Abstract
The capacity to generate myriad differentiated cell types, including neurons, from human embryonic stem cell (hESC) lines offers great potential for developing cell-based therapies and also for increasing our understanding of human developmental mechanisms. In addition, the emerging development of this technology as an experimental tool represents a potential opportunity for neuroscientists interested in mechanisms of neuroprotection and neurodegeneration. Potentially unlimited generation of well-defined functional neurons from hES and patient specific induced pluripotent (iPS) cells offers new systems to study disease mechanisms, signalling pathways and receptor pharmacology within a human cellular environment. Such systems may help in overcoming interspecies differences. Far from replacing rodent in vivo and primary culture systems, hES and iPS cell-derived neurons offer a complementary resource to overcome issues of interspecies differences, accelerate drug discovery, study of disease mechanism as well as provide basic insight into human neuronal physiology.
Introduction
Model organisms such as rodents have contributed greatly to our understanding of how the CNS functions in health and disease. Through the use of whole animal experiments and the study of ex vivo preparations and cultures of primary neurons and glial cells, detailed mechanisms of how neurons function and respond to environmental and traumatic stimuli have been elucidated. These findings are translatable to the human condition, given the conservation of gene orthologs and neuronal organisation, and also of CNS structure and development. Insights gained from rodent studies have revealed new therapeutic targets and treatment strategies in many diseases, including those afflicting the CNS. Despite this, there remains room for improved or complementary experimental models in which to study human disease mechanisms or cytoprotective strategies.
Human embryonic stem cells (hESCs) are pluripotent cell lines derived from the inner cell mass of the blastocyst. They are karyotypically normal and are capable of unlimited proliferation in their pluripotent state (1). HESCs can be readily neuralised generating neural stem cells (NSCs) and their functional derivatives including neurons (2, 3). A key advantage of hESC derived NSCs (hESC-NSCs) over alternative sources of human NSCs are that hESC-NSCs retain developmental competence to patterning signals and thus can be directed to regional neuronal subtypes. Critically, cells specified this way in vitro retain their imposed positional identity and differentiate appropriately when transplanted in vivo. This ability to reliably generate scaleable and enriched numbers of functional human neurons offers a tremendous experimental opportunity to study mechanisms of neurotoxicity and neuroprotection (4).
Generation of regionally specified neurons from hESCs as an experimental resource
The ability to direct neuroectodermal differentiation from hESCs has progressed rapidly in the last decade and reflects the near “default” nature of neural induction from ESCs when grown in conditions with limited extrinsic signalling. The most widely applied neuralising systems employ spontaneous differentiation with subsequent growth factor mediated propagation of neural precursors. Refinements include addition of retinoic acid to hESC aggregates, antagonism of the nodal / SMAD signalling pathway or co-culture with stromal cells and conditioned media (1, 5-7). Resulting hES-derived neural precursors can be propagated and expanded long-term (over 150 days) in adherent or substrate free conditions under defined conditions supplemented with mitogens such as EGF/FGF2 (8, 9). Subsequent differentiation of neural precursors upon plating onto substrate and withdrawal of mitogens confirms neuronal and glial potential. Importantly, hES-NSCs display a temporal determination of differentiation, consistent with neural development, with neurogenesis preceding gliogenesis. Early precursors thus represent an attractive population from which to generate region specific neurons through application of developmentally based signals. Neurons generated from hESCs under chemically defined conditions in the absence of exogenous morphogens including retinoic acid tend to differentiate towards an anterior positional identity (10) as has been found for mouse hESCs (11). However, the use of morphogens or the activation/inhibition of key developmental pathways enables functional neurons of different positional identities to be created. For example, we recently showed that inhibition of activin/nodal signaling results in hESC-NPCs of a strongly posterior/spinal cord positional identity to be generated (12). Classical anterior NPC markers such as PAX6 and OTX2 are suppressed while posterior markers such as HOXC8 and HOXB6 are up-regulated. These neurons fire TTX-sensitive action potentials and express functional AMPA receptors and NMDARs as assessed electrophysiologically and by Ca2+ imaging (12). Moreover they exhibit TTX-sensitive spontaneous excitatory postsynaptic currents and TTX-insensitive miniature EPSCs which were blocked by the AMPAR antagonist CNQX, confirming presence of synaptic AMPA receptors. Furthermore, these neurons are sensitive to both peroxide and glutamate-induced death (Fig. 1).
Fig. 1.
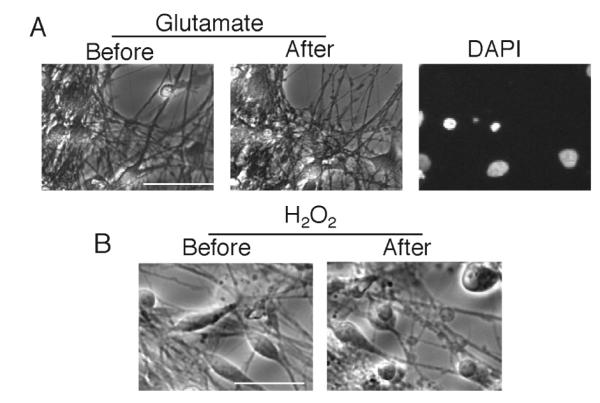
Neurons derived from hESCs as described (12) were treated for 8 h with glutamate (200 μM) or H2O2 (200 μM). Pictures before/after exposure were taken. Both insults led to nuclear pyknosis that was preceded by neuritic beading. Scale bar 50 μm.
Other laboratories have created specific neuronal types with markers consistent with midbrain dopamingergic neurons, spinal motor neurons and striatal neurons (3, 13-16). Recognising that all such populations although enriched, remain heterogeneous, there is a need for additional selection methods to further purify neuronal sub-type lineages. Sub-type specific reporter lines are one such method. While a key aim of positionally specifying hESC-NPCs is to work towards the generation of cell-based therapies for diseases that target a sub-population of cells, they will also provide a valuable resource for research into those very same diseases, (4). Future application of regional specification will be particularly powerful in attempting to understand disease specificty when applied to induced disease-specific pluripotent stem cells (iPS cells). The emergence of iPS cells, through manipulation of somatic cells by overexpression of key transcription factors to generate embryonic stem (ES) like cells, offers additional experimental and therapeutic possibilities. Combining somatic cell reprogramming technology to generate patient specific iPS cell lines carrying disease-causing mutations, with neural developmental insights offers the unique opportunity to develop bespoke human in vitro models of disease. Indeed, recent studies have confirmed the utility of such an approach with resulting neuronal cells displaying a disease phenotype as well as being an unique assay for drug discovery and testing (17, 18). Neural precusors were generated from an iPS cell line developed from fibroblasts taken from a spinal muscular atrophy patient, a disease characterised by selective loss of lower motor neurons caused by mutation in the survival motor neuron 1 gene (SMN1) gene. Compared to a wild-type iPS cell line, SMA-iPS cells were equally amenable to differentiation into immature motor neurons, but numbers of motor neurons observed at later timepoints post-differentiation were much lower in the SMA-iPS cell cultures, compared to wild-type, indicative of either impaired production or selective degeneration of motor neurons (17). In another recent example, iPS cells were generated from a patient with familial dysautonomia (FD), a fatal peripheral neuropathy, caused by a point mutation in the IKBKAP gene leading to depletion of autonomic and sensory neurons. FD-iPS cell-derived neural precursors have particularly low levels of IKBKAP, mis-splicing of IKBKAP, and defects in neurogenic differentiation and migration behaviour (18). Together these studies demonstrate that in vitro developmentally based human neuronal culture systems can reproduce a phenotype normally observed in vivo in juvenile or adult life.
Species-specific differences in signaling and gene expression indicate a role for studying human neurons
Despite the relevance of rodent models to the human condition, there are differences between rodent and human cellular systems that have the potential to cloud the inferences we can take from rodent studies. These differences should not be overstated, but nevertheless point to the benefit of having hESC-derived neurons as an additional tool for probing toxic and protective signaling pathways, validating therapeutic targets, and aiding drug discovery (19). Important interspecies differences are also evident in the signalling requirements underlying pluripotency of mouse and human ES cultures. Specifically mouse ES cells require activation of the JAK/STAT pathway through LIF whereas there is no requirement of LIF for human ES cell maintenance. Human ES cells are dependent on activin/nodal and FGF signalling. As outlined below, interspecies differences in transcriptional responses represent a potential point of difference. However, anecdotal differences in many aspects of cell biology have been reported, leading one to speculate that other unknown differences await detection.
Evolutionary turnover of transcription factor binding sites
Endogenous neuroprotective pathways, such as those triggered by synaptic NMDA receptor activity, or neurotrophic factors, exert their effect in part via changes in gene expression (20, 21). Through the up-regulation of pro-survival genes and the suppression of pro-death genes, the affected neurons can gain resistance to subsequent insults. Other less advantageous effects, such as those of disease-causing agents, or harmful side-effects of drugs can rely significantly on effects on intracellular signalling pathways causing changes in gene expression. Whilst many key factors mediating these processes are likely to be conserved in rodent and human systems, there may be some differences. At the transcriptional regulation level there is evidence for turnover of transcriptional regulatory elements. A significant fraction of functional human transcription factor binding sites are not functional in rodents (22). A more recent study on a number of functionally conserved tissue-specific transcription factors in mouse and human primary hepatocytes showed that the conservation of transcription factor occupancy in orthologous mouse and human gene promoters could be highly variable (23). Interestingly, while interspecies differences in binding events were very large, the location of binding events within two different human systems: primary human hepatocytes and a human hepatocarcinoma cell line HepG2, was highly conserved (23). These observations all strengthen the case for employing hESC-derived neurons to study transcriptional responses alongside rodent neurons in order to identify interspecies differences and thus home in on the most human-relevant pathways that could (for example) be explored further in rodent in vivo models.
There are many potential reasons why transcription factor binding events may not be conserved between species, such as non-equivalent developmental stages, or interspecies differences in transcription factor expression levels or even specificity. However, gene promoter sequence, as opposed to any differences in transcriptional machinery appears to be the dominant factor directing species-specific transcription (24). One recent study involved analysis of transcription factor binding events and the resulting gene expression associated with human chromosome 21 when carried by an aneuploid mouse strain (24). Study of hepatocytes from these mice compared to human ones revealed that human-specific binding and expression patterns were recapitulated in the mouse cells. Thus, genetic sequence is the primary determinant of the transcriptional response, rather than cellular environment.
Collectively, these findings have implications for trying to predict the transcriptional response in a human cell to a particular stimulus by studying the response in a rodent cell. In an example directly relevant to our research, we recently identified a gene, sulfiredoxin that is regulated by synaptic NMDAR activity in rat neurons via two AP-1 sites (25). The induction of this gene contributed to the enhanced antioxidant defenses found in electrically active rat neurons. However, one of these sites has been lost in the primate lineage, despite being well conserved among other mammals. While the loss of this site may have only a small effect on the inducibility of sulfiredoxin in human neurons, it would certainly be of importance to study the activity-dependent induction of this gene in hESC-derived forebrain neurons.
Other potential sources of interspecies differences
Post-transcriptionally, species-specific differences in the intron/exon pattern of genes and of alternative splicing patterns can lead to the products of conserved genes becoming functionally different (26, 27). There are species-specific differences in the expression and distribution of different isoforms of the human and mouse Tau genes (MAPT) (28). Species-specific differences in splicing of the Na+/Ca2+ transporter 1 (NCX1) gene have been reported (29). The NCX genes are important for neuronal Ca2+ homeostasis, particularly when substantial Ca2+ influx takes place, for example following an excitotoxic insult. Indeed, calpain-mediated cleavage and inactivation of the NCX3 gene contributes to the delayed Ca2+ deregulation and subsequent neuronal death (30) although there is no evidence of interspecies differences in its processing.
Post-translationally, protein-protein interactions can also vary, as can the distribution of gene products and the substrate profile of an enzyme. For example, while the C-terminal PDZ ligand of the human somatostatin receptor 3 interacts with the PDZ protein MUPP1, the corresponding ligand of the rat receptor does not (31). PDZ ligand interactions are important in the organization of neuron-specific macromolecular complexes such as the NMDA receptor signaling complex (32, 33). Coupling of the NMDA receptor NR2 subunit to toxic nitric oxide production and activation of pro-death p38 signaling is mediated by PDZ domains on PSD95 and neuronal nitric oxide synthase, and a PDZ ligand on NR2 (34-36) (though this is completely conserved between rodents and humans).
The activation of caspases is central to the initiation and execution of apoptosis (37). Apoptotic-like neuronal death or the activation of apoptotic biochemical cascades (e.g. caspases) are proposed to be associated with certain neurodegenerative diseases, such as Alzheimer’s and Parkinson’s Disease (38-41) where they are implicated both in neuronal death and also proximal events such as cleavage of amyloid precursor peptide and tau (41). As would be expected for a cellular mechanism as important as apoptosis, this is highly conserved within mammals. Nevertheless there do exist species-specific (human vs. mouse) differences in they way caspases process their targets, and even in whether a protein is a caspase target at all (42).
Of direct importance to therapeutics is the fact that some pharmacological compounds show large species-specific differences. Specific differences between rodent and human channel/receptor pharmacology are documented in cases such as the P2X receptor (43) the TRPV1 channel (44) and the TRPA1 receptor (45). The potential for species-specific differences motivates studies employing the human receptor expressed ectopically in a heterologous system, such as a recent study on the effect of the NMDA receptor antagonist memantine on human NR2A-containing NMDARs expressed in HEK293 cells (46). While worthwhile, such approaches can only study a single recombinant receptor, while native receptors may have varying subunit compositions and may be associated with post-translational modification and/or accessory proteins that alter the pharmacology of certain compounds.
Conclusions
While hESC-derived neurons are unlikely to replace any of the established experimental systems for studying neurotoxicity and neuroprotection, they have a valuable complementary role to play, and may reduce the overall use of animals in research. The experimental testing of a hypothesis in rodent in vitro and in vivo systems and also in hESC-derived neurons increases the burden of proof that a particular mechanism is relevant to the human organism, since each system has different limitations. Other in vitro uses for hESC-derived neurons include analysing the effects of neuroprotective receptor agonists/antagonists on native human receptors in a human cellular environment. The scalable nature of hESC systems means that hESC-derived neurons have the potential to be used in screening assays, potentially aiding drug discovery for neuroprotective compounds (19). The functional characterisation of hESC-derived neurons is developing exponentially, as are protocols for generating increasingly well defined region- or phenotype-specific neuronal populations. Aside from their much-vaunted potential for cell-based therapies, hESC-derived neurons have the potential to become an important tool for molecular neuroscientists.
References
- 1.Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23(6):699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- 2.Dhara SK, Stice SL. Neural differentiation of human embryonic stem cells. J Cell Biochem. 2008;105(3):633–40. doi: 10.1002/jcb.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Zeng X, Chen J, Deng X, Liu Y, Rao MS, Cadet JL, Freed WJ. An in vitro model of human dopaminergic neurons derived from embryonic stem cells: MPP+ toxicity and GDNF neuroprotection. Neuropsychopharmacology. 2006;31(12):2708–15. doi: 10.1038/sj.npp.1301125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krencik R, Zhang SC. Stem cell neural differentiation: A model for chemical biology. Curr Opin Chem Biol. 2006;10(6):592–7. doi: 10.1016/j.cbpa.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313(1):107–17. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3(9):e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joannides AJ, Fiore-Heriche C, Battersby AA, Athauda-Arachchi P, Bouhon IA, Williams L, Westmore K, Kemp PJ, Compston A, Allen ND, Chandran S. A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells. 2007;25(3):731–7. doi: 10.1634/stemcells.2006-0562. [DOI] [PubMed] [Google Scholar]
- 10.Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25(6):1511–20. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8(3):288–96. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 12.Patani R, Compston DA, Puddifoot C, Wyllie DJ, Hardingham GE, Allen ND, Chandran S. Activin/Nodal inhibition alone accelerates highly efficient neural conversion from human embryonic stem cells and imposes a caudal positional identity. PLoS ONE. 2009 doi: 10.1371/journal.pone.0007327. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23(6):781–90. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin S, Dalton S, Stice SL. Human motor neuron differentiation from human embryonic stem cells. Stem Cells Dev. 2005;14(3):266–9. doi: 10.1089/scd.2005.14.266. [DOI] [PubMed] [Google Scholar]
- 15.Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26(4):886–93. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aubry L, Bugi A, Lefort N, Rousseau F, Peschanski M, Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci U S A. 2008;105(43):16707–12. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebert AD, Yu J, Rose FF, Jr., Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, Viale A, Tabar V, Sadelain M, Studer L. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461(7262):402–6. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cezar GG. Can human embryonic stem cells contribute to the discovery of safer and more effective drugs? Curr Opin Chem Biol. 2007;11(4):405–9. doi: 10.1016/j.cbpa.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11(3):297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 21.Papadia S, Hardingham GE. The Dichotomy of NMDA Receptor Signaling. Neuroscientist. 2007 doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dermitzakis ET, Clark AG. Evolution of transcription factor binding sites in Mammalian gene regulatory regions: conservation and turnover. Mol Biol Evol. 2002;19(7):1114–21. doi: 10.1093/oxfordjournals.molbev.a004169. [DOI] [PubMed] [Google Scholar]
- 23.Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, Rolfe PA, Conboy CM, Gifford DK, Fraenkel E. Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat Genet. 2007;39(6):730–2. doi: 10.1038/ng2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson MD, Barbosa-Morais NL, Schmidt D, Conboy CM, Vanes L, Tybulewicz VL, Fisher EM, Tavare S, Odom DT. Species-specific transcription in mice carrying human chromosome 21. Science. 2008;322(5900):434–8. doi: 10.1126/science.1160930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11(4):476–87. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy SW, Gilbert W. The evolution of spliceosomal introns: patterns, puzzles and progress. Nat Rev Genet. 2006;7(3):211–21. doi: 10.1038/nrg1807. [DOI] [PubMed] [Google Scholar]
- 27.Laurell H, Grober J, Vindis C, Lacombe T, Dauzats M, Holm C, Langin D. Species-specific alternative splicing generates a catalytically inactive form of human hormone-sensitive lipase. Biochem J. 1997;328(Pt 1):137–43. doi: 10.1042/bj3280137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMillan P, Korvatska E, Poorkaj P, Evstafjeva Z, Robinson L, Greenup L, Leverenz J, Schellenberg GD, D’Souza I. Tau isoform regulation is region- and cell-specific in mouse brain. J Comp Neurol. 2008;511(6):788–803. doi: 10.1002/cne.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Eylen F, Bollen A, Herchuelz A. NCX1 Na/Ca exchanger splice variants in pancreatic islet cells. J Endocrinol. 2001;168(3):517–26. doi: 10.1677/joe.0.1680517. [DOI] [PubMed] [Google Scholar]
- 30.Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38(2 Suppl):674–6. doi: 10.1161/01.STR.0000256294.46009.29. [DOI] [PubMed] [Google Scholar]
- 31.Liew CW, Vockel M, Glassmeier G, Brandner JM, Fernandez-Ballester GJ, Schwarz JR, Schulz S, Buck F, Serrano L, Richter D, Kreienkamp HJ. Interaction of the human somatostatin receptor 3 with the multiple PDZ domain protein MUPP1 enables somatostatin to control permeability of epithelial tight junctions. FEBS Lett. 2009;583(1):49–54. doi: 10.1016/j.febslet.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 32.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5(10):771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 33.Collins MO, Grant SG. Supramolecular signalling complexes in the nervous system. Subcell Biochem. 2007;43:185–207. doi: 10.1007/978-1-4020-5943-8_9. [DOI] [PubMed] [Google Scholar]
- 34.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298(5594):846–50. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 35.Cao J, Viholainen JI, Dart C, Warwick HK, Leyland ML, Courtney MJ. The PSD95-nNOS interface: a target for inhibition of excitotoxic p38 stress-activated protein kinase activation and cell death. J Cell Biol. 2005;168(1):117–26. doi: 10.1083/jcb.200407024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soriano FX, Martel MA, Papadia S, Vaslin A, Baxter P, Rickman C, Forder J, Tymianski M, Duncan R, Aarts M, Clarke P, Wyllie DJ, Hardingham GE. Specific targeting of pro-death NMDA receptor signals with differing reliance on the NR2B PDZ ligand. J Neurosci. 2008;28(42):10696–710. doi: 10.1523/JNEUROSCI.1207-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier P, Vousden KH. Lucifer’s labyrinth--ten years of path finding in cell death. Mol Cell. 2007;28(5):746–54. doi: 10.1016/j.molcel.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, Yang L, Beal MF, Andersen JK. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease. J Neurosci. 2001;21(24):9519–28. doi: 10.1523/JNEUROSCI.21-24-09519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattson MP. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal. 2006;8(11-12):1997–2006. doi: 10.1089/ars.2006.8.1997. [DOI] [PubMed] [Google Scholar]
- 40.Ribe EM, Serrano-Saiz E, Akpan N, Troy CM. Mechanisms of neuronal death in disease: defining the models and the players. Biochem J. 2008;415(2):165–82. doi: 10.1042/BJ20081118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohn TT, Head E. Caspases as therapeutic targets in Alzheimer’s disease: is it time to “cut” to the chase? Int J Clin Exp Pathol. 2009;2(2):108–18. [PMC free article] [PubMed] [Google Scholar]
- 42.Ussat S, Werner U, Adam-Klages S. Species-specific differences in the usage of several caspase substrates. Biochem Biophys Res Commun. 2002;297(5):1186–90. doi: 10.1016/s0006-291x(02)02358-6. [DOI] [PubMed] [Google Scholar]
- 43.Tittle RK, Hume RI. Opposite effects of zinc on human and rat P2X2 receptors. J Neurosci. 2008;28(44):11131–40. doi: 10.1523/JNEUROSCI.2763-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips E, Reeve A, Bevan S, McIntyre P. Identification of species-specific determinants of the action of the antagonist capsazepine and the agonist PPAHV on TRPV1. J Biol Chem. 2004;279(17):17165–72. doi: 10.1074/jbc.M313328200. [DOI] [PubMed] [Google Scholar]
- 45.Klionsky L, Tamir R, Gao B, Wang W, Immke DC, Nishimura N, Gavva NR. Species-specific pharmacology of Trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol Pain. 2007;3:39. doi: 10.1186/1744-8069-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilling KE, Jatzke C, Hechenberger M, Parsons CG. Potency, voltage-dependency, agonist concentration-dependency, blocking kinetics and partial untrapping of the uncompetitive N-methyl-D-aspartate (NMDA) channel blocker memantine at human NMDA (GluN1/GluN2A) receptors. Neuropharmacology. 2009;56(5):866–75. doi: 10.1016/j.neuropharm.2009.01.012. [DOI] [PubMed] [Google Scholar]


