Abstract
Background
Herpes simplex virus (HSV) suppressive therapy reduces genital and plasma HIV-1 RNA over periods up to three months but the longer-term effect is unknown.
Methods
484 HIV-1 and HSV-2 seropositive Tanzanian women aged 16-35 years were enrolled in a randomised placebo-controlled trial of acyclovir 400mg bid. Cervico-vaginal lavage and blood samples were collected at 6m, 12m and 24m for quantification of genital and plasma HIV-1 RNA, and genital HSV DNA. Primary outcomes were detection and quantity of cervico-vaginal HIV-1 RNA at 6m.
Results
At 6m, there was little difference between acyclovir and placebo arms for cervico-vaginal HIV-1 RNA detection [88/213 (41.3%) vs. 84/191 (44.0%); odds ratio (OR)=0.90, 95% confidence interval (CI):0.60-1.33], HSV DNA detection [20/213 (9.4%) vs. 22/191 (11.5%); OR=0.80, 95%CI:0.42-1.51], genital HIV or HSV viral loads or plasma HIV-1 RNA load. Estimated median adherence was 91%. There was a suggestion of an impact on cervico-vaginal HIV-1 RNA detection among women with estimated adherence ≥90% (OR=0.74, 95%CI:0.50-1.09) when data from all three visits were included.
Conclusions
Acyclovir 400mg bid is unlikely to be a useful long-term intervention to reduce HIV transmission. The lack of effect on HIV may be due to suboptimal adherence or treatment regimen. (www.controlled-trials.com; ISRCTN35385041)
Keywords: HSV-2, HIV, genital shedding, Tanzania, randomized controlled trial, suppressive therapy, acyclovir
Introduction
Genital ulceration is a risk factor for HIV transmission [1]. The most frequent cause of genital ulcer disease is herpes simplex virus type 2 (HSV-2) [2]. Subclinical HSV-2 reactivation has also been associated with increased genital HIV load in most observational studies [3-6]. In vitro studies suggest that some HSV proteins increase HIV replication [7-12] providing biological plausibility for a protective effect of HSV suppressive therapy on HIV infectiousness.
Proof-of-concept randomised controlled trials (RCTs) of 1-3 months duration among HIV/HSV-2 co-infected individuals found that HSV suppressive therapy (valacyclovir 500mg b.i.d or acyclovir 800mg b.i.d) reduced genital HIV-1 RNA detection and genital and plasma viral loads [13-17]. Two trials of acyclovir 400mg b.i.d have shown less effect, one finding no reduction in cervico-vaginal HIV-1 RNA detection [18] and another a reduction in frequency of cervico-vaginal HIV-1 RNA detection and plasma HIV-1 RNA load but no effect on genital HIV-1 load among those shedding [19].
Here we report the results of a placebo-controlled RCT of the effect of acyclovir 400mg b.i.d on genital and plasma HIV-1 RNA over 24 months.
Methods
Study Participants
Women aged 16-35 years working in recreational facilities or self-employed as food handlers in 19 communities in northwest Tanzania were invited to attend a mobile study clinic for HSV-2 and HIV-1 antibody screening, as previously described [20]. Women returned 8–12 weeks later for their HSV-2 results and were eligible for enrolment if they were HSV-2 seropositive, lived in a study community and would not be travelling at the time of the next visit, were not pregnant or breastfeeding and were not planning pregnancy or to move outside the study communities in the next two years. Women were not enrolled if they did not understand the key trial concepts, had a history of epilepsy or were too ill to participate. Trial details have been published previously [21]. Women were enrolled regardless of HIV status and knowledge of HIV status was not required. In total, 1305 women (821 HIV seronegative and 484 HIV seropositive) were randomised in 3 phases (January-April 2004, July-September 2004 and January-May 2006) and followed for up to 30 months. There was no effect of acyclovir 400mg b.i.d on HIV acquisition (rate ratio=1.08; 95% confidence interval(CI), 0.64 to 1.83) [21]. In this paper we report the effects on genital and plasma HIV-1 RNA among the HIV-positive participants.
Trial procedures
Following informed consent, women were enrolled into the trial and biological samples including blood, urine, genital samples and a cervico-vaginal lavage (CVL) were taken [22]. Genital ulcers were swabbed. Participants were randomised to oral acyclovir (400mg b.i.d) or matching placebo [21]. Participants attended the study clinic every 3 months and were given a further supply of study tablets. Participants were asked to avoid vaginal cleansing before attending visits and were tested for pregnancy if it was suspected. Pregnant women were withdrawn from study tablets. Participants who chose to withdraw or who were withdrawn from tablets remained in the trial for follow-up where possible. At each visit, symptoms of reproductive tract infections (RTIs) were treated syndromically according to Tanzanian national guidelines. Women with laboratory-confirmed RTIs (with the exception of asymptomatic bacterial vaginosis) were offered treatment. Genital ulcers were swabbed. Women were offered family planning, condoms and risk reduction counselling, and voluntary counselling and testing for HIV. Every 6 months, a blood sample was taken for plasma viral load (PVL) and, with the exception of the 18m visit, genital samples and a CVL were collected. Following results from a trial of short duration HSV suppressive therapy reporting a reduction in frequency and quantity of genital HIV-1 RNA and in plasma HIV-1 RNA load [15], additional blood samples were taken for CD4 counts at 30m for phase I participants, at 24 and 30m for phase 2 and at 6 and 12m for phase 3 participants.
Adherence was estimated by tablet count at each visit. Women were given specific additional counselling and advice if adherence problems were identified (through comparison of tablet counts and elapsed days) or if tablets were reported missed, damaged or lost. Adherence was estimated for the preceding 3 month period and defined as unknown if a participant had not attended the previous visit, forgot to bring tablets to the clinic or brought back too few tablets and reported them as lost, stolen or damaged. Approximately one month after each scheduled visit, a mobile adherence-support team visited participants. A random sample of 161 urine specimens (80 and 81 from acyclovir and placebo arms, respectively), either collected by the adherence team between the 6 and 9m scheduled visits or from the 6, 12 and 24m scheduled visits were tested using high-performance liquid chromatography [23] for acyclovir by an independent laboratory.
An independent data monitoring committee periodically reviewed trial data including serious adverse events (SAEs) [24] which were collected at each visit.
Free anti-retroviral therapy (ART) became available in regional and district hospitals in the study area during the trial. HIV-positive women knowing their status were referred to HIV care and treatment centres for assessment and were offered transport if requested. At the end of the trial, travel costs were provided to enable them to attend their centre for the next 12-18 months. HIV-positive pregnant women were provided with transport to reach the nearest centre offering prevention of mother-to-child HIV transmission services.
Laboratory analyses
Screening sera were tested for HSV-2 antibodies and screening and randomisation sera for HIV-1 antibodies [20]. Randomisation sera were tested for syphilis and vaginal and cervical swabs were tested for other RTIs as previously described [25, 26]. CD4 T lymphocytes were quantified in blood samples (COULTER® Manual CD4 Kit, Beckman Coulter, California, USA). Genital ulcer swabs were tested for T. pallidum, HSV and Haemophilus ducreyi by PCR [27-31].
CVLs were centrifuged (3500 rpm for 10 minutes), separated, stored at −20°C for 2-3 weeks and then at −80°C before transport with plasma samples to Inserm U743, France. CVL supernatants were visually examined for blood. Nucleic acids were extracted from CVL supernatants and plasma (NucliSens® miniMAG and later easyMAG extraction system, bioMérieux, Marcy l'Etoile, France); and CVL cell pellets (QIAamp DNA Blood Mini Kit. Qiagen, Courtabeouf, France).
Genital and plasma HIV-1 RNA was quantified by in-house real-time PCR with a forward primer three nucleic acids shorter than previously reported [32] to facilitate detection of more HIV-1 sub-types. HSV DNA was quantified in CVL supernatants by in-house real-time PCR [33]. HSV DNA positive samples from randomisation were typed [34] and all were HSV-2. Y-chromosome DNA (Quantifiler™Y Human Male DNA Quantification kit, Applied Biosystems, Courtaboeuf, France) was tested for using nucleic acids from the CVL cell pellet. Quantifications were carried out using Applied Biosystems 7300 Real-time PCR System (Applied Biosystems). The laboratory registered with an external blinded quality control programme for HIV-1 RNA (Quality Control for Molecular Diagnostics, UK). A commercial unblinded standard was used to confirm the HSV DNA assay (HSV-1/HSV-2 Clear QC Panel, Argene, France).
Statistical methods
The primary objective was to determine the effect of HSV suppressive therapy on the detection and quantity of cervico-vaginal HIV-1 RNA at 6m. The original sample size of 1000 HSV-2 seropositive women was powered to determine effects on HIV acquisition among HIV-negative participants [21]. HIV prevalence was estimated at 40% [35]. Assuming 400 HIV-positive participants and 15% loss to follow-up (LTFU) or censored for pregnancy at 6m, we had over 95% power to detect a reduction in detectable genital HIV-1 RNA at 6m from 50% in the placebo [5, 36] to 30% in the acyclovir arm and a 0.5 log reduction in HIV-1 RNA load in women shedding HIV-1 RNA assuming a standard deviation of 0.8 log [4].
The primary analysis was a modified intention-to-treat (mITT) analysis where women were censored at the earliest of LTFU, death, positive pregnancy test or end of follow-up. Primary outcomes were detection and quantity of cervico-vaginal HIV-1 RNA. Secondary outcomes were detection and quantity of cervico-vaginal HSV DNA, plasma HIV-1 RNA load, GUD presence on examination and CD4 count. Genital viral load outcomes were compared only in women with detectable shedding. The primary analysis time-point was 6m. Secondary analysis time-points were 12m and 24m.
Logistic and linear regression models were used to analyse binary and continuous outcomes, respectively. For binary outcomes, the primary measure of effect was an unadjusted odds ratio (OR). For viral load outcomes, the primary measure of effect was mean difference of log viral load between arms, adjusted for baseline plasma HIV-1 RNA or baseline HSV DNA detection as appropriate. Adjustment was made for baseline plasma HIV-1 RNA load since this provides a more stable measure than cervico-vaginal HIV-1 RNA.
Data from the three follow-up visits (6, 12 and 24m) were combined to increase power assuming a constant effect over time and analysed using general estimating equations (GEE) regression models to allow for within-woman correlation. The log CD4 count was analysed combining data from different visits using GEE linear regression. Participants were censored at ART initiation for plasma HIV-1 RNA and CD4 outcomes only. The secondary analyses were ITT (including pregnant women attending follow-up) and an analysis stratified by adherence.
An a priori restricted analysis excluding samples with visible blood was carried out for the primary outcomes. Pre-specified subgroup analyses were performed using interaction tests to determine whether the effect of acyclovir on cervico-vaginal HIV-1 RNA differed according to baseline cervico-vaginal HIV-1 RNA detection, baseline detection of both cervico-vaginal HIV-1 RNA and HSV DNA, plasma HIV-1 RNA levels, Y-chromosome detection at follow-up and STI presence at follow-up.
Quantitation thresholds were 360 copies/ml for cervico-vaginal HIV-1 RNA and 300 copies/ml for cervico-vaginal HSV DNA and plasma HIV-1 RNA. For cervico-vaginal shedding, viral loads below threshold were classed as undetectable. Plasma HIV-1 RNA below the threshold was allocated half the threshold value for analysis.
Data were double entered and verified in Dbase IV (dataBase Intelligence) and analyses were conducted using Stata 10.0 (Stata Corporation, College Station, Texas, USA).
Ethics
The protocol was approved by the Medical Research Coordinating Committee of Tanzania and the London School of Hygiene & Tropical Medicine Ethics Committee. The trial was registered with Current Controlled Trials (ISRCTN35385041).
Results
Enrolment
In total, 2719 women were screened for HSV-2 and HIV-1 antibodies. Of 751 dually seropositive women, 465 (61.9%) returned for the randomisation visit, were eligible for enrolment and consented to participate. In addition, 19 women seroconverting to HIV-1 between screening and randomisation were eligible. In total, 484 women were enrolled (253 randomised to acyclovir and 231 to placebo; Figure 1).
Figure 1.
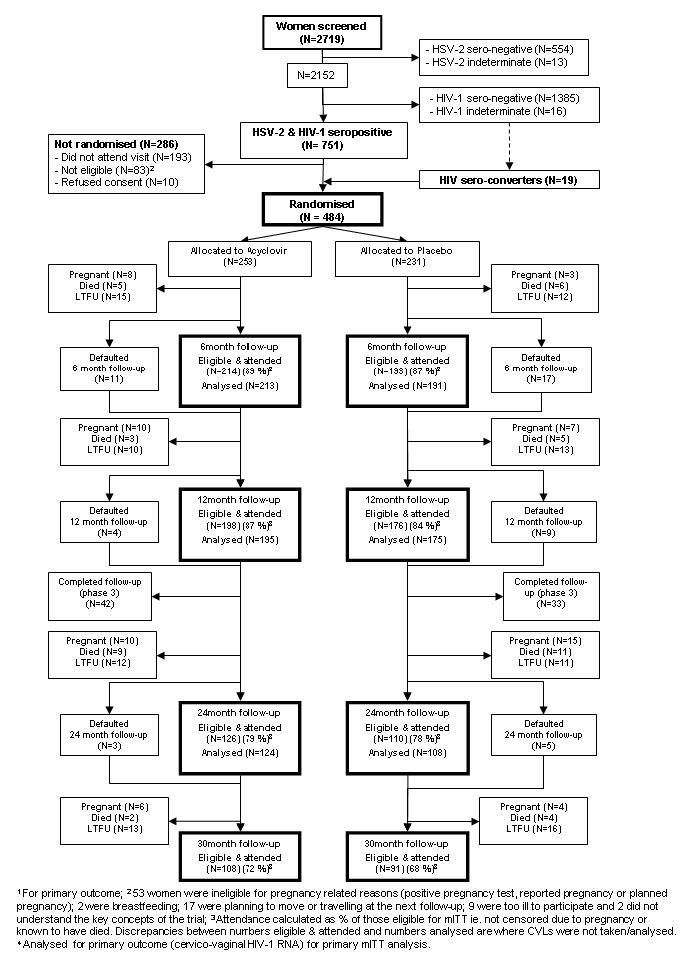
Flowchart to show enrolment and follow-up of the cohort to 30 months, mITT analysis
Cohort characteristics at enrolment
Participants' median age was 28 years (IQR, 25–32). Ninety (18.6%) women were married or living as married and 223 (46.1%) worked as local food handlers (Table 1). Approximately one-third (31.2%) of participants reported a history of genital ulceration or blisters. RTIs were prevalent. Cervico-vaginal HIV-1 RNA and HSV DNA were detected in 52.6% and 14.5% of CVLs, respectively.
Table 1.
Characteristics of the trial participants at enrolment by treatment allocation
| Characteristic | Acyclovir (N=253) No. (%) |
Placebo (N=231) No. (%) |
Total (N=484) No. (%) |
|---|---|---|---|
|
Socio-demographic characteristics
| |||
| Median age (IQR), years | 28 (25–31) | 29 (25–32) | 28 (25–32) |
|
Marital status | |||
| Unmarried | 58 (22.9) | 46 (19.9) | 104 (21.5) |
| Married/living as married | 49 (19.4) | 41 (17.8) | 90 (18.6) |
| Divorced/Separated | 133 (52.6) | 124 (53.7) | 257 (53.1) |
| Widowed | 13 (5.1) | 20 (8.7) | 33 (6.8) |
|
Education level | |||
| Less than primary | 67 (26.5) | 57 (24.7) | 124 (25.6) |
| Primary | 172 (68.0) | 155 (67.1) | 327 (67.6) |
| Secondary | 12 (4.7) | 18 (7.8) | 30 (6.2) |
| Other | 2 (0.8) | 1 (0.4) | 3 (0.6) |
|
| |||
| Literate | 205 (81.0) | 179 (77.5) | 384 (79.3) |
|
Type of work facility | |||
| Local food handler | 108 (42.7) | 115 (49.8) | 223 (46.1) |
| Guesthouse | 31 (12.3) | 17 (7.4) | 48 (9.9) |
| Bar | 66 (26.1) | 39 (16.9) | 105 (21.7) |
| Local brew seller | 17 (6.7) | 21 (9.1) | 38 (7.9) |
| Restaurant/café/grocery | 31 (12.3) | 39 (16.9) | 70 (14.5) |
|
| |||
|
Travelled for > 1 week in 3 months prior to enrolment |
69 (27.3) | 56 (24.2) | 125 (25.8) |
|
Behavioural characteristics | |||
| Median age at first sex (IQR), yrs 1 | 16 (15–17) | 16 (15–18) | 16 (15–18) |
|
Number of sexual partners in lifetime | |||
| 1-4 | 55 (21.7) | 84 (36.4) | 139 (28.7) |
| 5-9 | 89 (35.2) | 69 (29.9) | 158 (32.6) |
| 10-19 | 59 (23.3) | 44 (19.1) | 103 (21.3) |
| 20+ | 50 (19.8) | 34 (14.7) | 84 (17.4) |
|
Number of sexual partners in last 3 months | |||
| None | 10 (4.0) | 10 (4.3) | 20 (4.1) |
| 1 | 129 (51.0) | 119 (51.5) | 248 (51.2) |
| 2–3 | 97 (38.3) | 87 (37.7) | 184 (38.0) |
| ≥ 4 | 17 (6.7) | 15 (6.5) | 32 (6.6) |
|
No. of times cleanses vagina per day 1 | |||
| Doesn't cleanse | 84 (33.2) | 77 (33.3) | 161 (33.3) |
| 1 | 7 (2.8) | 18 (7.8) | 25 (5.2) |
| 2 | 54 (21.4) | 56 (24.4) | 110 (22.8) |
| ≥ 3 | 107 (42.5) | 79 (34.3) | 186 (38.6) |
|
Clinical characteristics | |||
| Frequency of ulcers 1 | |||
| Monthly | 29 (11.5) | 14 (6.1) | 43 (8.9) |
| Every 3 months | 18 (7.1) | 15 (6.6) | 33 (6.9) |
| Once-twice a year | 17 (6.7) | 15 (6.6) | 32 (6.6) |
| Only had them once or twice | 27 (10.7) | 14 (6.1) | 41 (8.5) |
| No history of genital ulcers / blisters | 162 (64.0) | 171 (74.7) | 333 (69.1) |
|
| |||
| Episode of GUD in the last year 1 | 69 (27.4) | 48 (20.9) | 117 (24.3) |
|
| |||
|
Ulcers / blisters / vesicles seen on examination |
15 (5.9) | 13 (5.6) | 28 (5.8) |
|
Biological characteristics | |||
| Biological presence of RTI/STI | |||
| Neisseria gonorrhoeae 2 | 13 (5.1) | 21 (9.1) | 34 (7.0) |
| Chlamydia trachomatis 2 | 17 (6.7) | 12 (5.2) | 29 (6.0) |
| Trichomonas vaginalis 2,3 | 96 (37.9) | 76 (33.0) | 172 (35.6) |
| Bacterial vaginosis 4 | 172 (69.9) | 152 (67.9) | 324 (68.9) |
| Candida albicans 2 | 30 (11.9) | 21 (9.1) | 51 (10.6) |
| Active syphilis 5 | 41 (16.2) | 34 (14.7) | 75 (15.5) |
|
| |||
| Aetiology of genital ulcer 6 | (N=12) | (N=10) | (N=22) |
|
| |||
| Negative for all pathogens | 11 (91.7) | 7 (70.0) | 18 (81.8) |
|
| |||
| T. pallidum | 0 | 0 | 0 |
|
| |||
| H. ducreyi | 0 | 0 | 0 |
|
| |||
| HSV-2 positive | 1 (8.3) | 3 (30.0) | 4 (18.2) |
|
| |||
|
Mean Plasma HIV load (SD); log copies/ml 2,7 |
4.44 (1.07) | 4.56 (1.10) | 4.50 (1.09) |
|
Cervico-vaginal HIV-1 RNA | |||
| Detected 2 | 132 (52.4) | 122 (52.8) | 254 (52.6) |
| Mean quantity (SD) – log copies/ml 8 | 3.60 (0.72) | 3.74 (0.76) | 3.67 (0.74) |
|
Cervico-vaginal HSV-2 DNA | |||
| Detected 2 | 40 (15.9) | 30 (13.0) | 70 (14.5) |
| Mean quantity (SD) – log copies/ml 8 | 3.98 (1.16) | 3.87 (1.11) | 3.93 (1.13) |
Two missing values
One missing value
As detected by wet prep or culture
14 missing values (slides were unclassifiable)
RPR+ & TPPA+ or FTA+
Ulcer swabs were only taken for 22 of the 28 participants diagnosed with GUD
Participants with undetectable viral load were assigned a value of half the threshold of detection
Among those with detectable virus.
In total, 279 (57.6%) women accepted VCT at enrolment and a further 68 (14.0%) during follow-up.
Follow-up
Follow-up for the primary outcome (cervico-vaginal HIV-1 RNA) is shown in Figure 1. Overall, 88%, 86% and 79% of women eligible for the mITT analysis attended the 6m, 12m and 24m visits, respectively. Fifty-two women (10.7%) were censored for pregnancy before 24m, 39 (8.1%) died and 81 (16.9%) were LTFU for other reasons including 20 (4.1%) who requested trial withdrawal. For plasma HIV-1 RNA and CD4 outcomes, 7 women were censored at ART initiation before 6m (4 in the acyclovir and 3 in the placebo arm, respectively), 3 between 6m and 12m (1 and 2, respectively) and 14 between 12m and 24m (8 and 6, respectively).
Effect of acyclovir on cervico-vaginal HIV-1 RNA
There was little evidence of an effect of acyclovir on detection or quantity of cervico-vaginal HIV-1 RNA (Table 2). At 6m, the prevalence of cervico-vaginal HIV-1 RNA detection was 41.3% and 44.0% in the acyclovir and placebo arms respectively (OR=0.90; 95%CI:0.60 to 1.33). There was little difference in HIV-1 RNA quantity by arm (adjusted mean difference=−0.01 log10 copies/mL, 95%CI:-0.20 to 0.19). Similar results were seen at 12 and 24m (Table 2) and in the secondary ITT analysis (data not shown). Combining data from all three visits, there was a suggestion of an impact on HIV-1 RNA detection (OR=0.82, 95%CI: 0.61 to 1.11), which was stronger among samples without visible blood (OR=0.71; 95%CI:0.51 to 0.97; Table 2), but there was little difference in quantity in this sub-group.
Table 2.
The impact of acyclovir on cervico-vaginal HIV-1 RNA, by treatment arm (modified intention to treat analysis)
| Detectable cervico-vaginal HIV-1 RNA | Mean quantity of cervico-vaginal HIV-1 RNA1 (log copies/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acyclovir | Placebo | Unadjusted OR (95%CI) |
Adjusted OR (95%CI)2 |
P-value3 | Acyclovir | Placebo | Unadjusted regression coefficient (95%CI) |
Adjusted regression coefficient (95%CI)2 |
P-value3 | |
| no./N (%) | no./N (%) | Mean (SE) | Mean (SE) | |||||||
| 6 months | 88/213 (41.3) |
84/191 (44.0) |
0.90 (0.60, 1.33) |
0.89 (0.57, 1.39)4 |
0.59 | 3.58 (0.07) | 3.57 (0.08) | 0.01 (−0.20, 0.21) |
−0.01 (−0.20, 0.19) |
0.95 |
| 12 months | 92/195 (47.2) |
88/175 (50.3) |
0.88 (0.59, 1.33) |
0.84 (0.53, 1.33) |
0.55 | 3.69 (0.07) | 3.63 (0.08) | 0.06 (−0.15, 0.27) |
0.06 (−0.14, 0.26) |
0.53 |
| 24 months | 58/124 (46.8) |
62/108 (57.4) |
0.65 (0.39, 1.10) |
0.56 (0.32, 0.98) |
0.11 | 3.77 (0.11) | 3.67 (0.10) | 0.10 (−0.20, 0.40) |
0.06 (−0.23, 0.36) |
0.67 |
| Repeated measures analysis (6, 12 and 24 months) | ||||||||||
| All samples | 238/532 5 (44.7) |
234/474 6 (49.4) |
0.82 (0.61, 1.11) |
0.79 (0.58, 1.07)4 |
0.19 | 3.67 (0.05) | 3.62 (0.05) | 0.04 (−0.11, 0.18) |
0.03 (−0.11, 0.16) |
0.70 |
|
Samples without visible blood |
173/440 7 (39.3) |
183/385 8 (47.5) |
0.71 (0.51, 0.97) |
0.69 (0.49, 0.97)4 |
0.035 | 3.64 (0.06) | 3.57 (0.06) | 0.07 (−0.09, 0.24) |
0.06 (−0.10, 0.22) |
0.47 |
|
≥ 90% adherence |
134/304 9 (44.1) |
131/250 10 (52.4) |
0.74 (0.50, 1.09) |
0.67 (0.44, 1.01)4 |
0.12 | 3.64 (0.07) | 3.62 (0.07) | 0.00 (−0.18, 0.19) |
−0.03 (−0.21, 0.15) |
0.75 |
|
<90% / unknown adherence |
104/228 11 (45.6) |
103/224 12 (46.0) |
0.96 (0.64, 1.44) |
0.94 (0.62, 1.43) |
0.85 | 3.71 (0.07) | 3.61 (0.08) | 0.09 (−0.11, 0.29) |
0.10 (−0.10, 0.30) |
0.34 |
CVL not taken/analysed for one, three and two women in the acyclovir arm and for two, one and two women in the placebo arm, at 6, 12 and 24 months respectively.
Among those with detectable HIV-1 RNA
Adjusted for baseline plasma HIV RNA load (log10 copies/ml)
For primary measures of effect – unadjusted OR and adjusted regression coefficient
Adjusted measure of effect excludes one women in the acyclovir arm who has no baseline PVL measurement
532 visits in 221 women
474 visits in 204 women
440 visits in 214 women
385 visits in 194 women
304 visits in 173 women
250 visits in 146 women
228 visits in 144 women
224 visits in 144 women.
There was no evidence that the impact of acyclovir differed between the categories of the pre-specified subgroups.
Effect of acyclovir on cervico-vaginal HSV DNA and GUD
At 6m, HSV DNA was detected in 9.4% compared to 11.5% of women in the acyclovir and placebo arms respectively (OR=0.80, 95%CI:0.42 to 1.51, Table 3). Similar results were observed at 12 and 24m, over the whole follow-up period (Table 3) and in the secondary ITT analysis (data not shown). There was little difference in HSV DNA quantity (Table 3).
Table 3.
The impact of acyclovir on cervico-vaginal HSV DNA, by treatment arm (modified intention to treat analysis)
| Detectable cervico-vaginal HSV DNA | Mean quantity of cervico-vaginal HSV DNA1 (log copies/ml) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acyclovir | Placebo | Unadjusted OR (95%CI) |
Adjusted OR (95%CI)2 |
P-value3 | Acyclovir | Placebo | Unadjusted regression coefficient (95%CI) |
Adjusted regression coefficient (95%CI)2 |
P-value3 | |
| no./N (%) | no./N (%) | Mean (SE) | Mean (SE) | |||||||
| 6 months | 20/213 (9.4) |
22/191 (11.5) |
0.80 (0.42, 1.51) |
0.79 (0.42, 1.51)4 |
0.49 | 4.40 (0.31) | 4.03 (0.28) | 0.37 (−0.46, 1.20) |
0.38 (−0.45, 1.21) |
0.36 |
| 12 months | 22/195 (11.3) |
23/175 (13.1) |
0.84 (0.45, 1.57) |
0.82 (0.44, 1.53) |
0.58 | 3.92 (0.25) | 4.12 (0.27) | −0.20 (−0.95, 0.55) |
−0.17 (−0.92, 0.57) |
0.64 |
| 24 months | 16/124 (12.9) |
11/108 (10.2) |
1.31 (0.58, 2.95) |
1.31 (0.58, 2.96) |
0.52 | 4.19 (0.34) | 4.03 (0.31) | 0.16 (−0.84, 1.16) |
0.29 (−0.66, 1.24) |
0.54 |
| Repeated measures analysis (6, 12 and 24 months) | ||||||||||
| All samples | 58/532 5 (10.9) |
56/474 6 (11.8) |
0.91 (0.61, 1.37) |
0.90 (0.60, 1.36) 4 |
0.65 | 4.16 (0.19) | 4.07 (0.17) | 0.03 (−0.44, 0.50) |
0.08 (−0.38, 0.55) |
0.73 |
|
≥ 90% adherence |
22/304 7 (7.2) |
29/250 8 (11.6) |
0.60 (0.33, 1.09) |
0.60 (0.33, 1.08) 4 |
0.090 | 4.28 (0.28) | 3.71 (0.18) | 0.57 (−0.07, 1.21) |
0.51 (−0.11, 1.13) |
0.11 |
|
<90% / unknown adherence |
36/228 9 (15.8) |
27/224 10 (12.1) |
1.38 (0.81, 2.35) |
1.35 (0.80, 2.30) |
0.24 | 4.09 (0.25) | 4.46 (0.27) | −0.37 (−1.05, 0.31) |
−0.16 (−0.86, 0.54) |
0.64 |
CVL not taken/analysed for one, three and two women in the acyclovir arm and for two, one and two women in the placebo arm, at 6, 12 and 24 months respectively.
Among those with detectable HSV DNA
Adjusted for baseline presence of cervico-vaginal HSV DNA
For primary measures of effect – unadjusted OR and adjusted regression coefficient
Adjusted measures of effect exclude one women in the acyclovir arm who has no baseline HSV shedding measurement
532 visits in 221 women
474 visits in 204 women
304 visits in 173 women
250 visits in 146 women
228 visits in 144 women
224 visits in 144 women.
Although there were few clinically diagnosed GUD episodes during follow-up there was a reduction in the acyclovir arm [8/769 (1.0%) vs. 18/688 (2.6%) of visits; OR=0.40, 95%CI:0.15 to 1.04]. There were 4 HSV-2 PCR positive ulcers in the acyclovir arm (of 7 swabbed, 57%) and 10 (of 16 swabbed, 63%) in the placebo arm (OR=0.34, 95%CI:0.09 to 1.29).
Adherence
Median estimated adherence, by tablet count, for the three month periods preceding each analysis point was 91.9% (IQR:64.0 to 99.5%) (Table 4). Using this estimate, unknown adherence (in approximately 20% women) was assumed to be low. Acyclovir was detected in 56% of the 80 urines tested from 69 women in the acyclovir arm.
Table 4.
Estimated adherence (measured by pill count) within the 3 months preceding analysis points for those included in the primary (mITT) analysis by treatment allocation
| Adherence | 3–6 months | 9–12 months | 21–24 months | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Acyclovir (N=213) |
Placebo (N=191) |
Acyclovir (N=195) |
Placebo (N=175) |
Acyclovir (N=124) |
Placebo (N=108) |
|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| ≥ 90% | 116 (54.5) | 99 (51.8) | 120 (61.5) | 96 (54.9) | 68 (54.8) | 55 (50.9) |
| 75 – 89% | 28 (13.2) | 32 (16.8) | 31 (15.9) | 29 (16.6) | 21 (16.9) | 21 (19.4) |
| < 75% | 13 (6.1) | 23 (12.0) | 13 (6.7) | 18 (10.3) | 8 (6.5) | 7 (6.5) |
| Unknown - tablets lost, stolen, damaged or participant forgot to bring them to the clinic |
44 (20.7) | 28 (14.7) | 27 (13.8) | 24 (13.7) | 23 (18.5) | 20 (18.5) |
| Unknown - defaulted previous visit |
12 (5.6) | 9 (4.7) | 4 (2.1) | 8 (4.6) | 4 (3.2) | 5 (4.6) |
|
| ||||||
| % (IQR) | % (IQR) | % (IQR) | % (IQR) | % (IQR) | % (IQR) | |
| Median1 | 92.3 (50.0-100) |
90.6 (55.9-98.1) |
94.5 (76.5-100) |
91.6 (68.5-99.4) |
91.7 (62.9-99.8) |
91.1 (54.3-98.6) |
Unknown adherence assumed to be zero.
Effect of acyclovir on cervico-vaginal HIV RNA and HSV DNA stratified by adherence
Among women with estimated adherence ≥90%, there was some evidence of an effect of acyclovir on HSV DNA detection (repeated measures analysis: 7.2% vs. 11.6% of visits; OR=0.60; 95%CI:0.33 to 1.09; Table 3; P-interaction=0.045) and, to a lesser extent, on HIV-1 RNA detection (44% vs. 52% of visits; OR=0.74; 95%CI:0.50 to 1.09; Table 2; P-interaction=0.40). However, there was no evidence for an effect on HSV DNA or HIV-1 RNA loads in women with estimated adherence ≥90%.
Effect of acyclovir on plasma HIV-1 RNA and CD4 cell count
Plasma HIV-1 RNA loads were similar in both arms (Table 5). At 6m the adjusted mean difference was 0.04 log copies/ml (95%CI:-0.07 to 0.15). Results were similar at all visits and there was no evidence for an effect in those with estimated adherence ≥90% (Table 5).
Table 5.
The impact of acyclovir on plasma HIV-1 RNA & CD4 count, by treatment arm (modified intention to treat analysis)
| Acyclovir | Placebo | Unadjusted regression coefficient (95%CI) |
Adjusted regression coefficient (95%CI)1 |
P-value2 | |
|---|---|---|---|---|---|
| Plasma HIV-1 RNA load (log copies/ml) | |||||
| Mean (SE) | Mean (SE) | ||||
| 6 months | (N=210) | (N=189) | 0.04 (−0.16, 0.24) |
0.043 (−0.07, 0.15) |
0.49 |
| 4.32 (0.07) | 4.28 (0.08) | ||||
| 12 months | (N=192) | (N=170) | 0.05 (−0.16, 0.27) |
0.03 (−0.11, 0.16) |
0.69 |
| 4.26 (0.07) | 4.21 (0.08) | ||||
| 24 months | (N=117) | (N=102) | 0.05 (−0.23, 0.33) |
−0.04 (−0.23, 0.15) |
0.69 |
| 4.48 (0.09) | 4.43(0.11) | ||||
| Repeated measures analysis (6, 12 and 24 months) | |||||
|
All samples |
(N=519)5 | (N=461)6 | 0.07 (−0.18, 0.20) |
0.02 (−0.09, 0.13) |
0.69 |
| 4.33 (0.07) | 4.29 (0.07) | ||||
|
≥ 90% adherence |
(N=293)7 | (N=244)8 | −0.01 (−0.23, 0.21) |
−0.02 (−0.15, 0.10) |
0.72 |
| 4.29 (0.08) | 4.31 (0.08) | ||||
|
<90% / unknown adherence |
(N=226)9 | (N=217)10 | 0.06 (−0.19, 0.30) |
0.09 (−0.05, 0.23) |
0.23 |
| 4.40 (0.08) | 4.26 (0.10) | ||||
| CD4 count (cells/μl) | |||||
|
Geometric mean (95%CI) |
Geometric mean (95%CI) |
||||
| All women | (N=181)11 | (N=151)12 | 0.11 (0.03, 0.19) 13 |
0.009 | |
| 346 (313, 381) | 271 (237, 310) | ||||
|
≥ 90% adherence |
(N=93)14 | (N=94)15 | 0.13 (0.03, 0.23) 13 |
0.008 | |
| 355 (309, 407) | 263 (223, 311) | ||||
|
<90% / unknown adherence |
(N=88)16 | (N=57)17 | 0.07 (−0.04, 0.19) 13 |
0.22 | |
| 336 (291, 388) | 284 (227, 357) | ||||
Adjusted for baseline plasma HIV RNA load (log10 copies/ml)
For primary measure of effect – adjusted regression coefficient
Adjusted measure of effect excludes one women in the acyclovir arm who has no baseline PVL measurement
519 visits in 218 women
461 visits in 201 women
293 visits in 169 women
244 visits in 143 women
226 visits in 147 women
217 visits in 140 women
181 visits in 131 women
151 visits in 112 women
regression coefficient for log CD4 count
93 visits in 78 women
94 visits in 72 women
88 visits in 82 women
57 visits in 55 women.
Conversely, there was evidence of an effect of acyclovir on CD4 cell counts. Geometric mean CD4 counts were 346 cells/μl in the acyclovir and 271 cells/μl in the placebo arm (Table 5; mean difference=0.11 log cells/μl; 95%CI:0.03 to 0.19).
Serious adverse events
In total, 147 SAEs were documented in 52 women (20.6%) in the acyclovir and 59 women (25.5%) in the placebo arm. These were mainly malaria, or HIV-related and no SAE was attributed to acyclovir. There were 48 deaths (20 and 28 in the acyclovir and placebo arms, respectively), 30 life-threatening illnesses (16 and 14, respectively) and 69 hospitalisations (33 and 36, respectively).
Discussion
We assessed the effect of providing HSV suppressive therapy (acyclovir 400mg b.i.d) for up to 24 months and found little impact on the prevalence or quantity of either genital HIV-1 RNA or HSV DNA or on plasma HIV-1 RNA. These results contrast with previous RCTs assessing suppressive therapy regimes of valacyclovir 500mg b.i.d or acyclovir 800mg b.i.d which reported substantial reductions in genital (0.2-0.5 log10 copies/ml) and plasma (0.3-0.5 log10 copies/ml) HIV-1 RNA [13-17] over periods of up to 3 months. A similar reduction in plasma HIV-1 RNA was seen in two RCTs using acyclovir 400mg b.i.d (0.34 and 0.25 log10 copies/ml) [19, 37]. The latter examined the effect of this regimen over 24 months. However, trials of acyclovir 400mg b.i.d [18, 19] have generally reported weaker effects on genital HIV-1 RNA and HSV DNA. This may be partially attributable to different regimens but suboptimal adherence may also have been a factor in one trial [18].
The difference between our results and previous trial findings is likely to be due mainly to a combination of sub-optimal adherence and the regimen used. There was some evidence that for HSV DNA, and to a lesser extent HIV-1 RNA, the effect of acyclovir was better in those with estimated adherence ≥90%. However, the absence of a convincing effect on HSV DNA overall and the weak effect in this sub-group may suggest that adherence was lower than estimated. Median estimated adherence, over 90% at each visit, was estimated using tablet counts, which does not guarantee that tablets were actually taken. Results from the urine analysis where only 56% samples had detectable acyclovir would support this. The mobile clinics, quarterly clinic and adherence support visits and more mobile trial population made achieving high adherence especially challenging in this trial compared to others among HIV infected individuals [13-17, 19]. Alternatively, adherence estimates may be reasonably accurate but participants may not have been adhering to the 12 hourly regimen. The short half-life of acyclovir (~3 hours [38]) means that effective HSV-2 suppression may require very high compliance with a twice-daily regimen which may be difficult to sustain, especially in asymptomatic or mobile individuals. In contrast, valacyclovir, which is broken down into acyclovir following absorption, has an oral bioavailability of 3.5-5.5 times that of acyclovir [39], and trough plasma acyclovir concentrations were approximately twice as high in individuals taking valacyclovir (500mg b.i.d) as acyclovir (400mg b.i.d) in a trial comparing the two regimens [40]. A trial comparing the effect of valacyclovir 1g b.i.d and acyclovir 400mg b.i.d on genital and plasma HIV is underway in the US (www.clinicaltrials.gov; NCT00527618).
Other factors that may help explain the results include stage of HSV-2 infection. Most (82%) of swabbed ulcers at baseline had no identified aetiology: this is higher than reported in other studies [41] and may suggest some misidentification of ulcers by the clinicians at this time. In contrast we observed few GUD episodes during follow-up, which may suggest this cohort acquired HSV-2 infection several years before the trial since frequency of GUD recurrences decreases over time [42]. HSV shedding in the placebo arm was also less frequent than in other trials although rates are difficult to compare because of different methods of sample collection and analysis. Other RTIs were also prevalent in this population which may have diluted any HSV-2 associated effects on HIV. Finally, most participants practised vaginal cleansing and at 23% of visits, cleansing within the previous 3 hours was reported. This may have affected cervico-vaginal HIV RNA and HSV DNA detection, but excluding these women does not affect results and this does not explain the absence of effect on plasma HIV-1 RNA.
Errors in labelling or drug resistance are unlikely to explain our results since quality control checks confirmed labelling and quality of the acyclovir tablets and acyclovir resistance is rare, remaining below 5% in HIV seropositive individuals [43]. Further work to examine acyclovir resistance in this cohort is underway.
Trial protocol differences may partly explain the difference in shedding results. Genital HIV-1 RNA loads show greater variability when collected by CVL than swab [44]. Of other RCTs using CVLs [14, 18, 19], one found no effect of suppressive therapy on HIV-1 RNA detection [18] and another [19] reported an effect on frequency of HIV-1 RNA detection but not quantity despite a reduction in plasma HIV-1 RNA. However, these trials also used acyclovir 400mg b.i.d making it difficult to disentangle the effects of regimen and sampling method. Some trials did not examine menstruating women [15, 19]. By including these samples our results may be underestimated. Our analysis restricted to samples without visible blood found a 25% reduction in HIV-1 RNA detection but no effect on quantity. However, there was no evidence for a substantial effect on HSV DNA and although recent in vitro data suggest acyclovir may directly inhibit HIV-1 reverse transcriptase [45, 46], this is not suggested by our absence of an effect on PVL.
We did, however, find some evidence for a difference in CD4 counts between treatment arms. One other trial examined the effect on CD4 but found no effect [19]. Our result should be interpreted cautiously due to of the lack of impact on other endpoints, and may be due to chance or baseline imbalance since there are no baseline CD4 counts.
A trial of acyclovir 400mg b.i.d recently reported no effect on HIV transmission from HIV positive to HIV negative partners despite a 0.25 log reduction in plasma HIV-1 RNA and a 73% reduction in clinical HSV-2 reactivations [37]. Further trials are needed to investigate the effect of HSV suppressive therapy on CD4 counts and whether this may be translated into any clinical benefit. Our results suggest that the regimen of acyclovir 400mg b.i.d is unlikely to substantially reduce HIV transmission or plasma HIV-1 RNA levels in a high-risk mobile population where it may be difficult to achieve the high levels of adherence that appear necessary for HSV-2 suppression.
Acknowledgements
We thank Rebecca Balira (NIMR Mwanza Centre, Tanzania), Louise Knight, Ian Hambleton and Tobias Chirwa (LSHTM, UK & NIMR Mwanza Centre, Tanzania) for data & statistical support; Maxime Lecerf, Cecile Lefebvre (Inserm U743, Paris France), Tania Crucitti and colleagues (ITM, Antwerp, Belgium) and Michel Alary (University of Laval, Quebec, Canada) for help with laboratory assays.
We thank the women who participated in the trial, the HSV trial team and staff at AMREF & NIMR Mwanza Centre.
Funding for the study was provided by the Wellcome Trust, the UK Medical Research Council (MRC) and the UK Department for International Development.
Appendix
Investigators
African Medical & Research Foundation, Mwanza, Tanzania - Kokugonza Mugeye, D Watson-Jones (principal investigator); National Institute for Medical Research, Mwanza, Tanzania – Kathy Baisley, John Changalucha, Dean Everett, Louise Knight, Clare Tanton; London School of Hygiene & Tropical Medicine, UK – Kathy Baisley, Tobias Chirwa, Tim Clayton, Dean Everett, Ian Hambleton, Richard Hayes, Louise Knight, David Ross, Clare Tanton, D Watson-Jones, Helen Weiss; Inserm U743 & Université Paris Descartes, Paris, France – Laurent Belec; Laboratoire de Microbiologie, Hôpital Saint Louis, 75010 Paris, France - Jerome Le Goff;
Trial Steering Committee
MRC Clinical Trials Unit, London, UK - A Nunn; University College, London, UK – F Cowan; Harvard School of Public Health, Boston, USA - S Kapiga, Ifakara Health Research & Development Centre, Dar es Salaam, Tanzania - H Mshinda; Data Monitoring Committee: MRC Clinical Trials Unit, London, UK - D Dunn; MRC/UVRI, Entebbe, Uganda - Heiner Grosskurth; AMANET, Dar es Salaam, Tanzania – W. Kilama.
Footnotes
None of the authors have a commercial or other association that might pose a conflict of interest.
Preliminary findings have been presented at the International AIDS Society Conference, Sydney, July 22-25th 2007 (Title: A randomized controlled trial in Tanzania to assess the impact of HSV2 suppressive therapy on genital HIV viral load among HSV2 and HIV1 seropositive women. Poster presentation. Presenting author: C Tanton. Abstract number TUPEC011) and the 17th meeting of the International Society for Sexually Transmitted Disease Research (ISSTDR), Seattle, July 29 – August 1 2007. (Title: A randomized controlled trial in Tanzania to assess the impact of HSV2 suppressive therapy on genital HIV viral load among HSV2 and HIV1 seropositive women. Presenting author: C Tanton. Abstract number O-099).
References
- 1.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 2.Celum C, Levine R, Weaver M, Wald A. Genital herpes and human immunodeficiency virus: double trouble. Bull World Health Organ. 2004;82:447–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, McClelland RS, Corey L, et al. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: a randomized clinical trial. J Infect Dis. 2004;189:1466–71. doi: 10.1086/383049. [DOI] [PubMed] [Google Scholar]
- 4.Mbopi-Keou FX, Gresenguet G, Mayaud P, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000;182:1090–6. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 5.McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16:2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Cowan FF, Pascoe SJ, Barlow KL, et al. Association of genital shedding of herpes simplex virus type 2 and HIV-1 among sex workers in rural Zimbabwe. AIDS. 2006;20:261–7. doi: 10.1097/01.aids.0000198086.39831.4a. [DOI] [PubMed] [Google Scholar]
- 7.Golden MP, Kim S, Hammer SM, et al. Activation of human immunodeficiency virus by herpes simplex virus. J Infect Dis. 1992;166:494–9. doi: 10.1093/infdis/166.3.494. [DOI] [PubMed] [Google Scholar]
- 8.Kucera LS, Leake E, Iyer N, Raben D, Myrvik QN. Human immunodeficiency virus type 1 (HIV-1) and herpes simplex virus type 2 (HSV-2) can coinfect and simultaneously replicate in the same human CD4+ cell: effect of coinfection on infectious HSV-2 and HIV-1 replication. AIDS Res Hum Retroviruses. 1990;6:641–7. doi: 10.1089/aid.1990.6.641. [DOI] [PubMed] [Google Scholar]
- 9.Margolis DM, Ostrove JM, Straus SE. HSV-1 activation of HIV-1 transcription is augmented by a cellular protein that binds near the initiator element. Virology. 1993;192:370–4. doi: 10.1006/viro.1993.1046. [DOI] [PubMed] [Google Scholar]
- 10.Mosca JD, Bednarik DP, Raj NB, et al. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987;84:7408–12. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heng MC, Heng SY, Allen SG. Co-infection and synergy of human immunodeficiency virus-1 and herpes simplex virus-1. Lancet. 1994;343:255–8. doi: 10.1016/s0140-6736(94)91110-x. [DOI] [PubMed] [Google Scholar]
- 12.Diaz JJ, Dodon MD, Schaerer-Uthurralt N, et al. Post-transcriptional transactivation of human retroviral envelope glycoprotein expression by herpes simplex virus Us11 protein. Nature. 1996;379:273–7. doi: 10.1038/379273a0. [DOI] [PubMed] [Google Scholar]
- 13.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunne EF, Whitehead S, Sternberg M, et al. Suppressive Acyclovir Therapy Reduces HIV Cervicovaginal Shedding in HIV- and HSV-2-Infected Women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49:77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 15.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 16.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–8. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 17.Zuckerman RA, Lucchetti A, Whittington WL, et al. HSV suppression reduces seminal HIV-1 levels in HIV-1/HSV-2 co-infected men who have sex with men. AIDS. 2009;23:479–83. doi: 10.1097/QAD.0b013e328326ca62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowan FM, Pascoe SJ, Barlow KL, et al. A randomised placebo-controlled trial to explore the effect of suppressive therapy with acyclovir on genital shedding of HIV-1 and herpes simplex virus type 2 among Zimbabwean sex workers. Sex Transm Infect. 2008;84:548–53. doi: 10.1136/sti.2008.031153. [DOI] [PubMed] [Google Scholar]
- 19.Delany S, Mlaba N, Clayton T, et al. Impact of aciclovir on genital and plasma HIV-1 RNA in HSV-2/HIV-1 co-infected women: a randomized placebo-controlled trial in South Africa. AIDS. 2009;23:461–9. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson-Jones D, Weiss HA, Rusizoka M, et al. Risk factors for herpes simplex virus type 2 and HIV among women at high risk in northwestern Tanzania: preparing for an HSV-2 intervention trial. J Acquir Immune Defic Syndr. 2007;46:631–42. doi: 10.1097/QAI.0b013e31815b2d9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belec L, Meillet D, Levy M, Georges A, Tevi-Benissan C, Pillot J. Dilution assessment of cervicovaginal secretions obtained by vaginal washing for immunological assays. Clin Diagn Lab Immunol. 1995;2:57–61. doi: 10.1128/cdli.2.1.57-61.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loregian A, Gatti R, Palu G, De Palo EF. Separation methods for acyclovir and related antiviral compounds. J Chromatogr B Biomed Sci Appl. 2001;764:289–311. doi: 10.1016/s0378-4347(01)00379-6. [DOI] [PubMed] [Google Scholar]
- 24.ICH Guideline for good clinical practice E6(R1) 1996 [Google Scholar]
- 25.Baisley K, Changalucha J, Weiss HA, et al. Bacterial vaginosis in female facility workers in north-western Tanzania: prevalence and risk factors. Sex Transm Infect. 2009;85:370–5. doi: 10.1136/sti.2008.035543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang XF, Song B, Tu YY, Tong JZ, Faul JL, Bai H. Rapid detection of glycoprotein G gene for the diagnosis and typing of herpes simplex virus infection in genital herpes. Sex Transm Infect. 1999;75:396–7. doi: 10.1136/sti.75.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1995;171:857–63. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 29.Orle KA, Gates CA, Martin DH, Body BA, Weiss JB. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J Clin Microbiol. 1996;34:49–54. doi: 10.1128/jcm.34.1.49-54.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noordhoek GT, Wolters EC, de Jonge ME, van Embden JD. Detection by polymerase chain reaction of Treponema pallidum DNA in cerebrospinal fluid from neurosyphilis patients before and after antibiotic treatment. J Clin Microbiol. 1991;29:1976–84. doi: 10.1128/jcm.29.9.1976-1984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu XX, Rossau R, Jannes G, Ballard R, Laga M, Van Dyck E. The rrs (16S)-rrl (23S) ribosomal intergenic spacer region as a target for the detection of Haemophilus ducreyi by a heminested-PCR assay. Microbiology. 1998;144(Pt 4):1013–9. doi: 10.1099/00221287-144-4-1013. [DOI] [PubMed] [Google Scholar]
- 32.Rouet F, Ekouevi DK, Chaix ML, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol. 2005;43:2709–17. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kessler HH, Muhlbauer G, Rinner B, et al. Detection of Herpes simplex virus DNA by real-time PCR. J Clin Microbiol. 2000;38:2638–42. doi: 10.1128/jcm.38.7.2638-2642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrows J, Nitsche A, Bayly B, Walker E, Higgins G, Kok T. Detection and subtyping of Herpes simplex virus in clinical samples by LightCycler PCR, enzyme immunoassay and cell culture. BMC Microbiol. 2002;2:12. doi: 10.1186/1471-2180-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clift S, Anemona A, Watson-Jones D, et al. Variations of HIV and STI prevalences within communities neighbouring new goldmines in Tanzania: importance for intervention design. Sex Transm Infect. 2003;79:307–12. doi: 10.1136/sti.79.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mostad SB, Kreiss JK, Ryncarz AJ, et al. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J Infect Dis. 2000;181:58–63. doi: 10.1086/315188. [DOI] [PubMed] [Google Scholar]
- 37.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–439. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Miranda P, Blum MR. Pharmacokinetics of acyclovir after intravenous and oral administration. J Antimicrob Chemother. 1983;12(Suppl B):29–37. doi: 10.1093/jac/12.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- 39.Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39:2759–64. doi: 10.1128/aac.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conant MA, Schacker TW, Murphy RL, Gold J, Crutchfield LT, Crooks RJ. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV-infected individuals: two randomized trials. Int J STD AIDS. 2002;13:12–21. doi: 10.1258/0956462021924550. [DOI] [PubMed] [Google Scholar]
- 41.Paz-Bailey G, Ramaswamy M, Hawkes SJ, Geretti AM. Herpes simplex virus type 2: epidemiology and management options in developing countries. Sex Transm Infect. 2007;83:16–22. doi: 10.1136/sti.2006.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wald A, Zeh J, Selke S, Ashley RL, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770–5. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 43.Strick LB, Wald A, Celum C. Management of herpes simplex virus type 2 infection in HIV type 1-infected persons. Clin Infect Dis. 2006;43:347–56. doi: 10.1086/505496. [DOI] [PubMed] [Google Scholar]
- 44.Coombs RW, Wright DJ, Reichelderfer PS, et al. Variation of human immunodeficiency virus type 1 viral RNA levels in the female genital tract: implications for applying measurements to individual women. J Infect Dis. 2001;184:1187–91. doi: 10.1086/323660. [DOI] [PubMed] [Google Scholar]
- 45.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4:260–70. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMahon MA, Siliciano JD, Lai J, et al. The antiherpetic drug acyclovir inhibits HIV replication and selects the V75I reverse transcriptase multidrug resistance mutation. J Biol Chem. 2008;283:31289–93. doi: 10.1074/jbc.C800188200. [DOI] [PMC free article] [PubMed] [Google Scholar]


