Abstract
There is a long-standing paradox that N-methyl-D-aspartate receptors (NMDARs) can both promote neuronal health and kill neurons. Recent studies show that NMDAR-induced responses depend on the receptor location: stimulation of synaptic NMDARs, acting primarily through nuclear Ca2+ signaling, leads to the build-up of a neuroprotective ‘shield’, whereas stimulation of extrasynaptic NMDARs promotes cell death. These differences result from the activation of distinct genomic programmes and opposing actions on intracellular signalling pathways. Perturbations in the balance between synaptic and extrasynaptic NMDAR activity contribute to neuronal dysfunction in acute ischaemia and Huntington’s disease and could be a common theme in the aetiology of neurodegenerative diseases. Neuroprotective therapies should aim to both enhance the effect of synaptic activity and disrupt extrasynaptic NMDAR-dependent death signalling.
Introduction
NMDARs (N-methyl-D-aspartate (NMDA) receptors) are cation channels that are gated by the neurotransmitter glutamate. They are essential mediators of many forms of synaptic plasticity and also mediate aspects of development and synaptic transmission1, 2. In addition to their synaptic localization, NMDARs are found at extrasynaptic and perisynaptic sites, which can be defined immunohistochemically (e.g. distal from the post-synaptic density, PSD) and electrophysiologically (e.g. out of reach of normal synaptic glutamate release, see Box 1). In immature hippocampal neurons, extrasynaptic NMDARs can represent up to three quarters of all NMDARs3. Although the proportion of synaptically located NMDARs increases with development, a significant population of NMDARs remain extrasynaptic in adulthood3-6. The exact location of extrasynaptic NMDARs had not been thoroughly addressed until recently6. Only around one quarter of extrasynaptic NMDARs in adult hippocampal slices are classified as being perisynaptic (that is, within 100 nm of the PSD), the rest being localized on dendrites or on non-perisynaptic parts of the spine. Of the dendritically localized extrasynaptic NMDARs, around one third are adjacent to glia-like processes, and around half are adjacent to axons. The physiological function of extrasynaptic NMDARs is not fully understood, but their activation by glutamate spillover may contribute to long-term depression 7-9. This Review will focus on the emerging role of extrasynaptic NMDARs in pathological scenarios, in contrast with the demonstrated survival-promoting effects of synaptic NMDAR activity.
BOX1. Studying and defining synaptic and extrasynaptic NMDARs.
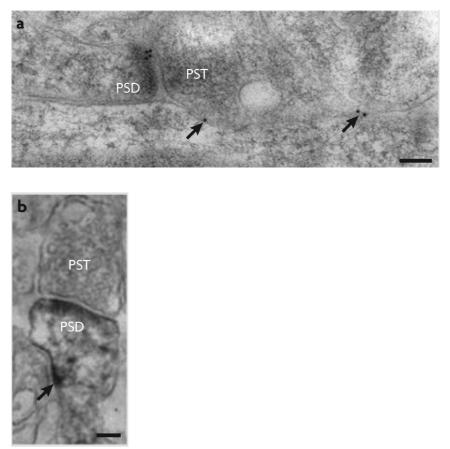
Synaptic N-methyl-D-aspartate receptors (NMDARs) are classically defined as functional receptors that are activated by glutamate released during low frequency synaptic events 116, 150-153. This could include low-frequency evoked events, spontaneous synaptic events and action potential-independent synaptic release of single quanta of glutamate. Extrasynaptic NMDARs — that is, receptors that are not activated during low-frequency synaptic events — can be found at various locations 6, including on the cell body, the dendritic shaft, the neck of the dendritic spine and also adjacent to the post-synaptic density (often referred to as perisynaptic) (see the figure, which shows examples of immuno-electron-micrograph (EM) images of extrasynaptic NMDARs on the dendritic shaft (A) and dendritic spine (B) in the hippocampal CA1 area of an adult rat ; the NMDARs are labelled with immunogold (A) or immunoperoxidase (B) using an NR1 antibody). Notably, in cultured neurons a proportion of NMDARs have the capacity to move into and out of synaptic sites 113, 151, 154, particularly GluN2B-containing NMDARs in young neurons 113 (although other studies have concluded that the mobility of GluN2B- and GluN2A-containing NMDARs is similar 151). However, such NMDAR mobility was not observed in acute slices, raising the question as to whether mobility is more restricted in vivo 116.
To study signalling associated with synaptic and extrasynaptic populations of NMDARs, the receptors must be isolated for electrophysiological characterization, and stimulation paradigms should be able to preferentially activate either pool. Preferential activation of synaptic NMDARs in cultured hippocampal or cortical neurons can be initiated via pharmacological removal of the GABAA receptor-mediated inhibition from the network. This induces bursts of firing activity and results in tetrodoxin (TTX)-sensitive NMDAR-dependent Ca2+ transients 50. A large portion of NMDARs are not activated by this stimulation paradigm and are presumed to be predominantly extrasynaptic NMDARs 38. Other techniques, such as the stimulation of afferents, are more suited to slice preparations.
The general principle applied in studies of extrasynaptic NMDARs and the resultant downstream signalling is to irreversibly block synaptic receptors before bath-applying the agonist (glutamate or NMDA), so that the agonist will activate only the extrasynaptic receptors. Synaptic NMDARs can be blocked by selectively inducing synaptic glutamate release under conditions that activate synaptic NMDARs, but in the presence of the open channel blocker MK-801 (blockade is essentially irreversible over the timescale of most experiments). Brief pulses of high K+ or episodes of firing activity, or simply allowing spontaneous action potential-dependent synaptic events can, in the presence of MK-801, induce open channel blockade of synaptic NMDARs 38, 43, 115, 155. A slightly more stringent approach involves only blocking those NMDARs that are exposed to glutamate upon release of single quanta of glutamate 43. The isolation of extrasynaptic NMDAR currents in hippocampal slice CA1 neurons can involve a similar strategy: MK-801 open-channel blockade of synaptic NMDARs activated by low-frequency stimulation of the stratum radiatum, followed by local uncaging of glutamate to activate extrasynaptic NMDARs 116. Note that strategies aimed at selectively blocking synaptic NMDARs require confirmation that little or no activation of extrasynaptic NMDARs takes place over the timeframe of synaptic stimulation – such activation could be caused by astrocytic glutamate release or elevated ambient glutamate due to nearby cellular damage.
We first discuss the background to the field of excitotoxicity, the dichotomous nature of NMDAR signalling, and the evidence that receptor location plays a role in this dichotomy. We then outline the molecular mechanisms that underlie synaptic NMDAR-dependent neuroprotection and the various pathways that are coupled preferentially to extrasynaptic NMDARs. After discussing the potential basis for differential signalling by synaptic versus extrasynaptic NMDARs we review recent studies that address the relevance of synaptic versus extrasynaptic NMDAR signalling in acute and chronic neurological disorders, focusing on stroke and Huntington’s disease. Finally we examine prospects for therapies designed to selectively target extrasynaptic NMDAR signalling while sparing synaptic signalling.
The ‘NMDAR paradox’
The neurotoxicity of sustained glutamate exposure to retinal neurons was first reported over 50 years ago10, at around the same time as the excitatory properties of glutamate were being characterized11. Later, Olney showed that glutamate toxicity was not restricted to retinal neurons, and coined the term “excitotoxicity” 12. Choi subsequently demonstrated that Ca2+ entry was a key mediator of glutamate excitotoxicity, and that the NMDAR was the primary source of toxic Ca2+ influx 13-15. Shortly after, it was proposed that Ca2+ entry through NMDARs was particularly effective at killing neurons compared to entry through other channels 16. At the same time, evidence was accumulating that implicated glutamate toxicity and NMDAR activity in acute neurological trauma such as stroke, as well as circumstantial evidence pointing to a role for glutamate toxicity and NMDAR activity in chronic neurodegenerative diseases, including Huntington’s disease 17-19. Since then, the work of many laboratories have advanced our understanding of how glutamate dyshomeostasis, ionic imbalance and abnormal NMDAR activity can contribute to such disorders 19-24.
The destructive effects of NMDAR activity are in striking contrast to the observation that the survival of several types of neuron is dependent on synaptic NMDAR activity and function25-27. Elimination of NMDAR activity in vivo causes widespread apoptosis and enhances trauma-induced injury in developing neurons28-32. In the adult CNS, NMDAR blockade exacerbates neuron loss when applied after traumatic brain injury (TBI) or during ongoing neurodegeneration33, and impairs the survival of newborn neurons in the adult dentate gyrus34. Thus, NMDARs are important for cell survival but can also be harmful and kill neurons. For many years it was thought that the degree of Ca2+ entry though NMDARs was solely responsible for these differences in cellular outcome: moderate levels of NMDAR activity were considered to be beneficial for neurons, whereas excessive activation of NMDARs, with subsequent ‘Ca2+ overload’ of the neurons, was deleterious. According to this model, neuronal responses to NMDAR activity followed a bell-shaped curve, where both too much and too little was potentially harmful35-37.
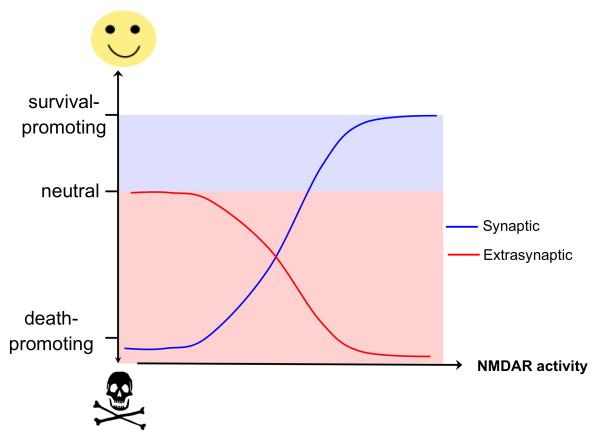
This view may be subject to revision in light of recent work that has revealed an alternative explanation for the ‘NMDAR paradox’, namely that it is the location of the NMDAR that also influences whether it is coupled to pro-death or pro-survival signals. According to this new model, synaptic NMDARs are neuroprotective, whereas extrasynaptic NMDARs preferentially initiate cell death pathways. Thus, the fate of neurons is not determined solely by the degree of overall NMDAR activity but by the extent to which synaptic and extrasynaptic NMDARs are activated. This may be best described with an ‘χ-shaped’ graph in which an ascending curve that represents increased neuroprotection due to increased synaptic NMDAR activity is superimposed on a descending curve that illustrates the progressive decrease in neuroprotection due to increasing extrasynaptic NMDAR activity (Figure 1). According to this concept, it is not the ‘Ca2+-overload’ per se that is the sole determinant of toxicity but rather the Ca2+ flow though NMDARs located outside the synapse that is particularly harmful to neurons. Synaptic NMDAR activity is by nature phasic and extrasynaptic activation is achieved by chronic agonism, but when equivalent overall Ca2+ concentrations are triggered by synaptic and extrasynaptic NMDARs, they cause strikingly different downstream events38. Ca2+ influx evoked by intense synaptic NMDAR activation is not only well tolerated by hippocampal neurons (i.e. it causes no perturbations to mitochondrial membrane potential), it is also the trigger for genomic processes that render neurons more resistant to apoptotic and oxidative insults38 (see below). In sharp contrast, comparable Ca2+ transients induced by activation of extrasynaptic NMDARs — either on their own or in the presence of synaptic NMDAR activation — trigger mitochondrial dysfunction and cell death38.
Figure 1. χ-shaped model of NMDAR-dependent excitotoxicity.
The schematic illustrates the opposing effects of increasing synaptic and extrasynaptic N-methyl-D-aspartate receptor (NMDAR) activity on neuronal survival and resistance to trauma. Hypoactivity of synaptic NMDARs is harmful to neurons. Enhancing synaptic NMDAR activity triggers multiple neuroprotective pathways and this promotes neuronal survival. Low levels of activation of extrasynaptic NMDARs have no effects on neuronal survival but increasing the level of extrasynaptic NMDAR activity activates cell death pathways and exacerbates certain neurodegenerative processes, thus reducing neuronal survival.
The distinct synaptic versus extrasynaptic NMDAR signalling pathways that lead to survival and death, respectively, were initially characterized in hippocampal neurons, and subsequently were confirmed to exist in cortical neurons39. Recent studies have shown that a selective activation of extrasynaptic NMDARs in hippocampal neurons triggers the same amount of cell death as activation of all NMDARs (synaptic and extrasynaptic) despite the fact that extrasynaptic NMDARs elicit a smaller initial Ca2+ elevation40, highlighting the importance of extrasynaptic NMDARs in excitotoxicity. Moreover, several studies have demonstrated that certain effects of extrasynaptic NMDAR signalling dominate over effects of synaptic signalling38, 41-43. However, under chronic conditions of NMDAR activation, the balance between synaptic and extrasynaptic NMDAR activity is important 44 and can influence the point at which escalating levels of bath-applied NMDA or glutamate become neurotoxic. For example, bath application of low concentrations of NMDA can induce firing activity and so preferentially activate synaptic pathways and activity-dependent neuroprotection, whereas higher levels of NMDA suppress firing, resulting in extrasynaptic pathways dominating 45.
Synaptic NMDAR-dependent neuroprotection
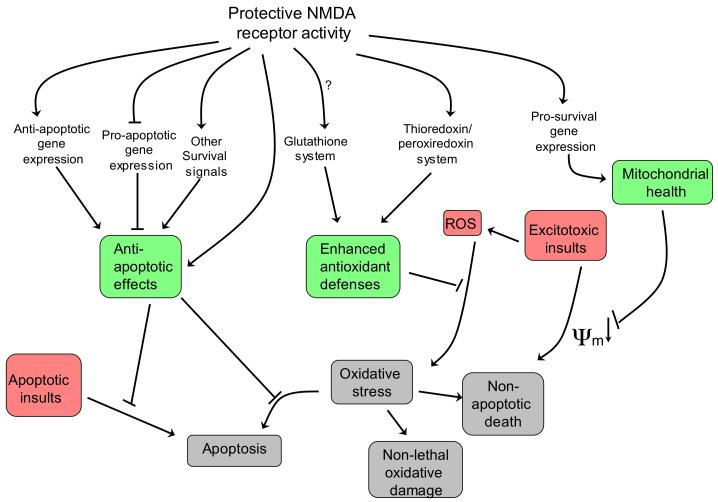
According to the theory of neuronal health46, neurons can exist in a spectrum of states ranging from fully functioning and resilient at one extreme to dysfunctional and vulnerable to insults at the other extreme. The position of the neuron within this spectrum is influenced by a multitude of signals and stimuli, but is known to be shifted towards health and robustness by synaptic NMDAR activity. An episode of synaptic NMDAR activity promotes neuroprotection that lasts beyond the duration of the episode and continues after most signalling pathways are no longer active47. This long-lasting form of ‘acquired neuroprotection’ predominantly results from changes in gene expression that have effects at multiple levels within the cell (Figure 2) such as enhancement of mitochondrial health, boosting of antioxidant defences and suppression of caspase activation, all of which in turn serve to preserve neuronal function and improve viability in the face of insults.
Figure 2. Neuroprotective pathways activated by synaptic NMDAR activity.
Changes in gene expression underlie the long-lasting neuroprotection exerted by activation of synaptic N-methyl-D-aspartate receptors (NMDARs). Changes include both the up-regulation of protective genes — including anti-apoptotic genes, pro-survival genes and genes involved in antioxidant responses — and the transcriptional suppression of pro-death genes. This restricts the apoptotic potential of the neuron, reduces vulnerability of mitochondria to insult-induced depolarization, and boosts intrinsic antioxidant defences (these three effects are shaded in green). These changes boost neuronal resistance to a range of traumata, including apoptotic, excitotoxic and oxidative insults (shaded in red), and thereby prevent harmful outcomes (shaded in grey) such as apoptotic and non-apoptotic cell death and cell dysfunction.ψm refers to mitochondrial membrane potential. ROS, reactive oxygen species.
Induction of survival genes
Ca2+ signalling is a major mediator of the dialogue between the synapse and cell nucleus. It has been known for over 20 years that seizure activity triggers transcriptional responses in neurons 48, as do physiological patterns of synaptic NMDAR activity, such as those that promote synaptic potentiation 49. Ca2+ entry through synaptic NMDARs is augmented by release from internal stores, after which Ca2+ is transduced to the cell soma where it invades the nucleus 50. Nuclear Ca2+ is one of the most potent activators of neuronal gene expression. Transcriptome analyses have revealed that in hippocampal neurons nearly 200 genes are controlled by nuclear Ca2+ signalling42, 51.
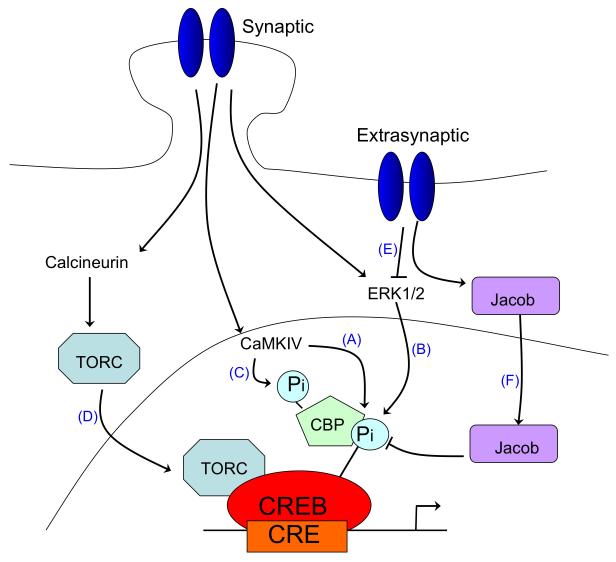
One important target of nuclear Ca2+ is the nuclear Ca2+/calmodulin-dependent protein (CaM) kinase IV and the transcription factor cyclic-AMP response element binding protein (CREB, Figure 3a–pathways A-D)52-58. CREB is the prototypical signal-regulated transcription factor, whose role in neuronal survival, as well as in several other processes (including synaptic plasticity, addiction, neurogenesis, and learning and memory), is well documented59-62,63. In hippocampal neurons, CREB-dependent gene expression is causally linked to the long-lasting phase of activity-dependent neuroprotection against apoptotic and excitotoxic insults47, 64. This form of acquired neuroprotection is dependent on nuclear Ca2+ signalling47, which is consistent with the known role for nuclear Ca2+ in CREB activation50, 57, 65. In recent years, the genomic programmes that underlie acquired neuroprotection have been uncovered42, 51. Among the pool of nuclear Ca2+-regulated genes, a core set of ten genes, termed Activity-regulated inhibitors of death (AID) genes have been identified, which have been shown to provide neurons with a broad-spectrum neuroprotective ‘shield’, both in cell culture and in animal models of neurodegeneration51. Some of the AID genes, which include the genes encoding activating transcription factor 3 (Atf3), B-cell translocation gene 2 (Btg2), B-cell lymphoma 6 (Bcl6), growth arrest and DNA damage induced gene 45 beta (GADD45beta), growth arrest and DNA damage induced gene 45 gamma (GADD45gamma), Inhibin beta-A (Inhba), Interferon activated gene 202B (Ifi202B), neuronal PAS domain protein 4 (Npas4), nerve growth factor IB (also known as nuclear receptor subfamily 4, group A, member 1) (Nr4a1), and serine protease inhibitor B2 (Serpinb2), seem to be potential CREB target genes and may provide neuroprotection through a common process that renders mitochondria more resistant to cellular stress and toxic insults (Figure 4a)42, 51, 66, 67. Another target of synaptic NMDAR and nuclear Ca2+-CREB signalling is the gene that encodes the neurotrophin brain-derived neurotrophic factor (Bdnf)38, 51, 68, 69. BDNF has neuroprotective properties70 and can rescue neurons from NMDAR blockade-induced neuronal death71.
Figure 3. Opposing effects of synaptic vs. extrasynaptic NMDAR signalling on gene expression.
A. Opposing effects of synaptic vs. extrasynaptic NMDAR signalling on CREB-dependent gene expression. The activation of cyclic-AMP response element binding protein (CREB)-dependent gene expression by synaptic N-methyl-D-aspartate receptor (NMDAR) activity is a multi-step process. CREB must be phosphorylated at serine-133 in order to recruit its coactivator CREB binding protein (CBP) 52, 54; this phosphorylation is mediated by the fast-acting nuclear CaM kinase pathway (A) and the slower acting, longer lasting Ras-ERK1/2 pathway (B) 50, 156, 157, both of which are promoted by activation of synaptic NMDARs CBP is subject to Ca2+-mediated transactivation by nuclear Ca2+ dependent CaM kinase IV 58, 86, which phosphorylates CBP at serine-301 (C) 158. In addition, nuclear translocation of transducer of regulated CREB activity (TORC) is a key step in CREB activation. Synaptic NMDAR-induced Ca2+ signals promote TORC import into the nucleus via calcineurin-dependent dephosphorylation (D) 159, 160. TORC acts at least in part by assisting in the recruitment of CBP to CREB 161 (not shown). In contrast to these CREB-activating signals of synaptic NMDARs, extrasynaptic NMDARs suppress CREB activity 38, 89 through inactivation of the Ras-ERK1/2 pathway (E) 41 and the nuclear translocation of Jacob, which promotes CREB dephosphorylation (F) 90.
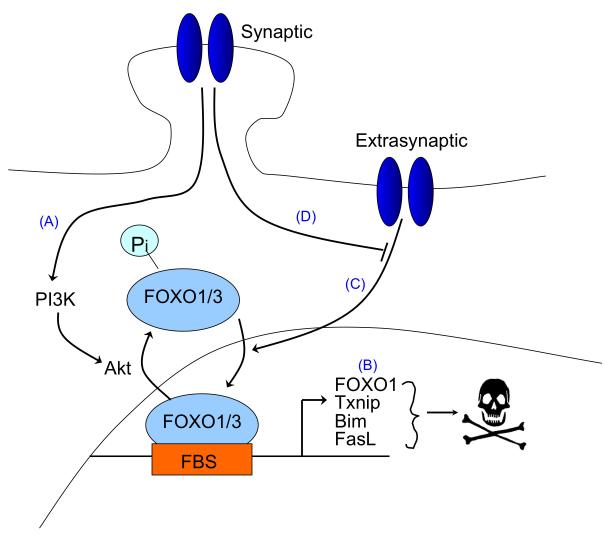
B. Opposing effects of synaptic vs. extrasynaptic NMDAR signalling on FOXO-dependent gene expression. Synaptic NMDAR activity suppresses FOXO activity by promoting the Akt-mediated phosphorylation and nuclear export of FOXOs (A) 43, of which FOXO1 and FOXO3 are the predominant neuronal subtypes. FOXO1 is also regulated transcriptionally by FOXOs 78 and thus signals that cause FOXO export also result in FOXO1 transcription being suppressed. By contrast, bath activation of NMDARs, which also triggers extrasynaptic NMDAR activity, stimulates FOXO nuclear import (B), an event that contributes, through promoting transcription of pro-death genes, to excitotoxic cell death. 77 Synaptic NMDAR activity can exert a long-lasting block on this import signal (C) 77, the mechanistic basis of which remains unclear.
Figure 4. Synaptic NMDAR-dependent transcriptional changes have both anti-apoptotic and anti-oxidant effects.
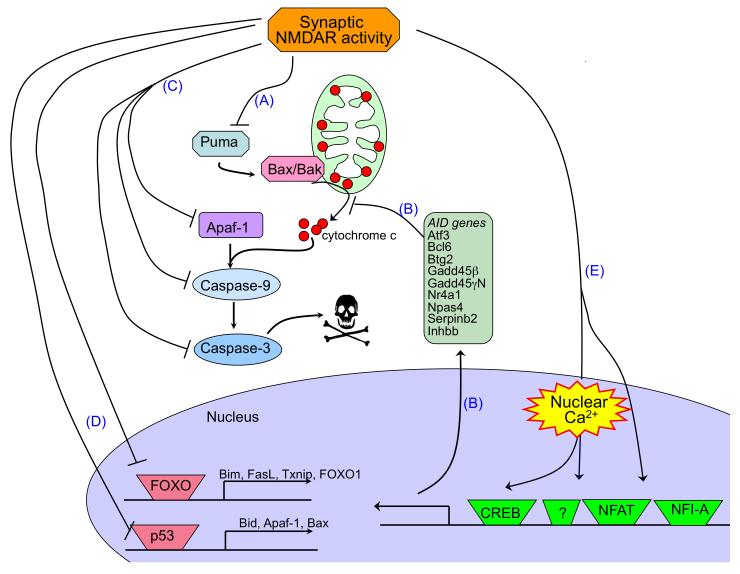
A) Synaptic NMDAR signalling suppresses the intrinsic apoptosis cascade at multiple levels. Synaptic N-methyl-D-aspartate receptor (NMDAR) activity protects neurons against apoptosis in a manner that is independent of the type of cellular insult because it suppresses the core apoptotic pathway. Transcriptional suppression of the BH3-only domain gene Puma by activation of synaptic NMDARs is a key event upstream of cytochrome c release (A) 66, 67. Up-regulation of a battery of nuclear Ca2+-regulated genes (green box) is also strongly protective 47, 51 and probably also acts upstream of cytochrome c release, as it inhibits insult-induced mitochondrial membrane depolarization (B). Downstream of cytochrome c release, transcriptional suppression of components of the caspase cascade, such as Apaf-1, Caspase-9 and Caspase-3, also slows the apoptotic process (C) or raises the amount of mitochondrial cytochrome c release that is required to initiate apoptosis (as cytochrome c’s cytoplasmic target is Apaf-1) 67. Synaptic NMDAR activity also induces inactivation of the pro-death transcription factors FOXO and p53 (D) 43, 66, 78, and the subsequent suppression of the genes whose expression they regulate. Finally, transcription factors that have been shown to be important targets for NMDAR-dependent pro-survival signals are shown, namely CREB, NFAT and NFI-A (E) 47, 72, 73.
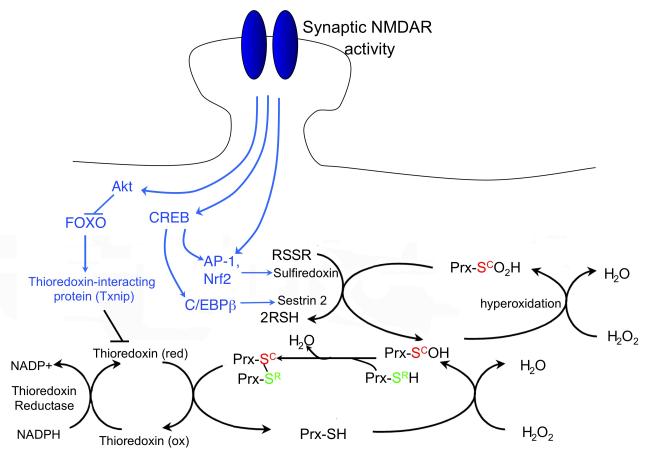
B) Synaptic NMDAR signalling boosts intrinsic antioxidant defences. A schematic showing the effect of synaptic activity-induced changes in transcription on the thioredoxin (Trx)-peroxiredoxin (Prx) system. The dotted arrows indicate activity-dependent signalling events, which are collectively shaded in green. Briefly, reduction of peroxide levels is executed via the transfer of electrons from NADPH to peroxides through the redox-active sulfhydryl (-SH) groups of thioredoxin reductase, Trx and Prxs (the major two-cysteine Prx sub-type is shown). The catalytic redox-active cysteine residue of Prx (Sc, red) reduces peroxide, and is oxidized to cysteine sulfenic acid (SCOH). The resolving cysteine (SR, green) of a different Prx molecule then forms a disulfide bond with Prx-SCOH, thereby eliminating H2O. This intermolecular disulfide bond is then reduced by Trx, whose two-cysteine active site is reduced by Trx reductase. However, if levels of peroxide are too high, the – SCOH group becomes hyperoxidized to –SO2H (cysteine sulfinic acid). –SO2H is not a substrate for the resolving cysteine –SCH or for Trx. Instead, sulfiredoxin (and possibly sestrin 2) catalyses the reduction of hyperoxidized Prx-SO2H, returning it to the Trx cycle. Synaptic activity triggers the events shown to both enhance Trx activity and boost the reduction of hyperoxidized Prxs. These include the transcriptional activation of sulfiredoxin and sestrin 2, and the transcriptional suppression of Txnip, the gene encoding a the Trx inhibitor thioredoxin-interacting protein (Txnip). Bim (bcl2-interacting mediator of cell death); Fasl (fas antigen ligand); Apaf1 (apoptotic peptidase activating factor 1); CREB (cAMP responsive element binding protein); NFAT (nuclear factor of activated T-cells); NFI-A (nuclear factor I/A); Atf3 (activating transcription factor 3); Bcl6 (B-cell leukemia/lymphoma 6); Btg2 (B-cell translocation gene 2); Gadd45beta, GADD45gamma (growth arrest and DNA damage induced gene 45 gamma and beta, respectively); Nr4a1 (nuclear receptor subfamily 4, group A, member 1); Npas4 (neuronal PAS domain protein 4); Inhbb (Inhibin beta-A).
Although CREB is a key mediator of nuclear Ca2+-dependent neuroprotection, other transcription factors implicated in mediating NMDAR-dependent survival may also be partly controlled by nuclear Ca2+, such as nuclear factor of activated T-cells (NFAT72). By contrast, NMDAR-dependent activation of other neuroprotective factors such as nuclear factor I, subtype A (NFI-A) might occur independently of nuclear Ca2+ elevation (its activation being dependent on the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway and nitric oxide (NO) synthesis73).
Suppression of death genes
A second mechanism through which synaptic NMDAR activity restricts the apoptotic potential of a neuron involves the transcriptional suppression of core components of the intrinsic apoptosis cascade (Figure 4a)66, 67. Synaptic NMDAR activity suppresses the expression of the pro-apoptotic Bcl-2 homology domain3 (BH3)-only member gene Puma in vitro and in vivo66, 67. Puma expression is both sufficient to induce cell death and necessary for apoptotic death in response to NMDAR blockade67. As with activation of CREB, suppression of Puma is specific to synaptic NMDAR signalling, as bath activation of all NMDARs (synaptic and extrasynaptic) fails to influence Puma expression. Suppression of Puma inhibits the apoptotic cascade upstream of cytochrome c release, but auxiliary protective mechanisms also exist downstream, as the genes encoding the core apoptosome components apoptotic protease activating factor 1 (Apaf-1) and pro-caspase-9 are also subject to transcriptional suppression by synaptic NMDAR activity66, 67. The net effect of all these transcriptional changes is that the activation of caspase-9 and downstream executioner caspases is very limited, apoptosis is inhibited, and the neurons remain viable and electrically active 67.
Synaptic NMDAR activity also suppresses the expression and/or activity of important pro-death transcription factors (Figure 3b, Figure 4a). The forkhead box O (FOXO) class of transcription factors can promote neuronal death following excitotoxic injury, trophic factor withdrawal and oxidative stress74-77. Known pro-death FOXO target genes include the BH3-only genes Noxa, Bcl2-like protein 11 (Bcl2l11, also known as Bim) and Puma, Fas ligand (Fasl) and thioredoxin-interacting protein (Txnip). In conditions of trophic deprivation, in which FOXOs are constitutively located in the nucleus, synaptic NMDAR activity promotes sustained activation of the Akt pathway, leading to the phosphorylation and nuclear export of FOXO and the subsequent inactivation of FOXO target genes, which includes Foxo1 (Foxo1 itself was recently identified as a FOXO target gene43, 78, Figure 3b–pathway A). Furthermore, synaptic NMDAR activity induces a long-lasting inhibition of FOXO nuclear import77 (Figure 3b–pathway D). This long-lasting blockade was shown to require nuclear CaM kinase IV activation and de novo gene expression, although the exact mechanism by which this leads to blockade of FOXO nuclear import remains unclear. Thus, synaptic NMDAR activity both promotes the nuclear export of FOXOs and the transcriptional suppression of Foxo1 and places a long-lasting blockade on FOXO nuclear translocation (Figure 3b). p53 is another pro-apoptotic transcription factor that is subject to negative transcriptional regulation by synaptic NMDAR activity and that may contribute to the suppression of Apaf-166, although p53-independent routes to Apaf-1 expression also exist67.
Protection against oxidative stress
In addition to apoptosis-suppressing effects, synaptic NMDAR activity also enhances antioxidant defences43 (Figure 2, Figure 4b), which contributes to neuroprotection against oxidative insults. NMDAR blockade in vivo triggers neuronal death that is associated with protein carbonylation (a marker of oxidative stress), which is suggestive of a role for physiological NMDAR activity in supporting antioxidant defences. Synaptic NMDAR activity also controls neuronal vulnerability to oxidative stress-induced death in vitro43. It triggers a number of changes to the thioredoxin-peroxiredoxin system that contribute to this neuroprotective effect. Specifically, synaptic activity enhances thioredoxin activity and facilitates the reduction of hyperoxidized peroxiredoxins, which are an important class of antioxidant enzymes. These effects are mediated by a coordinated programme of gene expression changes, some of which have been characterized43. These changes include the transcriptional suppression of the FOXO target gene Txnip, which is dynamically regulated by NMDAR activity in vitro and in vivo. Also, enhanced reduction of hyperoxidized peroxiredoxins is associated with transcriptional activation of two genes, Srxn1 (sulfiredoxin) and Sesn2 (sestrin 2). The products of these genes can mediate this reaction79-81, with sulfiredoxin induction likely to be more important82, 83. Trans-synaptic stimulation of synaptic NMDARs is crucial for boosting antioxidant defences, as chronic bath activation of all (synaptic and extrasynaptic) NMDARs failed to trigger the aforementioned transcriptional changes or protect against oxidative insults.
The gene expression changes (Figure 4b) do not account for all the antioxidant effects of synaptic NMDAR activity, raising the possibility that other antioxidant systems, such as the glutathione system, may also be subject to control by synaptic NMDAR activity.
Pro-death signalling by extrasynaptic NMDARs
NMDAR-dependent neuron death is mediated by a variety of pathways, the relative contribution of which may depend on the cell type, the developmental stage or intensity of the excitotoxic insult20, 84. Recent studies have found that certain pro-death pathways or events are preferentially activated by extrasynaptic NMDARs compared to synaptic ones (Figure 5). As discussed below, in some cases this involves direct antagonism of synaptic NMDAR-activated survival pathways, whereas in others it involves the triggering of events/pathways that are simply not activated by synaptic NMDARs.
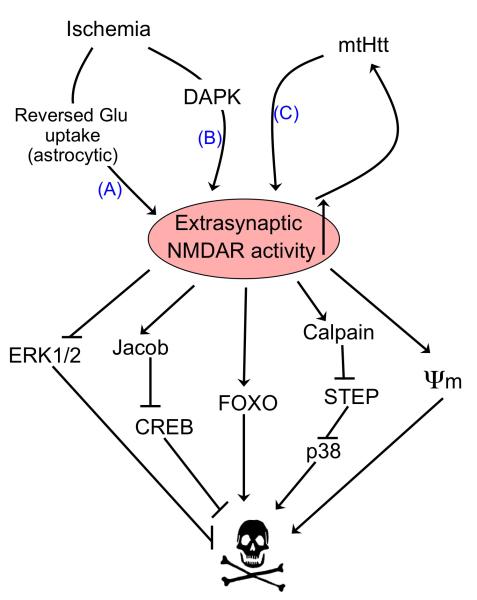
Figure 5. Activators and effectors of extrasynaptic NMDAR activity.
Ischaemia results in activation of extrasynaptic N-methyl-D-aspartate receptor (NMDAR) activation through reversed glutamate uptake from astrocytes (A) 130. Extrasynaptic currents are also preferentially enhanced by ischaemia-induced death-associated protein kinase (DAPK) activation (B) 131, as well as by mutant Huntingtin (mtHtt) in a mouse model of Huntington’s disease (C) 132. Increased extrasynaptic (but not synaptic) NMDAR activity in turn preferentially activates a number of pro-death pathways. ψm refers to mitochondrial membrane potential, which is disrupted by extrasynaptic NMDAR activity.
CREB shut-off
Bath activation of NMDARs has long been known to be a poor activator of CRE-dependent gene expression in hippocampal neurons, compared to Ca2+ entry though voltage-gated Ca2+ channels (although activation of the serum response element (SRE) was equally good)85. This could be explained by the fact that bath activation of NMDARs induces CREB-activating phosphorylation of CREB at serine-133, followed shortly afterwards by a strong CREB-dephosphorylation signal 86, 87. By contrast, activation of voltage-gated Ca2+ channels induces sustained CREB phosphorylation 86. The fact that NMDARs seemed to be poor inducers of CRE-dependent gene expression when they were bath-activated was at apparent odds with the fact that NMDARs were known to induce activation of CRE-dependent gene expression following electrical activity 49, 88. These historical observations can be explained by the observation that synaptic NMDAR activity promotes sustained CREB phosphorylation, whereas extrasynaptic NMDARs are coupled to a dominant CREB dephosphorylating signalling pathway 38, 89 (Figure 3a). When all (synaptic and extrasynaptic) NMDARs are activated by bath-applied glutamate or NMDA, the extrasynaptic pathway dominates, resulting in weak CREB activation 38, 85, 86. The only exception is when low levels of bath-applied NMDA induce firing activity, in which case synaptic signalling dominates and CREB activation is relatively strong 45.
Recently, insight into the mechanistic basis of this differential signalling was uncovered and found to be centred on Jacob, a binding partner of the neuronal Ca2+ binding protein caldendrin90. Jacob, when localized to the nucleus, causes CREB dephosphorylation and also promotes a loss of synaptic contacts. Jacob contains a nuclear localization signal and its nuclear import requires importin-α binding. This binding and nuclear import is prevented by caldendrin, which binds Jacob in a Ca2+-dependent manner. Synaptic NMDAR activity was shown to promote caldendrin-dependent retention of Jacob outside of the nucleus, whereas extrasynaptic NMDAR activity promoted the nuclear accumulation of Jacob, with subsequent effects on neuronal survival 90 (Figure 3a–pathway F). As the coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated and absent in very young neurons 89, it will be of interest to know whether there is a developmental switch that activates the Jacob pathway.
ERK1/2 inactivation
The ERK1/2 pathway, which can support activation of CREB and inactivate the pro-death protein Bcl-2-associated death promoter (BAD) has been implicated in NMDAR-dependent neuroprotection,26. Synaptic NMDAR activity promotes sustained ERK activity47, 91, whereas activation of all NMDARs by bath application of NMDA results in ERK activation that is followed by inactivation91-93. The basis for this bi-directional control of ERKs turned out to be due to the opposing actions of synaptic versus extrasynaptic NMDARs41. Synaptic NMDARs couple to activation, whereas extrasynaptic NMDARs promote ERK dephosphorylation and subsequent inactivation in both hippocampal41 and cortical neurons39 (Figure 3a–pathway E). Consistent with this, extrasynaptic NMDARs strongly inactivate Ras (upstream of ERK) 93. Thus, synaptic and extrasynaptic NMDARs are mutually antagonistic with regard to Ras-ERK signalling. However, ERK1/2 inactivation by extrasynaptic NMDAR is stimulus-specific: although extrasynaptic NMDARs exert an ERK1/2 shut-off signal that is dominant over that triggered by activation of synaptic NMDARs, it does not antagonize ERK1/2 activity induced by BDNF94.
FOXO activation
Synaptic NMDAR activity inactivates pro-death FOXOs at multiple levels (see above and Figure 3b), whereas extrasynaptic NMDAR activity does the opposite. Chronic activation of all (synaptic and extrasynaptic NMDARs) does not result in FOXO export from the nucleus, because activation of Akt is not sustained43 (Sustained activation of the Akt pathway leads to the phosphorylation and nuclear export of FOXO). More recently, it was shown that extrasynaptic NMDAR-evoked signals not only fail to induce FOXO nuclear export, but instead promote the nuclear import of FOXOs that are normally sequestered in the cytoplasm77 (Figure 3b–pathway C). This activation of FOXOs subsequently contributes to NMDAR-dependent neuronal death. Thus, the activation status of FOXOs is highly sensitive to the balance between synaptic and extrasynaptic NMDAR activity (Figure 3b).
Calpain activation and STEP cleavage
Activation of extrasynaptic NMDARs strongly activate calpains, whereas synaptic NMDAR activity does not95. In agreement with this, the classical calpain substrate fodrin and Na+/Ca2+ exchanger 3 (NCX3)95 are cleaved following extrasynaptic, but not synaptic, NMDAR activity. NCX3 is known to be cleaved by calpains in animal models of stroke, leading to delayed Ca2+ deregulation and neuronal death96. Another substrate for extrasynaptic NMDAR-activated calpains is striatal enriched tyrosine phosphatase (STEP), which is cleaved into a form that cannot interact with its normal substrates. One such substrate is p38 mitogen-activated protein (MAP) kinase, which is negatively regulated by STEP, is enriched at extrasynaptic membrane fractions, and is known to contribute to death induced by chronic NMDA or glutamate exposure in cerebellar granule cells and cortical neurons95, 97-99. Inhibition of calpain-mediated STEP cleavage is sufficient to inhibit NMDAR-dependent p38 activation and can protect neurons from glutamate toxicity and oxygen-glucose deprivation95. Thus calpain — coupled to STEP cleavage and subsequent p38 activation — represents another pro-death cascade that selectively activated by extrasynaptic NMDARs (Figure 5). One important caveat to the role of p38 in excitotoxicity is that its influence on survival and death is heavily context-dependent. For example, it can promote neuroprotection through activation of myocyte enhancer factor 2 (MEF2) 100 and indeed can be activated by neuroprotective, synaptic NMDAR activity 99, 101. Thus, its contribution to neuronal death in excitotoxicity may rely on interactions with other events that are triggered by the excitotoxic insult.
The observation that p38 is disinhibited by extrasynaptic NMDAR activity through STEP cleavage also raises the question as to why extrasynaptic NMDARs fail to activate ERK1/2, as STEP is also a powerful negative regulator of ERKs95, 102. It is possible that other, dominant ERK-inactivating signals may be triggered in parallel (such as those discussed above), overriding any effects of STEP cleavage.
Gene expression changes specifically induced by extrasynaptic NMDAR activity
As a result of these or other differences in signalling, extrasynaptic NMDAR activity induces a very different programme of gene expression compared to synaptic NMDARs42. As new gene expression contributes to at least some forms of excitotoxic neuronal death, these changes are likely to be functionally important. Although the identity of such genes remains to be elucidated, they may include FOXO target genes or other factors targeted by extrasynaptic signals. For example, Clca1, the gene that encodes Ca2+ -activated chloride channel regulator 1, is selectively activated by extrasynaptic NMDAR activity in cell culture and in a stroke model, and its overexpression is sufficient to kill neurons42, 103, although the mechanism by which its expression is induced is unclear.
New developments and ongoing questions
Control of nuclear architecture by synaptic and extrasynaptic NMDARs
In addition to differentially controlling specific transcription-regulating events, synaptic and extrasynaptic NMDARs may also more globally affect signal processing towards and within the cell nucleus by regulating nuclear architecture. A structural analysis of hippocampal nuclei using a novel algorithm for three-dimensional reconstruction uncovered that - in contrast to what is commonly thought and what is depicted in virtually all textbooks - many cell nuclei are not round or near-spherical organelles but have a very complex, highly infolded shape 104. The structure of the nucleus is dynamic and signal-regulated, with synaptic and extrasynaptic NMDARs having opposing effects: stimulation of synaptic NMDARs increases the number of infolded nuclei, whereas the infolded shape is rapidly lost following Ca2+ flux through extrasynaptic NMDARs. Thus, neurons translate NMDAR signals into changes in nuclear geometry, providing a means for an activity-dependent modulation of nucleo-cytoplasmic exchange of molecules and ions 104. The changes in nuclear shape and consequent rearrangements of the nuclear architecture and spatial re-organization of chromosome territories may affect chromatin structures and accessibility and could have profound effects on gene expression and genome stability. In addition, infolded and near-spherical nuclei have similar volumes but infolded nuclei have a larger surface and larger number of nuclear pore complexes 104. Given that actively transcribed DNA segments may be located near nuclear pore complexes 105, 106, a possible increase in the number of nuclear pore complexes in infolded nuclei following synaptic NMDAR activation may enhance the capacity of the nucleus for transcription-coupled anchoring of promoter regions to nuclear pore complexes. Synaptic NMDAR-induced structural plasticity of the nucleus may therefore represent an adaptation to a metabolically more active state with an increased demand for gene expression.
The basis for differential synaptic versus extrasynaptic NMDAR signalling: outstanding questions
The differences in signalling between synaptic and extrasynaptic NMDARs could theoretically be due to one of three factors, or a combination thereof. Firstly, they could be due to differences in the make-up of the NMDAR signalling complexes at extrasynaptic compared to synaptic locations. The components that make up the NMDAR signalling complex in the post-synaptic density are the subject of intense study107, but the molecules that associate with extrasynaptic NMDARs have been poorly characterized. Some proteins that are found in synapses and that are linked to synaptic NMDARs, such as the membrane-associated guanylate kinases (MAGUKs) postsynaptic density protein (PSD)-95 and PSD-93, discs, large homolog 1 (Drosophila) (DLG1, also known as SAP97, and discs, large homolog 3 (Drosophila) (DLG3, also known as SAP102), are also linked to extrasynaptic NMDARs. In 3-week old hippocampal cultures, around 20% of extrasynaptic NMDARs were found to be associated with PSD-95 and a further 20% with SAP1026. Association of extrasynaptic GluN1 NMDAR subunits with MAGUKs was also observed by double-labelling immuno-gold electron microscopy in adult hippocampal slices 6. This is of particular interest since MAGUKs have been implicated in coupling the NMDAR to downstream death pathways such as excessive nitric oxide production 108, 109. Other studies suggest the possibility of a specific association of extrasynaptic NMDARs with certain proteins. For example, it has been postulated that, as caldendrin is localised to the post-synaptic density, only trans-synaptic activation of synaptic NMDARs trigger local Ca2+ transients that are high enough to promote caldendrin-mediated Jacob retention90. Alternatively, the preferential localization of p38 (but not ERK1/2) at extrasynaptic membranes may explain its preferential activation by extrasynaptic NMDAR-dependent STEP cleavage95. It is likely that other, yet to be identified proteins associate differentially with synaptic/extrasynaptic NMDARs.
Secondly, receptor subunit composition could play a role in the differences in signalling between synaptic and extrasynaptic NMDARs. The cytoplasmic C-termini of the GluN2 subunits have undergone considerable sequence divergence through evolution, which potentially allows for differential association of signalling molecules. During development GluN2B is the predominant subunit at all synaptic and extrasynaptic locations in forebrain neurons, and GluN2A expression in the rodent CNS begins at the end of the first post-natal week110, 111. In the adult forebrain both the GluN2A and GluN2B subunits are prevalent. GluN2A becomes incorporated into synaptic NMDARs 112 and has been reported to become enriched at synapses compared to extrasynaptic locations, with GluN2B more abundant at extrasynaptic locations113, 114. No studies, however, have demonstrated a clear-cut spatial demarcation. Indeed, GluN2A subunits have been identified at extrasynaptic sites in cultured neurons115 and the subunit compositions of synaptic and extrasynaptic NMDARs in 3-week-old acute hippocampal slices have been found to be very similar116. Moreover, immunofluorescence and immuno-gold studies have also identified both GluN2A and GluN2B at extrasynaptic locations6.
Nevertheless, subunit differences could have a contributing effect if GluN2A- and GluN2B-containing NMDARs are coupled differently to pro-survival and pro-death pathways. Indeed, GluN2B or GluN2A-containing NMDARs have been proposed to promote neuronal death and neuronal survival, respectively117. However, the specificity of the GluN2A-specific antagonist used in this study117 has been questioned, particularly when employed in vivo or in non-steady state conditions118-122. Moreover, it has been shown that GluN2B- and GluN2A-containing NMDARs can both promote survival and death43, 114, 123. Whether they do so with differing efficiencies, however, remains unclear as a direct comparison has yet to be made. Indeed, attempting to do so would be challenging given the lack of a sufficiently selective GluN2A-specific antagonist. Also, the fact that GluN2B and GluN2A are expressed at different relative levels in development can cloud the issue, as developmental changes in NMDAR signalling can be misinterpreted as being due to a switch in GluN2 subunit when there are in fact many other potential explanations. Another important issue is that receptors can exist in a tri-heteromeric form that contains both a GluN2A and a GluN2B subunit 124, meaning that 2A- and 2B-dependent signals can theoretically be transduced from the same channel. Whether the GluN2 subunit (and particularly its C-terminus) influences whether an activated NMDAR will promote either survival or death will require more sophisticated approaches in order to be fully resolved.
A final potential factor contributing to differential signalling could be the way in which synaptic and extrasynaptic NMDAR pools are activated. Synaptic NMDARs are generally transiently and intensely activated by the trans-synaptic release of glutamate, while extrasynaptic NMDARs are typically activated chronically, by elevated levels of ambient glutamate. Subtle differences in the spatial properties of intracellular Ca2+ transients evoked by these stimuli could differentially affect signalling, even if the overall Ca2+ load were similar. If trans-synaptic versus chronic activation of NMDARs is a contributing factor in differential synaptic vs. extrasynaptic NMDAR signalling, this raises the question as to whether synaptic NMDARs can contribute to excitotoxic neuronal death under certain circumstances (e.g. chronic activation). Against this idea is the observation that prior blockade of synaptic NMDARs was not protective against NMDA-induced death 40. Nevertheless, it is a possibility that under certain conditions where extrasynaptic NMDARs are chronically active, that chronic activation of synaptic NMDARs may contribute to a toxic Ca2+ load. Conversely, it is unclear whether extrasynaptic NMDARs can contribute to neuroprotective signalling under certain circumstances. For example, if strong synaptic activity results in the transient trans-synaptic activation of a portion of perisynaptic or extrasynaptic receptors (as has been shown 125), does this contribute to neuroprotective signalling or antagonize those promoted by synaptic NMDAR activation? It is also not clear what proportion of a cell’s extrasynaptic NMDAR population need to be activated in order to promote pro-death signalling.
Another, related question is whether extrasynaptic NMDARs at different locations (dendritic, perisynaptic, cell body) signal differently. Studies have shown NMDARs demonstrating mobility between synaptic and extrasynaptic locations 113, 151, 154 (see Box 1), and it remains to be determined whether these receptors alter their signalling properties upon switching their location. One possibility is that an NMDAR moving (for example) between an extrasynaptic to a synaptic site, encounters a different biochemical environment and so associates with a different complement of downstream signalling molecules. However, the extent to which NMDARs are mobile in vivo remains to be clarified in the light of recent observations that synaptic and extrasynaptic NMDARs from stable pools in acute slices 116.
Addressing the above questions will enhance our knowledge about the molecular basis for differential synaptic vs. extrasynaptic NMDAR signalling, and enhance our understanding of the precise conditions that determine the balance between protective and destructive NMDAR signalling.
Clinical implications
Extrasynaptic NMDARs in ischaemic stroke
Inappropriate levels of NMDAR activity can contribute to neuronal death or dysfunction in a number of paradigms, including acute excitotoxic insults (e.g. stroke, TBI) and certain neurodegenerative diseases, such as HD and AD (reviewed in23, 24, 126). Recent work has specifically implicated extrasynaptic NMDAR activation in the aetio-pathophysiology associated with an acute and a chronic form of neuronal dysfunction (ischaemic stroke and HD, respectively).
During an ischaemic episode, extracellular glutamate accumulates due to synaptic release and impairment or reversal of uptake mechanisms22, 127, and neurons become depolarized, relieving the Mg2+ blockade of NMDARs. These events have the combined effect of causing strong activation of NMDARs128, possibly including those located outside the synapse. The consequences of reversed uptake can be studied in culture through the use of the transportable glutamate transport inhibitor L-trans-pyrrolidine-2,4-dicarboxylate (PDC), which causes reversed glutamate uptake through hetero-exchange129. In neuron/astrocyte co-cultures, PDC treatment causes glutamate efflux and excitotoxicity due to an activation of extrasynaptic NMDARs that mainly arises from reversed astrocytic uptake130. Interestingly, a significant proportion of extrasynaptic NMDARs in vivo are located adjacent to glia6, raising the possibility that astrocytic glutamate release may lead to activation of these receptors.
A recent study has demonstrated that extrasynaptic NMDAR activity may be selectively enhanced in ischaemia via an intracellular signalling cascade131. Ischaemia recruits death-associated protein kinase (DAPK1) directly to the C-terminal tail of GluN2B, leading to phosphorylation of the channel and increased single-channel conductance. The effect is specific to GluN2B-containing NMDARs and results in a selective enhancement of extrasynaptic NMDAR currents131, which is not observed in DAPK1-deficient neurons. Moreover, peptide-mediated disruption of DAPK1–NMDAR interactions was shown to protect neurons against oxygen-glucose deprivation in vitro and ischaemic damage in vivo131. Taken together, these studies indicate that extrasynaptic NMDAR activity contributes to ischaemic neuron loss.
Synaptic and extrasynaptic NMDARs in Huntington’s Disease
Recent studies show that the relationship between extrasynaptic NMDAR signalling and mutant Huntingtin (mtHtt) may be a key factor in HD progression44, 132 (Figure 6a). One study focused on the presymptomatic YAC128 transgenic mouse, which expresses a mutant huntingtin (mtHtt) that contains 128 CAG repeats, as compared to a YAC18 control mouse which expresses Htt with a non-pathological CAG repeat length132. Although expression of mtHtt had no effect on synaptic NMDAR activity, YAC128 transgenic mice showed both an increase in extrasynaptic NMDAR current and in extrasynaptic NMDAR expression specifically in striatal medium spiny neurons. This enhanced extrasynaptic NMDAR activity was dominated by GluN2B-containing NMDARs and persisted in aged YAC128 mice132. Consistent with the previously mentioned coupling of extrasynaptic NMDAR activity to CREB dephosphorylation and inactivation 38, reduced CREB phosphorylation was observed in the striatum of YAC128 mice132.
Figure 6. Clinical relevance of the balance between synaptic and extrasynaptic NMDAR activity.
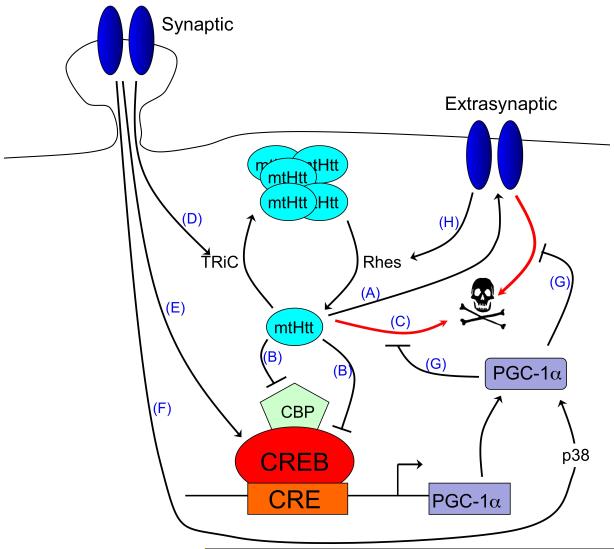
A. The balance of synaptic vs. extrasynaptic NMDAR signalling influences HD pathology. Mutant Huntingtin (mtHtt) promotes neuronal dysfunction and death by enhancing the expression and activity of extrasynaptic N-methyl-D-aspartate receptors (NMDARs) (A) 132 and by directly blocking CREB/CBP-dependent PGC-1α transcription (B) 134, 139, 140, as well as through other toxic mechanisms (C). Synaptic NMDAR activity opposes mtHtt toxicity by promoting TRiC-dependent aggregation of mtHtt into non-toxic inclusions (D) 44. Synaptic NMDAR activity also activates CREB-dependent PGC-1α transcription (E) 44, 101, and enhances PGC-1α activity post-translationally via p38 (F)101. PGC-1α itself protects neurons against mtHtt toxicity and extrasynaptic-NMDAR-dependent excitotoxicity (G) 44, 101, 162. Extrasynaptic NMDAR activity reduces the formation of non-toxic mtHtt inclusions by enhancing the expression of Rhes, a GTPase known to sumoylate and disaggregate mtHtt (H) 44. In addition, extrasynaptic NMDAR signals suppress CREB-dependent gene expression 38 (not shown).
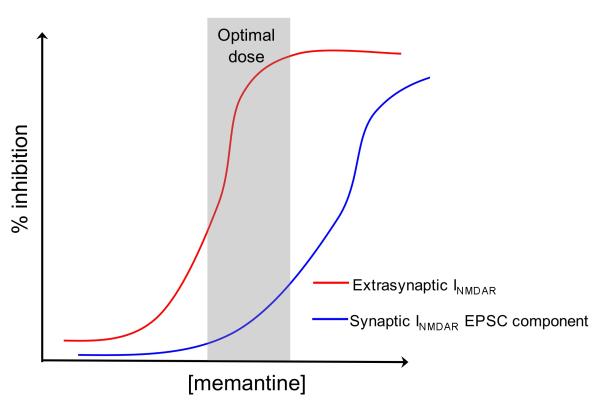
B. Effect of memantine on NMDARs. Low doses of memantine preferentially block the chronic activation of extrasynaptic NMDARs (red line) by elevated levels of glutamate, which may occur as a result of ischaemia or impaired glutamate homeostasis associated with other disease processes. Low levels of memantine spare the trans-synaptic activation of synaptic NMDARs (blue line). This specificity of the effect of memantine has been demonstrated electrophysiologically 126, 147 as well as by studying downstream pro-death and pro-survival signalling 39, 43, 44, 132.
In addition to the influence of mtHtt on extrasynaptic NMDAR function, a recent study has demonstrated that synaptic and extrasynaptic NMDAR signalling have distinct and opposing effects on the neurotoxicity of mtHtt itself. Synaptic NMDAR activity reduces mtHtt toxicity by increasing the formation of non-toxic mtHtt inclusions by a process involving the transcriptional up-regulation of the chaperonin TRiC (T complex-1 ring complex) subunit TCP1. TCP1 knock-down or blocking of synaptic activity reduced the formation of inclusions, rendering neurons vulnerable to mtHtt-induced neuronal death. This mtHtt-induced neuronal death is itself dependent on extrasynaptic NMDAR activity44, which is consistent with the upregulation of extrasynaptic NMDAR currents that have been observed in YAC128 mice132 and, indeed, with the large body of literature implicating excessive NMDAR-dependent activity in HD pathology23.
In addition to the enhancement of extrasynaptic NMDAR activity by mtHtt and in mediating mtHtt-induced excitotoxic death, extrasynaptic NMDARs may also serve as up-stream regulators of mtHtt toxicity44. Extrasynaptic NMDAR activity was found to support the expression of Rhes, a small GTPase that controls the sumoylation of mtHtt, a modification that promotes mtHtt toxicity and prevents its aggregation133.
Part of the toxic effects of mtHtt and excessive extrasynaptic NMDAR activity in HD pathology may be attributable to the suppression of pro-survival CREB target gene expression. As stated above, extrasynaptic NMDAR activity is coupled to a CREB-inactivating dephosphorylation signal38. Consistent with this, CREB phosphorylation levels are reduced in the striatum of YAC128 mice (where extrasynaptic NMDAR activity is elevated)132. Also, mtHtt can suppress CREB-mediated gene expression by sequestering the CREB coactivator CBP — an effect that contributes to mtHtt toxicity134, 135. A key neuroprotective CREB target gene whose underexpression is implicated in HD pathogenesis is the transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator 1a (PGC-1α)136. In neurons, PGC-1α regulates both mitochondrial density and antioxidant defences, and controls vulnerability to excitotoxic insults by these or other mechanisms101, 137, 138. MtHtt interferes with CREB-dependent PGC-1α expression, and this leads to mitochondrial dysfunction and metabolic defects139, 140. Indeed, PGC-1α expression is lower in the striatum of human HD patients, but not in the cerebellum or hippocampus, which is consistent with the brain areas associated with HD pathology. As a further exacerbating factor, the striatum seems to be particularly vulnerable to loss of PGC-1α expression141. Overexpression of PGC-1α protects both cortical and striatal neurons from mtHtt- and 3-nitropropanoic acid-induced cell death, and lentiviral transduction of PGC-1α into the striatum reduces striatal loss in a mouse model of HD44, 138-140.
Synaptic activity acts as a positive regulator of PGC-1α expression, both directly — via CREB activation — and indirectly — by inhibiting mtHtt-dependent suppression of PGC-1α (due to the aforementioned TCP1-dependent aggregation of mtHtt)44, 101. Moreover, synaptic activity enhances PGC-1α’s transactivating capacity through a post-translational, p38-dependent mechanism101. Taken together, the balance between synaptic and extrasynaptic NMDAR activity controls the toxicity of mtHtt and is itself controlled by mtHtt (Figure 6a). This reciprocal, feed-forward control may play a key role in HD progression and indicates that a selective blockade of extrasynaptic NMDARs and sparing of synaptic NMDAR activity offers a potentially attractive therapeutic strategy, as outlined below44, 132.
Therapeutic targeting of extrasynaptic NMDARs
Despite evidence from animal studies that have implicated NMDARs in ischaemic brain damage, clinical trials of NMDAR antagonists for stroke and TBI have failed due to poor tolerance and efficacy25, 142. The important role of NMDARs in the CNS may mean that for many antagonists the maximum tolerated dose may not be therapeutically effective, as many unacceptable side-effects are not off-target effects but are due to blockade of synaptic NMDAR activity. Evidence that NMDAR activity can exert a neuroprotective effect has led to suggestions that it may even promote recovery in the post-reperfusion phase, and prevent delayed neuronal loss in the penumbra25, 143. Dichotomous signalling by NMDARs is also evident in juvenile TBI. Here, treatment with NMDAR antagonists reduces primary excitotoxic death but exacerbate secondary apoptosis, resulting in increased overall death30. In adult TBI, the NMDAR has also been proposed to rapidly switch between ‘destructive’ and ‘recovery’ roles144, 145. As such, antagonists may be protective in early stages of the insult progression but not later, highlighting the need for more specific anti-excitotoxic strategies in which the physiological and neuroprotective roles of NMDARs are considered. One promising strategy is the use of antagonists that preferentially block NMDARs in excitotoxic scenarios and of pathologically-activated therapeutics, which are activated only in the pathological state146. As an example of the former, memantine is a clinically well-tolerated NMDAR antagonist with pharmacological properties that are suited to the blockade of chronic NMDAR activity in pathological situations without interfering with normal synaptic function146.
Memantine is an open channel blocker with a fast off-rate. Its uncompetitive nature results in an effective blockade of chronic extrasynaptic NMDAR activity that is triggered by elevated levels of glutamate126. Interestingly, due to its fast off-rate and voltage-dependent binding properties memantine, when used at low doses, does not accumulate in the channel and so does not substantially interfere with normal synaptic NMDAR activity 126, 147. As such, it was proposed that low doses of memantine would not substantially affect the trans-synaptic activation of synaptic NMDARs while allowing blockade of chronic extrasynaptic NMDAR activation. This discrimination was recently demonstrated definitively in a study on autaptic hippocampal cultures148. This study showed that the relative selectivity for extrasynaptic NMDARs is sensitive to the concentration of memantine used, as increasing the levels of memantine can lead to blockade of synaptic NMDARs (Figure 6b). Note, however, that it has been shown that memantine also preferentially antagonizes GluN2C- and GluN2D-containing NMDARs over GluN2A- and GluN2B-containing NMDARs under physiological levels of Mg2+ 149. This should be taken into account when interpreting the effects of memantine in systems in which GluN2C/D levels are substantial.
Nevertheless, the usefulness of memantine in preferentially uncoupling the NMDAR from pro-death extrasynaptic NMDAR signalling has also been shown39, 43. For example, low doses of memantine not only block NMDAR-dependent neuronal death that is induced by toxic doses of NMDA without interfering with trans-synaptic NMDAR-dependent activation of neuroprotective pathways; it does so in a manner which does not induce neuronal degeneration in vivo, unlike ‘conventional’ antagonists such as MK-80143. The in vivo use of memantine to antagonize neurodegenerative pathways that were characterized in vitro has been elegantly demonstrated in the context of HD pathology44, 132. Treatment of YAC128 mice with low dose memantine (1 mg/kg) from 2 months of age restored striatal phospho-CREB levels to a level similar to that in wild-type animals, and prevented the motor learning deficits that are normally apparent at 4 months of age132. A similar dosing regime also prevented striatal loss in YAC128 mice at 12 months and prevented motor deficits44. The protective effects of low-dose memantine were in sharp contrast with the effects of a much higher dose (30 mg/kg), which blocks all NMDARs: an exacerbation of striatal cell loss and motor deficits44. Given that memantine is a well-tolerated drug that has already been approved for clinical use, it will be of great interest to see whether clinical trials of memantine for HD reveal a reduction or delay of symptoms.
Concluding remarks
The survival and well-being of neurons requires synaptic activity and a dialogue between the synapse and the nucleus. Ca2+ signals, initiated by synaptic NMDARs, propagate inward to the nucleus and control genomic programmes that lead to a decrease in mitochondrial vulnerability, reduced expression of pro-apoptotic factors including caspases, and a strengthening of antioxidant defence systems. This form of acquired neuroprotection increases the ‘neuronal survival reserve’ that enables neurons to cope with harmful conditions in order to fulfil their function as computational units within neural circuits. Extrasynaptic NMDARs antagonize the pro-survival signalling of synaptic NMDARs by disturbing the communication between the synapse and the nucleus at various levels. Cell death associated with neurodegenerative diseases (including AD and HD) may be partly due to an imbalance of synaptic and extrasynaptic NMDAR signalling caused by synapse loss, failure to transduce Ca2+ signals from the synapse to the nucleus, or by redistributions of NMDARs from synaptic to extrasynaptic site. The antagonistic signalling of synaptic and extrasynaptic NMDARs provides a novel conceptual basis for future developments of neuroprotective therapies.
Acknowledgements
We thank Dr Karen Bell and Dr. C. Peter Bengston for comments on the manuscript. Work in the authors’ laboratories is supported by the Wellcome Trust, the MRC, the BBSRC, the Royal Society (GEH) and by the Alexander von Humboldt Foundation, the ERC Advanced Grant, the Deutsche Forschungsgemeinschaft, the EU Network of Excellence NeuroNE, and the EU Project GRIPANNT (HB). GEH is a MRC Senior Non-Clinical Research Fellow. HB is a member of the Excellence Cluster CellNetworks at Heidelberg University.
References
- 1.Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv Neurol. 1999;79:133–44. [PubMed] [Google Scholar]
- 2.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. The Journal of Neuroscience. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenmund C, Feltz A, Westbrook GL. Synaptic NMDA receptor channels have a low open probability. J Neurosci. 1995;15:2788–95. doi: 10.1523/JNEUROSCI.15-04-02788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cottrell JR, Dube GR, Egles C, Liu G. Distribution, density, and clustering of functional glutamate receptors before and after synaptogenesis in hippocampal neurons. J Neurophysiol. 2000;84:1573–87. doi: 10.1152/jn.2000.84.3.1573. [DOI] [PubMed] [Google Scholar]
- 6.Petralia RS, et al. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167:68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18:3158–70. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massey PV, et al. Differential roles of GluN2A and GluN2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–8. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao JP, Constantine-Paton M. GluN2A-/- mice lack long-term potentiation but retain NMDA receptor and L-type Ca2+ channel-dependent long-term depression in the juvenile superior colliculus. J Neurosci. 2007;27:13649–54. doi: 10.1523/JNEUROSCI.3153-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957;58:193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 11.Curtis DR, Phillis JW, Watkins JC. Chemical excitation of spinal neurones. Nature. 1959;183:611–612. doi: 10.1038/183611a0. [DOI] [PubMed] [Google Scholar]
- 12.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–21. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 13.Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–79. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–68. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8:185–96. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tymianski M, Charlton MP, Carlen PL, Tator CH. Source specificity of earlly calcium neurotoxicity in cultures embryonic spinal neurons. Journal of Neuroscience. 1993;13:2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann Neurol. 1986;19:105–11. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 18.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 19.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. The New England Journal of Medicine. 1994;330:613–621. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 20.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–68. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson TM, Zhang J, Dawson VL, Snyder SH. Nitric oxide: cellular regulation and neuronal injury. Prog Brain Res. 1994;103:365–9. doi: 10.1016/s0079-6123(08)61150-4. [DOI] [PubMed] [Google Scholar]
- 22.Camacho A, Massieu L. Role of glutamate transporters in the clearance and release of glutamate during ischemia and its relation to neuronal death. Arch Med Res. 2006;37:11–8. doi: 10.1016/j.arcmed.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s disease. Prog Neurobiol. 2007;81:272–93. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–55. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? The Lancet Neurology. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 26.Hetman M, Kharebava G. Survival signaling pathways activated by NMDA receptors. Curr Top Med Chem. 2006;6:787–99. doi: 10.2174/156802606777057553. [DOI] [PubMed] [Google Scholar]
- 27.Hardingham GE. Pro-survival signalling from the NMDA receptor. Biochem Soc Trans. 2006;34:936–8. doi: 10.1042/BST0340936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gould E, Cameron HA, McEwen BS. Blockade of NMDA receptors increases cell death and birth in the developing rat dentate gyrus. Journal of Comparitive Neurology. 1994;340:551–565. doi: 10.1002/cne.903400408. [DOI] [PubMed] [Google Scholar]
- 29.Ikonomidou C, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 30.Pohl D, et al. NMDA antagonists and apoptotic cell death triggered by head trauma in developing rat brain. Proceedings of the National Academy of Sciences USA. 1999;96:2508–2513. doi: 10.1073/pnas.96.5.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monti B, Contestabile A. Blockade of the NMDA receptor increases developmental apoptotic elimination of granule neurons and activates caspases in the rat cerebellum. Eur J Neurosci. 2000;12:3117–23. doi: 10.1046/j.1460-9568.2000.00189.x. [DOI] [PubMed] [Google Scholar]
- 32.Adams SM, de Rivero Vaccari JC, Corriveau RA. Pronounced cell death in the absence of NMDA receptors in the developing somatosensory thalamus. J Neurosci. 2004;24:9441–50. doi: 10.1523/JNEUROSCI.3290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikonomidou C, Stefovska V, Turski L. Neuronal death enhanced by N-methyl-D-aspartate antagonists. Proc Natl Acad Sci U S A. 2000;97:12885–90. doi: 10.1073/pnas.220412197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–33. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 35.Lipton SA, Kater SB. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989;12:265–70. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- 36.Lipton SA, Nakanishi N. Shakespeare in Love - with NMDA receptors? Nature Medicine. 1999;5:270–271. doi: 10.1038/6481. [DOI] [PubMed] [Google Scholar]
- 37.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–9. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 38.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 39.Leveille F, et al. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors. Faseb J. 2008;22:4258–71. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- 40.Stanika RI, et al. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A. 2009;106:9854–9. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanov A, et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the ERK activity in cultured rat hippocampal neurons. J Physiol. 2006;572.3:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang SJ, et al. Decoding NMDA Receptor Signaling: Identification of Genomic Programs Specifying Neuronal Survival and Death. Neuron. 2007;53:549–62. doi: 10.1016/j.neuron.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 43.Papadia S, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–87. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto S, et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15:1407–13. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soriano FX, et al. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J Neurosci. 2006;26:4509–18. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isacson O. On neuronal health. Trends Neurosci. 1993;16:306–8. doi: 10.1016/0166-2236(93)90104-t. [DOI] [PubMed] [Google Scholar]
- 47.Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25:4279–87. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–7. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 49.Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–6. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- 50.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–7. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 51.Zhang SJ, et al. Nuclear calcium signaling controls expression of a large gene pool: identification of a gene program for acquired neuroprotection induced by synaptic activity. PLoS Genet. 2009;5:e1000604. doi: 10.1371/journal.pgen.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chrivia JC, et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 53.Enslen H, et al. Characterization of Ca2+/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J Biol Chem. 1994;269:15520–7. [PubMed] [Google Scholar]
- 54.Kwok RPS, et al. Nuclear protein CBP is coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 55.Matthews RP, et al. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Molecular and Cellular Biology. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- 57.Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–5. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 58.Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–9. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- 59.Bonni A, et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 60.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 61.Lonze BE, Riccio A, Cohen S, Ginty DD. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- 62.Mantamadiotis T, et al. Disruption of CREB function in brain leads to neurodegeneration. Nature Genetics. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- 63.Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Lee B, Butcher GQ, Hoyt KR, Impey S, Obrietan K. Activity-Dependent Neuroprotection and cAMP Response Element-Binding Protein (CREB): Kinase Coupling, Stimulus Intensity, and Temporal Regulation of CREB Phosphorylation at Serine 133. J Neurosci. 2005;25:1137–1148. doi: 10.1523/JNEUROSCI.4288-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Limback-Stokin K, Korzus E, Nagaoka-Yasuda R, Mayford M. Nuclear calcium/calmodulin regulates memory consolidation. J Neurosci. 2004;24:10858–67. doi: 10.1523/JNEUROSCI.1022-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau D, Bading H. Synaptic activity-mediated suppression of p53 and induction of nuclear calcium-regulated neuroprotective genes promote survival through inhibition of mitochondrial permeability transition. J Neurosci. 2009;29:4420–9. doi: 10.1523/JNEUROSCI.0802-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leveille F, et al. Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci. 2010;30:2623–35. doi: 10.1523/JNEUROSCI.5115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Favaron M, et al. NMDA-stimulated expression of BDNF mRNA in cultured cerebellar granule neurones. Neuroreport. 1993;4:1171–4. [PubMed] [Google Scholar]
- 69.Jiang X, et al. The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem. 2005;94:713–22. doi: 10.1111/j.1471-4159.2005.03200.x. [DOI] [PubMed] [Google Scholar]
- 70.Thoenen H, Barde YA, Davies AM, Johnson JE. Neurotrophic factors and neuronal death. Ciba Found Symp. 1987;126:82–95. doi: 10.1002/9780470513422.ch6. [DOI] [PubMed] [Google Scholar]
- 71.Hansen HH, et al. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol Dis. 2004;16:440–53. doi: 10.1016/j.nbd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Vashishta A, et al. Nuclear factor of activated T-cells isoform c4 (NFATc4/NFAT3) as a mediator of antiapoptotic transcription in NMDA receptor-stimulated cortical neurons. J Neurosci. 2009;29:15331–40. doi: 10.1523/JNEUROSCI.4873-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng S, et al. NMDA-induced neuronal survival is mediated through nuclear factor I-A in mice. J Clin Invest. 2010 doi: 10.1172/JCI33144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shinoda S, et al. Bim regulation may determine hippocampal vulnerability after injurious seizures and in temporal lobe epilepsy. J Clin Invest. 2004;113:1059–68. doi: 10.1172/JCI19971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lehtinen MK, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 76.Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–36. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dick O, Bading H. Synaptic activity and nuclear calcium signaling protects hippocampal neurons from death signal-associated nuclear translocation of FoxO3a induced by extrasynaptic NMDA receptors. J Biol Chem. 2010 doi: 10.1074/jbc.M110.127654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Mubarak B, Soriano FX, Hardingham GE. Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels (Austin) 2009;3 doi: 10.4161/chan.3.4.9381. DOI: 10.4161/chan.3.4.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Biteau B, Labarre J, Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–4. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 80.Chang TS, et al. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–1001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 81.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 82.Soriano FX, et al. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhee SG, Woo HA, Bae SH, Park S. Sestrin 2 Is Not a Reductase for Cysteine Sulfinic Acid of Peroxiredoxins. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2008.2360. [Epub ahead of print], PMID: 19113821. [DOI] [PubMed] [Google Scholar]
- 84.Hardingham GE. Coupling of the NMDA receptor to neuroprotective and neurodestructive events. Biochem Soc Trans. 2009;37:1147–60. doi: 10.1042/BST0371147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 86.Hardingham GE, Chawla S, Cruzalegui FH, Bading H. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron. 1999;22:789–98. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- 87.Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. Journal of Neuroscience. 2000;20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghosh A, Greenberg ME. Calcium signalling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 89.Hardingham GE, Bading H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim Biophys Acta. 2002;1600:148–53. doi: 10.1016/s1570-9639(02)00455-7. [DOI] [PubMed] [Google Scholar]
- 90.Dieterich DC, et al. Caldendrin-Jacob: a protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol. 2008;6:e34. doi: 10.1371/journal.pbio.0060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J Biol Chem. 2001;276:2627–36. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- 92.Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- 93.Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of GluN2A- and GluN2B- containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–60. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 94.Mulholland PJ, Luong NT, Woodward JJ, Chandler LJ. Brain-derived neurotrophic factor activation of extracellular signal-regulated kinase is autonomous from the dominant extrasynaptic NMDA receptor extracellular signal-regulated kinase shutoff pathway. Neuroscience. 2008;151:419–27. doi: 10.1016/j.neuroscience.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu J, et al. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–43. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bano D, et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–85. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 97.Kawasaki H, et al. Activation and involvement of p38 mitogen-activated protein kinase in glutamate-induced apoptosis in rat cerebellar granule cells. J Biol Chem. 1997;272:18518–21. doi: 10.1074/jbc.272.30.18518. [DOI] [PubMed] [Google Scholar]
- 98.Cao J, et al. Distinct requirements for p38alpha and c-Jun N-terminal kinase stress-activated protein kinases in different forms of apoptotic neuronal death. J Biol Chem. 2004;279:35903–13. doi: 10.1074/jbc.M402353200. [DOI] [PubMed] [Google Scholar]
- 99.Soriano FX, et al. Specific targeting of pro-death NMDA receptor signals with differing reliance on the GluN2B PDZ ligand. J Neurosci. 2008;28:10696–710. doi: 10.1523/JNEUROSCI.1207-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Okamoto S, Krainc D, Sherman K, Lipton SA. Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc Natl Acad Sci U S A. 2000;97:7561–6. doi: 10.1073/pnas.130502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soriano FX, Leveille F, Papadia S, Bell KF, Hardingham GE. Neuronal activity controls the antagonistic balance between PGC-1α and SMRT in regulating antioxidant defences. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3568. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- 103.Wahl AS, et al. Hypoxic/ischemic conditions induce expression of the putative pro-death gene Clca1 via activation of extrasynaptic N-methyl-D-aspartate receptors. Neuroscience. 2009;158:344–52. doi: 10.1016/j.neuroscience.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Wittmann M, et al. Synaptic activity induces dramatic changes in the geometry of the cell nucleus: interplay between nuclear structure, histone H3 phosphorylation, and nuclear calcium signaling. J Neurosci. 2009;29:14687–700. doi: 10.1523/JNEUROSCI.1160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taddei A, et al. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature. 2006;441:774–8. doi: 10.1038/nature04845. [DOI] [PubMed] [Google Scholar]
- 106.Akhtar A, Gasser SM. The nuclear envelope and transcriptional control. Nat Rev Genet. 2007;8:507–17. doi: 10.1038/nrg2122. [DOI] [PubMed] [Google Scholar]
- 107.Collins MO, Grant SG. Supramolecular signalling complexes in the nervous system. Subcell Biochem. 2007;43:185–207. doi: 10.1007/978-1-4020-5943-8_9. [DOI] [PubMed] [Google Scholar]
- 108.Aarts M, et al. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–50. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 109.Cao J, et al. The PSD95-nNOS interface: a target for inhibition of excitotoxic p38 stress-activated protein kinase activation and cell death. J Cell Biol. 2005;168:117–26. doi: 10.1083/jcb.200407024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–7. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- 111.Zhong J, Russell SL, Pritchett DB, Molinoff PB, Williams K. Expression of mRNAs encoding subunits of the N-methyl-D-aspartate receptor in cultured cortical neurons. Mol Pharmacol. 1994;45:846–53. [PubMed] [Google Scholar]
- 112.Steigerwald F, et al. C-Terminal truncation of GluN2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–81. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Groc L, et al. NMDA receptor surface mobility depends on GluN2A-2B subunits. Proc Natl Acad Sci U S A. 2006;103:18769–74. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martel M, Wyllie DJ, Hardingham GE. In developing hippocampal neurons, GluN2B-containing NMDA receptors can mediate signalling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience. 2009;158:334–43. doi: 10.1016/j.neuroscience.2008.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor GluN2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–34. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- 116.Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–19. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–57. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weitlauf C, et al. Activation of GluN2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–90. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berberich S, et al. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–10. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–3. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frizelle PA, Chen PE, Wyllie DJA. Equilibrium constants for NVP-AAM077 acting at recombinant NR1/GluN2A and NR1/GluN2B NMDA receptors: implications for studies of synaptic transmission. Mol Pharmacol. 2006;70:1022–32. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- 122.de Marchena J, et al. NMDA Receptor Antagonists Reveal Age-Dependent Differences in the Properties of Visual Cortical Plasticity. J Neurophysiol. 2008;100:1936–48. doi: 10.1152/jn.90290.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.von Engelhardt J, et al. Excitotoxicity in vitro by GluN2A- and GluN2B-containing NMDA receptors. Neuropharmacology. 2007;53:10–7. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 124.Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–74. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 125.Harris AZ, Pettit DL. Recruiting extrasynaptic NMDA receptors augments synaptic signaling. J Neurophysiol. 2008;99:524–33. doi: 10.1152/jn.01169.2007. [DOI] [PubMed] [Google Scholar]
- 126.Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–26. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- 127.Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- 128.Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–9. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- 129.Waagepetersen HS, Shimamoto K, Schousboe A. Comparison of effects of DL-threo-beta-benzyloxyaspartate (DL-TBOA) and L-trans-pyrrolidine-2,4-dicarboxylate (t-2,4-PDC) on uptake and release of [3h]D-aspartate in astrocytes and glutamatergic neurons. Neurochem Res. 2001;26:661–6. doi: 10.1023/a:1010939304104. [DOI] [PubMed] [Google Scholar]
- 130.Gouix E, et al. Reverse glial glutamate uptake triggers neuronal cell death through extrasynaptic NMDA receptor activation. Mol Cell Neurosci. 2009;40:463–73. doi: 10.1016/j.mcn.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 131.Tu W, et al. DAPK1 interaction with NMDA receptor GluN2B subunits mediates brain damage in stroke. Cell. 2010;140:222–34. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milnerwood A, et al. Early increase in extrasynaptic NMDA receptor signalling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron. 2010;65:178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 133.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–30. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nucifora FC, Jr., et al. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science. 2001;291:2423–8. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 135.Jiang H, et al. Depletion of CBP is directly linked with cellular toxicity caused by mutant huntingtin. Neurobiol Dis. 2006;23:543–51. doi: 10.1016/j.nbd.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 136.McGill JK, Beal MF. PGC-1alpha, a new therapeutic target in Huntington’s disease? Cell. 2006;127:465–8. doi: 10.1016/j.cell.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 137.St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 138.Wareski P, et al. PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem. 2009;284:21379–85. doi: 10.1074/jbc.M109.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weydt P, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metab. 2006;4:349–62. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 140.Cui L, et al. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 141.Lin J, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–35. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 142.Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 143.Albers GW, Goldstein LB, Hall D, Lesko LM. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. Jama. 2001;286:2673–82. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- 144.Biegon A, et al. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci U S A. 2004;101:5117–22. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yaka R, et al. D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. Faseb J. 2007;21:2033–41. doi: 10.1096/fj.06-7856com. [DOI] [PubMed] [Google Scholar]
- 146.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–8. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 147.Chen HS, et al. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–32. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- 148.Xia P, Chen HS, Zhang D, Lipton SA. Memantine Preferentially Blocks Extrasynaptic Over Synaptic NMDA Receptor Currents in Hippocampal Autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci. 2009;29:2774–9. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22:2165–73. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–64. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 152.Clark BA, Cull-Candy SG. Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. J Neurosci. 2002;22:4428–36. doi: 10.1523/JNEUROSCI.22-11-04428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Scimemi A, Fine A, Kullmann DM, Rusakov DA. GluN2B-containing receptors mediate cross talk among hippocampal synapses. J Neurosci. 2004;24:4767–77. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Groc L, Bard L, Choquet D. Surface trafficking of N-methyl-D-aspartate receptors: physiological and pathological perspectives. Neuroscience. 2009;158:4–18. doi: 10.1016/j.neuroscience.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 155.Bengtson CP, Dick O, Bading H. A quantitative method to assess extrasynaptic NMDA receptor function in the protective effect of synaptic activity against neurotoxicity. BMC Neurosci. 2008;9:11. doi: 10.1186/1471-2202-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A. 2001;98:2808–13. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4:565–6. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- 158.Impey S, et al. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron. 2002;34:235–44. doi: 10.1016/s0896-6273(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 159.Screaton RA, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 160.Kovacs KA, et al. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proc Natl Acad Sci U S A. 2007;104:4700–5. doi: 10.1073/pnas.0607524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ravnskjaer K, et al. Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. EMBO J. 2007;26:2880–9. doi: 10.1038/sj.emboj.7601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Luo Y, Zhu W, Jia J, Zhang C, Xu Y. NMDA receptor dependent PGC-1alpha up-regulation protects the cortical neuron against oxygen-glucose deprivation/reperfusion injury. J Mol Neurosci. 2009;39:262–8. doi: 10.1007/s12031-009-9196-5. [DOI] [PubMed] [Google Scholar]