Abstract
Neutrophils are recruited to sites of injury but their timely removal is thought to be vital to prevent exacerbating inflammation. In addition, the recognition of apoptotic cells by cells of the innate immune system provides potent anti-inflammatory and anti-immunogenic signals. In this paper we describe how human neutrophils dying by apoptosis or necrosis release anti-inflammatory peptides, the alpha defensins. This family of small cationic peptides, effectively inhibits the secretion of multiple pro-inflammatory cytokines and nitric oxide from macrophages, the main innate immune cell found at sites of chronic inflammation. In addition, the systemic administration of necrotic neutrophil supernatants and alpha defensins protects mice from a murine model of peritonitis. Hence their effects may be far reaching and serve to kill microbes whilst regulating a potentially tissue destructive inflammatory response.
Keywords: Macrophage, Apoptosis, Neutrophil, Inflammation
Introduction
Polymorphonuclear cells (PMNs) are the most abundant type of leukocyte, rapidly recruited to sites of inflammation by pathogen-derived stimuli or host derived danger signals (1). Subsequent activation of PMN triggers the release of reactive oxygen species and an arsenal of non-specific cytotoxic compounds. This has led researchers to consider that the safe disposal of neutrophils as early as possible is essential to the maintenance of immunological homeostasis and the resolution of inflammation (2). However the data that exposes the pathogenic role of late apoptotic and necrotic neutrophils is conflicting. Elastase, which is released by necrotic neutrophils, has been reported to induce resting macrophages to secrete pro-inflammatory cytokines (3). In contrast, other studies indicate that necrotic neutrophils are phagocytosed by macrophages in a non-phlogistic manner and even down regulate CD80, CD86 and CD40 on immature DC, rendering them unable to induce T cell proliferation in an MLR (4, 5). In addition whilst necrotic cell lines are able to induce DC maturation, necrotic primary cells are not (6-8), suggesting that necrotic cells cannot by themselves be considered dangerous, without reference to the cell type and the way in which they are exposed to the immune system.
Defensins are widely distributed in nature, being expressed by leukocytes and epithelial cells lining the environmental interface. They are divided into alpha and beta defensins based on their tertiary structure, which has a characteristic six cysteine motif; pairing to form three intramolecular disulphide bonds. α-Defensins are small cationic and amphipathic peptides with a molecular weight of 3–5 kDa (9). Of the six α-defensins, four (HNP1-4) are major constituents of human neutrophils, where they are found stored in the azurophilic (primary) granules. The other two (HD5-6) are expressed in the Paneth cells, which are secretory epithelial cells located in the small intestinal crypts (10). Whilst rats and rabbits express neutrophil α-defensins, mice do not; but they do express homologues of human HD5-6 in the Paneth cells, known as cryptidins (11). The secretion of α-defensins by epithelial cells is an important component of innate immunity. This is highlighted by mice that lack matrilysin-7 and cannot secrete active cryptidins, due to an inability to process Paneth cell α-defensin precursors. Despite the fact that they secrete a number of other antimicrobial molecules they are more susceptible to an oral challenge with a virulent strain of S. typhimurium and mount a more severe inflammatory response (11). In contrast mice transgenic for the human crypt α-defensin, HD-5, are protected from a normally lethal dose of Salmonella (12). Recently α-defensins have been reported to block the release of IL-1β from monocytes whilst having no effect on the release of TNF-α (13). Monocytes, which are found circulating in the blood, mature into macrophages upon egress from the circulation and entry into tissues. Here they interact with activated neutrophils in the absence of serum proteins that are known to inhibit α-defensin function (14, 15). In this paper we describe how α-defensins, released by dying and necrotic neutrophils exert a powerful anti-inflammatory effect on human macrophages whilst still maintaining significant anti-microbial activity.
Materials and Methods
Reagents
Purified HNP1-3 was supplied by Hycult biotechnology. Synthetic HNP1, linearized HNP1 and the D enantiomer of HNP was kindly provided by Prof Wuyuan Lu. Linear (or linearized) HNP1 is an unstructured form of the α-defensin, in which the six Cys residues have been replaced by Ala. LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES; MW 4493.33) was synthesized by N-(9-fluorenyl) methoxycarbonyl chemistry at the Nucleic Acid/Protein Service unit at the University of British Columbia (UBC; Vancouver, Canada), as described previously (Barlow et al, J. Leuk. Biol, 1996, 80:509-520). R-roscovitine, (R)-2-[[9-(1-methylethyl)-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-1-butanol (A.G. Scientific) was kindly provided by Prof A. Rossi and used at 20uM. HMDMs were stimulated with CD40 ligand (Peprotech, UK) at 3ug/ml and IFN-γ (Peprotech, UK) at 5ng/ml. LPS (Sigma) was used at 1ng/ml.
Mice
6-8 week old female C57BL/6, mice (Harlan-UK), were used at 8-9 weeks of age and were sex and age-matched within experiments. All experiments were covered by a Project License granted by the Home Office under the Animal (Scientific Procedures) Act 1986. Locally, this license was approved by the University of Edinburgh Ethical Review Committee.
Generation of apoptotic cells
Human neutrophils were extracted from peripheral blood of healthy volunteers, as described previously (16). Blood was separated using dextran sedimentation and a Percoll gradient. This yielded highly pure human neutrophils (>95%). Neutrophils were cultured in serum free IMDM supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 ug/ml streptomycin for various periods of time and the cell free medium collected and ultracentrifuged at 100,000g for 1 hour prior to using immediately or storing at −70°C. Necrotic neutrophils were generated from freshly isolated neutrophils by freeze thawing them 5 times after which no complete cells remained. Membranes were removed by ultracentrifuging them at 100,000g for 1 hour. In all in vitro experiments the number of neutrophils used was 12 × 106/ml. An equivalent number of necrotic neutrophils were generated by freeze thawing per ml of culture medium and the membrane free supernatant was used at this concentration. Necrotic thymocytes were generated from thymi removed from 6 week old syngeneic mice, teased into single cell suspensions and freeze thawed 5 times as described for necrotic neutrophils. Murine neutrophils were isolated from the bone marrow of syngeneic mice by percoll gradient and then treated in the same way as human neutrophils to obtain necrotic membrane free cell fractions at the same concentration.
Macrophage culture
Human monocytes were extracted from peripheral blood of healthy volunteers according to Lothian Research Ethics Committee approval (LREC/2001/4/56), using dextran sedimentation and a Percoll gradient as previously described (16). They were cultured in IMDM supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 ug/ml streptomycin, and 10% autologous platelet-rich plasma-derived serum. Mature macrophages were used on day 7. Murine bone marrow derived macrophages were cultured, as described previously (4) and used between day 7 and 10 of culture. All assays were done in serum free medium.
Depletion of α-defensins using R2 or dynabeads beads
R2 beads (Applied Biosystems), which bind hydrophobic proteins, were incubated with membrane free necrotic neutrophil supernatants for 2 hours. Beads were then removed following centrifugation and proteins in the supernatant or on the beads was analysed by NuPage 10% Bis-Tris gel with MES running buffer, Invitrogen. Proteins were visualized by silver stain. The band of peptides at 3-5 kd was cut out, reduced, alkylated and digested with trypsin. Chromatographic separation of tryptic digests was conducted by an Ultimate 3000 nanoLC system (Dionex, Mountain View, CA) and peptides were analyzed by an HCT Ultra PTM ion trap instrument (Bruker Daltonics) equipped with a nano-ESI source. Acquired spectra were analyzed using the MASCOT search engine (Matrix Science) (D. Compopiano and D. Clarke-University of Edinburgh). To specifically deplete NN of α-defensin, Dynabeads M280 coated with sheep anti mouse IgG (Invitrogen) were bound to mouse anti-human HNP 1-3 (Hycult) as per the manufacturers instructions. These anti-HNP 1-3 coated beads were then used to deplete NN of α-defensin and beads with bound α-defensin were removed with a magnet (BD Pharmingen). Complete and specific depletion of α-defensin was checked both by HNP 1-3 ELISA (Hycult) and by protein gel.
Tests of cell viability
LDH released from the cytoplasm of dying macrophages into the assay medium was used as a measure of membrane integrity and viability. A cell cytotoxicity colorimetric kit was used according to the manufacturers instructions (Sigma Aldrich) The assay utilizes NAD, reduced by the released lactate, which induces a colour change in a tetrazolium dye that can be detected using a spectrophotometric method. As a positive control the protein synthesis inhibitor cyclohexamide (10ug/ml) was used to reduce cell viability after 24 hours. The Alamar Blue assay is a sensitive non-radioactive means of measuring cell viability based on the addition of a fluorogenic redox indicator to cells in culture. When taken into cells, Alamar Blue becomes reduced and turns red. This reduced form of Alamar Blue is highly fluorescent. The extent of this conversion, which is a reflection of cell viability, was quantified by its optical density. Alamar Blue was used at a 1:10 dilution and added to the assay medium for the duration of the culture.
Eating assay
Cells were pretreated with α-defensin or a positive control puromycin known to reduce cell viability (Sigma /Aldrich at 50ug/ml). After 24 hours the HMDMs were washed and fresh medium containing 1.25×106 flourscent beads (Fluoresbrite Plain YG 3.0 micron microspheres; PolySciences, Inc. warrington, PA) was added per 0.5×106 HMDMs. After 1h unbound cells were removed and cells washed ×3 with PBS containing magnesium and calcium. Cells were removed from the wells using Trypsin/EDTA, washed again and resuspended in FACs buffer (PBS + 2% FCS) prior to analysis on a FACs machine.
Sterile Peritonitis
Peritonitis was induced by i.p. injection of 0.5 ml of 10% thioglychollate. Mice underwent peritoneal lavage at various time points following thioglychollate injection.
Bacterial in vitro and in vivo infections of macrophages
Murine BMDM were cultured as described above. Salmonella enterica serovar Typhimurium strain SL3261 (17), which was live or had been heat killed was added to murine BMDM at an MOI (multiple of infection) of 10:1 bacteria to macrophages. After 1 hour excess bacteria were washed away and Gentamicin at 100ug/ml was added for 1 hour to kill any residual extracellular bacteria. Cells were washed again and HNP1 or medium alone was added for various time-points after which supernatants were collected for cytokine estimation prior to lysing the macrophages with 1% Triton X for 15 minutes. Lysed cells containing live bacteria were collected and plated onto agar and incubated for 18 hours after which colonies were counted. In a similar way Pseudomonas Aeruginosa PA01 was added to HMDM at an MOI of 10. After 4 hours supernatants were collected prior to lysing the cells with 0.1% Triton X and the number of live colonies counted after a further 18 hours of culture.
ELISA
Supernatants collected after specified culture periods were analyzed for production of cytokines by a sandwich ELISA according to the manufacturers instruction (R&D systems, UK). HNP 1-3 was measured using an HNP1-3 ELISA according to the manufacturers instructions (Hycult Biotechnology). All experiments were performed in triplicate.
Statistics
Data are expressed, when appropriate, as mean and SEM. Significance was assessed using unpaired t tests, and p-values <0.05 were considered significant.
Results
Apoptotic neutrophils do not require contact to inhibit inflammatory macrophages
Our interest in a soluble factor released by dying human neutrophils was initiated by the observation that co-culture of apoptotic neutrophils separated from activated human monocyte derived macrophages (HMDMs) by transwells led to the inhibition of pro-inflammatory cytokine secretion (Fig 1 a/b). TGF-β is thought to play a pivotal role in the inhibition of HMDM TNF-α secretion by apoptotic neutrophils (18). However the addition of blocking anti-TGF-β to apoptotic neutrophils, in contact with LPS-stimulated HMDM had only a moderate inhibitory effect but no effect on CD40L/IFN-γ stimulated HMDM. Apoptotic cells generate apoptotic bodies (19), which may be able to pass through the pores of a transwell. To control for this we ultra-centrifuged supernatants derived from neutrophil cultures to remove apoptotic bodies and all membrane constituents. The active inhibitory factor contained within this neutrophil-conditioned medium (NCM) was released by dying neutrophils in a time-dependent manner. It was able significantly to inhibit the secretion of TNF-α from macrophages stimulated by both LPS and CD40L/IFN-γ by 4 hours after culture, when neutrophils are beginning to undergo apoptosis (Fig 1c/d). TGF-β measured in supernatants from CD40L/IFN-γ- and LPS-stimulated HMDM was not significantly raised whilst levels of IL-1β, IL-6, IL-8 and IL-10 and nitric oxide were all decreased (data not shown).
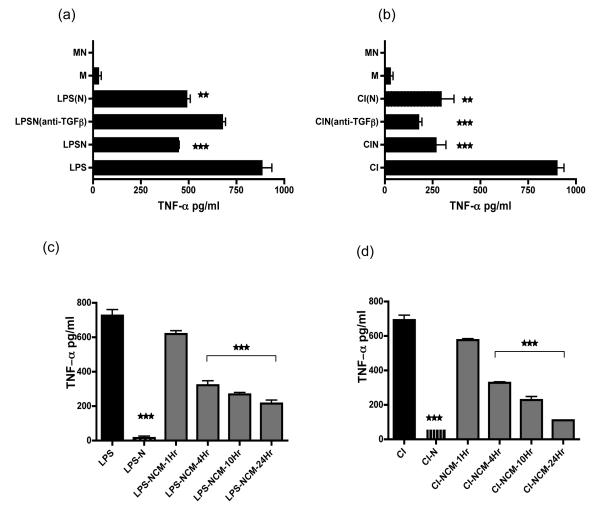
Figure 1. Neutrophils secrete a soluble anti-inflammatory factor.
(a+b) HMDM were stimulated with either LPS or CD40L/IFN-γ (CI) along with added apoptotic neutrophils (LPSN or CIN) for 18 hours, prior to harvesting culture supernatants for assay of TNF-α by ELISA. In triplicate wells anti-TGFβ was added to assess the role of TGFβ in mediating the immunosuppressive effect of apoptotic neutrophils [LPSN(anti-TGFβ) or CIN(anti-TGFβ)]. In addition apoptotic neutrophils were separated from activated HMDM by a transwell [LPS(N) or CI(N)] for the duration of the culture period. Macrophages alone (M) or unstimulated macrophages cultured with apoptotic neutrophils (MN) did not secrete TNF-α.
(c+d) Neutrophils were cultured for up to 24 hours, harvested at set timepoints and ultracentrifuged to remove cell membranes and apoptotic bodies. This neutrophil conditioned medium (NCM) was added (as 25% final vol) to LPS or CD40L/IFN-γ (CI) stimulated macrophages. After 18 hours of culture HMDM culture supernatants were harvested and assayed for TNF-α by ELISA.
Representative of 10 experiments performed with different human donors. Error bars represent SEM and significance of *** p ≤0.0002, ** p ≤0.002, * p ≤0.02.
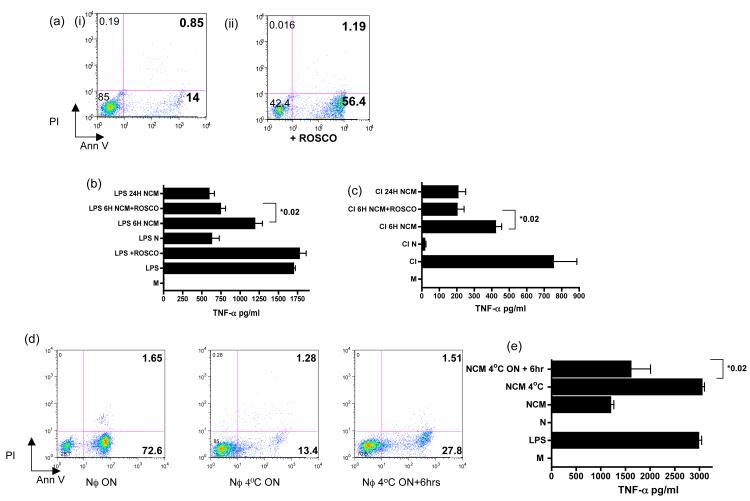
To ask if neutrophil apoptosis augmented the release of the soluble factor we cultured neutrophils in the presence of R-roscovitine, which is known to induce neutrophil apoptosis (20). Following 6 hours of culture the percentage of neutrophils positive for annexin-V increased from 14% to 56.4% (Fig 2a). R-roscovitine did not itself inhibit TNF-α secretion from LPS-stimulated HMDMs (Fig 2b). However culture supernatants from R-roscovitine-treated neutrophils inhibited pro-inflammatory cytokine secretion significantly more than untreated NCM (Fig 2b-c). In contrast, if apoptosis was inhibited, (by culturing neutrophils at 4°C overnight) (Fig 2d) the ability of the NCM was lacking in anti-inflammatory activity. If the same neutrophils were then allowed to undergo apoptosis for 6 hours by culturing at room temperature the inhibitory factor was released into the NCM (Fig 2e).
Figure 2. Neutrophils release an active anti-inflammatory factor as they become apoptotic.
(a-i-ii) Facs analysis of neutrophils cultured in 20uM Roscovitine (Rosco) for 6 hours and stained with Annexin V/PI to detect apoptotic and necrotic cells respectively. NCM from neutrophils cultured with or without Roscovitine for 6 hours was co-cultured with either CI (b) or LPS (c) stimulated macrophages for 18 hours after which macrophage culture supernatants were collected and tested for TNF-α content by ELISA. (d) FACS analysis of neutrophils stained with Annexin V/PI in which apoptosis was inhibited by culturing them at 4°C overnight (NCM 4°C) and then allowed to undergo apoptosis for 6 hrs by culturing at 37°C (NCM 4°C+6hr). Analysis of the ability of NCM from these 3 neutrophil populations to inhibit LPS activated HMDM cytokine secretion was assessed by ELISA following an 18 hour incubation with LPs stimulated macrophages (e). Data representative of 3 separate experiments with different donors. Error bars=SEM and **=p<0.02.
Necrotic neutrophils are also anti-inflammatory
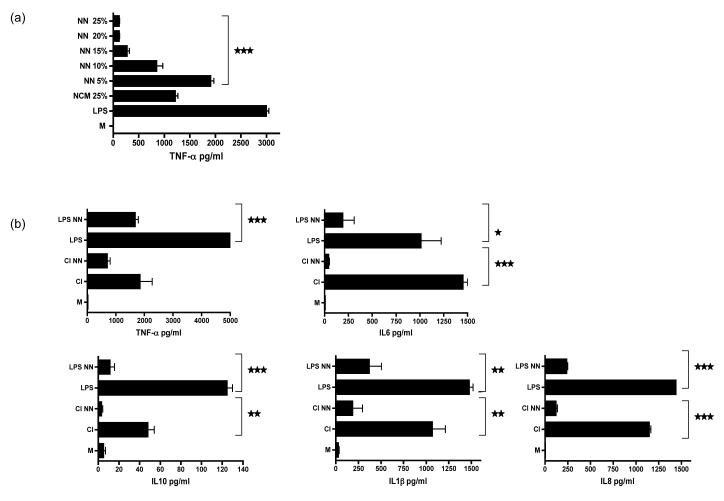
We wondered whether primary or secondary necrotic neutrophils would also release the active immunosuppressive factor. To generate necrotic neutrophils we freeze thawed fresh neutrophils 5 times after which more than 90% of the neutrophils had lysed (data not shown). These lysed cells were then ultracentrifuged to remove membranous material and the remaining necrotic neutrophil conditioned medium (NN) added to LPS stimulated macrophages. Titration of the NN revealed a dose dependent inhibition of TNF-α secretion by the activated macrophages, which was even more effective than using NCM at the same dilution (Fig 3a). The TNF-α ELISA was able to detect both mature and precursor TNF-α. In addition, TNF–α converting enzyme (TACE) levels were measured and found to be unchanged (data not shown). Multicytokine analysis confirmed that NN was also able to inhibit the production of cytokines including IL-6, IL-1β, IL-8, and IL-1 (Fig 3b). In addition NN also inhibited the generation of nitric oxide (Fig 4c). The concentration of TGF-β was either decreased or similar to stimulated cells (data not shown). Identical results were obtained using NN prepared from secondarily necrotic neutrophils that had previously undergone 24 hours of culture (data not shown).
Figure 3. Necrotic neutrophils are anti-inflammatory.
(a) Fresh neutrophils were freeze thawed and then ultracentrifuged to generate membrane-free necrotic neutrophil supernatants (NN). NN was titrated into cultures containing LPS activated HMDMs. This was compared with the ability of NCM at a final vol:vol of 25% released from apoptotic neutrophils to inhibit TNF-α secretion. TNF-α in the supernatants collected from these stimulated macrophages (after 18 hrs of culture) was quantified by ELISA.
(b) Multicytokine analysis of these supernatants to show that NN inhibited the secretion of a wide range of pro-inflammatory cytokines as well as IL-10 by activated macrophages stimulated with LPS or CD40L/IFN-γ (CI). *** p ≤0.0002, ** p ≤0.003, * p ≤0.03.error bars =SEM. Experiments representative of 1 from 5 using separate donors.
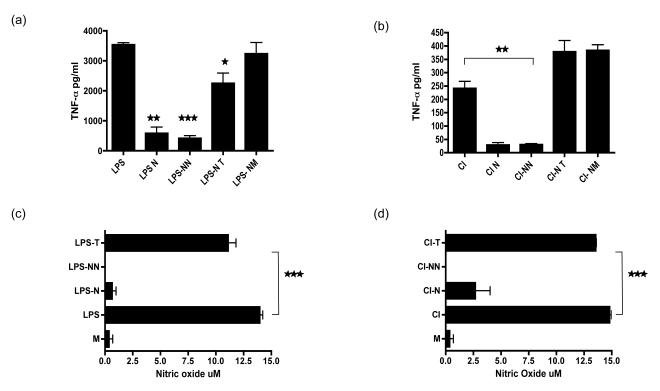
Figure 4. Necrotic neutrophils but not other necrotic cells are anti-inflammatory.
Apoptotic neutrophils (N), necrotic neutrophil supernatants (NN), necrotic thymocyte supernatants (NT) and necrotic supernatants from the tumour cell line Mutu (NM) were added to either LPS (a) or CD40L/IFN-γ (CI) (b) stimulated macrophages and culture supernatants collected 18 hours later were analysed by ELISA for TNF-α. Murine bone marrow derived macrophages were also stimulated with either LPS (c) or CD40L/IFN-γ [CI] (d) with added NN or NT and culture supernatants tested for NO by the Griess reaction. *** p ≤0.0001, ** p ≤0.002, * p ≤0.02.error bars =SEM.
Necrotic neutrophils but not other necrotic cells are anti-inflammatory
Necrotic cells are generally considered to pose a danger to the immune system resulting in auto-antibody generation and a breakdown in tolerance to self with subsequent autoimmunity (21-23). We were interested to know if necrotic neutrophils were unique in their ability to release a soluble anti-inflammatory factor or if this could be generalised to other primary cells or tumour cell lines. The anti-inflammatory activity of necrotic neutrophils was compared to supernatants from necrotic murine thymocytes (NT) and from the necrotic human tumour cell line, Mutu (NM). While necrotic thymocyte supernatants had a limited ability to suppress TNF-α secretion from LPS-stimulated HMDM, necrotic tumor cells had none (Fig 4a) and both necrotic thymocytes and tumour cells were pro-inflammatory to CD40L/IFN-γ-stimulated HMDMs (Fig 4b). In contrast NN was markedly anti-inflammatory inhibiting both TNF-α and nitric oxide (NO) generation (Fig 4c/d). This indicates that compared to the cells tested, the release of a soluble anti-inflammatory factor is specific to neutrophils.
Alpha defensins are the active anti-inflammatory factor released by apoptotic/necrotic neutrophils
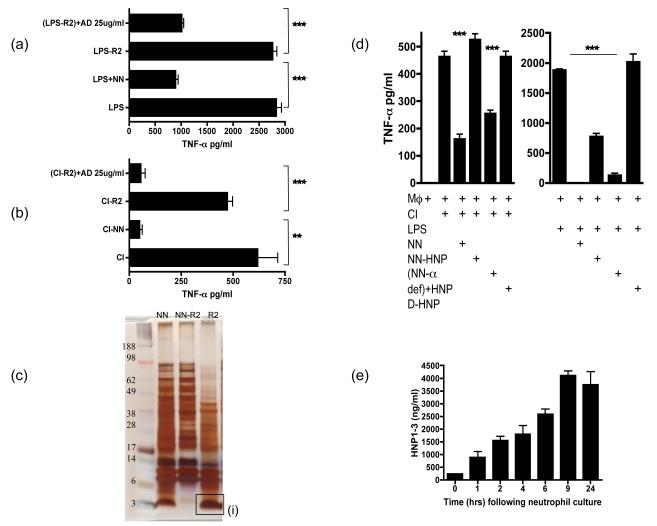
To delineate further the active immunosuppressive factor we tested the NN that had been depleted of hydrophobic proteins using R2 beads and found that depleted NN now lacked the ability to inhibit LPS (Fig 5a) or CD40L/IFN-γ (Fig 5b) stimulated HMDM release of TNF-α. The R2 beads had partially removed a range of proteins from the NN, but completely removed a band of proteins between 3-5kDa in size (Fig 5c). This band was digested and sequenced by MS/MS and found to be the anti-microbial peptide α-defensins (data not shown). When purified alpha defensins (AD) were added back to the R2 depleted NN the immunosuppressive activity of the NN was restored, indicating that one of the active inhibitory factors released by and contained within the neutrophils was α-defensins. However R2 beads removed a range of proteins from the NN and to ensure specificity, α-defensins were depleted from NN using anti-human HNP 1-3 bound to dynabeads. The complete removal of α-defensins was confirmed with an HNP1-3 ELISA whilst the specificity of the antibody bound beads was confirmed by protein gel analysis (data not shown). When α-defensins were specifically depleted from the NN the ability of NN to inhibit TNF-α production by CD40L/IFN-γ stimulated HMDMs was completely lost (Fig 5di), but was regained upon addition of HNP-1. However NN was still able to significantly inhibit TNF-α production by HMDMs stimulated with LPS (Fig 5dii) because the NN retained LL37, which is known to bind to LPS and inhibit its pro-inflammatory potential (24). When HNP-1 was added back though the full inhibitory capacity of the NN was restored.
Figure 5. Alpha defensins are one of the the active anti-inflammatory factors released by apoptotic/necrotic neutrophils.
LPS (a) or CI (b) stimulated HMDM were cultured with NN, NN depleted of hydrophobic molecules by R2 beads (NN-R2) and NN-R2 where α-defensins were added back at 25ug/ml [(NN-R2)+AD]. Culture supernatants were collected after 18 hours of culture and tested for TNF-α secretion by ELISA. (c) A protein gel of NN indicated the large number of proteins released by necrotic neutrophils (NN). NN were depleted of hydrophobic proteins by R2 beads (NN-R2) and the proteins bound to the R2 beads (R2) were identified. R2 beads completely depleted a large band of small proteins between 3-5kD. This band was digested and sequenced by HPLC and identified as the anti-microbial peptides, α-defensins. (d) The actual release of α-defensins over 24 hours by cultured neutrophils undergoing apoptosis was quantified by HNP 1-3 ELISA. (e) To ensure that the R2 beads had not depleted other anti-inflammatory factors, α-defensins in NN were specifically depleted using anti-HNP antibodies bound to dynabeads. HMDMs were then stimulated with CI or LPS along with added NN depleted of α-defensins (NN-α def) or depleted NN where HNP 1-3 has been added back at 25ug/ml (NN-α def)+HNP. As an additional control HMDMs were stimulated with CI or LPS in the presence of the D-enantiomer of HNP1-3, which lacks anti-inflammatiory activity and is protease resistant.. *** p ≤0.0001, ** p ≤0.04. error bars =SEM.
α-Defensins exist as 4 types in human neutrophils; human neutrophil peptides 1-4 (HNP1-4). HNP1-3 constitute more than 5% of the total cellular protein in human neutrophils and 99% of the total defensin content of neutrophils with traces of HNP4. We measured HNP1-3 released by neutrophils undergoing apoptosis in culture and found that the concentration of HNP1-3 increased progressively with time reaching a peak by 9 hours suggesting that the release of α-defensins is associated with ongoing neutrophil apoptosis (Fig 5e). The level of the α-defensins in necrotic neutrophil supernatants was consistently higher at between 8-15 +/− 0.45 ug/ml, depending on the human donor. We also assessed the concentration of HNP 1-3 in the synovial fluid of 12 patients suffering with a flare of rheumatoid arthritis undergoing arthrocentesis for an acutely swollen knee, which was found to range between 3-25ug/ml with an average of 12.4ug/ml indicating that the concentration reached in tissues is not dissimilar to that tested in our assays.
α-Defensins do not kill macrophages
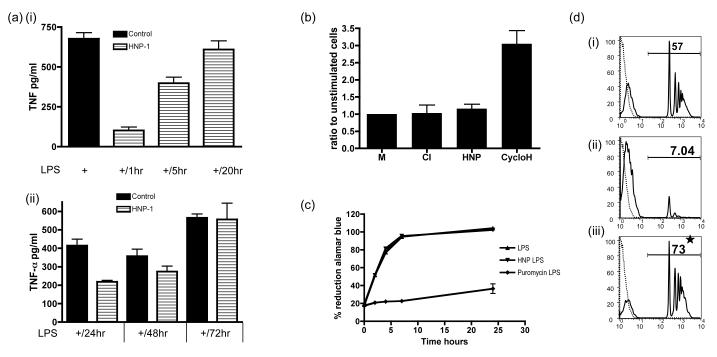
A number of reports have described how α-defensins are able to kill eukaryotic cells reviewed in (25). In contrast, L929 cells a murine fibroblast cell line, is resistant to killing by α-defensins (26). We asked if α-defensins decreased the cytokine production of macrophages through a delayed effect on cell viability. We found that α-defensin (25ug/ml) pre-treatment for 1 hour prior to stimulating HMDMs with LPS inhibited the ability of macrophages to generate TNF-α, but 20 hours following the removal of α-defensins they were able to secrete equivalent amounts of TNF-α when compared to untreated control macrophages (Fig 6ai). In addition HMDMs cultured in the presence of α-defensin for 24 hours were more refractory to stimulation with LPS and only fully recovered their ability to secrete TNF-α after 72 hours (Fig 6aii). However the fact that they do completely recover indicates that α-defensin treated macrophages, (which do not proliferate in culture) are still viable and able to respond to LPS as well as control cells after a period of time. In addition we performed LDH assays to assess the viability of macrophages after α-defensin treatment. Lactate dehydrogenase (LDH), which is released as cells die, was not significantly elevated when compared to both resting and CD40L/IFN-γ stimulated HMDMs (Fig 6b) after 24 hours of culture with α-defensins. We utilised an additional test of cell viability, the Alamar Blue assay, which relies on detecting the reduced form of Alamar Blue generated by reductase enzymes present in viable cells. When cells were cultured in the presence of the cytotoxic agent, puromycin for 24 hours and then stimulated with LPS a definite decrease in the reduction of Alamar blue is seen secondary to a reduction in cell viability. In contrast no change in reductive capacity is seen in HMDMs pretreated with α-defensin (25ug/ml) for the same length of time indicating that viability was maintained (Fig 6c). Finally we assessed the other main function of HMDMs, their ability to phagocytose (beads) following pre-treatment for 24 hours with either α-defensin or puromycin (Fig 6d). In comparison to control untreated HMDMs (6ci) puromycin treated HMDMs showed a reduction in the ability to phagocytose fluorescent beads (6cii); but HMDMs pre-treated with α-defensins had a significantly augmented phagocytic capacity when compared to untreated macrophages (6ciii), suggesting that α-defensins had functionally altered the macrophage to a pro-resolution, pro phagocytic phenotype.
Fig 6. Alpha defensins do not kill macrophages and actually enhance their phagocytic capacity.
(a) HMDMs were pre-treated with HNP1-3 for 1 hour (i) and then allowed to rest for 1 (+/1hr), 5 (+/5hr) or 20 hours (+/20hr) prior to stimulating them with LPS for a further 18 hours, after which supernatants were collected and tested for TNF-α by ELISA. (ii) The same as (i) but HMDMs were pre-treated with HNP 1-3 for 24 hours and then rested for 24 (+/24hr), 48 (+/48hr) or 72 hours (+/72hr) prior to stimulating with LPS. Control HMDMs were pre treated with vehicle alone for the same time period. (b)LDH levels were measured from supernatants taken from HMDMs stimulated with CD40/IFN-γ (CI), HNP1-3 (HNP) or cyclohexamide for 18 hours. (c) HMDMs were pre treated with HNP 1-3 or Puromycin for 24 hours prior to stimulating with LPS for a further 18 hours. Alamar blue was used to determine the the presence of reductive enzymes seen in viable cells. (d) Histograms of HMDMs that were treated with vehicle (i), puromycin (ii) or HNP1-3 (iii) for 24 hours, prior to adding fluorescent beads for 1 hour. Cells were washed, lifted from the cell culture plates and the degree of eating quantified by FACs (black line). Dashed line represents control HMDMs without added fluorescent beads. * p ≤0.01. error bars =SEM.
Alpha defensins inhibit the pro-inflammatory cytokine production by macrophages in the presence of both live and dead whole bacteria
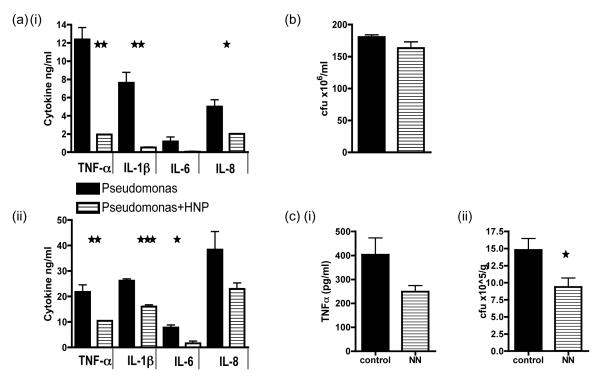
We went on to ask if α-defensins were able to inhibit macrophage pro-inflammatory function and still inhibit the growth of bacteria. We first looked at the response of HMDMs to infection with the human opportunistic pathogen Pseudomonas Aeruginosa PA01. HMDMs infected with live bacteria (Fig 7ai) at a MOI of 10 and treated with α-defensins or with an equivalent number of dead whole bacteria (Fig 7aii) also showed an inhibited secretion of TNF-α, IL-8, IL-6 and IL-1β. In spite of the reduced pro-inflammatory cytokine secretion bacterial counts were not increased when compared to control infected HMDMs (Fig 7b). Hence α-defensin treatment inhibits an excessive pro-inflammatory cytokine response from the HMDM despite the presence of both live and dead Pseudomonas Aeruginosa PA01, but this does not subsequently allow for excessive pathogen replication. We went on to ask if α-defensins could affect a murine model of infection. We used the murine pathogenic Salmonella enterica serovar Typhimurium strain SL3261 to infect mice and sacrificed them on day 7 at the height of infection (Fig 7c). We found that the administration of NN had a significant effect on reducing bacterial counts in the spleen (7ci) and also reduced TNF-α in the serum (7cii).
Fig 7. Alpha defensins can still inhibit pro-inflammatory cytokine secretion by HMDMs despite infection with whole bacteria.
(a) HMDMs were co-cultured with dead Pseudomonas Aeruginosa PA01 for 24 hours (i) or live bacteria (at an MOI of 10) for 4 hours (ii) after which culture supernatants were collected and cytokine secretion quantitated by ELISA. (b) For the live bacterial experiment HMDMs were lysed after 4 hours and bacteria cultured for a further 18 hours on agar prior to counting the number of live colonies. (c) Mice were injected with 10×6 of live Salmonella enterica serovar Typhimurium and PBS or NN was administered on days 0,1,2,4 and 6. Mice were sacrificed on day 7 and TNF-α was measured in the serum (i). In addition the number of live bacteria retrieved from lysed splenocytes after an overnight culture was calculated following a further 18 hours of culture on agar (ii). *** p ≤0.0004, ** p ≤0.003, * p ≤0.02. error bars =SEM
Alpha defensins but not LL37 inhibits both T cell mediated and LPS mediated activation of macrophages
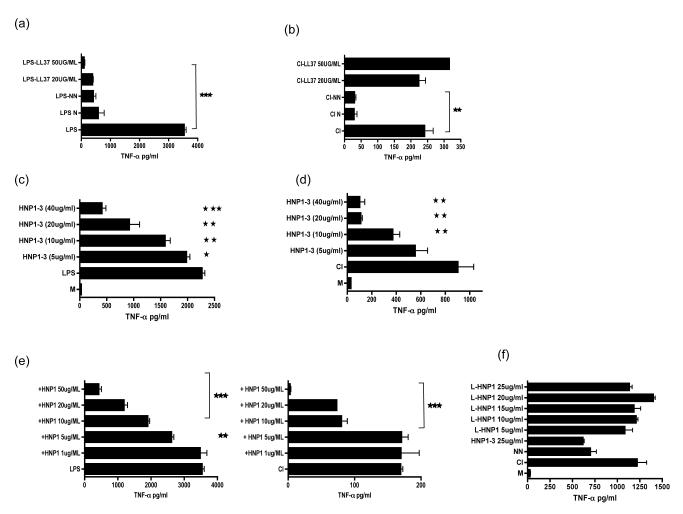
Neutrophils contain within the secondary granules cathelicidin, an anti microbial peptide of comparable electrophorectic mobility to α-defensins. LL37, the active fragment of the only human cathelicidin hCAP-18 is known to bind LPS and inhibit LPS mediated activation of macrophages (24, 27). To test the possibility that one of the inhibitory factors contained within the NN was LL37 we titrated LL37 into both LPS (Fig 8a) and CD40L/IFN-γ (Fig 8b) stimulated HMDMs and compared this with the ability of apoptotic neutrophils (N) or NN to inhibit TNF-α secretion. Whereas LL37 was able to inhibit TNF-α secretion from LPS activated HMDMs, it behaved as a pro-inflammatory peptide to CD40L/IFN-γ stimulated HMDMs. This indicates that LL37 is not the active factor that inhibits both CD40L/IFN-γ and LPS stimulated macrophages. We titrated purified HNP 1-3 into LPS (Fig 8c) or CD40L/IFN-γ (Fig 8d) stimulated HMDMs and found that this peptide preparation was able to significantly inhibit pro-inflammatory cytokine secretion by activated HMDMs. As HNP1 constitutes the major alpha defensin in the primary granules of neutrophils (25) we used synthetically-derived HNP-1, finding similar levels of immunosuppressive activity (Fig 8e). HNP2 and HNP3 were also able to significantly inhibit TNF-α secretion by LPS or CD40L/IFN-γ stimulated HMDMs (data not shown). The requirement for structural integrity of HNP1 was examined by comparing the ability of linearized α-defensin, to inhibit TNF-α secretion from CD40L/IFN-γ. HMDMs; this confirmed that the three dimensional structure of HNP-1 was essential for anti-inflammatory activity, which was completely lost when the peptide was linearized (Fig 8f).
Fig 8. Alpha defensins but not cathelicidins inhibit both T cell mediated and LPS mediated activation of macrophages.
HMDM were stimulated with either LPS (a) or CD40L/IFN-γ (CI) [b] and apoptotic neutrophils (N), necrotic neutrophil supernatants (NN) or LL37 at the indicated doses. In separate experiments HNP1-3 (c-d) or purified HNP-1 was titrated into CI or LPS activated HMDMs. The anti-inflammatory potential of synthetically derived HNP-1 that had been linearized was compared to α-defensins using CD40L/IFN-γ (CI) stimulated HMDMs. Culture supernatants were harvested after 18 hours and tested for TNF-α secretion by ELISA. *** p ≤0.0001, ** p ≤0.001, * p ≤0.01.error bars =SEM when compared to HMDMs treated with stimulus alone .
Alpha defensins do not affect the release of pro-inflammatory cytokines from macrophages
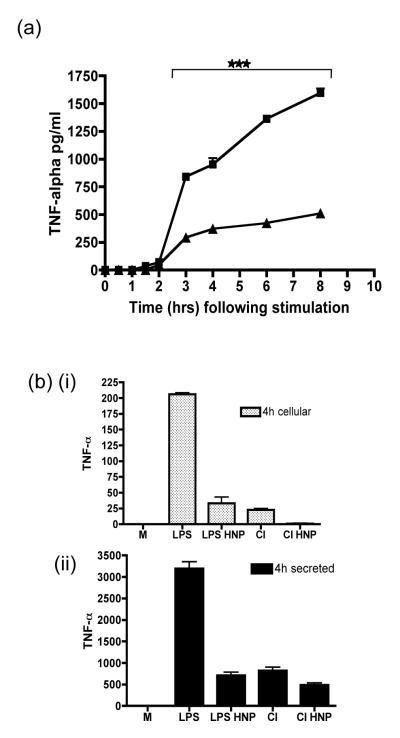
We asked if α-defensins elicited their anti-inflammatory properties via a direct effect on cell membranes preventing the release of cytokines contained within secretory vesicles of HMDMs. To address this we stimulated mature HMDMs with CD40L/IFN-γ plus or minus HNP1-3. At specified time-points culture supernatants were collected and analysed for TNF-α protein by ELISA. TNF–α levels climbed steadily after stimulation in control wells reaching a peak after 8 hours. However in stimulated and HNP1-3 treated wells TNF-α appeared to plateau soon after 3 hours and remained low for the duration of the experiment (Fig 9a). To ask if the TNF-α may be prevented from leaving the cells, macrophages were lysed at 4 hours after stimulation. The concentration of cytokines contained within the macrophage (Fig 9bi) and secreted into the culture medium was then compared by ELISA (Fig 9bii). No significant differences were seen in the ratio of secreted to retained TNF-α in either LPS or CD40L/IFN-γ stimulated HMDMs treated with α-defensins suggesting that TNF-α was not being sequestered within the macrophage. The low levels of NO found after α-defensin treatment would also be in keeping with our data as this is not stored in secretory vesicles (Fig 4c/d).
Figure 9. Alpha defensins do not inhibit the exocytosis of TNF-α.
(a) HMDM were stimulated with CD40L/IFNγ (CI) either alone or in the presence of HNP1-3 (25ug/ml) for the indicated times. TNF-α protein released by HMDMs was measured by ELISA. (b) HMDMs were stimulated with LPS or CD40L/IFN-γ (CI) and treated with HNP1-3 (25ug/ml) or vehicle alone. At 4 hours post stimulation culture supernatants (i) were harvested prior to lysis of the HMDMs to reveal TNF-α retained within the cells (ii). *** p ≤0.001, error bars =SEM.
Necrotic neutrophils and HNP-1 protect mice from experimental inflammation
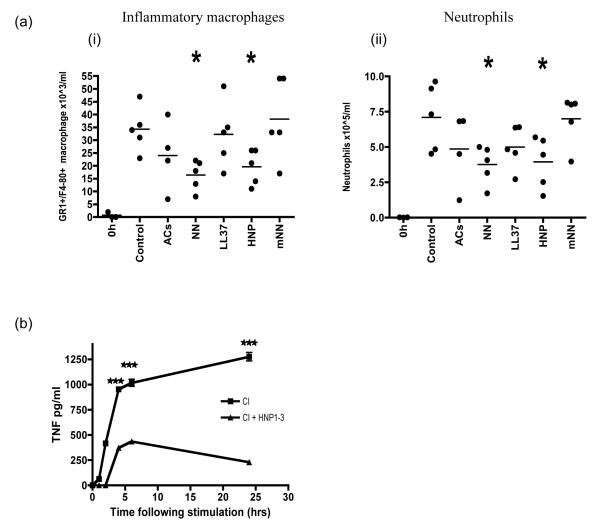
To assess the local effect of α-defensins on an established inflammatory response in vivo we used the thioglychollate model of peritonitis and found HNP1 and NN reduced the cellular infiltrate of neutrophils and macrophages (Fig 10a). We did not find a significant reduction in the inflammatory cell influx using necrotic mouse neutrophils (prepared in an identical way to human NN and at the same concentration), which lack α-defensins nor did the injection of whole AC or LL37 at 5 ug/ml affect the accumulation of inflammatory cells. In separate experiments to test the possibility that the reduced influx of inflammatory cells was secondary to the inhibition of resident peritoneal macrophages, these cells were isolated from the peritoneum of untreated mice, adhered to plastic overnight and stimulated with CD40L/IFN-γ along with added α-defensins (Fig 10b). Resident peritoneal macrophages treated with α-defensin were completely unable to respond to the stimulus and secrete TNF-α. Identical results were obtained following LPS stimulation (data not shown).
Figure 10. Necrotic neutrophils and α-defensins protect mice from experimental inflammation.
Sterile peritonitis was induced by injecting thioglychollate, along with either PBS, apoptotic cells (AC), necrotic human neutrophils (NN), LL37, HNP1-3 or mouse necrotic neutrophils (mNN). After 4 hours peritoneal lavages were used to isolate inflammatory GR1+F480+ macrophages (a) and neutrophils (b), which were characterised by FACS and compared to cell numbers in control mice with peritonitis given PBS. Experiment is representative of 2 separate expts with 5 mice per group. (c) In separate experiments resting murine peritoneal macrophages were isolated and stimulated in vitro with CD40/IFN-γ along with α-defensins and supernatants were collected at various timepoints and tested for TNF-α. *** p ≤0.001, * p ≤0.01, error bars =SEM.
Discussion
It is currently widely believed that macrophages must engulf apoptotic neutrophils before they become necrotic to prevent the release into the tissues of potentially toxic and immunogenic intracellular substances (28). We have now discovered that both apoptotic and necrotic neutrophils elicit a profound anti-inflammatory response in macrophages that does not require cell contact. We have identified the anti-inflammatory mediator they release as α-defensins. The α-defensins inhibit macrophage pro-inflammatory function driven both by the microbial cell wall constituent LPS and a T cell surrogate stimulus CD40L/IFN-γ. When HMDMs are infected with Pseudomonas α-defensins effectively prevented the macrophages from inducing an exaggerated pro-inflammatory cytokine response, whilst not compromising the ability of macrophages to keep bacterial viability in check. This was mirrored in an in vivo model of infection with the pathogenic Salmonella Typhimurium using NN where both bacterial cell counts and serum TNF-α measured at the height of the infection were reduced.
Alpha-defensins are released by neutrophils as early as 4 hours after in vitro culture and continue to be released reaching a peak when neutrophil apoptosis is established. Importantly, the α-defensins are also released from necrotic cells when they disintegrate, explaining the protective effect of necrotic neutrophils when injected in vivo in a murine model of inflammation and infection. The finding that human NN, (which contains α-defensins) were able to reduce the influx of neutrophils and inflammatory macrophages in a murine model of peritonitis was surprising given that they have been shown to be chemotactic for immature dendritic cells and lymphocytes, though interestingly do not activate them (29). In addition necrotic human neutrophil supernatants were devoid of membranous products (following ultracentrifugation) but were otherwise replete with preformed enzymes that would be expected to be pro-inflammatory in their own right (1, 3). In contrast murine necrotic neutrophils, which do not contain α-defensins but are otherwise similar to human neutrophils did not affect the influx of inflammatory cells into the peritoneum suggesting that this effect was specific for the presence of the peptide (30). The effect of α-defensins on macrophages may be specific as α-defensin treatment did not inhibit the activation of human neutrophils by TNF-α, as measured by the loss of surface CD62L (L-selectin) and CD11b upregulation. Myeloperoxidase release from these activated neutrophils and the degranulation of murine peritoneal mast cells was also unaffected (data not shown). One may speculate that the reduced influx of inflammatory cells in the peritoneum may relate to an initial dampening of the inflammatory response of resident macrophages normally seen when the irritant and innate immune stimulus, thioglychollate is administered. This in turn would lead to a reduction in cellular influx of neutrophils and inflammatory macrophages. In support of this, in vitro experiments on resting resident peritoneal macrophages that have been stimulated with α-defensins show that they are completely inhibited from responding to concomitant stimulation with CD40/IFN-γ and this inhibition may override any chemotactic effect of α-defensin alone. α-Defensins have recently been shown to inhibit specifically the secretion of IL-1β by monocytes attesting to their anti-inflammatory role (13). Interestingly recent reports have linked the absence of intestinal Paneth cell α-defensins to chronic colitis seen both in animal models and in humans with Crohn’s disease (11, 31). As Crohn’s disease is likely due to an aberrant response to commensal bacteria which normally pose no risk to healthy adults (32-34), one could speculate that the lack of these α-defensins may deprive these patients not only of an antimicrobial peptide but also of an important anti-inflammatory and immuno-regulatory signal in the distal small intestine (13). Indeed the effect of α-defensins on macrophages, reducing the secretion of multiple pro-inflammatory cytokines whilst checking the growth of bacteria attests to its ability to prevent an excessively pro-inflammatory macrophage response whilst not sacrificing its ability to function as an antimicrobial peptide. The mechanism by which α-defensins inhibit such a broad swathe of pro-inflammatory cytokines and NO is unknown. As an anti-microbial peptide, they induce pores in bacterial membranes but the exact means by which they kill microbes remains a mystery (35). Analysis of treated macrophages used in our assays showed no evidence of macrophage apoptosis following culture with α-defensins. Prolonged treatment for up to 24 hours with α-defensins did not result in a delayed decrease in viability as measured by Alamar blue and LDH assays. In addition macrophages regained the ability to respond to pro-inflammatory stimuli producing equivalent amounts of TNF-α, compared to control untreated macrophages following a delay that was proportional to the time that they had been initially exposed to α-defensins. Pre-treatment with α-defensins led to an increase in phagocytic capacity, which suggests that they do not simply inhibit macrophage function but alter it to a pro-phagocytic pro-resolution phenotype. Time-course studies of secreted TNF-α indicate that released cytokine fails to ever reach control levels following α-defensin treatment but lysates of cells did not contain TNF-α, suggesting that it was not prevented from leaving the cells as Shi et al have found specifically for IL-1β in monocytes. We would speculate that α-defensins may affect the translation of pro-inflammatory cytokines through an effect on mRNA stability or alternatively through the inhibition of the pro-inflammatory transcription factor, nuclear factor-kB. Future work clearly needs to elucidate the molecular mechanism by which they inhibit the inflammatory phenotype of macrophages and to ask if this could be useful as a therapeutic option in autoimmune diseases such as rheumatoid arthritis in which the inflammatory macrophage mediates the final assault on normal healthy tissue.
Currently, the prevailing view is that necrotic cells present danger signals to the immune system; for instance necrotic fibroblasts are found to be immunostimulatory to DC (36, 37). Thus, it is generally accepted that the presence of necrotic cells, especially neutrophils, is pro-inflammatory (3, 28). This is despite reports of the inhibitory effect of necrotic neutrophils on dendritic cell (DC) maturation and the ability of macrophages to respond to necrotic neutrophils in a non-phlogistic way, (4, 38). It seems likely that not all necrotic cells pose a danger. Our data clearly shows that necrotic human neutrophils are, in fact, anti-inflammatory and if a macrophage encounters such a cell its ability to secrete pro-inflammatory cytokines and NO is inhibited, whilst its ability to phagocytose material is increased. Thus, neutrophil necrosis at sites of inflammation far from driving the process, initiates its resolution.
Tissue resident macrophages are among the first cells to detect microorganisms that have crossed an epithelial barrier. They then recruit large numbers of neutrophils, followed by blood monocytes that differentiate into macrophages upon entry into the affected tissue. Both cell types become activated, phagocytose microorganisms and in the case of neutrophils then undergo apoptosis. The presence of these apoptotic cells then alters the macrophage response, switching it from an inflammatory to a pro-resolution phenotype (39). If necrotic neutrophils were pro-inflammatory and if the ability of macrophages to phagocytose them was overwhelmed even temporarily; then the inevitable result would be further inflammation. In this scenario the immune system would be permanently poised on a knife-edge, dependent entirely upon the rate at which apoptotic neutrophils were removed. In our model, the finding that necrotic human neutrophils are uniquely anti-inflammatory attests to the importance of avoiding this catastrophic possibility. In fact during an inflammatory response the apoptosis of neutrophils (and subsequent interaction with macrophages), is correlated temporally with the resolution of inflammation (40, 41). Physiologically, α-defensins released by dying neutrophils may then exert potent anti-inflammatory effects on macrophages, providing the perfect counterbalance to the arsenal of cytotoxic compounds contained within them. The release of alpha defensins means that the pro-resolution effect of apoptotic/necrotic neutrophils on inflammatory macrophages is not limited to those cells the neutrophil specifically contacts. In conclusion, neutrophils secrete both an antimicrobial and an anti-inflammatory peptide as they die and undergo necrosis, so that even in death they continue to exert an immunomodulatory and anti-microbial phenotype fighting pathogens whilst preventing an excessive inflammatory response that would place healthy tissue at risk of further damage.
Acknowledgements
We would like to thank Professor Sir John Savill, Professor Adriano Rossi and Dr Simon Brown for their helpful suggestions and Dr Rengi Mathews for the collection of synovial fluid.
This work was supported by grants from the ARC (MG and KH), Wellcome Trust (DJD), MRC (PB) and EPSRC and RSE (DJC and DJC).
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
The authors have no conflicting financial interests.
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 3.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 4.Ren Y, Stuart L, Lindberg FP, Rosenkranz AR, Chen Y, Mayadas TN, Savill J. Nonphlogistic clearance of late apoptotic neutrophils by macrophages: efficient phagocytosis independent of beta 2 integrins. J Immunol. 2001;166:4743–4750. doi: 10.4049/jimmunol.166.7.4743. [DOI] [PubMed] [Google Scholar]
- 5.Clayton AR, Prue RL, Harper L, Drayson MT, Savage CO. Dendritic cell uptake of human apoptotic and necrotic neutrophils inhibits CD40, CD80, and CD86 expression and reduces allogeneic T cell responses: relevance to systemic vasculitis. Arthritis Rheum. 2003;48:2362–2374. doi: 10.1002/art.11130. [DOI] [PubMed] [Google Scholar]
- 6.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel RM. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci U S A. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronchetti A, Rovere P, Iezzi G, Galati G, Heltai S, Protti MP, Garancini MP, Manfredi AA, Rugarli C, Bellone M. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J Immunol. 1999;163:130–136. [PubMed] [Google Scholar]
- 9.Fellermann K, Stange EF. Defensins -- innate immunity at the epithelial frontier. Eur J Gastroenterol Hepatol. 2001;13:771–776. doi: 10.1097/00042737-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Ouellette AJ, Bevins CL. Paneth cell defensins and innate immunity of the small bowel. Inflamm Bowel Dis. 2001;7:43–50. doi: 10.1097/00054725-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 12.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Aono S, Lu W, Ouellette AJ, Hu X, Ji Y, Wang L, Lenz S, van Ginkel FW, Liles M, Dykstra C, Morrison EE, Elson CO. A novel role for defensins in intestinal homeostasis: regulation of IL-1beta secretion. J Immunol. 2007;179:1245–1253. doi: 10.4049/jimmunol.179.2.1245. [DOI] [PubMed] [Google Scholar]
- 14.Panyutich A, Ganz T. Activated alpha 2-macroglobulin is a principal defensin-binding protein. Am J Respir Cell Mol Biol. 1991;5:101–106. doi: 10.1165/ajrcmb/5.2.101. [DOI] [PubMed] [Google Scholar]
- 15.Panyutich AV, Hiemstra PS, van Wetering S, Ganz T. Human neutrophil defensin and serpins form complexes and inactivate each other. Am J Respir Cell Mol Biol. 1995;12:351–357. doi: 10.1165/ajrcmb.12.3.7873202. [DOI] [PubMed] [Google Scholar]
- 16.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr., Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 17.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 18.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–223. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 20.Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, Gray M, Crescenzi E, Martin MC, Brady HJ, Savill JS, Dransfield I, Haslett C. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- 21.Grossmayer GE, Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, Herrmann M. Removal of dying cells and systemic lupus erythematosus. Mod Rheumatol. 2005;15:383–390. doi: 10.1007/s10165-005-0430-x. [DOI] [PubMed] [Google Scholar]
- 22.Ma L, Chan KW, Trendell-Smith NJ, Wu A, Tian L, Lam AC, Chan AK, Lo CK, Chik S, Ko KH, To CK, Kam SK, Li XS, Yang CH, Leung SY, Ng MH, Stott DI, MacPherson GG, Huang FP. Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur J Immunol. 2005;35:3364–3375. doi: 10.1002/eji.200535192. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Molinaro C, Johnson N, Casiano CA. Secondary necrosis is a source of proteolytically modified forms of specific intracellular autoantigens: implications for systemic autoimmunity. Arthritis Rheum. 2001;44:2642–2652. doi: 10.1002/1529-0131(200111)44:11<2642::aid-art444>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Heumann D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells. J Immunol. 2001;167:3329–3338. doi: 10.4049/jimmunol.167.6.3329. [DOI] [PubMed] [Google Scholar]
- 25.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein AK, Ganz T, Nguyen TM, Selsted ME, Lehrer RI. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J Immunol. 1988;140:2686–2694. [PubMed] [Google Scholar]
- 27.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- 30.Eisenhauer PB, Lehrer RI. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, Shen B, Schaeffeler E, Schwab M, Linzmeier R, Feathers RW, Chu H, Lima H, Jr., Fellermann K, Ganz T, Stange EF, Bevins CL. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci U S A. 2005;102:18129–18134. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T, Kitani A, Strober W. NOD2 regulation of Toll-like receptor responses and the pathogenesis of Crohn’s disease. Gut. 2005;54:1515–1518. doi: 10.1136/gut.2005.071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Carpio DF, Zheng Y, Bruzzo P, Singh V, Ouaaz F, Medzhitov RM, Beg AA. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128–7135. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- 37.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 38.Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 39.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 40.Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232–237. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- 41.Ishii Y, Hashimoto K, Nomura A, Sakamoto T, Uchida Y, Ohtsuka M, Hasegawa S, Sagai M. Elimination of neutrophils by apoptosis during the resolution of acute pulmonary inflammation in rats. Lung. 1998;176:89–98. doi: 10.1007/pl00007597. [DOI] [PubMed] [Google Scholar]