity, and luminal accumulations of cells, including numerous shed airway epithelial cells with pyknotic nuclei (but no neutrophils) in many airways (Figures 7C and 7G).
Figure 7.
Detection of LL-37 and epithelial cell apoptosis in the murine airway after intratracheal instillation of peptide. Lung sections from mice given a 25-μl intratracheal bolus of water, as a control carrier vehicle (A, B, E, and F), or 2 mg/ml LL-37 solution (C, D, G, and H) were examined by light microscopy after staining with Mayer's haematoxylin (A, C, E, and G), by immunohistochemistry for the presence of LL-37 (B and D), or TUNEL analysis to assess apoptosis (F and H). Regular airway morphology was observed in the control animals (A and E). Irregular epithelial cell organization, loss of epithelial integrity (black arrows in G), and luminal accumulations of cells (black arrows in C), including numerous shed airway epithelial cells, were observed in the airways after exposure to high dose LL-37 (C and G). Widespread distribution of LL-37 in the airways and alveoli was demonstrated (green positive signal for FITC-labeled secondary antibody detecting anti-LL37 primary antibody) in mice treated with peptide (D), and no positive signal was detected in the control animals (B). In untreated mice, TUNEL-positive cells were observed scattered throughout the lung parenchyma (green signal), but with no positive signals in airway epithelial cells (F). In contrast, LL-37–treated mice demonstrated extensive apoptosis of airway epithelium by TUNEL assay (white arrows; H). The white bar represents 20 μm. Figures are representative of three lung sections each from two mice for each condition.
In addition, TUNEL analysis was performed to assess apoptosis, demonstrating scattered positive cells in the lung parenchyma of control untreated mice, but no positive signals in airway epithelial cells (Figure 7F). In contrast, in LL-37–treated mice, whole sections of conducting airway demonstrated positive signal by TUNEL in the entire epithelial lining (Figure 7H). No differences were observed between untreated mice and those exposed to 25 μl of distilled water intratracheally as a carrier control (data not shown). These in vivo studies demonstrate significant airway epithelial cell apoptosis in response to high dose LL-37 exposure, supporting the observations from the in vitro studies.
DISCUSSION
Many cationic peptides are able to kill a wide range of microorganisms in vitro, with varying degrees of effectiveness, but have been suggested to have limited cytotoxicity toward eukaryotic cells, based often on an examination of hemolysis in the absence of serum. The bovine cathelicidins BMAP-27 and BMAP-28, for example, have been shown to induce membrane permeabilization and apoptosis in eukaryotic cells as well as microbes, mediated by their hydrophobic C-terminal tails (28). This may not be a conserved property of all cathelicidins, as we have previously demonstrated that indolicidin and a bactenecin derivative Bac2A do not induce cell death in either an airway epithelial or monocyte-like cell line in the presence of FBS (46), while CRAMP, the murine homolog of LL-37, induced significantly less cell death than LL-37 in A549 cells. Although the sole human cathelicidin, LL-37, has been reported to induce cell death in peripheral blood leukocytes at concentrations above 125 μg/ml (25 μM) in the presence of 10% FBS (30), we previously demonstrated an absence of LL-37–induced cell death in primary human monocytes or lymphocytes at concentrations of peptide up to 50 μg/ml in the presence of 10% FBS (26, 47). LL-37–derived peptides have been demonstrated to induce membrane permeabilization and apoptosis in tumor cell lines (31); however, neither these peptides nor native LL-37 have a hydrophobic tail, and thus the mechanism of cell death induction might be different from that ascribed to the bovine cathelicidins.
We demonstrate here that LL-37 induced dose-dependent membrane permeabilization and apoptosis in airway epithelial cells, both in vitro in human A549 and 16HBE4o- cells, and in vivo in murine airway epithelial cells. Furthermore, LL-37 induced significantly more cell death in the absence of serum than in the presence of human serum, with FBS having a much more modest effect. This is in keeping with previous reports describing a protective effect of human serum on LL-37–exposed leukocytes (30), and the inhibition of the antimicrobial effects of this peptide as a consequence of binding to apolipoprotein A-1 (30, 43). LL-37–induced LDH release in these studies correlated with dose-dependent induction of TUNEL-positive cells and caspase activation, and likely represents cytolysis secondary to a failure to clear apoptotic cells in vitro. However, we also anticipate an early contributory release of LDH reflecting temporary and noncytotoxic membrane perturbation and permeabilization, as previously described in response to LL-37 (23, 38) and other cathelicidins (28). The significance of such an effect to the induction of apoptosis, and the precise mechanisms underlying these effects remain undetermined.
To date there have been a number of receptors associated with LL-37–induced immunomodulation, including FPRL-1 and EGFR on epithelial cells and P2X7 on monocytes, as well as unidentified high- and low-affinity receptors on mast cells (19, 20, 22). Inhibitors of EGFR associated tyrosine kinases blocked LL-37–induced IL-8 production in airway epithelial cells and partially inhibited IL-8 production from keratinocytes (22, 48). LL–37-mediated chemotaxis can be inhibited either by an agonist of the G protein–coupled receptor FPRL-1 or by pertussis toxin (19). However, other LL-37–mediated effects, such as MAP kinase activation and IL-8 production in monocytes, are not pertussis toxin–sensitive, indicating that LL-37 may mediate these events through other receptors or other mechanisms of action. LL-37–induced cell death does not appear to be linked to either EGFR or FPRL-1, implying induction by an alternative unidentified receptor or mechanism of action. A recent observation that LL-37 synthesized with D-amino acids (D-LL-37), which would be predicted to have the reversed three-dimensional structure, is a more potent inducer of IL-8 from keratinocytes than is the conventionally synthesized L-form, raised some interesting questions concerning whether LL-37 has a conventional receptor (48). Although most receptors do not recognize analogs made from D-amino acids, it has been proposed that receptors that bind a wide range of proteins and peptides might also be able to bind to peptides composed of D-amino acids by recognizing the lower-order structural properties of their ligands, specifically regions of segmental or helical amphipathicity (49, 50). LL-37 is an α-helical peptide with such regions of amphipathicity and thus might interact with a promiscuous binding protein.
While the precursor cathelicidin protein hCAP-18 is predominantly bound to low density protein/very low density lipoprotein particles in human serum (18), LL-37 has been previously demonstrated to bind predominantly to apolipoprotein A-1 (43, 51), a component of HDL in human serum. Indeed, it has been suggested that apolipoprotein A-1 acts as the main LL-37–binding protein (Kd of 0.6–2.4 μM) in human serum, and has been hypothesized to inhibit eukaryotic cell cytotoxicity (18, 43). These reports implicated apolipoprotein A-1 and HDL as candidate serum components responsible for the protective effects of human serum observed in our studies. The protective effects of human serum could not be replicated using apolipoprotein A-1. However, in contrast, physiologically relevant concentrations of HDL demonstrated an effective, dose-dependent inhibition of the LL-37–induced cell death, observed both in the absence of serum and in the presence of 10% FBS. Although these studies do not eliminate the possibility that other components of serum may also contribute to the protective effects of human serum in LL-37–exposed airway epithelial cells, they suggest that HDL could be a major contributor. HDL comprises a lipidic shell surrounding an apolipoprotein A-1 core and is present in human serum at a concentration range from 0.94–1.99 mg/ml (52). The failure of apolipoprotein A-1 to inhibit LL-37–induced cell death suggest that despite LL-37 binding capacity, it may need to be in its lipid-bound conformation to prevent these effects of this peptide. The binding of LL-37 to HDL may thus inhibit induction of cell death by circulating LL-37 in the blood, and thus be analogous to defensin (HNP-1) binding by activated α2-microglobulin (53).
A dose-dependent increase in TUNEL positive A549 and 16HBE4o- cells, indicative of apoptosis, was observed starting at 25–50 μg/ml LL-37 in the absence of serum or in the presence of FBS. In contrast, no TUNEL-positive cells were observed in the presence of human serum, even at LL-37 concentrations up to 100 μg/ml, correlating with the protective effects of human serum observed against LL-37–induced LDH release. In keeping with these in vitro observations in the presence of LL-37, a dramatic loss of airway epithelial architecture and integrity was observed in the mouse lung exposed to high dose LL-37, accompanied by shed epithelial cells and extensive areas of TUNEL-positive airway epithelium. LL-37–induced apoptosis in vitro occurred through caspase activation, as demonstrated by dose-dependent activation of caspase 3, and inhibition with a caspase 3 inhibitor in epithelial cells exposed to LL-37. However, the precise mechanism by which LL-37 induced epithelial cell apoptosis, and the role of caspase 3 activation, remains to be fully elucidated.
We have previously described the receptor-mediated uptake and perinuclear localization of LL-37 by epithelial cells in the presence of FBS (38). Here it was demonstrated that this process is also dependent upon the type of serum present. In the absence of serum (the condition under which maximal cell death was observed), LL-37 localized to the nucleus, often in what appeared to be dividing cells. Interestingly, in such cells actin could not be visualized using Alexa-conjugated phalloidin (Figure 3) due presumably to the binding of LL-37 to actin (54) and probably reflecting high uptake of LL-37. In the presence of the protective level of human serum, no LL-37 was detected inside cells, in marked contrast to the cytoplasmic and perinuclear localization of LL-37 observed in A549 lung epithelial cells in the presence of FBS. When LL-37 was observed in the presence of human serum, it was detected only in cell-associated aggregates. This data is consistent with the possibility that cellular, and particularly nuclear uptake of LL-37 is associated with the induction of cell death in epithelial cells.
We also demonstrated that the LL-37–induced transcription and release of the neutrophil chemokine IL-8 by epithelial cells is inhibited in the presence of 10% human serum. Although this cytokine response has previously shown to be an MAPK-dependent, receptor-mediated process involving EGFR (22, 48), our previous results (42) indicated that it was dependent on LL-37 uptake, which did not occur in the presence of human serum. However, there is probably not a cause-and-effect relationship between the induction of cell death and IL-8 expression based on parallel observations in other cell types. Exposure of primary human monocytes to LL-37 in the presence of FBS also induced MAPK-dependent transcription and release of chemokines IL-8 and MCP-1, but did so in the absence of any detectable cell death (21). Indeed, under serum-free conditions, where cell death was observed in response to LL-37 (42), monocytes did not undergo LL-37–dependent activation of MAPK (21), indicating a requirement of serum components for this signaling process to occur. Furthermore, LL-37–induced MAPK signaling and chemokine expression in primary human monocytes was also observed in the presence of human serum (D. M. E. Bowdish and coworkers, unpublished data), and LL-37–induced expression of MCP-1 was observed in whole human blood (3).
These data suggest that epithelial cells may be inherently more sensitive to LL-37–induced cell death than are peripheral blood mononuclear cells. While serum antagonizes the effects of LL-37, epithelial cells generally reside in serum-free environments, and thus may be acutely sensitive to high concentrations of LL-37 encountered during infection, inflammation, or injury, such as observed in the airway surface liquid during lung infection (14). Apoptosis of infected cells has been demonstrated to be important in the clearance of infection at the lung, bladder, and corneal epithelium (55-57). For example, the epithelial cell internalization of Pseudomonas aeruginosa after lipid platform reorganization, and subsequent apoptosis of these epithelial cells, has been demonstrated to be essential for clearance of infection and survival in a mouse model of pulmonary infection (55, 58). In this context, the apoptosis observed in response to delivery of high dose LL-37 into the serum-free environment of the murine lung may represent an exaggerated host defense response. We propose that LL-37 may alter the balance of pro- and anti-apoptotic gene expression in epithelial cells to initiate a “pre-apoptotic” state in which cells are primed for apoptosis to contribute to host defense against acute infection, complemented by an LL-37–induced enhancement of wound healing for resolution of inflammation (5, 25). This may occur without initiating a proinflammatory response, as we have previously demonstrated in studies performed in parallel that instillation of LL-37 in the mouse lung results in increased levels of the chemokine MCP-1 but not the proinflammatory cytokine TNF-α (3). However, these data also raise questions about the effects of long-term exposure to high levels of LL-37. This could be of particular significance in the neutrophil-dominated chronic inflammation associated with cystic fibrosis, in which raised pulmonary levels of LL-37 have been correlated with severity of lung disease (59). In addition, failure by epithelial cells to internalize P. aeruginosa via the cystic fibrosis transmembrane conductance regulator protein has been implicated as the cause of pulmonary colonization with this pathogen in cystic fibrosis (60). In this context, LL-37–enhanced apoptosis of these epithelial cells would not augment pathogen clearance and may even be detrimental.
We have demonstrated that airway epithelial cells are sensitive to LL-37–induced membrane permeabilization and apoptosis in vitro and in vivo, in a manner that can be inhibited by human serum and involves the activation of caspase pathways. The cellular internalization of LL-37 and expression of IL-8 correlate with peptide-induced cell death in these cells, and can also be inhibited by human serum. The protective effects of human serum can be replicated by using HDL; however, FBS is unable to inhibit LL-37–induced cell death, IL-8 release, or peptide uptake by epithelial cells. These data indicate the need to establish the most appropriate serum environment for specific cells types in vitro in the study of host defense peptides. The clear distinction between the responses of airway epithelial cells and mononuclear leukocytes to LL-37 exposure indicate that given concentrations of this, and other, host defense peptides in vivo may have quite contrasting consequences at different sites in the body. Further studies will be required to fully establish the physiologically relevant roles of this multipotent immunomodulator in health and in disease states, and to determine the relevance of these observations to therapeutic application of analogous peptides.
Acknowledgments
The authors thank Pieter S. Hiemstra and G. Sandra Tjabringa for providing the LL-37 antibody, and David Speert, Monisha Scott, Annett Rozek, and Danika Goosney for advice and assistance.
Financial support was provided from the Canadian Institutes of Health Research (CIHR). Y.E.L. was the recipient of a Canadian Cystic Fibrosis Foundation Student-ship; D.M.E.B. is supported by a CIHR studentship; R.E.W.H. holds a Canada Research Chair; and D.J.D. was funded by a Wellcome Trust UK, International Prize Travelling Research Fellowship (060168), and the Norman Salvesen Emphysema Research Trust.
Footnotes
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 2.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1:141–150. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 4.Welling MM, Hiemstra PS, van den Barselaar MT, Paulusma-Annema A, Nibbering PH, Pauwels EK, Calame W. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. J Clin Invest. 1998;102:1583–1590. doi: 10.1172/JCI3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koczulla R, Von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukumoto K, Nagaoka I, Yamataka A, Kobayashi H, Yanai T, Kato Y, Miyano T. Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr Surg Int. 2005;21:20–24. doi: 10.1007/s00383-004-1256-x. [DOI] [PubMed] [Google Scholar]
- 7.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial Peptide chemoattracts leukocytes using formyl Peptide receptor-like 1/mouse formyl Peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 8.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 10.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 11.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson GH. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 14.Schaller-Bals S, Schulze A, Bals R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med. 2002;165:992–995. doi: 10.1164/ajrccm.165.7.200110-020. [DOI] [PubMed] [Google Scholar]
- 15.Armogida SA, Yannaras NM, Melton AL, Srivastava MD. Identification and quantification of innate immune system mediators in human breast milk. Allergy Asthma Proc. 2004;25:297–304. [PubMed] [Google Scholar]
- 16.Bals R, Wilson JM. Cathelicidins-a family of multifunctional antimicrobial peptides. Cell Mol Life Sci. 2003;60:711–720. doi: 10.1007/s00018-003-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen O, Bratt T, Johnsen AH, Madsen MT, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J Biol Chem. 1999;274:22445–22451. doi: 10.1074/jbc.274.32.22445. [DOI] [PubMed] [Google Scholar]
- 19.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowdish DM, Davidson DJ, Speert DP, Hancock RE. The human cationic peptide LL-37 induces activation of the extracellular signal-regulated kinase and p38 kinase pathways in primary human monocytes. J Immunol. 2004;172:3758–3765. doi: 10.4049/jimmunol.172.6.3758. [DOI] [PubMed] [Google Scholar]
- 22.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 23.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 24.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 26.Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, Hancock RE, Speert DP. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172:1146–1156. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 27.Skerlavaj B, Gennaro R, Bagella L, Merluzzi L, Risso A, Zanetti M. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J Biol Chem. 1996;271:28375–28381. doi: 10.1074/jbc.271.45.28375. [DOI] [PubMed] [Google Scholar]
- 28.Risso A, Zanetti M, Gennaro R. Cytotoxicity and apoptosis mediated by two peptides of innate immunity. Cell Immunol. 1998;189:107–115. doi: 10.1006/cimm.1998.1358. [DOI] [PubMed] [Google Scholar]
- 29.Risso A, Braidot E, Sordano MC, Vianello A, Macri F, Skerlavaj B, Zanetti M, Gennaro R, Bernardi P. BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol Cell Biol. 2002;22:1926–1935. doi: 10.1128/MCB.22.6.1926-1935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson J, Gudmundsson GH, Rottenberg ME, Berndt KD, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 31.Okumura K, Itoh A, Isogai E, Hirose K, Hosokawa Y, Abiko Y, Shibata T, Hirata M, Isogai H. C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SASH1 cells. Cancer Lett. 2004;212:185–194. doi: 10.1016/j.canlet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 34.Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- 36.Tsujimoto Y, Shimizu S, Eguchi Y, Kamiike W, Matsuda H. Bcl-2 and Bcl-xL block apoptosis as well as necrosis: possible involvement of common mediators in apoptotic and necrotic signal transduction pathways. Leukemia. 1997;11:380–382. [PubMed] [Google Scholar]
- 37.Denecker G, Vercammen D, Declercq W, Vandenabeele P. Apoptotic and necrotic cell death induced by death domain receptors. Cell Mol Life Sci. 2001;58:356–370. doi: 10.1007/PL00000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau YE, Rozek A, Scott MG, Goosney DL, Davidson DJ, Hancock RE. Interaction and cellular localization of the human host defense peptide LL-37 with lung epithelial cells. Infect Immun. 2005;73:583–591. doi: 10.1128/IAI.73.1.583-591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson DJ, Webb S, Teague P, Govan JR, Dorin JR. Lung pathology in response to repeated exposure to Staphylococcus aureus in congenic residual function cystic fibrosis mice does not increase in response to decreased CFTR levels or increased bacterial load. Pathobiology. 2004;71:152–158. doi: 10.1159/000076470. [DOI] [PubMed] [Google Scholar]
- 40.Davidson DJ, Kilanowski FM, Randell SH, Sheppard DN, Dorin JR. A primary culture model of differentiated murine tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 2000;279:L766–L778. doi: 10.1152/ajplung.2000.279.4.L766. (Corrigendum: Am J Physiol Lung Cell Mol Physiol 2001;280:La1) [DOI] [PubMed] [Google Scholar]
- 41.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 42.Bowdish DM, Davidson DJ, Lau YE, Lee K, Scott MG, Hancock RE. Impact of LL-37 on anti-infective immunity. J Leukoc Biol. 2005;77:451–459. doi: 10.1189/jlb.0704380. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Agerberth B, Lothgren A, Almstedt A, Johansson J. Apolipoprotein A-I binds and inhibits the human antibacterial/cytotoxic peptide LL-37. J Biol Chem. 1998;273:33115–33118. doi: 10.1074/jbc.273.50.33115. [DOI] [PubMed] [Google Scholar]
- 44.Geiser M, Zimmermann B, Baumann M, Cruz-Orive LM. Does lack of Cftr gene lead to developmental abnormalities in the lung? Exp Lung Res. 2000;26:551–564. doi: 10.1080/019021400750048090. [DOI] [PubMed] [Google Scholar]
- 45.Jayaraman S, Song Y, Vetrivel L, Shankar L, Verkman AS. Noninvasive in vivo fluorescence measurement of airway-surface liquid depth, salt concentration, and pH. J Clin Invest. 2001;107:317–324. doi: 10.1172/JCI11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowdish DM, Davidson DJ, Scott MG, Hancock RE. Immunomodulatory activities of small host defense peptides. Antimicrob Agents Chemother. 2005;49:1727–1732. doi: 10.1128/AAC.49.5.1727-1732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowdish DM, Davidson DJ, Hancock RE. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci. 2005;6:35–51. doi: 10.2174/1389203053027494. [DOI] [PubMed] [Google Scholar]
- 48.Braff MH, Hawkins MA, Nardo AD, Lopez-Garcia B, Howell MD, Wong C, Lin K, Streib JE, Dorschner R, Leung DY, et al. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005;174:4271–4278. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 49.Kersh GJ, Allen PM. Essential flexibility in the T-cell recognition of antigen. Nature. 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 50.Fisher PJ, Prendergast FG, Ehrhardt MR, Urbauer JL, Wand AJ, Sedarous SS, McCormick DJ, Buckley PJ. Calmodulin interacts with amphiphilic peptides composed of all D-amino acids. Nature. 1994;368:651–653. doi: 10.1038/368651a0. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Johansson J, Agerberth B, Jornvall H, Griffiths WJ. The antimicrobial peptide LL-37 binds to the human plasma protein apolipoprotein A-I. Rapid Commun Mass Spectrom. 2004;18:588–589. doi: 10.1002/rcm.1361. [DOI] [PubMed] [Google Scholar]
- 52.Sorci-Thomas MG, Thomas MJ. The effects of altered apolipoprotein A-I structure on plasma HDL concentration. Trends Cardiovasc Med. 2002;12:121–128. doi: 10.1016/s1050-1738(01)00163-3. [DOI] [PubMed] [Google Scholar]
- 53.Panyutich A, Ganz T. Activated α2-macroglobulin is a principal defensin-binding protein. Am J Respir Cell Mol Biol. 1991;5:101–106. doi: 10.1165/ajrcmb/5.2.101. [DOI] [PubMed] [Google Scholar]
- 54.Weiner DJ, Bucki R, Janmey PA. The antimicrobial activity of the cathelicidin LL37 is inhibited by F-actin bundles and restored by gelsolin. Am J Respir Cell Mol Biol. 2003;28:738–745. doi: 10.1165/rcmb.2002-0191OC. [DOI] [PubMed] [Google Scholar]
- 55.Grassme H, Kirschnek S, Riethmueller J, Riehle A, von Kurthy G, Lang F, Weller M, Gulbins E. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa. Science. 2000;290:527–530. doi: 10.1126/science.290.5491.527. [DOI] [PubMed] [Google Scholar]
- 56.Aronson M, Medalia O, Amichay D, Nativ O. Endotoxin-induced shedding of viable uroepithelial cells is an antimicrobial defense mechanism. Infect Immun. 1988;56:1615–1617. doi: 10.1128/iai.56.6.1615-1617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fleiszig SM, Zaidi TS, Pier GB. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect Immun. 1995;63:4072–4077. doi: 10.1128/iai.63.10.4072-4077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 59.Chen CI, Schaller-Bals S, Paul KP, Wahn U, Bals R. Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J Cyst Fibros. 2004;3:45–50. doi: 10.1016/j.jcf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Pier GB, Grout M, Zaidi TS, Olsen JC, Johnson LG, Yankaskas JR, Goldberg JB. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]