Abstract
Objective
This review focuses on the criteria for assessing osteoarthritis (OA) in the guinea pig at the macroscopic and microscopic levels, and recommends particular assessment criteria to assist standardization in the conduct and reporting of preclinical trails in guinea pig models of OA.
Methods
A review was conducted of all OA studies from 1958 until the present that utilized the guinea pig. The PubMed database was originally searched August 1, 2006 using the following search terms: guinea pig and osteoarthritis. We continued to check the database periodically throughout the process of preparing this chapter and the final search was conducted January 7, 2009. Additional studies were found in a review of abstracts from the OsteoArthritis Research Society International (OARSI) conferences, Orthopaedic Research Society (ORS) conferences, and literature related to histology in other preclinical models of OA reviewed for relevant references. Studies that described or used systems for guinea pig joint scoring on a macroscopic, microscopic, or ultrastructural basis were included in the final comprehensive summary and review. General recommendations regarding methods of OA assessment in the guinea pig were derived on the basis of a comparison across studies and an inter-rater reliability assessment of the recommended scoring system.
Results
A histochemical-histological scoring system (based on one first introduced by H. Mankin) is recommended for semi-quantitative histological assessment of OA in the guinea pig, due to its already widespread adoption, ease of use, similarity to scoring systems used for OA in humans, its achievable high inter-rater reliability, and its demonstrated correlation with synovial fluid biomarker concentrations. Specific recommendations are also provided for histological scoring of synovitis and scoring of macroscopic lesions of OA.
Conclusions
As summarized herein, a wealth of tools exist to aid both in the semi-quantitative and quantitative assessment of OA in the guinea pig and provide a means of comprehensively characterizing the whole joint organ. In an ongoing effort at standardization, we recommend specific criteria for assessing the guinea pig model of OA as part of an OARSI initiative, termed herein the OARSI-HISTOgp recommendations.
Introduction
Osteoarthritis (OA) in the guinea pig can be induced chemically and surgically, and can occur spontaneously (Table 1). Silverstein and Sokoloff were the first to report spontaneous OA-like changes in the guinea pig [1], although this report described only the bony manifestations of the disease and lacked information about articular cartilage alterations. Bendele et. al. next noted spontaneous OA-like changes in non-operated knees, contralateral to partial medial meniscectomized knees [2]. This observation was quickly followed by a characterization of spontaneous knee OA in the Hartley guinea pig [3], and an in-depth light and electron microscopic description, including an assessment of shoulder and elbow joints [4]. Coincident with these observations, the guinea pig continued to be utilized for surgical models of OA, induced by either medial meniscectomy [2, 5], or section of the lateral collateral ligament, with or without section of the medial collateral ligament [5].
Table 1.
Studies of osteoarthritis in the guinea pig that included microscopic or macroscopic evaluations.
| Types of OA Models | Intervention | References |
|---|---|---|
| Spontaneous | None (Spontaneous joint disease in OA prone Dunkin-Hartley compared with OA resistant Strains 2, 13 or GOHI) | [1, 3–6, 12, 15–19, 25, 37, 38, 56, 60, 72, 77, 78, 85, 94–97, 101, 104–113] [7, 8, 14, 20, 26–28, 30, 31, 45, 47, 57, 58, 69, 71, 79–82, 91, 98, 99, 102, 114–118] |
| Surgically Induced | Medial Meniscectomy | [2, 5, 21, 40, 42, 43, 61, 62, 74, 93, 119] |
| Anterior cruciate ligament transection | [36] | |
| Anterior cruciate ligament transection, medial collateral ligament section and partial medial meniscectomy | [120, 121] | |
| Anterior cruciate ligament transection and partial medial meniscectomy | [122, 123] | |
| Osteotomy | [13, 39] | |
| Ovariectomy | [10, 11] | |
| Patellar tendonotomy and patellectomy | [54] | |
| Lateral and/or medial collateral ligament section | [5] | |
| Myectomy +/− tendonotomy | [23, 76, 124] | |
| Unilateral sciatic neurectomy +/− meniscectomy | [125] | |
| Combination Surgically and Chemically Induced | Medial meniscectomy or Collateral ligament transaction +/− iodoacetate | [125] |
| Enzymatic/Chemically Induced | Iodoacetate (IA) | [126–128] |
| Papain (IA) | [33, 34, 129] | |
| Copper II bisglycinate (IA) | [35] | |
| Lipopolysaccharide (IA) | [130] | |
| Chondromucoprotein (IA) - reported to be antigenic and caused arthritis to develop but this study was performed prior to knowledge of the spontaneous development of OA in this animal | [131] | |
| Chymotrypsin (IA) ex situ | [46] | |
| Impact Models | Impact Loading | [132] |
| Other | Intravenous injection of mycobacteria | [32] |
| Intraplantar injection of adjuvant (heat killed mycobacterium tuberculosis) | [55] | |
| Cationic amidated bovine serum albumin (IA) (pI approximately 9.2) into the knee joint of immunized guinea pigs | [53] | |
| Lymphokine (IA) | [52] | |
| Genetically Induced | None |
IA=intra-articular injection
The majority of studies of OA in the guinea pig have utilized the outbred albino Dunkin-Hartley guinea pig, although a few other strains of guinea pig have been evaluated and display similar susceptibility to OA, such as the English Short Hair (Kraus and Huebner, unpublished observation), or lesser susceptibility to knee OA, including Strain 13 [6], Bristol Strain 2 [7], and the GOHI strain [8]. The evaluation of OA has been performed primarily in male animals, mainly because of a more rapid weight gain that results in more consistent pathological alterations [9]. However, spontaneous lesions have also been observed in female animals [4], and it has been reported that the progression of knee OA can be accelerated with ovariectomy [10, 11]. The attraction of the guinea pig as an OA model system is its histopathological similarity to human disease. The appearance of joint pathology in guinea pig and humans is both age-related and subject to a variety of well-recognized OA risk factors shared in common with humans including body weight [12], mechanical load [13], and high bone turnover [6]. Spontaneous lesions in the knee are bilaterally symmetric, and appear first, and are more pronounced, in the medial knee compartment in the area not covered by the meniscus. This corresponds to the pattern of localization of idiopathic, non-traumatic knee OA in humans. Femoral lesions lag behind the appearance of tibial lesions.
In addition to the histological similarities of OA between the guinea pig and humans, a variety of OA-related biomarkers, used to evaluate human OA, are detectable in guinea pig cartilage or body fluids [14]. The guinea pig is also amenable to evaluation by a variety of methods for assessing disease status including magnetic resonance imaging [15–22], micro-computed tomography [23–31], radiography [7, 32–39], and dual x-ray absorptiometry to quantify bone density [28, 40–43]. Joints are also large enough to enable cartilage harvest for molecular analyses and to accommodate cartilage and bone biomechanical analyses that can provide valuable quantitative outcomes to complement histological assessments [30, 44–47]. Due to the fact that guinea pigs are relatively sedentary, it may also be possible to simultaneously evaluate the effects of a therapeutic agent on both spontaneous knee OA and surgically induced OA in the guinea pig because unilateral knee meniscectomy does not appear to alter the course of spontaneous knee OA in the contralateral limb (Bendele, unpublished observation). Finally, much is known about the general care and manipulation of this animal [48], which facilitates the ease with which OA studies can be conducted. This review focuses on the methods of assessment (macroscopic, microscopic, and ultrastructural) of OA in this animal model system and recommends particular methods of assessment to assist standardization in the conduct and reporting of clinical trails in the guinea pig model of OA.
I. Anatomy and Joint Pathology
Anatomy
Although classified in the order Rodentia, the guinea pig has a phylogenetic origin closer to lagomorphs than rodents [49]. The guinea pig knee, like the human knee, is a diarthrodial joint composed of cartilage, bone, synovium, and supporting structures such as ligaments. The age-related pathological process known as OA affects all of these tissue components. Bone growth in the guinea pig ceases by 4 months of age, evinced by stable tibial condyle widths after 3 months of age [16]. However, skeletal maturity of the limb, defined by growth plate fusion, does not occur until between 7 months (femur) and 12–18 months (tibia) of age [50]. The structures of the guinea pig knee (at age 12 months) are shown in Figure 1.
Figure 1. Guinea pig knee joint structures.
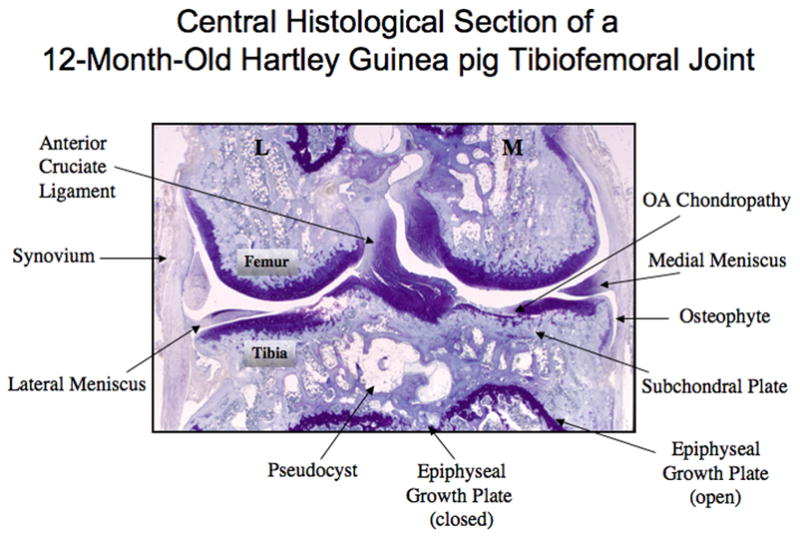
A frontal section through a 12-month-old guinea pig knee joint to demonstrate its structures and the landmarks and features typical of advanced OA at the center of the tibiofemoral joint (M=medial; L=lateral).
Pathology
The preferential occurrence of spontaneous OA in the medial compartment of the guinea pig knee is due to the fact that the medial aspect of the knee is loaded predominantly in the guinea pig as in humans [3]. Cartilage degeneration is characterized by the breakdown of type II collagen and the loss of proteoglycan. Changes in bone associated with the development of OA include subchondral bone thickening, osteophyte formation, and alterations in trabecular bone. The OA-related changes in cartilage and bone are evident microscopically after 2 months with a steady age-related worsening in joint pathology by histological appearance (Figure 2A), apparent upon quantification of histological changes using the histochemical-histological scoring system proposed herein (Figure 2B). Even earlier pathological changes are apparent at 2 months of age in the form of collagen fibril disruption detected by polarized light microscopy (imaging sections stained with picrosirius red)[51]. The synovium has been characterized in only a few studies in the guinea pig [12, 33, 34, 52–57] but should not be overlooked. Like other rodents, guinea pigs are prone to meniscal calcification [25, 58]. The calcification of the menisci is due to basic calcium phosphate crystal deposition that becomes apparent at 2-months of age. This calcification is theorized to contribute to OA by altering joint loading and producing a pro-inflammatory stimulus upon shedding of crystals shed into the joint [58].
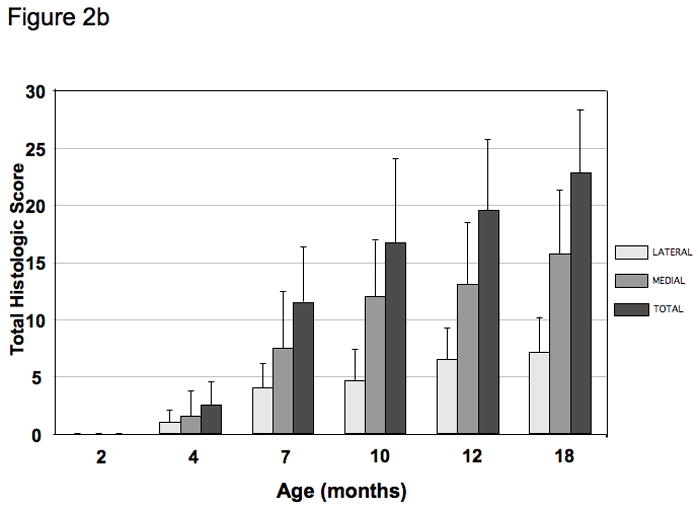
Figure 2. The timecourse of spontaneous histological OA in the Hartley guinea pig knee.
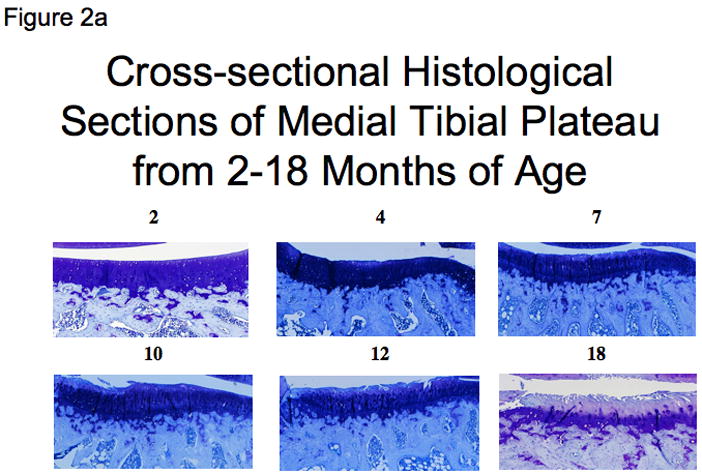
a) Representative sections of the central medial tibial plateau stained with toluidine blue for each age group (ages 2 to 18 months). Scores of cartilage structure (CS) and proteoglycan content (PG) using the recommended scoring system for the regions shown is as follows: 2 months CS=0/PG=0; 4 months CS=2/PG=1; 7 months CS=5/PG=4; 10 months CS=5/PG=5; 12 months CS=8/PG=5; and 18 months CS=8/PG=6.
b) Mean histological scores of the knee demonstrating increasing OA severity with age in this model system (N=6 for each group ages 2–12 months and N=10 for 18 month age group). Error bars = SD.
II. Scoring of Alterations in Joint Structures
II. a. Macroscopic scoring (Staging of the disease process)
Macroscopic images of guinea pig knees are shown in Figures 3A and 3B, demonstrating the overall joint dimensions (1 × 1 cm) and varying severities of lesions. Macroscopic scoring, both with [58–60] and without [61, 62] Indian ink staining has been utilized in the guinea pig. The use of Indian ink on the cartilage surface is a method of quantifying depth of cartilage loss in the macroscopic scoring process. The use of Indian ink staining has also been advocated prior to tissue fixation and sectioning as an aid to histological interpretation; Indian ink particles enter fissures that communicate with the synovial space and do not appear subsequently in artifactual irregularities created during the fixation and sectioning process that occur after the Indian ink staining [63]. The results of scoring of Indian ink stained sections have been expressed semi-quantitatively as the percentage of surface with overt superficial fissuring or fibrillation and the percentage of surface with bone exposure.
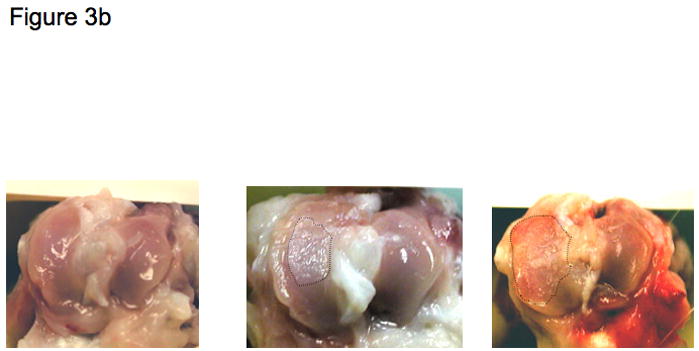
Figure 3. Macroscopic images of the guinea pig knee joint.
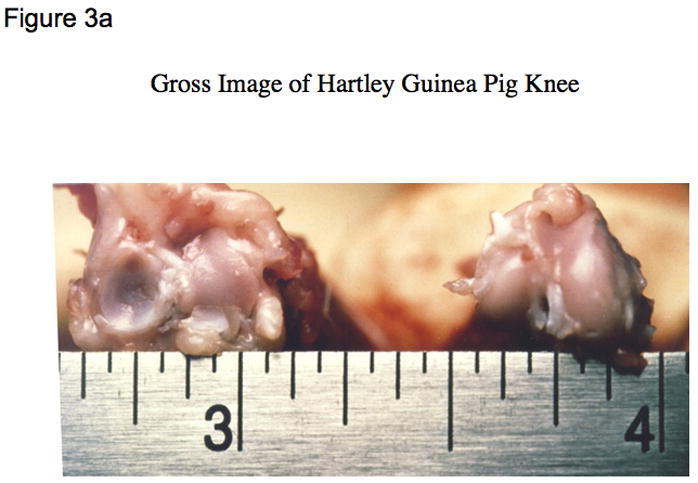
a) Guinea pig knee joint demonstrating normal dimensions of approximately 1 cm x 1 cm (width x depth);
b) Arial views of the tibial plateau oriented (medial on left, lateral on right) with macroscopic lesions of increasing severity from no lesions (left panel), to moderate surface fissuring (middle panel), and severe full depth erosion to bone (right panel); the diseased areas in the middle and right panels are indicated by the dotted demarcated region.
Indian ink staining method
As published by Richardson et al [59] and used subsequently by Tessier et al [18], the cartilage surface is painted, for 15 seconds, with a 20% (v/v) dilution of blue Indian ink (Parker, Quink) in phosphate buffered saline containing protease inhibitors. Others have successfully used 10% (v/v) dilution of Indian ink without protease inhibitors (J DeGroot unpublished). Any excess is blotted off with a moist cotton swab. For frozen joints, the specimens are thawed by immersion in phosphate buffered saline, pH 7.4 for 20 minutes prior to the application of Indian ink.
Semi-quantitative scoring methods
The severity of histological OA can be assessed semi-quantitatively or quantitatively with the aid of digital images of the cartilage surfaces and image analysis software [59, 60], expressing the damaged area (that took up stain) as a percentage of the surface area of cartilage. The Outerbridge semi-quantitative scoring scheme [64], or a variation thereof, has been a mainstay of macroscopic scoring for many years for human and animal studies. The intra-observer and inter-observer kappa coefficients were reported to be 0.80 and 0.52 when cartilage damage was graded arthroscopically in humans with a mean inter-observer kappa coefficient of 0.72 between two physicians in practice 5 years or more, thus demonstrating good reliability for trained graders [65].
For guinea pig studies, we recommend a variation of the Outerbridge classification that has been used in rabbit experimental OA [66] and is amenable to guinea pig studies as summarized in Table 2.
Table 2.
Semiquantitative macroscopic scoring of cartilage.
| Parameter | Parameter (per OARSI Atlas recommended nomenclature) | Grade | Original Outerbridge Description [64] | Recommended method: Description by Pelletier [66] |
|---|---|---|---|---|
| Normal | Normal | 0 | Normal appearance | Normal appearing surface |
| Fibrillation | Fissures-into superficial zone | 1 | Softening and swelling | Minimal fibrillation (fraying, fissuring or flaking) or a slight yellowish discoloration of the surface |
| Erosion-Superficial or Middle | Erosion-into superficial or middle zones | 2 | Fragmentation and fissuring involving an area of less than half an inch (~1.25 cm) in diameter | Erosion extending into superficial or middle layers |
| Erosion-Deep | Erosion-into deep zone | 3 | Fragmentation and fissuring in an area greater than half an inch (~1.25 cm) in diameter | Erosion extending into the deep layers |
| Bone Exposure | Erosion-full depth | 4 | Erosion of cartilage down to bone | Erosion extending to the subchondral bone |
| Lesion Area* | Estimated surface or measured area (mm2) | |||
| Total Joint Score | Sum of (grade x lesion area) for all involved and scored areas |
The scoring of lesion depth (grade 0–4) is performed with the aid of a dissecting microscope. The scoring of lesion area is performed with the aid of calipers (or image analysis software on digital images).
II. b. Microscopic scoring of cartilage alterations (Grading of cartilage degeneration)
Methods of fixation, embedding, and decalcification
An extensive review of the literature has revealed that the most frequently used method of preparing guinea pig joints for histological evaluation of OA is fixation in 10% buffered formalin, decalcification in either a solution of 10% EDTA (in 0.1 M phosphate buffer, pH 7–8 for approximately 8–10 weeks, solution changed once a week) or 5% formic acid (diluted in water, for approximately 1 week), and embedding in paraffin. Approximately halfway through decalcification, the process can be hastened by transecting the joints in half coronally with a sharp razor blade (using the tibial collateral ligament as a guide), then returning the joint halves to the decalcification solution. While EDTA decalcification is optimal for proteoglycan preservation and highly recommended for sections destined for in situ hybridization [67], formic acid decalcification has the advantage of being rapid. Decalcification is adequate when an aliquot of the decalcification solution no longer produces a calcium precipitate upon addition of ammonium oxalate using the method of Rosen [68]. Briefly, remove a 0.5 ml aliquot of the EDTA decalcification solution and add 10 ml of a citrate-phosphate buffer (0.2 M citric acid and 0.16 M dibasic potassium phosphate, pH 3.2–3.6 achieved with NaOH), and 2.5 ml of saturated ammonium oxalate (5% solution) and vortex. The failure to form a cloudy white precipitate over the course of 20 minutes on two successive days of testing indicates decalcification is complete. When decalcification is complete, the whole joint is embedded in paraffin and sectioned (usually front to back), or the two halves of the joint are embedded (in paraffin) to facilitate production of central sections.
For simple histological scoring we recommend the use of formic acid decalcification for its ease and time efficiency.
Methods of sectioning
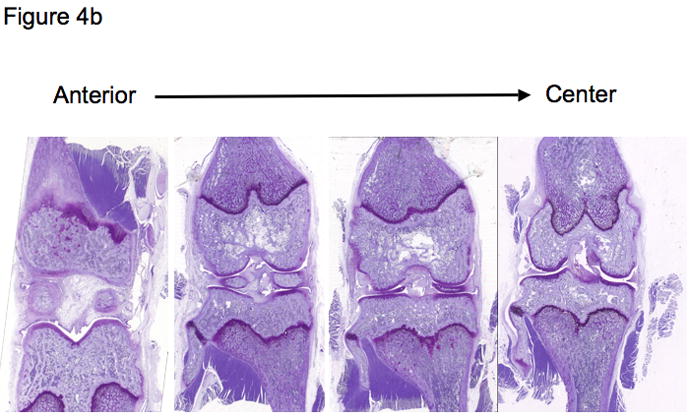
Although some studies have used sagittal sections through the knee joint in order to separate the medial and lateral compartments, frontal (coronal) sections are feasible in a joint of this size and optimal for assessing all surfaces of the femorotibial joint simultaneously and in their correct anatomic arrangement. When using a spontaneous model of OA, it is important to grade histological damage in the central section of the joint to assess the site of maximal pathology and to compare animals in a consistent manner. This can be accomplished by observing anatomical landmarks characteristic of a central section, such as the anterior cruciate ligament insertion site as well as the medial and lateral menisci (see Figures 4A and 4B). If OA is induced surgically, it may be necessary to verify the lesion location by macroscopic evaluation in order to direct microscopic scoring to the appropriate region, or to section and histologically examine the entire joint. Paraffin embedded sections are cut at 5–8 μm on a microtome along the coronal plane of limbs fixed in a slightly flexed position as described by Cashin [35] and shown in Figure 5. A clever alternate method utilized for studies involving bone histomorphometry calls for the preparation of thick (300 μm) methylmethacrylate sections for histomorphometry with subsequent use of these thick sections to generate thin (5 μm) sections for histology [69].
Figure 4. Landmarks of the central region of the guinea pig tibiofemoral joint.
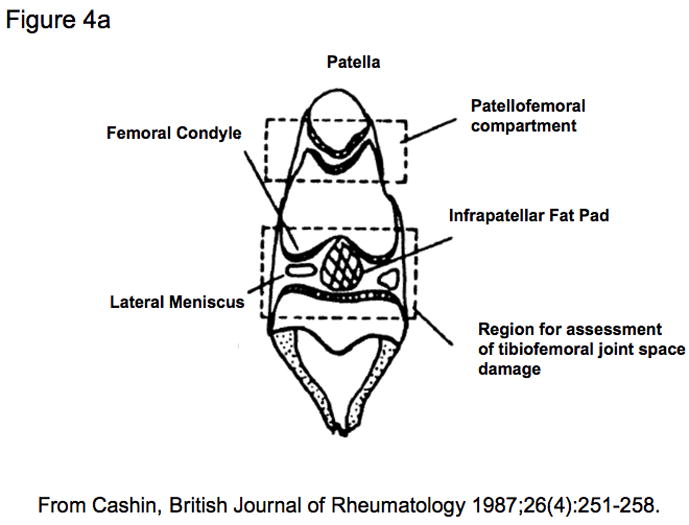
a) A schematic representation of a centrally sectioned guinea pig knee joint based upon Cashin [35] and reproduced by permission of Oxford University Press;
b) A series of 4 sections taken at 200 μm intervals in the coronal plane, spanning the anterior of the joint through to the center. Note the elongated appearance of the lateral meniscus (on left) and the more rounded appearance of the medial meniscus (on right) when the center of the knee joint is attained.
Figure 5. Plane of sectioning of the guinea pig knee joint.
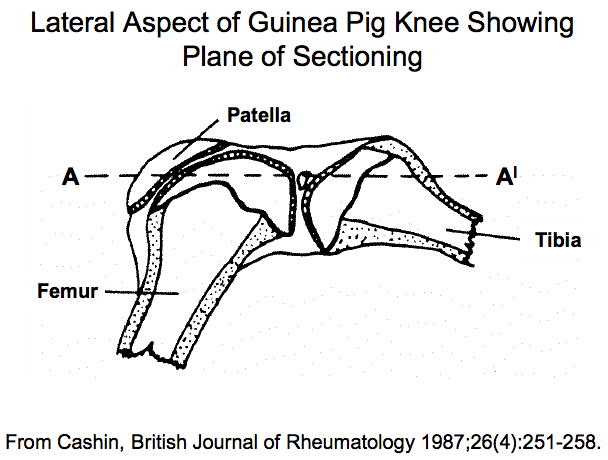
The lateral aspect of the guinea pig knee showing the plane of sectioning (A-A′) based on Cashin [35] and reproduced by permission of Oxford University Press. Note that the knee is in slight flexion.
For simple histological scoring we recommend 5–8 μm paraffin sections be used.
Methods of staining
Both Safranin O and Toluidine blue are cationic dyes that stain proteoglycans as well as glycosaminoglycans [Toluidine Blue (0.04%) in 0.1 M sodium acetate buffer, pH4; Safranin-O (0.04%) in 0.1 M sodium acetate buffer, pH4]. Toluidine blue has been reported to provide more intense staining, due to the fact that it has a higher affinity for the sulfur in cartilage compared to Safranin O [70]. Although Safranin O staining is proportional to proteoglycan content in normal cartilage, it has been reported that it is not a sensitive indicator of proteoglycan content in cartilage in which glycosaminoglycans have been depleted [70].
For simple histological scoring of proteoglycan content, we recommend staining with Toluidine Blue
Standardization procedures for semi-quantitative scoring
For assessment of spontaneous OA in the guinea pig, it is sufficient to score the site of maximal OA at the joint center, encompassing the region not covered by the meniscus (Figures 1, 4A and 4B). However, as noted above, assessing the maximal extent of surgically induced OA may require a survey of the entire joint due to localization of the lesions in other than the central location. For spontaneous OA, several studies have relied on scoring a single central section. Brismar et al defined the central portion of the condyle, corresponding to an area of 3–4 mm2, as the middle of 5 sections (taken at intervals of 250 μm) without cruciate ligament remnants [71]. Hyttinen et al [72] adopted a sectioning and scoring system previously used in mice [73] that relied on the mean of the grades of three sections: the first was from the level of insertion of the anterior cruciate ligament, the second 200 μm distant, and the third 400 μm posterior to the first one, thereby grading an area that approximated the central third of the tibial condyles. For the evaluation of guinea pig OA, the relative merits are currently unknown of grading a single central section versus several sections encompassing the middle third of the joint.
Grading should be performed blinded to the age and intervention, and the number of graders reported. Grading of the central section can be performed by two blinded observers and averaged [14]; alternatively, 3–4 central sections can be graded by a single observer and the scores averaged [3, 74]. Scores should be tabulated for the various quadrants or condyles separately (medial tibia, medial femur, lateral tibia, and lateral femur) to capture data for site-specific changes, since the disease manifests to varying degrees at these different sites. These scores can be used to calculate compartmental (medial and lateral) and total joint scores.
For simple histological scoring of spontaneous OA in the guinea pig, we recommend two blinded observers, and scoring of one intact central section. For scoring of surgically induced OA, step through sections encompassing the entire joint are required to insure accurate lesion localization.
Semi-quantitative scoring systems
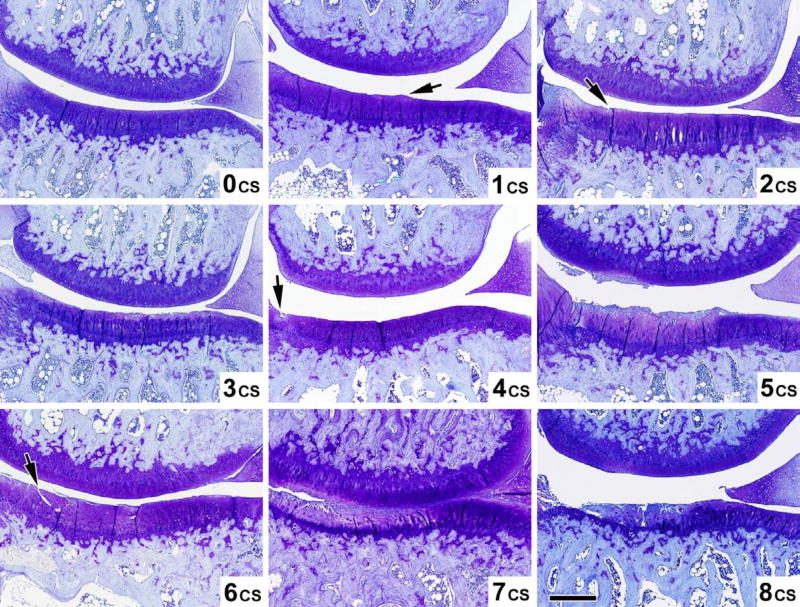
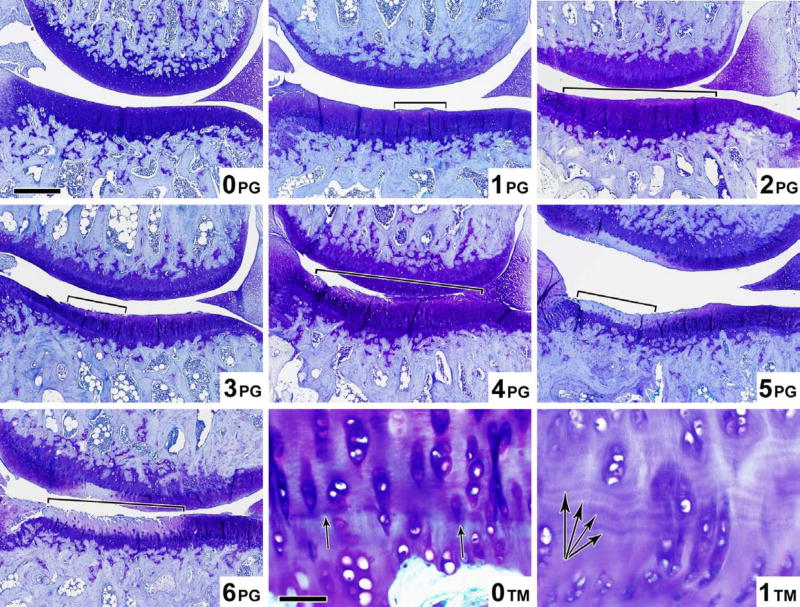
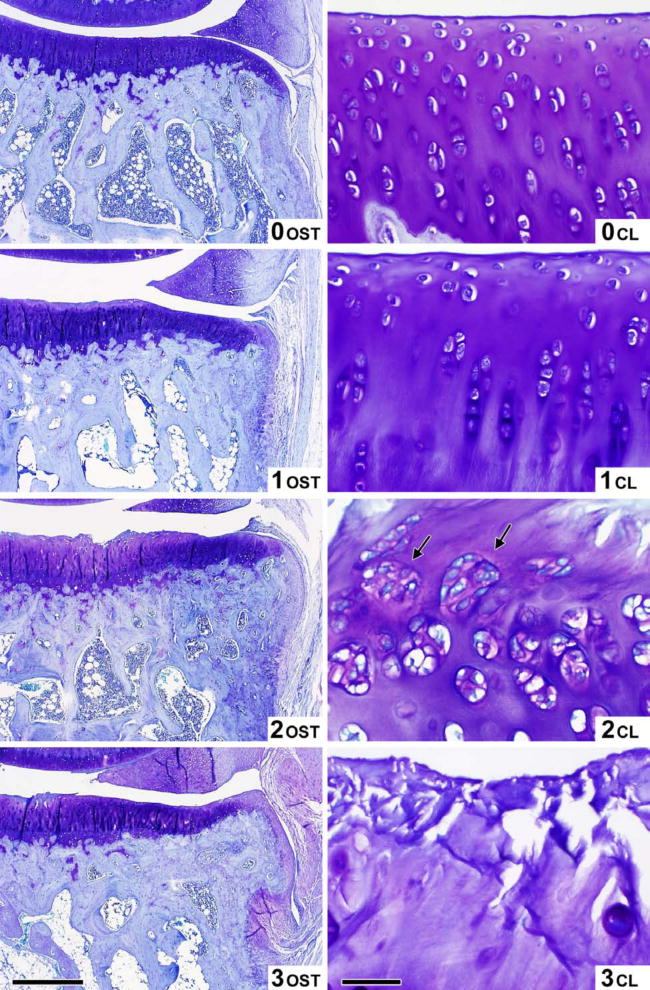
Five distinct semi-quantitative scoring schemes have been employed in guinea pig OA studies (summarized in Tables 3 and 4 for ease of comparison). The majority of studies performed in the guinea pig to date use a scoring scheme based on one first introduced by Mankin in 1971 [75] or some adaptation of this (Table 4) made to better conform to the features of OA in the guinea pig [6, 7, 14, 19, 20, 26, 27, 31, 36, 38, 43, 45, 60, 61, 69, 76–82]. The scoring scheme in Table 4 was an adaptation developed by Carlson [83] for studies in primates, but has been used successfully by numerous investigators to evaluate guinea pig knee OA [69, 82, 84]. This scoring scheme permits site-specific and separate scoring of cartilage structure and proteoglycan content. Accurate scoring requires the appropriate identification of the zones of articular cartilage as depicted in Figure 6. Variations in the feature of tidemark integrity are not very prominent in spontaneous OA. Recently, Mapp, et. al. characterized osteochondral and synovial angiogenesis in the spontaneous model of OA and concluded that guinea pigs display low vascularity throughout the development of the disease [57] however, this feature may be of importance for surgically induced OA, as described in the rat meniscal transection model [57], and for drug intervention trials. Therefore, scoring of tidemark duplication and penetration by vasculature can be considered optional components of the scoring method, dependent on the range of variation in these parameters observed with the particular intervention. Similarly, the cellularity feature of the original Mankin scoring system can be assessed (Figure 7c), however, differentiation of scores 0 and 1 can be difficult in the guinea pig model due to the high density of chondrocytes present in the articular cartilage. In addition, osteophyte and synovitis grading, as well as bone histomorphometry, as described below, can complement the scoring scheme to provide a comprehensive assessment of the whole joint organ. This approach is an attractive one for more sensitively evaluating target-specific effects of drugs in a clinical trial setting. Figure 7a-c demonstrates the entire range of lesions of various grades for all aspects of the recommended scoring scheme as described in Table 4, with mean histological scores shown for each image based on an assessment by two independent experienced graders.
Table 3.
Semiquantitative methods of histological assessment of knee OA in the guinea pig.
| Author (Reference) | Description |
|---|---|
| Modified Mankin [75] with modifications proposed by Carlson [83], Fini [69], and Kobayashi [61] | See Table 4 |
| Modified Colombo method [119] developed by Colombo [133] and modified by Kikuchi [134] | Range 0–24: loss of superficial layer 0–4, erosion of cartilage 0–4, fibrillation and/or fissures 0–4, loss of stainable proteoglycan 0–4, disorganization of chondrocytes 0–4, loss of chondrocytes 0–4 |
| Modified Williams method developed by Williams et al [87] and modified and applied to guinea pig knee OA by Han et al [56] | Range 0–27: articular surface fibrillation 0–4; cellularity 1–4; Safranin-O staining 1–5; osteophyte formation 0–5; brood clusters 1–5; synovial membrane 0–4). A score ≤ 6 for any one surface (medial femoral condyle, medial tibial plateau, distal tibia, and talus) was considered normal. |
| Lapvetelainen [73] method for mice adopted for guinea pig by Hyttinen et al [72] | Range 0–16 for all 4 condyles combined: cartilage intact and articular surfaces smooth = grade 0; superficial fibrillation of the cartilage = grade 1; lesion extends into the deep zone of uncalcified cartilage = grade 2; lesion surpasses the tidemark and affects the calcified cartilage, in the superficial zone a loss of cells and in the deep zone formation of clones observed = grade 3; no cartilage tissue left between the two adjacent bone ends = grade 4. |
| Bendele [54] | Cartilage alterations ranging from none, to minimal, focal, moderate, marked or severe scored 0–5; this score was multiplied by the depth of the lesion scored on the basis of involving one-third, two-thirds, or all of the surface score 1–3; osteophytes small, medium or large scored 1–3; synovial membrane proliferation minimal, focal, moderate or marked scored 1–4. |
Table 4.
Recommended semiquantitative histochemical-histologic scoring scheme for knee joint evaluation in guinea pigs.
| Parameter | Grade | Description |
|---|---|---|
| Articular Cartilage Structure | 0 | Normal, smooth, uninterrupted surface |
| 1 | Mild surface irregularities (undulations) | |
| 2 | Irregular surface, 1–3 superficial clefts (fissures) | |
| 3 | >3 fissures and/or loss of cartilage in the superficial zone | |
| 4 | 1–3 fissures extending into the middle zone | |
| 5 | >3 fissures and/or loss of cartilage extending into the middle zone | |
| 6 | 1–3 fissures extending into the deep zone | |
| 7 | >3 fissures extending into the deep zone and/or loss of cartilage to deep zone | |
| 8 | Fissures or loss of cartilage extending to the zone of calcified cartilage | |
| Proteoglycan Content (staining by toluidine blue) | 0 | Uniform throughout articular cartilage |
| 1 | Decreased in superficial zone only and for <half the length of the condyle or plateau | |
| 2 | Decreased in superficial zone for half the length or greater of the condyle or plateau | |
| 3 | Decreased in superficial and middle zones for <half the length of the condyle or plateau | |
| 4 | Decreased in superficial and middle zones for half the length or greater of the condyle or plateau | |
| 5 | Decreased in all 3 zones for <half the length of the condyle or plateau | |
| 6 | Decreased in all 3 zones for half the length or greater of the condyle or plateau | |
| Cellularity | 0 | Normal (1/2 cells/lacuna) |
| 1 | Diffuse/slight hypercellularity | |
| 2 | Regions of hypercellularity and clustering | |
| 3 | Diffuse hypocellularity | |
| Tidemark Integrity | 0 | Intact/single tidemark |
| 1 | Crossed by vessels/duplication of tidemark | |
| Additional Features* | ||
| Osteophyte | 0 | No osteophyte present |
| 1 | Small osteophyte | |
| 2 | Medium-sized osteophyte | |
| 3 | Large osteophyte | |
| Osteophytes are tallied for three joint margins (medial and lateral tibial plateau, and lateral femoral condyle) | ||
| Total score 0–9 | ||
Examples of varying severity for each of the parameters above are shown in Figures 7a-c.
Scoring system modified from one originally introduce by Mankin [75] whose original scoring system ranged from 0–14 (for structure 0–6, cells 0–3, safranin O staining 0–4, and tidemark integrity 0–1; where 0 has normal cartilage and >10 has severe cartilage degeneration). The semiquantitative histological scoring scheme in this Table was developed by Carlson [83] for studies in primates but has been used successfully by numerous investigators to evaluate guinea pig knee OA. It has been used in truncated form, assessing cartilage structure and proteoglycan content only [84], and in full form, assessing cellularity and tidemark integrity in addition to structure and proteoglycan content [69]. Scale: range 0–18 (for structure 0–8, proteoglycan content 0–6, cellularity 0–3, tidemark integrity 0–1). A further modification has been employed by Kobayashi et al [61]: range 0–12 (for cartilage structure 0–5, proteoglycan content 0–4, cellularity 0–3). This modified scheme with osteophyte scoring has been used by Kraus et al [81]. In the original usage, fissures were referred to as clefts.
These features supplemental the scoring system modified from the one first introduced by Mankin.
Figure 6. Zones of articular cartilage.
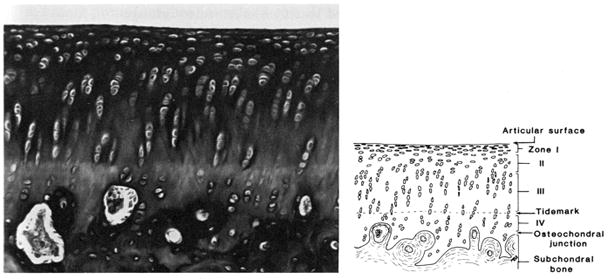
Accurate scoring requires the ability to distinguish the four zones of articular cartilage as depicted here: I-superficial zone, II-middle zone, III-deep zone, and IV-calcified cartilage zone, and the tidemark separating zones III and IV. Reproduced by permission of Dr. James Williams.
Figure 7. Representative images of guinea pig knees (aged 2–18 months) demonstrating varying severities of histological lesions.
Examples of lesions scored by the recommended histochemical-histological scoring scheme. The mean score generated by two experts are shown in the bottom right of each panel in Figures 7a-c.
Figure 7a. Cartilage structure (CS) scoring atlas. Microscopic toluidine blue stained images of the guinea pig knee joint demonstrating the 9 levels (scores 0–8) of cartilage structure (CS) corresponding to the criteria in Table 4. Magnification bar 500 μm.
Figure 7b. Proteoglycan content (PG) and Tidemark™ scoring atlas. Microscopic toluidine blue stained images of the guinea pig knee joint demonstrating the 7 levels (scores 0–6) of proteoglycan content (PG) (Magnification bar = 500 μm), and the 2 levels (scores 0–1) of Tidemark ™ integrity (Magnification bar = 50 μm) corresponding to the criteria in Table 4.
Figure 7c. Osteophyte (OST) and Cellularity (CL) scoring atlas. Microscopic toluidine blue stained images of the guinea pig knee joint demonstrating the 4 levels (scores 0–3) of osteophyte (OST) (Magnification bar = 250 μm), and the 4 levels (scores 0–3) of Cellularity (CL) (Magnification bar = 50 μm) corresponding to the criteria in Table 4.
For semiquantitative scoring of histological OA in the guinea pig, we recommend the histochemical-histological scoring system summarized in Table 4 with grading of cartilage structure (Figure 7a) and proteoglycan content (Figure 7b). Grading of tidemark for duplication or vascular penetration (Figure 7b), cellularity (Figure 7c), and osteophyte (Figure 7c), are optional features and are recommended for inclusion when the intervention warrants it on the basis of potential for change in these parameters with the intervention.
Quantitative scoring systems
Cartilage histomorphometry has been used to quantitatively assess OA in the guinea pig [69, 74]. Measured parameters have included chondrocyte density [74], cartilage thickness, cartilage surface fibrillation index [74, 85], cartilage proteoglycan content [34, 86], and cartilage volume [85] (Table 5).
Table 5.
Histomorphometry of cartilage and bone.
| Parameter | Description | References |
|---|---|---|
| Cartilage | ||
| Cartilage thickness | μm at magnification 20x although measurements at 80x more readily yielded significant differences in the study by Pastoureau et al, 2003 | [69, 74, 91, 119] |
| Average cartilage thickness | Ratio of cartilage volume divided by the projection of the corresponding surface area | [71] |
| Cartilage surface fibrillation index | Ratio of the length of the cartilage surface border divided by the length of a standardized measured area x 100 (%) at a magnification of 80x | [39, 69, 74, 85, 91, 101] |
| Cartilage volume | Point counting of hematoxylin and eosin stained paraffin section at a magnification of x50 with a conventional grid | [85] |
| Height of calcified cartilage | Measured on 6–8 non-decalcified plastic-embedded step sections of the central compartment stained with toluidine blue | [101] |
| Proteoglycan content ratio | Ratio of superficial zone to deep zone intensity (optical density of greyscale image) of safranin O staining in a standardized measured area x 100 (%) at a magnification of 80x | [74] |
| Proteoglycan content by Critical Electrolyte Method (CEC) | Stain sections with alcian blue 8 GS in the presence of increasing molar concentrations of MgCl2 (0, 0.10, 0.15. 0.25, 0.35, 0.45, 0.55, 0.65, 0.75, 0.85, and 1.00), which compete with the dye solution (0.05 % alcian blue in 0.025 M acetate buffer, pH 5.6) for anionic sites until all dye is displaced from the substrate. The value of the CEC is the mean of the two concentrations of MgCl2 between which the alcian blue staining disappears. | [34, 86] |
| Chondrocyte density | Manual count of cells divided by the area of the standardized region at a magnification of 200x | [74] |
| Subchondral Bone Plate | ||
| Plate volume fraction | Bone volume per total plate volume (%) per Ding; bone volume in standardized region in the upper half of the epiphysis per Pastoureau | [26, 27, 74, 92] |
| Plate thickness | (μm); measured from cartilage bone interface to the top of the epiphyseal marrow space--mean of 10 measurements perpendicular to the articular surface per Huebner et al; thickness (μm) in a standardized region in the upper half of the epiphysis per Pastoureau | [6, 26, 27, 30, 69, 74, 91] based on definition of Carlson [83] |
| Plate surface density | Plate surface to total plate volume (mm−1) | [26, 27] |
| Plate perforation | Mean pore size (μm) | [26, 27] |
| Surface density of capillaries | mm2/mm3 subchondral bone | [101] |
| Subchondral Cancellous Bone* | ||
| Bone volume fraction | Bone voxel per total specimen voxel (%) | [26, 27, 30, 69, 71, 91] |
| Trabecular thickness | Measured in μm | [26, 27, 30, 69, 91] |
| Structure model index | How plate-like, rod-like or combination the structure is | [26, 27, 30] |
| Architectural anisotropy | Ratio of the eigenvalue of the primary direction divided by the eigenvalue of the tertiary direction | [26, 27, 30] |
| Connectivity density | Number of multiple connected trabeculae per volume (mm−3) | [26, 27, 30] |
| Bone surface density | Bone surface area per total volume of specimen (mm−1) | [26, 27, 30, 31] |
| 3-D trabecular spacing or separation | (μm), at a magnification of 50x | [26, 27, 30, 31, 69, 91] |
| Trabecular number | Number per mm | [69, 91] |
| Fractal dimensionality | Trabecular texture analysis | [31] |
| Subchondral Cortical Bone | ||
| Bone volume fraction | Bone volume per total specimen volume (%) | [26, 27] |
| Cortical thickness | (μm) | [26, 27, 30, 31] |
| Bone surface density | Bone surface area per total volume of specimen (mm−1) | [26, 27] |
| Cortical bone porosity | Cavity/perforation (%) | [26, 27] |
| Cross-sectional area of cortex | Mean value obtained from subchondral cortical bone (mm2) | [26, 27, 30] |
Quantitative histological assessment is not required for routine assessment of OA severity, however these methods can provide complementary information tailored to the needs of the investigation.
II. c. Microscopic scoring of synovial alterations (Grading of synoviopathy)
Representative images of the guinea pig knee are shown in Figure 8 demonstrating varying severities of synovitis. Three methods could be found, used in six published studies, that graded synovitis semi-quantitatively as a separate entity from cartilage and bone alterations in the guinea pig joint [12, 33, 34, 54, 56, 57] (summarized in Table 6). The method of Williams et al [87], developed in dog and used for a guinea pig study by Han et al [56] is a simple 5 level (0–4) grading scheme based on synovial hyperplasia. In the study by Han et al [56], synovial hyperplasia was reported in only 3 of 8 animals with spontaneous OA, and in the study by Mapp, et al [57] in only 3 of 24 animals. Therefore, this histological parameter is likely to be of greater importance in surgical models of OA than in spontaneous OA. In addition, a more detailed method, published by Pelletier et al [88] for canine studies, although not yet applied to guinea pig, is preferred for clinical trial testing of a drug with an anti-inflammatory mechanism of action because the scale is broader and more detailed, potentially providing a means of discerning small changes in this parameter with treatment.
Figure 8. Examples of synovitis associated with spontaneous OA in the Hartley guinea pig knee joint.
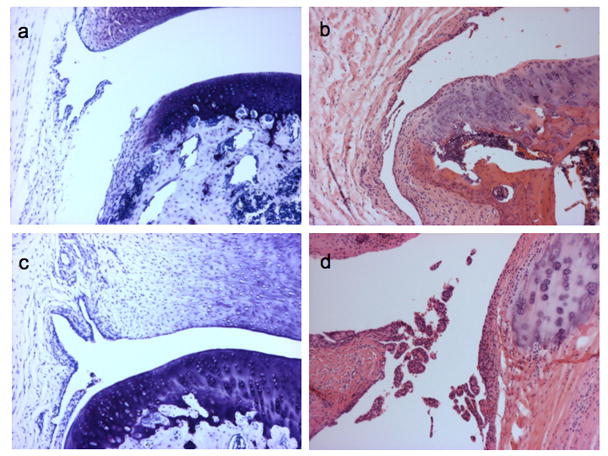
Representative images (100x) of synovitis scored according to the recommended scoring system in Table 6. Sections stained with toluidine blue (a and c) or Hematoxylin and Eosin (b and d). Normal synovium from a 60 day old animal (a); Synovitis scores of 3 in a 145 day old animal (b) and a 180 day old animal (c) based on scoring synovial hyperplasia=1 (3–5 layers), villous hyperplasia=1, and scattered infiltrate=1. Synovitis score of 7 in a 2 year old animal (d) (100x) based on scoring synovial hyperplasia=2 (≥6 layers), villous hyperplasia=3, and infiltrate=2.
Table 6.
Semiquantitative methods of grading synovitis in the guinea pig.
| Parameter | Scoring System | Comments | References |
|---|---|---|---|
| Recommended Scoring System | |||
| Severity of Synovitis | Range 0–10: synovial lining cell hyperplasia 0 = 1–2 layers of cells 1 = 3–5 layers of cells 2 = ≥ 6 layers of cells; villous hyperplasia 0 = not present 1 = few, scattered and short 2 = marked (finger-like projections) 3 = marked and diffuse; degree of cellular infiltration by perivascular lymphocytes and mononuclear cells 0 = normal 5 = marked cellular infiltration mixed with lymphoid follicles). |
Based on rabbit joints and the Pond Nuki dog model of OA. Grading performed on hematoxylin and eosin stained paraffin (5 μm) sections. (5 μm) sections. Two synovial membrane specimens were examined for each compartment. The highest score from each compartment was recorded and the average score for the two compartments considered the whole knee score. | Pelletier [66, 88]; And Mapp [57] |
| Other Scoring Systems | |||
| Cellularity Score | Range 0–4: 1 = solitary 2 = small number 3 = moderate number 4 = large number |
Grading performed on frozen sections of 3×3 mm specimens of synovial membrane and the highest grade in any section of the synovial membrane was recorded for the joint. | Kopp [33]; and Mejersjo [34] |
| Synovial Membrane Hyperplasia | Range 0–4: 0 = Decrease in the number of lining cells 1 = normal 2 = slight increase in number of layers (4–6) 3 = > 6 layers of lining cells 4 = Increase of lining cells with inflammatory cells present |
Developed in dog and used in the guinea pig by Han, 2002. Grading performed on hematoxylin and eosin stained paraffin (8 μm) sections. Scoring done separately for medial femoral condylar and medial tibial plateau compartments. | Williams [87]; and Han [56] |
| Severity of Proliferative Changes | Range 0–4: 1 = minimal 2 = mild 3 = moderate 4 = marked |
Grading performed on hematoxylin and eosin stained paraffin (8 μm) sections. | Bendele [12, 54] |
For synovitis assessment we recommend the more detailed scoring system of Pelletier [66, 88], summarized in Table 6, for its comprehensiveness, need for only haematoxylin and eosin staining, and cross-species utility demonstrated by its previous use in canine [88], rabbit [66], and human [89] studies.
II. d. Microscopic scoring of bone alterations
As summarized in Table 5, there has been a wealth of bone histomorphometric measurements made in the guinea pig model of OA. Bone histomorphometry has been performed with the aid of micro-computerized tomography (micro-CT) on guinea pig knees or on polymethylmethacrylate embedded thick (300 μm thick) sections. The American Society of Bone and Mineral Research devised a unified system of terminology for bone histomorphometry [90], intended to standardize reporting of bone histomorphometric data. This document serves as a source of standardized terms and abbreviations and a universally applicable method for reporting all data. Several bone histomorphometric studies in the guinea pig have published results in conformity with this nomenclature [26, 27, 30, 31, 69, 91] (Table 5). Methods for cartilage and bone histomorphometry have been published by Pastoureau et al [74, 92] including analyses utilizing an automated computerized system. They demonstrate the feasibility of this more quantitative method for histological characterization of OA in the guinea pig model. Currently, there is no agreed upon core set of elements that is most indicative of disease severity and progression.
Quantitative histomorphological assessment is not required for routine assessment of OA severity, however these methods can provide complementary information tailored to the needs of the investigation.
II. e. Microscopic scoring of ultrastructural alterations
Changes in joint cartilage ultrastructure have also been characterized by electron microscopy by a total of eleven studies [4, 10, 56, 72, 93–99], and graded qualitatively [10]. In addition, there have been several studies in which the ultrastructural properties of type II collagen fibers have been assessed by birefringence under polarized light microscopy [51, 56, 72] demonstrating the utility of ultrastructural assessment to detect early alterations of cartilage.
II. f. Scoring of other alterations - osteophytes, menisci, ligaments, cysts
Osteophyte
These should be scored separately from cartilage structure and proteoglycan content as they reflect a separate reparative process characterized by distinct stages of chondrocyte differentiation within the osteophyte [100], and distinct from cartilage degeneration. One published sum score tabulated osteophytes for 3 joint margins: range 0–9 (medial tibial plateau 0–3, lateral tibial plateau 0–3, lateral femoral condyle 0–3) (see Table 4) [81]. Osteophyte of the medial femoral condyle was not scored because it could not be reliably distinguished from normal variation of the femoral margin. Patellar osteophytes have been indirectly quantified by measuring patellar perimeter on a lateral radiograph [7]. Another method of scoring osteophytes relied on a range of 0–3 based on the μm measurement from the original tidemark using an ocular micrometer [9]. Finally, the growth of tibial osteophytes has been estimated by the increase in length of the medial tibial plateau in coronal sections using Bézier curve fitting [16]. This is done by measuring the distance along the length of the medial tibial plateau, from the tip of the intercondylar eminence to the osteochondral junction on the medial aspect of the joint. Alternatively, the volume of osteophytes (mm3) has been quantified [101].
For purposes of general histological scoring we recommend the scoring of osteophytes at the margins of the joint on a 0–3 scale based on the photomicrographs provided in Figure 7c.
Other anatomical features that do not currently form part of a standardized histological scoring system include scoring of meniscus, ligaments and subchondral cysts and are described below.
Meniscus
Significant ossification of the meniscus has been reported to increase in the guinea pig with age and is thought to contribute to the pathogenesis of OA by altering mechanical forces in the joint [25]. The qualitative assessment of meniscal calcification has been demonstrated with von Kossa’s specific stain for calcium [58]. In addition to meniscal ossification, it is possible to observe decreased proteoglycan content of the meniscus.
Ligaments
The central mid-portion of the posterior cruciate ligament in the Dunkin-Hartley has been shown to form a fibrocartilage matrix by the age of 3 months [97]. Proteoglycans were demonstrated by electron microscopy through fixation of the ligament in the presence of the cationic dye cuprolinic blue. It has been reported that the Dunkin Hartley guinea pig also has significant laxity of the anterior cruciate ligament and that this may predispose this strain to early onset of OA [102].
Subchondral cysts
These structures, termed pseudocysts, have been noted to be an early feature of the disease process in the guinea pig. They appear on the femoral and tibial surfaces of the knee joint in the regions of the cruciate ligament insertions [16, 18, 37]. Although the femoral pseudocyst is initially the larger, the rate of increase of the tibial pseudocyst is greater [16]. Pseudocysts are not observed at other locations within the joint [16]. The volume of cysts (mm3) in epiphyseal bone has been quantified as a parameter of OA [101].
III. Evaluation of sources of variability
III. a. Intra- and interobserver validation study
Inter-rater reliability of histological grading with the recommended histochemical-histological scoring system
A set of 20 guinea pig knee joint histological sections were graded by 12 independent observers using the recommended scoring scheme described in Table 4. The intra-class correlation coefficients (ICCs) depicting the inter-rater reliability of grading the features of the scoring system is shown in Table 7. Two graders (JH, VBK) were co-authors of this review and considered, for the purposes of this validation exercise, to be paired expert guinea pig histology graders, as they had graded sections together for many years. An additional 7 graders (“trained novices”) underwent a 30-minute training session, and then were asked to grade the same 20 sections for the three main features of cartilage structure, proteoglycan content, and osteophyte. Training consisted of a group introduction and review (in this order) of Figures 5, 4a, 1, and 6, followed by a review of the appropriate panels in Figures 7a-c. The three “untrained” experts are experienced histologists who did not undergo a group training session together. Two of these three graders however work together and their scores are shown in the “untrained” paired experts column; one of these two is an experienced joint histologist and the other a skin histopathologist who was briefly instructed on how to score the sections. The ICCs were highest for cartilage structure and proteoglycan content and highest for paired graders who have some common understanding of the grading scheme. Total novices performed surprisingly well after only a very brief training session. The inter-rater reliability of novices would likely be improved with a discussion period following the first training, followed by egarding for purposes of training. Of note, the greatest variability was found for grading of cellularity and tidemark, the features with the lowest ICC values among expert graders.
Table 7.
Inter-rater reliability of guinea pig histological grading using the recommended scoring system (Intraclass Correlation Coefficients*).
| Histological Feature | Guinea Pig Experts (n=2) | Trained Novices (n=7) | “Untrained” Experts (n=3) | “Untrained” Paired Experts (n=2) |
|---|---|---|---|---|
| Cartilage Structure | 0.95 (0.88, 0.98) | 0.79 (0.69, 0.91) | 0.59 (0.32, 0.79) | 0.92 (0.81, 0.97) |
| Proteoglycan Content | 0.91 (0.79, 0.96) | 0.74 (0.60, 0.87) | 0.49 (0.22, 0.73) | 0.90 (0.76, 0.96) |
| Osteophytes | 0.85 (0.66, 0.94) | 0.66 (0.50, 0.82) | 0.80 (0.55, 0.91) | 0.80 (0.55, 0.91) |
| Cellularity | 0.47 (0.05, 0.75) | ND | 0.59 (0.34, 0.79) | 0.82 (0.60, 0.92) |
| Tidemark | 0.63 (0.27, 0.84) | ND | 0.23 (−0.04, 0.53) | 0.24 (−0.22, 0.61) |
Values are Intraclass Correlation Coefficients (ICCs) for multiple graders of 20 histological sections each.
95% Confidence intervals for each ICC are listed below in parentheses.
ND=not determined
III. b. Variability due to animal housing
Effects of animal housing on semi-quantitative histological outcome
To evaluate potential effects of single versus multiple housing on histological severity of knee OA in the Hartley guinea pig, the histological results of four independent studies were analyzed. Two studies involved individual housing of animals (n=26), and two studies involved paired housing of animals (n=14). All animals were assessed for histological severity of knee OA at 12 months of age using the recommended histochemical-histological scoring system. Separate scores were computed for medial tibial cartilage structure and proteoglycan content, medial compartment cartilage structure and proteoglycan content, and total histological severity of OA (sum of cartilage structure and proteoglycan content scores for all four compartments). For each parameter, paired housing resulted in mean histological scores of approximately 2.5 units higher than the same parameter assessed in singly housed animals (Figure 9). This difference was significant for all histological scores of the medial tibia (p<0.05) and medial compartment (p<0.05). The presumed reason for the difference of OA in paired versus singly housed animals is due to differences in physical activity levels. For this reason, when feasible, we recommend housing in groups.
Figure 9.
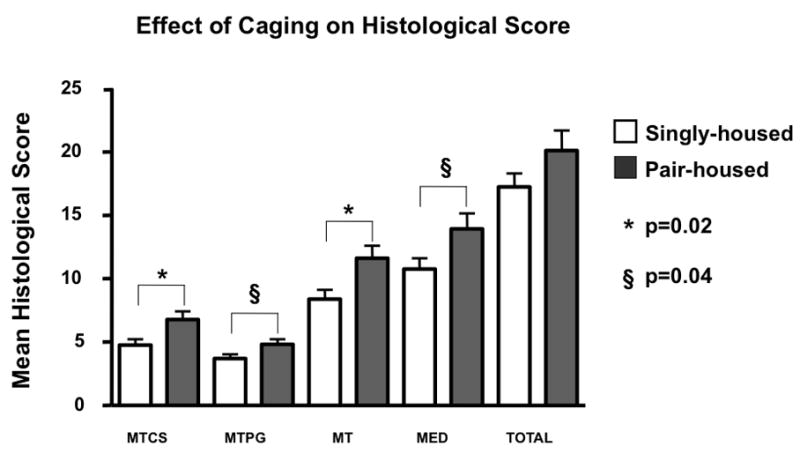
Effect of caging on guinea pig knee joint histological OA severity. Animals were housed either singly (n=26, white bars), or in pairs (n=14, dark bars) from 2 to 12 months of age. Paraffin central sections of these knee joints (aged 12 months) were scored using the recommended scoring scheme to assess cartilage structure (CS) and proteoglycan content (PG) or a combination of both (Total), of the medial tibia (MT) or entire medial compartment (MED). A comparison of paired housing with single housing by the non-parametric Mann Whitney test, was associated with slight but significantly greater histological scores in the paired-housed animals compared with single-housed animals (*p=0.02; § p =0.04, respectively). Error bars = SEM.
Discussion
The process of recommending a particular histological scoring system for common use must take into consideration several factors: 1) ease of use and inter-rater and intra-rater reliability, 2) ready adoption or familiarity by investigators in the field of musculoskeletal and joint diseases, 3) potential utility across various animal systems, and 4) demonstrated validity through a correlation of histological findings with a wholly different outcome measure, such as synovial fluid biomarker concentrations, which provide reassurance that the histological joint scoring is accurate and fully representative of the pathological process. The guinea pig provides a model system in which synovial fluid harvest is feasible and in which synovial fluid biomarker measurements can be performed. Correlations have been demonstrated between severity of histological OA assessed by the recommended scoring system (sum of cartilage structure and proteoglycan content scores) and several synovial fluid biomarkers, namely cartilage oligomeric matrix protein and keratan sulfate [6, 14, 81]. Our estimations of the number of animals required in a clinical trial differs somewhat by outcome measure. For instance, to provide 80% power to detect a significant change in response to a hypothetical treatment capable of achieving a 30% treatment difference compared to untreated animals, with statistical significance of p=0.05, histological scoring requires 16 animals per group, synovial fluid cartilage oligomeric matrix protein requires 12 animals per group, and synovial fluid keratan sulfate requires 11 animals per group [103]. Thus, it is apparent that in this model system, a significant treatment effect can be realized using a relatively small number of animals. In summary, we recommend the OARSI-HISTOgp histochemical-histological scoring system in Table 4 for semi-quantitative histological assessment of OA in the guinea pig, due to its already widespread adoption, ease of use, the achievable high inter-rater reliability, similarity to the scoring system most often used to assess OA in humans, and its demonstrated correlation with synovial fluid biomarker concentrations. In summary, as demonstrated by this review, a wealth of tools exist to facilitate the semi-quantitative and quantitative assessment of OA in the guinea pig to achieve a comprehensive characterization of the whole joint organ.
Acknowledgments
We wish to thank the following individuals for their expert assistance with the manuscript: JM Williams (Chicago, USA) and Cathy Carlson (Minnesota, USA). We also wish to thank Karen Whitney for her invaluable assistance in obtaining the many journal articles necessary for this review. The photomicrograph and printing costs were supported by an unrestricted educational grant to OARSI by Bayer, Expanscience, Genzyme, Lilly, MerckSerono, Novartis, Pfizer, SanofiAventis, Servier, and Wyeth. The work performed was not influenced at any stage by the support provided.
Supported in part by the NIH/NIAMS (P01 AR50245)
Footnotes
Disclosures: Sub-Coordinator Virginia B Kraus is employed by Duke University Medical Center
- Janet L. Huebner is employed by Duke University Medical Center
- Jeroen DeGroot is employed by TNO, an organization for applied scientific research
- Alison Bendele is Director of Bolder BioPATH, a contract research laboratory for preclinical studies of arthritis
- Coordinator Thomas Aigner is employed by the University of Leipzig
Conflict of interest statement: No author has any conflict of interest related to this work. The work performed was not influenced at any stage by the support provided.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Virginia B Kraus, Durham, USA.
Janet L. Huebner, Durham, USA
Jeroen DeGroot, Leiden, Netherlands.
Alison Bendele, Bolder BioPATH, Colorado, USA.
References
- 1.Silverstein E, Sokoloff L. Natural history of degenerative joint disease in small animals. 5. Osteoarthritis in guinea pigs. Arthritis Rheum. 1958;1:82–86. doi: 10.1002/art.1780010112. [DOI] [PubMed] [Google Scholar]
- 2.Bendele AM. Progressive chronic osteoarthritis in femorotibial joints of partial medial meniscectomized guinea pigs. Vet Pathol. 1987;24:444–448. doi: 10.1177/030098588702400512. [DOI] [PubMed] [Google Scholar]
- 3.Bendele A, Hulman J. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum. 1988;31:561–565. doi: 10.1002/art.1780310416. [DOI] [PubMed] [Google Scholar]
- 4.Bendele AM, White SL, Hulman JF. Osteoarthritis in guinea pigs: histopathologic and scanning electron microscopic features. Lab Animal Sci. 1989;39:115–121. [PubMed] [Google Scholar]
- 5.Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea pigs. J Exp Pathol (Oxford) 1990;71:279–293. [PMC free article] [PubMed] [Google Scholar]
- 6.Huebner JL, Hanes MA, Beekman B, TeKoppele JM, Kraus VB. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthritis Cartilage. 2002;10:758–767. doi: 10.1053/joca.2002.0821. [DOI] [PubMed] [Google Scholar]
- 7.Anderson-MacKenzie JM, Quasnichka HL, Starr RL, Lewis EJ, Billingham ME, Bailey AJ. Fundamental subchondral bone changes in spontaneous knee osteoarthritis. Int J Biochem Cell Biol. 2005;37:224–236. doi: 10.1016/j.biocel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Eyre D, Arsenault L, Nussli S, Hunziker E. 3-D subchondral bone evaluation of guinea pig spontaneous OA and the effects of hyaluronan in this model. Trans Orthopaedic Res Soc. 2007:47. [Google Scholar]
- 9.Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1:363–376. [PubMed] [Google Scholar]
- 10.Dai G, Li J, Liu X, Liu Q, Liu C. The relationship of the expression of estrogen receptor in cartilage cell and osteoarthritis induced by bilateral ovariectomy in guinea pig. J Huazhong Univ Sci Technolog Med Sci. 2005;25:683–686. doi: 10.1007/BF02896170. [DOI] [PubMed] [Google Scholar]
- 11.Dai G, Wang S, Li J, Liu C, Liu Q. The validity of osteoarthritis model induced by bilateral ovariectomy in guinea pig. J Huazhong Univ Sci Technolog Med Sci. 2006;26:716–719. doi: 10.1007/s11596-006-0624-2. [DOI] [PubMed] [Google Scholar]
- 12.Bendele AM, Hulman JF. Effects of body weight restriction on the development and progression of spontaneous osteoarthritis in guinea pigs. Arthritis & Rheumatism. 1991;34:1180–1184. doi: 10.1002/art.1780340916. [DOI] [PubMed] [Google Scholar]
- 13.Wei L, de Bri E, Lundberg A, Svensson O. Mechanical load and primary guinea pig osteoarthrosis. Acta Orthop Scand. 1998;69:351–357. doi: 10.3109/17453679808999046. [DOI] [PubMed] [Google Scholar]
- 14.Huebner JL, Kraus VB. Assessment of the utility of biomarkers of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2006;14:923–930. doi: 10.1016/j.joca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Tyler JA, Watson PJ, Koh HL, Herrod NJ, Robson M, Hall LD. Detection and monitoring of progressive degeneration of osteoarthritic cartilage by MRI. Acta Orthop Scand Suppl. 1995;266:130–138. [PubMed] [Google Scholar]
- 16.Watson PJ, Hall LD, Malcolm A, Tyler JA. Degenerative joint disease in the guinea pig. Use of magnetic resonance imaging to monitor progression of bone pathology. Arthritis Rheum. 1996;39:1327–1337. doi: 10.1002/art.1780390810. [DOI] [PubMed] [Google Scholar]
- 17.Watson PJ, Carpenter TA, Hall LD, Tyler JA. MR protocols for imaging the guinea pig knee. Magn Reson Imaging. 1997;15:957–970. doi: 10.1016/s0730-725x(97)00113-6. [DOI] [PubMed] [Google Scholar]
- 18.Tessier JJ, Bowyer J, Brownrigg NJ, Peers IS, Westwood FR, Waterton JC, et al. Characterisation of the guinea pig model of osteoarthritis by in vivo three-dimensional magnetic resonance imaging. Osteoarthritis Cartilage. 2003;11:845–853. doi: 10.1016/s1063-4584(03)00162-6. [DOI] [PubMed] [Google Scholar]
- 19.Bowyer J, Browning N, Tessier J, Pickford R, Horrocks M, Waterton J, et al. Correlation between MRI cartilage volume and urinary biomarkers of cartilage destruciton in the guinea pig model of osteoarthritis. Osteo Cartilage. 2003;11:187. [Google Scholar]
- 20.Lee J, Dyke J, Ballon D, Feldman F, Ciombor D, Aaron R, et al. Subchondral fluid dynamics in the Dunkin-Hartley guinea pig model of spontaneous osteoarthritis: use of a novel dynamic MRI technique. Trans Orthop Res Soc. 2005;30:1424. [Google Scholar]
- 21.Bolbos R, Benoit-Cattin H, Langlois JB, Chomel A, Chereul E, Odet C, et al. Knee cartilage thickness measurements using MRI: a 4(1/2)-month longitudinal study in the meniscectomized guinea pig model of OA. Osteoarthritis Cartilage. 2007;15:656–665. doi: 10.1016/j.joca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Bolbos R, Benoit-Cattin H, Langlois JB, Chomel A, Chereul E, Odet C, et al. Measurement of knee cartilage thickness using MRI: a reproducibility study in a meniscectomized guinea pig model of osteoarthritis. NMR Biomed. 2008;21:366–375. doi: 10.1002/nbm.1198. [DOI] [PubMed] [Google Scholar]
- 23.Layton MW, Goldstein SA, Goulet RW, Feldkamp LA, Kubinski DJ, Bole GG. Examination of subchondral bone architecture in experimental osteoarthritis by microscopic computed axial tomography. Arthritis Rheum. 1988;31:1400–1405. doi: 10.1002/art.1780311109. [DOI] [PubMed] [Google Scholar]
- 24.Dedrick DK, Goulet R, Huston L, Goldstein SA, Bole GG. Early bone changes in experimental osteoarthritis using microscopic computed tomography. J Rheumatol Suppl. 1991;27:44–45. [PubMed] [Google Scholar]
- 25.Kapadia RD, Badger AM, Levin JM, Swift B, Bhattacharyya A, Dodds RA, et al. Meniscal ossification in spontaneous osteoarthritis in the guinea-pig. Osteoarthritis Cartilage. 2000;8:374–377. doi: 10.1053/joca.1999.0312. [DOI] [PubMed] [Google Scholar]
- 26.Ding M, Christian Danielsen C, Hvid I. Effects of hyaluronan on three-dimensional microarchitecture of subchondral bone tissues in guinea pig primary osteoarthrosis. Bone. 2005;36:489–501. doi: 10.1016/j.bone.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Ding M, Danielsen CC, Hvid I. Age-related three-dimensional microarchitectural adaptations of subchondral bone tissues in guinea pig primary osteoarthrosis. Calcif Tissue Int. 2006;78:113–122. doi: 10.1007/s00223-005-0028-5. [DOI] [PubMed] [Google Scholar]
- 28.Muraoka T, Hagino H, Okano T, Enokida M, Teshima R. Role of subchondral bone in osteoarthritis development: a comparative study of two strains of guinea pigs with and without spontaneously occurring osteoarthritis. Arthritis Rheum. 2007;56:3366–3374. doi: 10.1002/art.22921. [DOI] [PubMed] [Google Scholar]
- 29.Vermeirsch H, Biermans R, Salmon PL, Meert TF. Evaluation of pain behavior and bone destruction in two arthritic models in guinea pig and rat. Pharmacol Biochem Behav. 2007;87:349–359. doi: 10.1016/j.pbb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Ding M, Danielsen CC, Hvid I. The effects of bone remodeling inhibition by alendronate on three-dimensional microarchitecture of subchondral bone tissues in guinea pig primary osteoarthrosis. Calcif Tissue Int. 2008;82:77–86. doi: 10.1007/s00223-007-9093-2. [DOI] [PubMed] [Google Scholar]
- 31.McDougall JJ, Andruski B, Schuelert N, Hallgrimsson B, Matyas JR. Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley guinea pigs. Pain. 2008 doi: 10.1016/j.pain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Kubin M, Kruml J. Experimental osteo-arthritis in guinea pigs produced by nonchromogenic unclassified mycobacteria. Acta Tuberc Pneumol Scand. 1964;44:159–167. [PubMed] [Google Scholar]
- 33.Kopp S, Mejersjo C, Clemensson E. Induction of osteoarthrosis in the guinea pig knee by papain. Oral Surg Oral Med Oral Pathol. 1983;55:259–266. doi: 10.1016/0030-4220(83)90325-0. [DOI] [PubMed] [Google Scholar]
- 34.Mejersjo C. Long-term development after treatment of mandibular dysfunction and osteoarthrosis. A clinical-radiographic follow-up and an animal experimental study. Swed Dent J Suppl. 1984;22:1–58. [PubMed] [Google Scholar]
- 35.Cashin CH. The induction of an erosive arthropathy in the guinea pig with copper II bisglycinate and its treatment with antirheumatic drugs. British Journal of Rheumatology. 1987;26:251–258. doi: 10.1093/rheumatology/26.4.251. [DOI] [PubMed] [Google Scholar]
- 36.Jimenez PA, Harlan PM, Chavarria AE, Haimes HB. Induction of osteoarthritis in guinea pigs by transection of the anterior cruciate ligament: radiographic and histopathological changes. Inflamm Res. 1995;44 (Suppl 2):S129–130. doi: 10.1007/BF01778296. [DOI] [PubMed] [Google Scholar]
- 37.Billingham M, Meijers M, Mahwinney B, Malcolm A. Spontaneous osteoarthritis in guinea pig: cartilage degeneration is preceded by loss of subchondral trabecular bone. Br J Rheum. 1996;S1:200. [Google Scholar]
- 38.Jimenez PA, Glasson SS, Trubetskoy OV, Haimes HB. Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: histologic, radiologic, and biochemical changes. Lab Anim Sci. 1997;47:598–601. [PubMed] [Google Scholar]
- 39.Wei L, Hjerpe A, Brismar BH, Svensson O. Effect of load on articular cartilage matrix and the development of guinea-pig osteoarthritis. Osteoarthritis Cartilage. 2001;9:447–453. doi: 10.1053/joca.2000.0411. [DOI] [PubMed] [Google Scholar]
- 40.Pastoureau PC, Chomel AC, Bonnet J. Evidence of early subchondral bone changes in the meniscectomized guinea pig. A densitometric study using dual-energy X-ray absorptiometry subregional analysis. Osteoarthritis Cart. 1999;7:466–473. doi: 10.1053/joca.1999.0241. [DOI] [PubMed] [Google Scholar]
- 41.Fink C, Cooper HJ, Huebner JL, Guilak F, Kraus VB. Precision and accuracy of a transportable dual-energy X-ray absorptiometry unit for bone mineral measurements in guinea pigs. Calcified Tissue International. 2002;70:164–169. doi: 10.1007/s00223-001-1063-5. [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Stuart K, Kandel R, Grynpas M. The effect of bisphosphonate on the development of bone changes in the guinea pig OA model. Osteo Cartilage. 2003;11:S29, 78. [Google Scholar]
- 43.Wang X, Kandel R, Grynpas M. Characterization of subchondral bone changes during the progression of osteoarthritis: a 6-month study in the partial medial meniscectomised guinea pig. Trans Orthop Res Soc. 2005;30:1434. [Google Scholar]
- 44.Flahiff CM, Narmoneva DA, Huebner JL, Kraus VB, Guilak F, Setton LA. Osmotic loading to determine the intrinsic material properties of guinea pig knee cartilage. J Biomech. 2002;35:1285–1290. doi: 10.1016/s0021-9290(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 45.Flahiff CM, Kraus VB, Huebner JL, Setton LA. Cartilage mechanics in the guinea pig model of osteoarthritis studied with an osmotic loading method. Osteoarthritis Cartilage. 2004;12:383–388. doi: 10.1016/j.joca.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Teeple E, Fleming BC, Mechrefe AP, Crisco JJ, Brady MF, Jay GD. Frictional properties of Hartley guinea pig knees with and without proteolytic disruption of the articular surfaces. Osteoarthritis Cartilage. 2007;15:309–315. doi: 10.1016/j.joca.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teeple E, Elsaid KA, Fleming BC, Jay GD, Aslani K, Crisco JJ, et al. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26:231–237. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terril L, Clemons D. The Laboratory Guinea Pig. Boca Raton: CRC Press; 1998. [Google Scholar]
- 49.D’Erchia A, Gissi C, Pesole G, Saccone C, Arnason U. The guinea pig is not a rodent. Nature. 1996;381:597–600. doi: 10.1038/381597a0. [DOI] [PubMed] [Google Scholar]
- 50.Zuck T. Age order of epiphyseal union in the guinea pig. The Anatomical Record. 1938;70:389–399. [Google Scholar]
- 51.Huebner JL, Williams JM, Kraus VB. The timecourse of collagen fibril disruption in primary guinea pig osteoarthritis. Trans Orthop Res Soc. 2003;28:748. [Google Scholar]
- 52.Limb GA, Brown KA, Wolstencroft RA, Ellis BA, Dumonde DC. The production of arthritis in the guinea-pig by intra-articular reaction between lymphokines and inflammatory leucocytes. Br J Exp Pathol. 1989;70:443–456. [PMC free article] [PubMed] [Google Scholar]
- 53.Yamashita N, Nakanishi I, Okada Y. Arthritis induced immunologically with cationic amidated bovine serum albumin in the guinea pig. A morphological and biochemical study on the destruction of articular cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60:57–66. doi: 10.1007/BF02899528. [DOI] [PubMed] [Google Scholar]
- 54.Bendele AM, Bendele RA, Hulman JF, Swann BP. Beneficial effects of treatment with diacerhein in guinea pigs with osteoarthritis. Rev Prat. 1996;46:S35–S39. [PubMed] [Google Scholar]
- 55.Hood VC, Cruwys SC, Urban L, Kidd BL. The neurogenic contribution to synovial leucocyte infiltration and other outcome measures in a guinea pig model of arthritis. Neurosci Lett. 2001;299:201–204. doi: 10.1016/s0304-3940(00)01790-0. [DOI] [PubMed] [Google Scholar]
- 56.Han B, Cole AA, Shen Y, Brodie T, Williams JM. Early alterations in the collagen meshwork and lesions in the ankles are associated with spontaneous osteoarthritis in guinea-pigs. Osteoarthritis Cartilage. 2002;10:778–784. doi: 10.1053/joca.2002.0822. [DOI] [PubMed] [Google Scholar]
- 57.Mapp PI, Avery PS, McWilliams DF, Bowyer J, Day C, Moores S, et al. Angiogenesis in two animal models of osteoarthritis. Osteoarthritis Cartilage. 2008;16:61–69. doi: 10.1016/j.joca.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 58.Cheung HS, Sallis JD, Demadis KD, Wierzbicki A. Phosphocitrate blocks calcification-induced articular joint degeneration in a guinea pig model. Arthritis Rheum. 2006;54:2452–2461. doi: 10.1002/art.22017. [DOI] [PubMed] [Google Scholar]
- 59.Richardson C, Bae W, Fazeli B, Filvaroff E, Sah R. Quantitative characterization of osteoarthritis in the guinea pig. Trans Orthop Res Soc. 2001:26. [Google Scholar]
- 60.Bowyer J, Tessier J, Brownrigg N, Pickford R, Westwood R, Waterton J, et al. Assessing disease modification in the guinea pig model of spontaneous OA. Trans Orthop Res Soc. 2003;28:759. [Google Scholar]
- 61.Kobayashi T, Notoya K, Naito T, Unno S, Nakamura A, Martel-Pelletier J, et al. Pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, reduces the progression of experimental osteoarthritis in guinea pigs. Arthritis Rheum. 2005;52:479–487. doi: 10.1002/art.20792. [DOI] [PubMed] [Google Scholar]
- 62.Miot-Noirault E, Vidal A, Pastoureau P, Bonafous J, Chomel A, Sarry L, et al. Early detection and monitoring of cartilage alteration in the experimental meniscectomised guinea pig model of osteoarthritis by (99m)Tc-NTP 15–5 scintigraphy. Eur J Nucl Med Mol Imaging. 2007 doi: 10.1007/s00259-006-0320-2. [DOI] [PubMed] [Google Scholar]
- 63.Meachim G. Light microscopy of Indian ink preparations of fibrillated cartilage. Ann Rheum Dis. 1972;31:457–464. doi: 10.1136/ard.31.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 65.Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the outerbridge classification for grading chondral lesions of the knee arthroscopically. Am J Sports Med. 2003;31:83–86. doi: 10.1177/03635465030310012601. [DOI] [PubMed] [Google Scholar]
- 66.Pelletier JP, Fernandes JC, Brunet J, Moldovan F, Schrier D, Flory C, et al. In vivo selective inhibition of mitogen-activated protein kinase kinase 1/2 in rabbit experimental osteoarthritis is associated with a reduction in the development of structural changes. Arthritis Rheum. 2003;48:1582–1593. doi: 10.1002/art.11014. [DOI] [PubMed] [Google Scholar]
- 67.Alers JC, Krijtenburg PJ, Vissers KJ, van Dekken H. Effect of bone decalcification procedures on DNA in situ hybridization and comparative genomic hybridization. EDTA is highly preferable to a routinely used acid decalcifier. J Histochem Cytochem. 1999;47:703–710. doi: 10.1177/002215549904700512. [DOI] [PubMed] [Google Scholar]
- 68.Rosen A. End-Point Determination in EDTA decalcification using Ammonium Oxalate. Stain Technol. 1981;56:48–49. doi: 10.3109/10520298109067275. [DOI] [PubMed] [Google Scholar]
- 69.Fini M, Giavaresi G, Torricelli P, Cavani F, Setti S, Cane V, et al. Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J Orthop Res. 2005;23:899–908. doi: 10.1016/j.orthres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Camplejohn KL, Allard SA. Limitations of safranin ‘O’ staining in proteoglycan-depleted cartilage demonstrated with monoclonal antibodies. Histochemistry. 1988;89:185–188. doi: 10.1007/BF00489922. [DOI] [PubMed] [Google Scholar]
- 71.Brismar BH, Lei W, Hjerpe A, Svensson O. The effect of body mass and physical activity on the development of guinea pig osteoarthrosis. Acta Orthop Scand. 2003;74:442–448. doi: 10.1080/00016470310017767. [DOI] [PubMed] [Google Scholar]
- 72.Hyttinen MM, Arokoski JP, Parkkinen JJ, Lammi MJ, Lapvetelainen T, Mauranen K, et al. Age matters: collagen birefringence of superficial articular cartilage is increased in young guinea-pigs but decreased in older animals after identical physiological type of joint loading. Osteoarthritis Cartilage. 2001;9:694–701. doi: 10.1053/joca.2001.0466. [DOI] [PubMed] [Google Scholar]
- 73.Lapvetelainen T, Nevalainen T, Parkkinen JJ, Arokoski J, Kiraly K, Hyttinen M, et al. Lifelong moderate running training increases the incidence and severity of osteoarthritis in the knee joint of C57BL mice. Anat Rec. 1995;242:159–165. doi: 10.1002/ar.1092420204. [DOI] [PubMed] [Google Scholar]
- 74.Pastoureau P, Leduc S, Chomel A, De Ceuninck F. Quantitative assessment of articular cartilage and subchondral bone histology in the meniscectomized guinea pig model of osteoarthritis. Osteoarthritis Cartilage. 2003;11:412–423. doi: 10.1016/s1063-4584(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 75.Mankin H, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone and Joint Surgery. 1971;53-A:523–537. [PubMed] [Google Scholar]
- 76.Arsever CL, Bole GG. Experimental osteoarthritis induced by selective myectomy and tendotomy. Arthritis Rheum. 1986;29:251–261. doi: 10.1002/art.1780290214. [DOI] [PubMed] [Google Scholar]
- 77.Ikeda T, Kubo T, Arai Y, Nakanishi T, Kobayashi K, Takahashi K, et al. Adenovirus mediated gene delivery to the joints of guinea pigs. J Rheumatol. 1998;25:1666–1673. [PubMed] [Google Scholar]
- 78.Ono M, Yamashita Y, Minamizaki T, Morio Y. Processes of osteophyte formation in guinea pigs with spontaneous osteoarthritis. Yonago Acta medica. 2000;43:131–140. [Google Scholar]
- 79.Lamers RJ, DeGroot J, Spies-Faber EJ, Jellema RH, Kraus VB, Verzijl N, et al. Identification of disease- and nutrient-related metabolic fingerprints in osteoarthritic Guinea pigs. J Nutr. 2003;133:1776–1780. doi: 10.1093/jn/133.6.1776. [DOI] [PubMed] [Google Scholar]
- 80.Ciombor DM, Aaron RK, Wang S, Simon B. Modification of osteoarthritis by pulsed electromagnetic field--a morphological study. Osteoarthritis Cartilage. 2003;11:455–462. doi: 10.1016/s1063-4584(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 81.Kraus VB, Huebner JL, Stabler T, Flahiff CM, Setton LA, Fink C, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50:1822–1831. doi: 10.1002/art.20291. [DOI] [PubMed] [Google Scholar]
- 82.Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/beta-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;88:264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 83.Carlson C, Loeser R, Purser C, Gardin J, Jerome C. Osteoarthritis in cynomolgus macaques III: Effects of age, gender, and subchondral bone thickness on the severity of disease. J Bone Miner Res. 1996;11:1209–1217. doi: 10.1002/jbmr.5650110904. [DOI] [PubMed] [Google Scholar]
- 84.Huebner J, Beekman B, TeKoppele J, Kraus V. Strain comparison in the guinea pig model of naturally occurring osteoarthritis. Trans Orthop Res Soc. 1999;24:198. [Google Scholar]
- 85.de Bri E, Reinholt FP, Svensson O. Primary osteoarthrosis in guinea pigs: a stereological study. J Orthop Res. 1995;13:769–776. doi: 10.1002/jor.1100130517. [DOI] [PubMed] [Google Scholar]
- 86.Scott J. Affinity, competition and specific interactions in the biochemistry and histochemistry of polyelectrolytes. Biochem Soc trans. 1973;1:787–806. [Google Scholar]
- 87.Williams JM, Ongchi DR, Thonar E-M. Repair of articular cartilage injury following intra-articular chymopapain-induced matrix proteoglycan loss. J Orthop Res. 1993;11:705–716. doi: 10.1002/jor.1100110513. [DOI] [PubMed] [Google Scholar]
- 88.Pelletier JP, Martel-Pelletier J, Ghandur-Mnaymneh L, Howell DS, Woessner JF., Jr Role of synovial membrane inflammation in cartilage matrix breakdown in the Pond-Nuki dog model of osteoarthritis. Arthritis Rheum. 1985;28:554–561. doi: 10.1002/art.1780280515. [DOI] [PubMed] [Google Scholar]
- 89.Oehler S, Neureiter D, Meyer-Scholten C, Aigner T. Subtyping of osteoarthritic synoviopathy. Clin Exp Rheumatol. 2002;20:633–640. [PubMed] [Google Scholar]
- 90.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 91.Fini M, Torricelli P, Giavaresi G, Aldini NN, Cavani F, Setti S, et al. Effect of pulsed electromagnetic field stimulation on knee cartilage, subchondral and epyphiseal trabecular bone of aged Dunkin Hartley guinea pigs. Biomed Pharmacother. 2007 doi: 10.1016/j.biopha.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Pastoureau P, Chomel A. Methods for cartilage and subchondral bone histomorphometry. Methods Mol Med. 2004;101:79–91. doi: 10.1385/1-59259-821-8:079. [DOI] [PubMed] [Google Scholar]
- 93.Bendele AM, White SL. Early histopathologic and ultrastructural alterations in femorotibial joints of partial medial meniscectomized guinea pigs. Vet Pathol. 1987;24:436–443. doi: 10.1177/030098588702400511. [DOI] [PubMed] [Google Scholar]
- 94.Hedlund H, de Bri E, Mengarelli-Widholm S, Reinholt FP, Svensson O. Ultrastructural changes in primary guinea pig osteoarthritis with special reference to collagen. Apmis. 1996;104:374–382. doi: 10.1111/j.1699-0463.1996.tb00730.x. [DOI] [PubMed] [Google Scholar]
- 95.de Bri E, Lei W, Reinholt FP, Mengarelli-Widholm S, Heingard D, Svensson O. Ultrastructural immunolocalization of bone sialoprotein in guinea-pig osteoarthritis. Osteoarthritis Cartilage. 1997;5:387–393. doi: 10.1016/s1063-4584(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 96.Helminen HJ, Hyttinen MM, Lammi MJ, Arokoski JP, Lapvetelainen T, Jurvelin J, et al. Regular joint loading in youth assists in the establishment and strengthening of the collagen network of articular cartilage and contributes to the prevention of osteoarthrosis later in life: a hypothesis. J Bone Miner Metab. 2000;18:245–257. doi: 10.1007/pl00010638. [DOI] [PubMed] [Google Scholar]
- 97.Young RD, Vaughan-Thomas A, Wardale RJ, Duance VC. Type II collagen deposition in cruciate ligament precedes osteoarthritis in the guinea pig knee. Osteoarthritis Cartilage. 2002;10:420–428. doi: 10.1053/joca.2002.0530. [DOI] [PubMed] [Google Scholar]
- 98.Wei L, Brismar BH, Hultenby K, Hjerpe A, Svensson O. Distribution of chondroitin 4-sulfate epitopes (2/B/6) in various zones and compartments of articular cartilage in guinea pig osteoarthrosis. Acta Orthop Scand. 2003;74:16–21. doi: 10.1080/00016470310013590. [DOI] [PubMed] [Google Scholar]
- 99.Johnson K, Svensson CI, Etten DV, Ghosh SS, Murphy AN, Powell HC, et al. Mediation of spontaneous knee osteoarthritis by progressive chondrocyte ATP depletion in Hartley guinea pigs. Arthritis & Rheumatism. 2004;50:1216–1225. doi: 10.1002/art.20149. [DOI] [PubMed] [Google Scholar]
- 100.Gelse K, Soder S, Eger W, Diemtar T, Aigner T. Osteophyte development--molecular characterization of differentiation stages. Osteoarthritis Cartilage. 2003;11:141–148. doi: 10.1053/joca.2002.0873. [DOI] [PubMed] [Google Scholar]
- 101.de Bri E, Jonsson K, Reinholt FP, Svensson O. Focal destruction and remodeling in guinea pig arthrosis. Acta Orthop Scand. 1996;67:498–504. doi: 10.3109/17453679608996676. [DOI] [PubMed] [Google Scholar]
- 102.Quasnichka HL, Anderson-MacKenzie JM, Tarlton JF, Sims TJ, Billingham ME, Bailey AJ. Cruciate ligament laxity and femoral intercondylar notch narrowing in early-stage knee osteoarthritis. Arthritis Rheum. 2005;52:3100–3109. doi: 10.1002/art.21340. [DOI] [PubMed] [Google Scholar]
- 103.Huebner JL, VV, Kraus VB. Synovial fluid biomarkers as predictors of therapeutic efficacy in a longitudinal analysis of the Hartley guinea pig model of naturally occurring osteoarthritis. Trans Orthop Res Soc. 2003;28:760. [Google Scholar]
- 104.Silberberg R, Stamp WG, Lesker PA, Hasler M. Aging changes in ultrastructure and enzymatic activity of articular cartilage of guinea pigs. J Gerontol. 1970;25:184–198. doi: 10.1093/geronj/25.3.184. [DOI] [PubMed] [Google Scholar]
- 105.Caterson B, Hughes C, Johnstone B, Mort J. Immunological markers of cartilage proteoglycan metabolism in animal and human osteoarthritis. In: Kuettner K, Schleyerbach R, Peyron J, Hascall V, editors. Articular Cartilage and Osteoarthritis. Weisbaden: Raven Press; 1992. pp. 415–427. [Google Scholar]
- 106.Watson PJ, Hall LD, Carpenter TA, Tyler JA. A magnetic resonance imaging study of joint degeneration in the guinea pig knee. Agents Actions Suppl. 1993;39:261–265. doi: 10.1007/978-3-0348-7442-7_32. [DOI] [PubMed] [Google Scholar]
- 107.Greenwald RA. Treatment of destructive arthritis disorders with MMP inhibitors. In: Greenwald RA, Golub LM, editors. Inhibition of Matrix Metalloproteinases. Vol. 732. Tampa, FL: The New York Academy of Sciences; 1994. pp. 181–198. [DOI] [PubMed] [Google Scholar]
- 108.Osborne D, Woodhouse S, Meacock R. Early changes in the sulfation of chondroitin in guinea-pig articular cartilage, a possible predictor of osteoarthritis. Osteoarthritis Cartilage. 1994;2:215–223. doi: 10.1016/s1063-4584(05)80071-8. [DOI] [PubMed] [Google Scholar]
- 109.Carney SL, Hicks CA, Tree B, Broadmore RJ. An in vivo investigation of the effect of anthraquinones on the turnover of aggrecans in spontaneous osteoarthritis in the guinea pig. Inflamm Res. 1995;44:182–186. doi: 10.1007/BF01782817. [DOI] [PubMed] [Google Scholar]
- 110.Wei L, Svensson O, Hjerpe A. Correlation of morphologic and biochemical changes in the natural history of spontaneous osteoarthrosis in guinea pigs. Arthritis Rheum. 1997;40:2075–2083. doi: 10.1002/art.1780401121. [DOI] [PubMed] [Google Scholar]
- 111.Huebner JL, Otterness IG, Freund EM, Caterson B, Kraus VB. Collagenase 1 and collagenase 3 expression in a guinea pig model of osteoarthritis.[comment] Arthritis & Rheumatism. 1998;41:877–890. doi: 10.1002/1529-0131(199805)41:5<877::AID-ART16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 112.de Bri E, Lei W, Svensson O, Chowdhury M, Moak SA, Greenwald RA. Effect of an inhibitor of matrix metalloproteinases on spontaneous osteoarthritis in guinea pigs. Adv Dent Res. 1998;12:82–85. doi: 10.1177/08959374980120012601. [DOI] [PubMed] [Google Scholar]
- 113.Bendele A, McComb J, Gould T, McAbee T, Sennello G, Chlipala E, et al. Animal models of arthritis: relevance to human disease. Toxicologic Pathology. 1999;27:134–142. doi: 10.1177/019262339902700125. [DOI] [PubMed] [Google Scholar]
- 114.Watanabe M, Atsugi T, Sato K, Nomura Y. Evaluation of shark collagen for chondroprotective action in guinea pig model of osteoarthritis. Osteo Cartilage. 2003;11:S30, 79. [Google Scholar]
- 115.Rothschild BM. Osteoarthritis as a complication of artificial environment: the Cavia (Guinea pig) story. Ann Rheum Dis. 2003;62:1022–1023. doi: 10.1136/ard.62.10.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burton DW, Foster M, Johnson KA, Hiramoto M, Deftos LJ, Terkeltaub R. Chondrocyte calcium-sensing receptor expression is up-regulated in early guinea pig knee osteoarthritis and modulates PTHrP, MMP-13, and TIMP-3 expression. Osteoarthritis Cartilage. 2005;13:395–404. doi: 10.1016/j.joca.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 117.Quasnichka HL, Anderson-Mackenzie JM, Bailey AJ. Subchondral bone and ligament changes precede cartilage degradation in guinea pig osteoarthritis. Biorheology. 2006;43:389–397. [PubMed] [Google Scholar]
- 118.Aaron RK, Dyke JP, Ciombor DM, Ballon D, Lee J, Jung E, et al. Perfusion abnormalities in subchondral bone associated with marrow edema, osteoarthritis, and avascular necrosis. Ann N Y Acad Sci. 2007;1117:124–137. doi: 10.1196/annals.1402.069. [DOI] [PubMed] [Google Scholar]
- 119.Sabatini M, Lesur C, Thomas M, Chomel A, Anract P, de Nanteuil G, et al. Effect of inhibition of matrix metalloproteinases on cartilage loss in vitro and in a guinea pig model of osteoarthritis. Arthritis Rheum. 2005;52:171–180. doi: 10.1002/art.20900. [DOI] [PubMed] [Google Scholar]
- 120.Schwartz ER, Leveille CR, Stevens JW, Oh WH. Proteoglycan structure and metabolism in normal and osteoarthritic cartilage of guinea pigs. Arthritis Rheum. 1981;24:1528–1539. doi: 10.1002/art.1780241212. [DOI] [PubMed] [Google Scholar]
- 121.Schwartz ER. The modulation of osteoarthritic development by vitamins C and E. International Journal for Vitamin and Nutrition Research, Supplement. 1984;26:141–146. [PubMed] [Google Scholar]
- 122.Baragi V, Schwartz E. Surgically induced osteoarthritis (OA): effect of long-term treatment with proxicam and indomethacin. Arthritis Rheum. 1986;29:S13, 18. [Google Scholar]
- 123.Gomis A, Miralles A, Schmidt RF, Belmonte C. Nociceptive nerve activity in an experimental model of knee joint osteoarthritis of the guinea pig: effect of intra-articular hyaluronan application. Pain. 2007;130:126–136. doi: 10.1016/j.pain.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 124.Layton MW, Arsever C, Bole GG. Use of the guinea pig myectomy osteoarthritis model in the examination of cartilage-synovium interactions. J Rheumatol. 1987;14(Spec No):125–126. [PubMed] [Google Scholar]
- 125.Bendele A, Bean J, Hulman J. Passive role of articular chondrocytes in the pathogenesis of acute meniscectomy-induced cartilage degeneration. Vet Pathol. 1991;28:207–215. doi: 10.1177/030098589102800304. [DOI] [PubMed] [Google Scholar]
- 126.Williams JM, Brandt KD. Immobilization ameliorates chemically-induced articular cartilage damage. Arthritis Rheum. 1984;27:208–216. doi: 10.1002/art.1780270213. [DOI] [PubMed] [Google Scholar]
- 127.Williams JM, Brandt KD. Benoxaprofen reduces osteophyte formation and fibrillation after articular cartilage injury. J Rheumatol. 1985;12:27–32. [PubMed] [Google Scholar]
- 128.Williams JM, Brandt KD. Triamcinolone hexacetonide protects against fibrillation and osteophyte formation following chemically induced articular cartilage damage. Arthritis Rheum. 1985;28:1267–1274. doi: 10.1002/art.1780281111. [DOI] [PubMed] [Google Scholar]
- 129.Tanaka H, Kitoh Y, Katsuramaki T, Tanaka M, Kitabayashi N, Fujimori S, et al. Effects of SL-1010 (sodium hyaluronate with high molecular weight) on experimental osteoarthritis induced by intra-articularly applied papain in guinea pigs. Nippon Yakurigaku Zasshi. 1992;100:77–86. doi: 10.1254/fpj.100.77. [DOI] [PubMed] [Google Scholar]
- 130.Yamada A, Uegaki A, Nakamura T, Ogawa K. ONO-4817, an orally active matrix metalloproteinase inhibitor, prevents lipopolysaccharide-induced proteoglycan release from the joint cartilage in guinea pigs. Inflammation Research. 2000;49:144–146. doi: 10.1007/s000110050573. [DOI] [PubMed] [Google Scholar]
- 131.Eguro H, Goldner JL. Antigenic properties of chondromucoprotein and inducibility of experimental arthritis by antichondromucoprotein immune globulin. J Bone Joint Surg Am. 1974;56:129–141. [PubMed] [Google Scholar]
- 132.Simon SR, Radin EL, Paul IL, Rose RM. The response of joints to impact loading. II. In vivo behavior of subchondral bone. J Biomech. 1972;5:267–272. doi: 10.1016/0021-9290(72)90042-5. [DOI] [PubMed] [Google Scholar]
- 133.Colombo C, Butler M, O’Byrne E, Hickman L, Swartzendruber D, Selwyn M, et al. A new model of osteoarthritis in rabbits. I. Development of knee joint pathology following lateral meniscectomy and section of the fibular collateral and sesamoid ligaments. Arthritis Rheum. 1983;26:875–886. doi: 10.1002/art.1780260709. [DOI] [PubMed] [Google Scholar]
- 134.Kikuchi T, Yamada H, Shimmei M. Effect of high molecular weight hyaluronan on cartilage degeneration in a rabbit model of osteoarthritis. Osteoarthritis Cartilage. 1996;4:99–110. doi: 10.1016/s1063-4584(05)80319-x. [DOI] [PubMed] [Google Scholar]