Summary
Six soil samples from natural and cultivated sites of Saudi Arabia were investigated for ciliate diversity, using the non-flooded Petri dish culture method, live observation, and silver impregnation. We identified 135 species, all new for the fauna of Saudi Arabia, of which seven were undescribed: Spathidium alqasabi nov. spec.; Enchelyodon alqasabi nov. spec.; Metauroleptus arabicus nov. gen., nov. spec.; Pseudohemisincirra arabica nov. gen., nov. spec.; Saudithrix terricola Berger, Al-Rasheid and Foissner, 2006; Oxytricha arabica nov. spec.; and Erimophrya monostyla nov. spec. Based on Spathidium alqasabi, S. seppelti foissneri Vd’ačný et al., 2006 and S. seppelti etoschense Foissner et al., 2002 are raised to species rank; for the latter, a new name is required to avoid homonymy: Spathidium fraterculum nov. nom. The new genus Metauroleptus, which possesses two long and two to three short ventral cirral rows, generates all dorsal kineties intrakinetally and produces caudal cirri exclusively in dorsal kinety 1. Metauroleptus belongs to the hypotrichs, while family classification remains doubtful. The same applies to the new hypotrich genus Pseudohemisincirra, which has frontoventral and transverse cirri, while buccal cirri and caudal cirri are absent. The number of species contained in Saudi Arabian soils, including sand dunes, is in the range reported from other regions of the earth, suggesting that ciliates are well adapted to dry habitats, possibly mainly by their ability to produce very resistant resting cysts, most surviving for a long time due to reduced metazoan predation.
Keywords: Adaptation, biodiversity, Metauroleptus nov. gen., protozoa, Pseudohemisincirra nov. gen., resting cysts, sand dunes, Tenerife
INTRODUCTION
While the marine and brackish ciliate faunas of the Saudi Arabian Gulf have been investigated in some detail (for reviews, see Al-Rasheid 1999, 2001), the soil protists of Arabia remained unknown, although some early studies are available from Palestine (Bodenheimer 1935) and the Algerian Sahara (Varga 1936). More recently, Foissner (1993) and Foissner et al. (2002) described two new species from Tunisia (Pseudocyrtolophosis terricola) and Egypt (Paragonostomum binucleatum). Thus, we performed a study on the diversity of soil ciliates in some representative natural and cultivated habitats of Saudi Arabia. Such data are important also in the current biogeographical discussion (Foissner 2006) because Saudi Arabia is in the transition zone of two main biogeographical regions (Müller 1981): the Holarctis and the Palaeotropis.
MATERIAL AND METHODS
The material is from Saudi Arabia, i.e., from the surroundings of the capital (Riyadh) and from sites along the main road from Riyadh to the Arab Gulf. Six samples were taken in February 1998, air-dried for one month, sealed in plastic bags, and investigated during June to December 1999. Sampling, taxonomic methods, and identification of species follow Foissner (1991) and Foissner et al. (2002); for details on terminology, see Foissner and Al-Rasheid (2006) and Foissner and Xu (2007). The samples were processed with the non-flooded Petri dish method, as described by Foissner et al. (2002), and species were identified in vivo and in silver preparations. Briefly, this method involves placing 50–500 g litter and mineral soil in a Petri dish (13–18 cm wide, 2–3 cm high) and saturating, but not flooding it, with distilled water. Such culture is analysed for ciliates by inspecting about 2 ml of the run-off on days 2, 7, 14, 21, and 28.
Site 1
About 40 km north of Riyadh, surroundings of the village of Al-Jubailah (Al-Dschubaila), 47° E 25° N. A vegetable field irrigated with hard ground water. Soil greyish when dry, pH 6.4 in water; not saline, but enriched with minerals. The upper 0–2 cm litter and soil layer under a weed bush was collected.
Site 2
About 150 km northwest of Riyadh, surroundings of the village of Al-Qasab (Al-Kasab), 45°30′ E 26° N. Pasture land in a semi-desert with many flowers and bushes. Soil very sandy, yellowred, pH 6.7, not saline, grass roots covered with a thick layer of mycorrhiza (?). The very sandy soil was sieved with a kitchen sieve to a depth of 15 cm to enrich the sample with fine roots and partially decomposed organic matter, mainly plant remnants.
Site 3
About 200 m distant from site 2. Soil saline (21‰) and covered with halophilous vegetation, pH 6.7 in water. The sample was collected as described for site 2.
Site 4
About 150 km east of Riyadh, a shifting, hardly vegetated sand dune in the surroundings of the village of Khurays, 48°10′ E 25°15′ N. In a small depression, where plant litter has accumulated, the sand was sieved to a depth of 20 cm to enrich the sample with fine roots and incompletely decomposed organic material; pH 6.2 in water, slightly saline (<5‰).
Site 5
Near site 4, but another, well-vegetated sand dune. Sampling as described at site 4, i.e., in a small depression where sand grains and organic debris were bound to crumbles by a massive growth of fungal hyphae; grass roots covered with a thick layer of mycorrhiza (?), similar as at site 2. Soil (sand) not saline, pH 6.0 in water.
Site 6
A leguminosae field in the Al-Hassa oasis, a few km east of the town Al-Hofuf (Al-Hufuf), 49°31′ E 25°20′ N. Soil very sandy, stongly penetrated by leguminosae roots, pH 6.0 in water. The upper 0–5 cm litter and soil (sand) layer was collected and enriched with litter from the surroundings.
RESULTS AND DISCUSSION
Faunistics
The six samples contained 135 identified ciliate species, all new for the fauna of Saudi Arabia. At least seven species were undescribed (Table 1): Spathidium alqasabi, Enchelyodon alqasabi, Saudithrix terricola Berger et al. (2006), Metauroleptus arabicus, Pseudohemisincirra arabica, Oxytricha arabica and Erimophyra monostyla. About 20 further species, half of which is likely undescribed, were noted in the protargol slides, but could not be described because of lack of live observations and/or sufficient specimens (Table 1). 135 (+20) species is a considerable number compared to the about 100 species found in 150 (!) samples of 1 ha of upland grassland in Southern Scotland (Esteban et al. 2006), the about 100 species reported from several sites of The Netherlands, Belgium and Denmark (Foissner and Al-Rasheid 2007), and the 87 species found in six samples from Singapore (Foissner 2008b). On the other hand, 80 to 140 species may be found in single samples (Foissner 1995; Foissner et al. 2002, 2005), showing that the Arabian sites are in the mid-range.
Table 1.
Ciliate species found in six soil samples from Saudi Arabia. For authors and dates of species, see Foissner (1998) and Foissner et al. (2002)
| Species | Sites | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Acaryophrya collaris | + | − | − | − | − | − |
| Acropisthium mutabile | + | − | − | − | − | − |
| Amphisiella australis | − | + | − | − | + | − |
| Amphisiella elegans | − | − | + | − | − | − |
| Amphisiella magnigranulosa | − | − | − | + | − | − |
| Apertospathula verruculifera | + | − | − | − | − | − |
| Apocyclidium obliquum | + | − | − | + | − | + |
| Apospathidium longicaudatum | + | − | + | − | + | − |
| Arcuospathidium lionotiforme | − | − | − | − | − | + |
| Arcuospathidium muscorum muscorum | + | − | − | − | + | − |
| Arcuospathidium namibiense tristicha | − | − | + | − | − | − |
| Armatospathula periarmata | − | − | − | − | + | − |
| Blepharisma hyalinum | − | − | − | + | − | − |
| Blepharisma steinii | + | − | − | + | − | − |
| Cinetochilum margaritaceum | − | − | − | + | − | − |
| Circinella filiformis | − | + | + | − | − | − |
| Clavoplites edaphicus | + | − | − | − | − | − |
| Colpoda aspera | + | + | − | + | + | + |
| Colpoda cucullus | + | + | + | + | + | + |
| Colpoda edaphoni | − | − | − | + | − | − |
| Colpoda formisanoi | − | − | − | + | − | − |
| Colpoda henneguyi | − | − | − | − | − | + |
| Colpoda inflata | + | + | + | + | + | + |
| Colpoda magna | + | − | − | − | − | − |
| Colpoda maupasi | + | + | + | + | + | + |
| Colpoda steinii | + | + | + | + | + | + |
| Colpodidium (Colpodidium) trichocystiferum | + | + | − | − | − | − |
| Condylostomides trinucleatus | + | − | − | − | − | − |
| Cyrtohymena citrina | + | − | − | − | − | + |
| Cyrtolophosis muscicola | + | − | − | − | − | − |
| Dileptus mucronatus | − | − | − | − | − | + |
| Dileptus visscheri | + | − | − | − | − | − |
| Drepanomonas pauciciliata | − | − | − | + | − | − |
| Drepanomonas sphagni | + | − | − | − | − | − |
| Enchelyodon alqasabi nov. spec. | − | − | + | − | − | − |
| Enchelyodon armatides | − | − | − | + | − | − |
| Enchelyodon nodosus | + | − | − | − | − | − |
| Enchelys geleii | − | + | − | − | − | − |
| Enchelys polynucleata | − | − | − | + | − | − |
| Epispathidium ascendens | + | − | − | − | − | − |
| Epispathidium polynucleatum | + | − | − | − | − | − |
| Erimophyra monostyla nov. spec. | + | + | − | − | − | + |
| Euplotopsis incisa | − | − | − | − | − | + |
| Euplotopsis muscicola | + | + | − | − | − | − |
| Exocolpoda augustini | − | + | + | + | + | − |
| Fuscheria nodosa | + | − | − | − | − | − |
| Fuscheria terricola | + | + | + | − | + | + |
| Gastrostyla steinii | + | − | − | − | − | − |
| Gonostomum affine | + | + | + | + | + | + |
| Grossglockneria acuta | − | + | − | + | − | − |
| Halteria grandinella | + | + | − | − | − | − |
| Hausmanniella patella | − | − | − | − | − | + |
| Hemiamphisiella terricola | − | − | − | − | − | + |
| Hemisincirra inquieta | + | − | − | + | − | + |
| Hemisincirra rariseta | − | + | − | − | − | − |
| Hemiurosoma terricola | + | − | − | − | − | − |
| Holostichides chardezi | − | − | − | − | − | + |
| Holostichides terricola | − | − | + | + | + | − |
| Homalogastra setosa | + | + | + | + | − | + |
| Keronopsis dieckmanni | − | − | − | − | − | + |
| Kuklikophrya ougandae | + | − | − | − | − | − |
| Lamtostyla halophila | − | − | + | − | − | − |
| Lamtostyla islandica | − | − | − | + | − | + |
| Leptopharynx costatus | + | + | − | + | + | + |
| Metauroleptus arabicus nov. spec. | − | + | − | − | − | − |
| Metopus gibbus | + | − | − | − | − | − |
| Metopus hasei | + | + | − | − | − | − |
| Metopus minor | + | − | − | − | − | − |
| Metopus ovalis | + | − | − | − | − | − |
| Metopus palaeformis | + | − | − | − | − | − |
| Monodinium perrieri | + | + | − | − | − | − |
| Mykophagophrys terricola | + | − | − | + | − | − |
| Nassula tuberculata | − | − | + | − | − | + |
| Nassulides pictus | + | − | − | − | − | − |
| Nivaliella plana | + | + | − | + | + | + |
| Odontochlamys alpestris biciliata | − | − | − | − | − | + |
| Odontochlamys convexa | + | + | + | − | − | − |
| Oxytricha arabica nov. spec. | − | + | − | − | + | − |
| Oxytricha elegans | + | − | − | − | − | − |
| Oxytricha granulifera | + | − | − | − | − | − |
| Oxytricha longa | + | + | − | − | − | − |
| Oxytricha setigera | + | − | − | − | − | − |
| Paraenchelys terricola | − | − | − | − | − | + |
| Parafurgasonia sorex | − | − | − | − | − | + |
| Paragonostomum binucleatum | − | + | − | − | − | − |
| Paragonostomum caudatum | + | − | − | + | − | + |
| Paragonostomum multinucleatum | − | − | + | − | − | − |
| Periholosticha paucicirrata | + | + | − | − | − | − |
| Plagiocampa difficilis | − | + | + | + | − | + |
| Plagiocampa ovata | + | − | − | − | − | − |
| Plagiocampa pentadactyla | + | − | − | − | − | − |
| Plagioicampa rouxi | + | − | − | − | − | − |
| Platyophrya macrostoma | − | + | − | + | − | + |
| Platyophrya spumacola spumacola | − | + | − | + | + | + |
| Platyophrya vorax | − | + | − | + | + | + |
| Plesiocaryon elongatum | + | − | + | + | + | + |
| Pleuroplites australis | − | − | − | − | − | + |
| Podophrya halophila | − | + | − | − | − | − |
| Protocyclidium muscicola | + | − | + | + | − | − |
| Protocyclidium terricola | + | + | − | + | − | − |
| Pseudochilodonopsis mutabilis | + | + | − | − | − | − |
| Pseudocohnilembus persalinus hexakineta | − | − | + | − | − | − |
| Pseudocyrtolophosis alpestris | + | − | − | + | − | − |
| Pseudohemisincirra arabica nov. spec. | − | + | − | − | − | − |
| Pseudoholophrya terricola | − | − | − | − | − | + |
| Pseudoplatyophrya nana | + | + | − | + | + | − |
| Pseudoplatyophrya saltans | + | − | − | − | − | − |
| Pseudourostyla franzi | − | − | − | + | − | − |
| Pseudovorticella sphagni | + | − | − | − | − | − |
| Rigidocortex octonucleatus | + | − | − | − | − | − |
| Rostrophrya fenestrata | − | − | + | − | − | − |
| Sagittaria hyalina | + | − | + | − | − | − |
| Sathrophilus muscorum | − | + | + | − | + | + |
| Saudithrix terricola nov. gen., nov. spec. | + | − | − | − | − | − |
| Semiplatyophrya foissneri | − | − | + | − | − | − |
| Spathidium alqasabi nov. spec. | − | − | + | − | − | − |
| Spathidium claviforme | + | + | + | + | − | − |
| Spathidium etoschense | − | − | − | − | − | + |
| Spathidium procerum | + | + | − | − | − | − |
| Spathidium spathula | + | − | − | − | − | − |
| Sterkiella histriomuscorum-complex | + | − | − | − | − | + |
| Stylonychia bifaria | + | − | − | − | − | − |
| Tachysoma humicola humicola | + | − | − | + | − | − |
| Terricirra matsusakai | + | − | − | − | + | − |
| Tetrahymena rostrata | + | − | − | − | − | + |
| Uroleptus notabilis | − | + | + | + | − | + |
| Urosoma emarginata | + | − | − | − | − | − |
| Urosomoida agiliformis | + | − | + | + | − | − |
| Urosomoida agilis | − | + | − | − | − | + |
| Urostyla grandis | + | − | − | − | − | − |
| Vorticella astyliformis | + | + | + | − | − | − |
| Vorticella infusionum | + | − | − | − | − | − |
| Wolfkosia loeffleri | + | − | − | − | − | − |
| Woodruffia rostrata | − | − | − | − | − | + |
| Woodruffides terricola | + | + | − | − | − | − |
|
| ||||||
| Number of species identified | 80 | 45 | 31 | 40 | 22 | 42 |
| New species | 2 | 4 | 3 | 0 | 1 | 1 |
| Number of unidentified taxa | 13 | 7 | 4 | 0 | 0 | 0 |
| Number of unidentified, likely undescribed taxa | 7 | 3 | 1 | 0 | 0 | 0 |
| Total number of taxa | 93 | 51 | 35 | 40 | 22 | 42 |
The richest samples were from a vegetable field and pasture land (samples 1, 2, 6; 80, 45, 42 species), while the natural sites 3, 4, and 5 contained only 22–40 species (Table 1). This is not surprising. Agriculture and pastures are set up on fertile soils, while the dry and/or saline sites are rather extreme (for details, see Foissner 1987b).
Sites 4 and 5 are from sand dunes and can be compared with similar sites globally (Table 2). Species richness in the Arabian dunes matches the general pattern, i.e., single samples provide 20–50 ciliate species (but see below). Further, ciliate abundances are usually high, i.e., a few days after rewetting the samples masses of ciliates appear, most possibly originating from resting cysts. The high species diversity is surprising because such numbers are widely found in various soils, for instance, in Central European meadows (Foissner 1987b) and natural forest stands (Foissner et al. 2005). Obviously, ciliates are adapted to desert conditions, mainly by their ability to form quickly a highly resistant dormant stage, the resting cyst, when the environmental conditions become detrimental. Further, a large group of soil ciliates, the Colpodea, contains mainly r-selected species adapted to extreme conditions (Foissner 1993, Lüftenegger et al. 1985). Last but not least, part of the rich diversity and high individual numbers might be caused by reduced metazoan competition and cyst predation in such extreme habitats.
Table 2.
Comparison of ciliate diversity in sand dunes of Arabia, Namibia, Australia, the USA, and Europe
| Regions/sites | Identified species | Number of unidentified species |
Number of new speciesa |
Total | References |
|---|---|---|---|---|---|
| Saudi Arabia, site 4 | 40 | 0 | 0 | 40 | this paper |
| Saudi Arabia, site 5 | 23 | 0 | 1 | 23 | this paper |
| Namibia, site 26 | 31 | 3 | 3 | 34 | Foissner et al. (2002) |
| Namibia, site 33 | 47 | 2 | 7 | 49 | Foissner et al. (2002) |
| Australia, site 5 | 36 | 0 | 3 | 36 | both from Blatterer and Foissner (1988) |
| Australia, site 9 | 34 | 8 | 2 | 42 | |
| USA, Utah | 26 | 0 | 1 | 26 | Foissner (1994) |
| The Netherlands, site 1, Europe | 41 | 0 | 3 | 41 | both from Foissner and Al-Rasheid (2007) |
| Norderney, Germany, Europe | 29 | ~10 | ? | 39 |
Saudi Arabia, site 5: Oxytricha arabica; Nambia, site 26: Paragonostomum caudatum, Plesiocaryon terricola, Spathidium namibicolar; Namibia, site 33: Diplites arenicola, Metacineta namibiensis, Paragonostomum binucleatum, Paragonostomum rarisetum, Protospathidium namibicola, Spathidium lanceoplites, Urosomoida deserticola; Australia, site 5: Coriplites australis, Phialinides australis, Pseudoplatyophrya saltans; Australia, site 9: Oxytricha granulifera quadricirrata, Rostrophryides australis; USA: Circinella arenicola; The Netherlands, site 1: Apobryophylhum schmidingeri, Dileptus n. sp., Keronopsis schminkei.
Foissner (1987b) and Foissner et al. (2002) showed that, due to methodological shortcomings, a single sample from a certain site provides only about one third of the species actually present, i.e., when the same site is investigated 10 or more times in the course of one or two years. This is supported by a recent study on 12 Austrian forest stands, which were investigated in spring and autumn (Foissner et al. 2005): 68 species (first sample)/ 75 (second sample)/ 92 (total), 73/ 58/ 87, 65/ 64 / 83, 39/ 34/ 52, 67/ 61/ 90, 36/ 33/ 45, 38/ 55/ 69, 28/ 49/ 54, 72/ 101/ 120, 55/ 60/ 86, 75/ 77/ 99, 60/ 63/ 81. Thus, the gain of species by the second sample ranges between 16% and 48%, on average 30%, i.e., only one third because the sites were investigated only two times. These values must be taken into account when interpreting the data from single samples. If we apply the “1/3 rule” to the richest site 1 (93 species) and to the poorest site 5 (22 species), 280 and 66 species can be expected, respectively. If we apply only 30%, still 133 and 31 species might be present. Likely, the true numbers lie between these extremes. Unfortunately, such extrapolations cannot be applied to the total number of species found in six or more samples because multiple samples from a certain, small area represent some sort of replication of a single site (Foissner et al. 2002). Nonetheless, the extrapolated values match literature data (Foissner 1987b, Foissner et al. 2002), showing that the Arabian samples are in the ordinary range.
Biogeographic aspects
Most of the new species discovered would not have been recognized 30–40 years ago, when ciliate alpha-taxonomy was much coarser and protargol impregnation and morphometry were not as widely used as today. But are these details really of significance at species level? As an example, we choose Oxytricha arabica and the cosmopolitan (?) O. lanceolata. Their size, cytology, cirral pattern, and morphometrics are highly similar, and thus the Oxytricha key of Berger (1999) guides to O. lanceolata. However, the dorsal kinetid (bristle) pattern is quite different. Of the three different features, the split of kinety 3 greatly influences the bristle pattern: while O. lanceolata belongs to the oxytrichids with four dorsal ciliary rows, O. arabica and the type species of Oxytricha belong to the group with five rows. As far as we know, this pattern is hardly variable and ontogenetically fixed (Berger 1999). Classifying the Arabian population as a distinct species is also supported by recent sequence data which show that Oxytricha is polyphyletic, and thus likely consists of much more species than recognized presently (Schmidt et al. 2007); many of these will be cryptic species like O. arabica. For instance, the study of Schmidt et al. (2007) shows that two populations of Oxytricha granulifera, virtually differing only in the shape of the cortical granules (globular vs. oblong), have rather different rRNA gene sequences, supporting the classification as distinct species, viz., O. granulifera and O. longigranulosa (for a review, see Berger 1999). A similar case has been reported in Glaucoma by Fried and Foissner (2007). Thus, inconspicuous differences in important features might be indicative of cryptic speciation.
Lumping and splitting of species greatly influences their perceived geographical range, one of the main problems in the distribution controversy of protists (for reviews, see Foissner 2006, 2008a). Most of the seven new species found in the six Arabian samples have been recorded only from their type locality, suggesting that some might have restricted distribution. A good candidate was Saudithrix terricola Berger et al., 2006, a flagship species with a size of about 270 × 100 μm. However, recently this species has been rediscovered in soil from the floodplain of the Yangtze River in Wuhan, China (Foissner 2007). This does not mean that S. terricola is a cosmopolitan, but the range is larger than originally expected. Two further species, previously known only from Venezuela, have been observed in the Arabian samples, viz., Armatospathula periarmata and Apertospathula verruculifera, both described in Foissner and Xu (2007). We rediscovered also some species as yet known only from Namibia, Southwest Africa (Foissner et al. 2002): Spathidium etoschense, Pseudocohnilembus persalinus hexakineta, Hemisincirra rariseta, and Rostrophrya fenestrata.
Obviously, investigations like the present one broaden the known geographical range of some species or of even supposed endemic flagships. However, usually the loss is less than the gain, that is, more undescribed species are found than described “endemics” are rediscovered (see this study and Foissner et al. 2005, Foissner and Al-Rasheid 2007, Foissner 2008b).
There is also a hot debate on the number of free-living ciliate species. While Finlay (2001) suggests 3,000–4,000 species, Foissner et al. (2008a) estimate 30,000 species. We believe that the later number is supported by, e.g., our data on the genus Erimophrya. Foissner et al. (2002) founded the genus with two species, E. glatzeli (type) and E. arenicola, both occurring in the dunes of the Namib Desert. Later, Foissner et al. (2005) described two further species from a Pinus nigra forest near Vienna, Austria: E. sylvatica and E. quadrinucleata. Now, a fifth species has been discovered, showing our ignorance on soil ciliate diversity: five new species were discovered in a single genus during the short period 2002–2008! All species obviously prefer meagre, extreme habitats, such as sand dunes, coniferous litter, and saline coastal soil.
Interestingly, the five species belong to three biogeographical regions: the Palaeotropis (E. glatzeli, E. arenicola), the Holarctis (E. sylvatica, E. quadrinucleata), and the transition zone of both (E. monostyla). Unfortunately, Erimophrya species are difficult to identify in vivo; further, they resemble several Urosomoida and Hemisincirra species. Thus, a definite conclusion about their biogeography is not yet possible. However, it is remarkable that, as yet, none of the species has been reported from outside its region.
Description of species
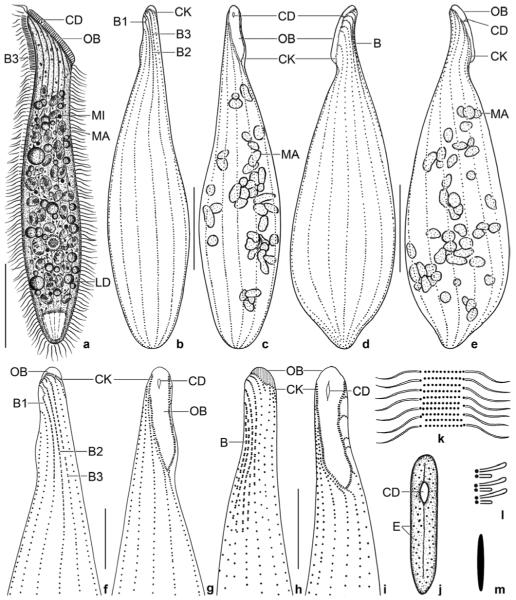
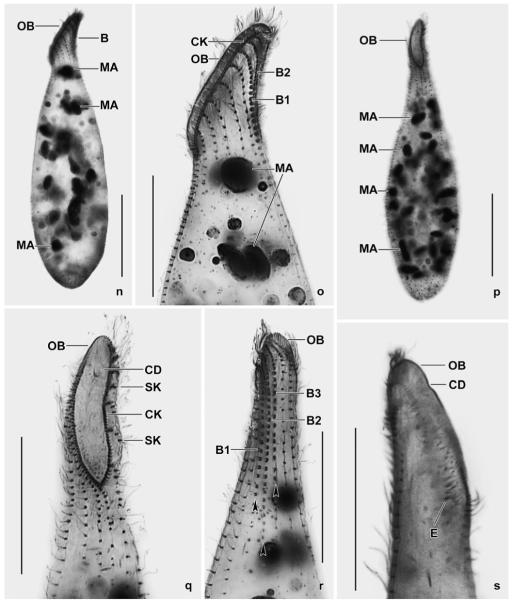
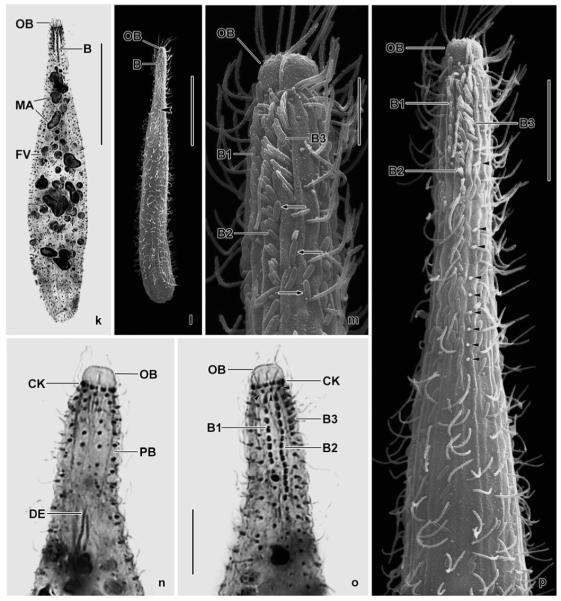
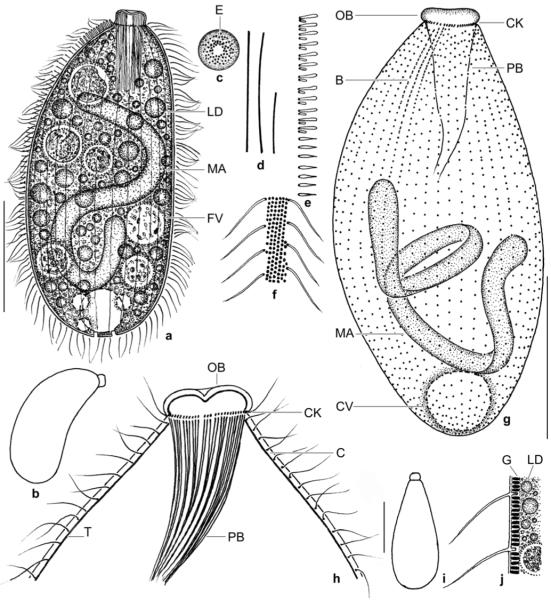
Spathidium alqasabi nov. spec. (Fig. 1a-s; Tables 3, 4)
Figs 1.
a–m. Spathidium alqasabi from life (a, j–m) and after protargol impregnation (b–i). a – right side view of a representative specimen, length 200 μm; b, c, f, g – ciliary pattern of dorsal and ventral side and macronucleus of holotype specimen, length 210 μm; d, e – infraciliature of left and right side of a strongly inflated specimen; h, i – dorsal and ventral view of a specimen with four dorsal brush rows; j – frontal view of oral bulge showing the conical depression; k – surface view showing cortical granulation; l – bristles of brush row 1; m – extrusome, length 4 μm. B (1–3) – dorsal brush (rows), CD – conical depression, CK – circumoral kinety, E – extrusomes, LD – lipid droplets, MA – macronucleus nodules, MI – micronucleus, OB – oral bulge. Scale bars: 50 μm (a–e) and 20 μm (f–i).
n–s. Spathidium alqasabi, morphology and ciliary pattern after protargol impregnation. n, o – left side views showing the scattered macronucleus nodules, the steep oral bulge, and part of the dorsal brush; p, q, s – ventral and lateral views showing the shape of the circumoral kinety, the scattered macronucleus nodules and, specifically, the conical depression near the dorsal end of the oral bulge; r – dorsal view with ends of dorsal brush rows marked by arrowheads. Rows 1 and 3 have a similar length, an unusual feature. B (1–3) – dorsal brush (rows), CD – conical depression in oral bulge, CK – circumoral kinety, E – extrusomes, MA – macronucleus nodules, OB – oral bulge, SK – somatic kineties. Scale bars: 50 μm (n, p) and 30 μm (o, q–s).
Table 3.
Morphometric data on Spathidium alqasabi. Data based on mounted, protargol-impregnated (Foissner 1991, protocol A), and randomly selected specimens from a non-flooded Petri dish culture. Measurements in μm. CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of individuals investigated, SD – standard deviation, SE – standard error of arithmetic mean, x̄ – arithmetic mean
| Characteristics | x̄ | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|
| Body, length | 171.3 | 174.0 | 20.8 | 4.3 | 12.2 | 122.0 | 205.0 | 23 |
| Body, width | 46.2 | 45.0 | 7.6 | 1.6 | 16.5 | 33.0 | 62.0 | 23 |
| Body length:width, ratio | 3.8 | 3.6 | 0.8 | 0.2 | 21.3 | 2.5 | 5.6 | 23 |
| Oral bulge, length | 30.2 | 30.0 | 3.7 | 0.8 | 12.3 | 24.0 | 40.0 | 22 |
| Oral bulge, height | 2.8 | 3.0 | - | - | - | 2.0 | 3.0 | 13 |
| Body length:length of oral bulge, ratio | 5.7 | 5.6 | 0.7 | 0.2 | 12.8 | 4.5 | 7.3 | 21 |
| Brush kinety 1, length a | 29.7 | 29.0 | 8.2 | 1.9 | 27.6 | 20.0 | 47.0 | 19 |
| Brush kinety 2, lengtha | 42.2 | 42.0 | 6.8 | 1.6 | 16.0 | 31.0 | 57.0 | 18 |
| Brush kinety 3, length a | 32.0 | 31.5 | 8.6 | 2.0 | 27.0 | 16.0 | 49.0 | 18 |
| Anterior body end to first macronucleus nodule, distance | 40.9 | 41.0 | 8.9 | 1.9 | - | 26.0 | 58.0 | 23 |
| Macronucleus nodules, length | 8.1 | 8.0 | 1.2 | 0.3 | 15.0 | 5.0 | 10.0 | 23 |
| Macronucleus nodules, width | 5.5 | 5.0 | 0.9 | 0.2 | 16.4 | 4.0 | 8.0 | 23 |
| Macronucleus nodules, number | 34.1 | 35.0 | 5.9 | 1.2 | 17.4 | 24.0 | 48.0 | 23 |
| Somatic kineties, number | 14.5 | 15.0 | 1.5 | 0.3 | 10.1 | 10.0 | 18.0 | 23 |
| Ciliated kinetids in a right side kinety, number | 93.4 | 93.0 | 11.9 | 2.9 | 12.7 | 72.0 | 115.0 | 17 |
| Dorsal brush rows, number | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 |
| Dikinetids in brush row 1, number | 20.7 | 21.0 | 3.7 | 0.9 | 17.9 | 15.0 | 29.0 | 19 |
| Dikinetids in brush row 2, number | 32.5 | 32.5 | 5.4 | 1.3 | 16.7 | 24.0 | 45.0 | 18 |
| Dikinetids in brush row 3, number | 21.2 | 22.0 | 5.6 | 1.3 | 26.6 | 11.0 | 32.0 | 18 |
Distance between circumoral kinety and last dikinetid of row.
Table 4.
Comparison of main features in four Spathidium seppelti-like species. SA – Spathidium alqasabi (Saudi Arabia), SFR – S. fraterculum c (from Foissner et al. 2002), SF – S. foissneri (from Vd’ačný et al. 2006), SS – Spathidium seppelti (from Petz and Foissner 1997)
| Characteristicsa | SA | SFRc | SF | SS |
|---|---|---|---|---|
| Body, length | 171.3 | 133.5 | 149.6 | 99.3 |
| Body, width | 46.2 | 25.1 | 25.7 | 24.8 |
| Body length:width, ratio | 3.8 | 5.4 | 5.9 | 4.0 |
| Oral bulge, length | 30.2 | 40.9 | 38.5 | 31.5 |
| Body length:length of oral bulge, ratio | 5.7 | 3.3 | 3.9 | 3.1 |
| Brush kinety 1, lengthb | 29.7 | 34.0 | 31.9 | 18.1 |
| Brush kinety 2, lengthb | 42.2 | 38.1 | 36.9 | 22.3 |
| Brush kinety 3, lengthb | 32.0 | 35.5 | 10.7 | 9.6 |
| Macronucleus nodules, numberd | 35 | 67 | 25 | >100 |
| Somatic kineties, numberd | 15.0 | 28.0 | 23.0 | 21.0 |
| Dikinetids in brush row 1, numberd | 21.0 | 27.0 | 30.0 | 13.0 |
| Dikinetids in brush row 2, numberd | 32.0 | 40.0 | 35.0 | 18.0 |
| Dikinetids in brush row 3, numberd | 22.0 | 27.0 | 10.0 | 8.0 |
| Extrusomes, shape and size | very slenderly ellipsoidal; 4–5 μm long |
rod-shaped; type I: 5–6 μm long, type II: 3 μm long |
rod-shaped to slightly ellipsoidal; 5 μm long |
rod-shaped; 3–4 μm long |
| Conical depression in oral bulge | present | absent | absent | present |
| Ecology | highly saline sand soil | slightly saline soil from Etosha Pan |
terrestrial moss from xero- thermic meadow |
algal ornithogenic soil |
| Type locality | Saudi Arabia | Namibia | Slovakia | Antarctica |
Data based on protargol-impregnated (Foissner’s method for S. alqasabi, S. foissneri, S. fraterculum; Wilbert’s method for S. seppelti) specimens from non-flooded Petri dish cultures. Except where noted, based on arithmetic means. Measurements in μm.
Distance between circumoral kinety and last dikinetid of row.
Spathidium seppelti etoschense in Foissner et al. (2002). The new name is required to avoid homonymy (see description of S. alqasabi).
Median values.
Diagnosis
Size about 200 × 35 μm in vivo. Shape typically spathidiid. Oral bulge with obconical depression and of about same length as body width. On average 34 ellipsoidal, scattered macronucleus nodules and several micronuclei. Extrusomes very slenderly ellipsoidal, about 5 μm long. On average 15 ciliary rows. Dorsal brush heterostichad occupying about 24% of body length; brush row 1 composed of 21 dikinetids, row 2 of 32, and row 3 of 21 dikinetids on average.
Type locality
Highly saline (21‰) sand soil in the surroundings of the village of Al-Qasab, about 150 km NW of the town of Riyadh, Saudi Arabia, 45°30′ E 26° N.
Type material
1 holotype and 5 paratype slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Upper Austrian Museum in Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Etymology
Named after the village the species was discovered.
Description
Size 140–240 × 25–50 μm, usually about 200 × 35 μm in vivo, while 171 × 46 μm in protargol preparations; length:width ratio also highly different, i.e., about 6:1 in vivo and 3.8:1 in protargol preparations, where specimens are heavily inflated due to insufficient fixation (Figs 1a, d, n; Table 3). Shape elongate spatulate, mid-body circular in cross-section, anterior region laterally flattened, hyaline, and with slightly convex oral bulge; posterior body portion slightly tapering (Figs 1a–e). On average 34 globular to broadly ellipsoidal macronucleus nodules scattered throughout cytoplasm (Figs 1a, c, e, n–p; Table 3). Micronuclei 1.5–2 μm across, scattered between macronucleus nodules, exact number difficult to determine because hardly distinguishable from similar-sized and impregnated cytoplasmic inclusions. Contractile vacuole in posterior body end, with several excretory pores in pole centre. Extrusomes attached to oral bulge, basically rod-shaped, but on detailed analysis very slenderly ellipsoidal, 4–5 μm long, impregnate faintly with the protargol method used (Figs 1m, s); developing extrusomes scattered in cytoplasm and heavily impregnated with protargol. Cortex very flexible, contains ca 10 rows of minute, about 0.5 × 0.2 μm-sized granules between each two ciliary rows (Fig. 1k). Cytoplasm colourless, more or less densely packed with lipid droplets 1–10 μm across and food vacuoles with granular contents, except for hyaline anterior portion. Likely feeds on ciliates.
Cilia about 10 μm long in vivo, densely spaced (≤2 μm) and arranged in an average of 15 ordinarily spaced (~7.3 μm), equidistant rows; widely spaced, i.e., ~9.5 μm in protargol preparations due to insufficient fixation; those of right side abutting to circumoral kinety at acute angles, while those of left side attached to circumoral kinety at right angles and with anterior end composed of 2–8 closely spaced cilia (Figs 1d, g, o, r). Dorsal brush three-rowed, a fourth row occurs in a few specimens (Fig. 1h); rather short because occupying on average only 24% of body length; heterostichad because row 1 about 30% shorter than longest row 2; inconspicuous because bristles merely up to 4 μm long and only slightly inflated distally. Rows 1 and 3 similar both in average length (~30 μm vs. 32 μm) and number of dikinetids (21 and 22), brush row 2 about 42 μm long and composed of an average of 32 dikinetids; row 3 continues to mid-body with a monokinetidal tail of about 2.5 μm long bristles. Anterior bristle of pairs of brush row 1 about 4 μm long, posterior bristle shortened by about 50% (Fig. 1l), length decreases to 2 μm posteriorly; bristles of rows 2 and 3 similar to those of row 1, but only 3 μm long (Figs 1b, d, f, n, o, r; Table 3).
Oral bulge of about same length as body width in vivo, whilst about one third shorter than average body width in protargol preparations because specimens became heavily inflated by the preparation procedures (Table 3); slightly convex when viewed laterally, oblong with bluntly pointed ventral end in frontal view (Figs 1a, c, g, i, q). A conical depression near dorsal end of bulge, recognizable both in vivo and after protargol impregnation (Figs 1a, c–e, g, i, j, q, s), seen in all appropriately oriented specimens (>30). Circumoral kinety elongate elliptical with acute proximal end, composed of closely spaced dikinetids associated with nematodesmata forming the oral basket (Figs 1c, g, i, p, q).
Occurrence and ecology
See Enchelyodon alqasabi.
Comparison with related species
Originally, we classified Spathidium alqasabi as a fourth subspecies of S. seppelti. However, there is no correlation of specific features between these taxa, suggesting that all represent distinct species (Table 4). For instance, S. alqasabi and S. seppelti both have a conical oral bulge depression, but differ considerably in body length (170 μm vs. 100 μm), the relative length of the oral bulge (5.7 vs. 3.1), the number of ciliary rows (15 vs. 21) and, especially, in the dorsal brush pattern (rows 1 and 3 of same length, a very unusual feature vs. of distinctly different length, as usual). A further example: the curious dorsal brush pattern (rows 1 and 3 of same length) is found in S. alqasabi and S. etoschense, but only S. alqasabi has an oral bulge depression.
Obviously, the four taxa represent cryptic species rather similar at first glance, but distinctly different on more detailed analysis. This is emphasized by the different geographic regions they originate (Table 4). Thus, we raise S. seppelti foissneri and S. seppelti etoschense to species rank – Spathidium foissneri Vd’ačný et al., 2006 nov. stat. and Spathidium fraterculum nov. nom. (for S. seppelti etoschense Foissner et al., 2002). The new name is required to avoid homonymy with Spathidium etoschense Foissner et al., 2002.
There are some similarities between S. alqasabi and Arcuospathidium multinucleatum Foissner, 1999, viz., body shape, scattered macronucleus nodules, conical depression in oral bulge, and a monokinetidal bristle tail of brush row 3 extending to mid-body. However, the ciliary pattern (spathidiid vs. arcuospathidiid) and the oral bulge (elliptical vs. narrowly cuneate) are completely different. Further, the dorsal brush of A. multinucleatum is isostichad, whilst that of S. alqasabi is heterostichad.
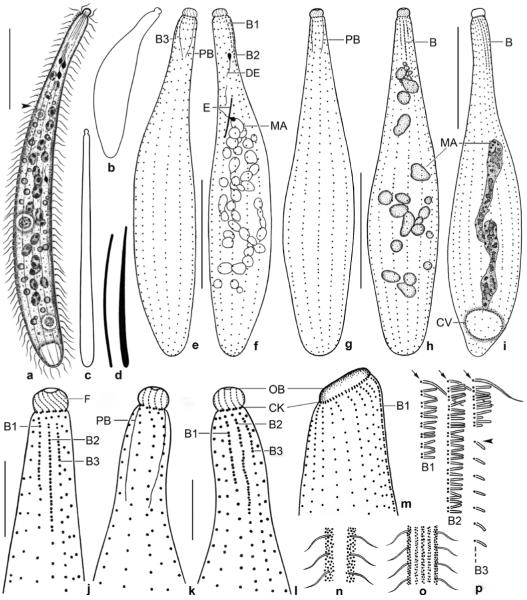
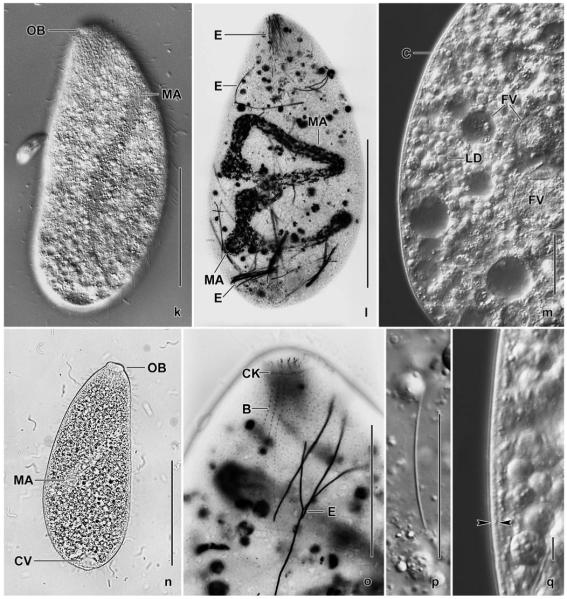
Enchelyodon alqasabi nov. spec. (Figs 2a–p, 3a–p; Table 5)
Figs 2.
a–p. Enchelyodon alqasabi (a–h, j–l, n–p), E. armatides (d, i; from Foissner et al. 2002), and Semispathidium armatum (m; from Foissner et al. 2002) from life (a, c, d, n–p) and after protargol impregnation (b, e–m). a – right side view of a representative specimen, length 180 μm. Arrowhead marks end of bristle tail of brush row 3; b – strongly contracted specimen; c – extreme shape variant; d – comparison of extrusomes of E. alqasabi (left; length 17 μm) and E. armatides (right; length 18 μm); e, f – ciliary pattern of right and left side and macronucleus of a paratype specimen; g, h, j – ciliary pattern of ventral and dorsal side and macronucleus of holotype specimen, length 120 μm; i – dorsolateral view of E. armatides, which has a tortuous macronucleus strand; k–m – ventral and dorsal view of a specimen with slightly curved ciliary rows, thus resembling Semispathidium (m); n, o – surface views showing cortical granulation in anterior third (n) and in mid-body (o); p – fine structure of dorsal brush. All rows commence with an ordinary monokinetid (arrows), and row 3 continues to second third of body with a monokinetidal bristle tail (arrowhead). B (1–3) – dorsal brush (rows), CK – circumoral kinety, CV – contractile vacuole, DE – developing extrusome, E – extrusome, F – oral bulge fibres, MA – macronucleus or macronucleus nodules, OB – oral bulge, PB – pharyngeal basket. Scale bars: 40 μm (a, e–i) and 10 μm (j–l).
Figs 3.
a–j. Enchelyodon alqasabi after protargol impregnation. a – body shape, infraciliature, and macronucleus pattern of a representative specimen; b–j – details of anterior body region of various specimens. Note developing extrusomes, inconspicuous pharyngeal basket, variation of oral bulge shape, and structure of dorsal brush. The dorsal brush is heterostichad, and the dikinetids of the circumoral kinety are composed of basal bodies side by side (j). Arrowheads in Fig. 3g, i mark the monokinetidal bristle tail of brush row 3. B – dorsal brush, B (1–3) – dorsal brush (rows), CK – circumoral kinety, DE – developing extrusomes, F – oral bulge fibres, MA – macronucleus nodules, OB – oral bulge, PB – pharyngeal basket. Scale bars: 50 μm (a) and 10 μm (b–j).
k–p. Enchelyodon alqasabi after protargol impregnation (k, n, o) and in the scanning electron microscope (l, m, p). k – dorsal view of holotype specimen showing dorsal brush and macronucleus nodules; l – general shape of a representative specimen. Arrowhead marks the end of the monokinetidal bristle tail of brush row 3; m, p – anterior body end of same specimen shown in (l). Note the structure of the dorsal brush and the cell surface covered by bacteria (arrows). Arrowheads mark the monokinetidal bristle tail of brush row 3; n, o – ventral and dorsal anterior body region of same specimen, showing the circumoral kinety with basal bodies of dikinetids side by side, the anteriorly slightly curved somatic kineties, and the short, slightly displaced anterior tail of the brush rows (arrowheads). B (1–3) – dorsal brush (rows), CK – circumoral kinety, DE – developing extrusomes, FV – food vacuole, MA – macronucleus nodules, OB – oral bulge, PB – pharyngeal basket. Scale bars: 40 μm (k, l), 10 μm (p, n, o), 5 μm (m).
Table 5.
Morphometric data on Enchelyodon alqasabi. Data based on mounted, protargol-impregnated (Foissner 1991, protocol A), and randomly selected specimens from a non-flooded Petri dish culture. Measurements in μm. CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of individuals investigated, SD – standard deviation, SE – standard error of arithmetic mean, x̄ – arithmetic mean
| Characteristics | x̄ | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|
| Body, length | 118.9 | 120.0 | 13.2 | 2.4 | 11.1 | 91.0 | 141.0 | 31 |
| Body, widtha | 24.4 | 24.5 | 4.7 | 0.9 | 19.3 | 18.0 | 35.0 | 30 |
| Body length:width, ratio | 5.0 | 4.8 | 1.1 | 0.2 | 22.4 | 2.7 | 7.3 | 31 |
| Oral bulge, width | 4.5 | 5.0 | - | - | - | 3.5 | 6.0 | 31 |
| Oral bulge, heigth | 3.1 | 3.0 | - | - | - | 2.5 | 4.5 | 31 |
| Anterior body to first macronucleus nodule, distance | 25.9 | 23.0 | 7.1 | 1.3 | 27.2 | 14.0 | 40.0 | 31 |
| Macronucleus nodules, length | 5.8 | 6.0 | 1.1 | 0.2 | 19.3 | 4.0 | 9.0 | 31 |
| Macronucleus nodules, width | 4.1 | 4.0 | 0.6 | 0.1 | 14.1 | 3.0 | 5.0 | 31 |
| Macronucleus nodules, number | 20.6 | 21.0 | 2.8 | 0.5 | 13.7 | 15.0 | 26.0 | 31 |
| Brush row 1, lengthb | 9.8 | 9.0 | 1.6 | 0.3 | 16.9 | 6.0 | 14.0 | 31 |
| Brush row 1, number of dikinetids | 7.2 | 7.0 | 1.0 | 0.2 | 14.2 | 5.0 | 9.0 | 31 |
| Brush row 2, lengthb | 15.9 | 16.0 | 2.5 | 0.4 | 15.5 | 11.0 | 20.0 | 30 |
| Brush row 2, number of dikinetids | 13.8 | 14.0 | 2.2 | 0.4 | 15.8 | 10.0 | 19.0 | 30 |
| Brush row 3, lengthb | 6.1 | 6.0 | 1.0 | 0.2 | 15.6 | 5.0 | 9.0 | 31 |
| Brush row 3, number of dikinetids | 4.7 | 5.0 | 0.8 | 0.1 | 16.3 | 4.0 | 7.0 | 31 |
| Number of ciliary rows (including brush) | 10.6 | 11.0 | 1.1 | 0.2 | 10.2 | 8.0 | 12.0 | 31 |
| Number of brush rows | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 31 |
| Kinetids in a ventral kinety, numberc | 52.3 | 54.0 | 7.7 | 2.0 | 14.7 | 37.0 | 64.0 | 15 |
One specimen with 41 μm excluded because caused by a big food vacuole.
Distance between circumoral kinety and last dikinetid of row.
Ciliated and unciliated kinetids (granules) were counted.
Diagnosis
Size about 180 × 20 μm in vivo, contractile up to one third of body length. Very slenderly clavate with conspicuous, hemispherical to slightly discoidal oral bulge about 5 μm across. On average 21 macronucleus nodules. Extrusomes rod-shaped, about 17 × 0.4 μm. On average 11 ciliary rows, three anteriorly differentiated to a heterostichad dorsal brush occupying about 10% of body length; brush row 1 composed of an average of 7 dikinetids, longest row 2 of 14, and row 3 of 5 dikinetids.
Type locality
Highly saline (21‰) sand soil in the surroundings of the village of Al-Qasab, about 150 km NW of the town of Riyadh, Saudi Arabia, 45°30′ E 26° N.
Type material
1 holotype and 5 paratype slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Upper Austrian Museum in Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Etymology
Named after the village the species was discovered.
Description
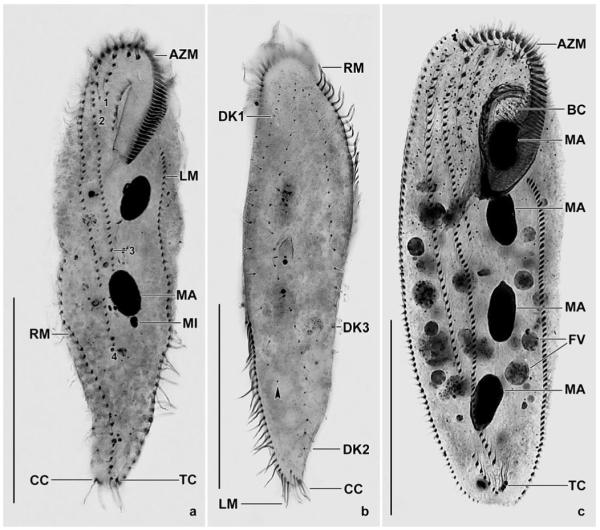
Size of extended specimens 130–230 × × 5–30 μm in vivo, usually about 180 × 20 μm, contracts slowly and about one third of body length under weak coverslip pressure or when taken up with the pipette; in protargol preparations on average only 120 × 25 μm and with a high width variability (CV 19%, Table 5), indicating about 35% length loss due to contraction, fixation and 15% preparation shrinkage. Shape in vivo and SEM preparations very slenderly clavate to almost cylindroidal (~9:1), unflattened (Figs 2a, c, 3l); in protargol preparations slenderly clavate or, when strongly contracted, bluntly fusiform (Figs 2b, e–h, 3a, k); narrow anterior third more or less distinctly curved and slightly inflated subapically, both in vivo and protargol preparations. On average 21 scattered macronucleus nodules; individual nodules globular to ellipsoidal, about 6 × 4 μm in size (Figs 2a, f, h, 3a, h, k; Table 5); number of micronuclei not known because indistinguishable from cytoplasmic inclusions. Contractile vacuole in posterior body end. Extrusomes attached to oral bulge, rod-shaped and slightly curved, about 17 × 0.4 μm in size and thus rather conspicuous in vivo (Fig. 2d), do not impregnate with the protargol method used; several fusiform developmental stages scattered in cytoplasm and heavily impregnated (Figs 2a, f, 3a, b, h, n). Cortex colourless and very flexible, definitely without scales (lepidosomes); cortical granules minute, i.e., about 0.3 μm across, but distinct in vivo because highly refractive, arranged in several rows between kineties in main body portion and scattered around kineties in anterior third (Figs 2n, o). Cytoplasm colourless, usually studded with lipid droplets 1–5 μm across and up to 40 μm-sized food vacuoles with granular or compact contents (Figs 2a, 3k); likely feeds on ciliates and, especially, heterotrophic flagellates (Polytomella). Glides slowly on microscope slide, rotates around main body axis when swimming.
Cilia about 7 μm long in vivo, ordinarily spaced (average ciliary distance 2–3 μm) and arranged in an average of 11 ordinarily spaced (~7 μm), meridional kineties slightly curved anteriorly in some specimens (Figs 2e–h, j–l, 3a, n, p). Dorsal brush three-rowed and heterostichad because row 3 distinctly shorter than row 2; inconspicuous because occupying only 10% of body length and bristles merely up to 3 μm long (Figs 2a, f, h, j, l, p, 3b, g–i, k, m, o, p; Table 5). Brush rows 1 and 3 conspicuously shorter than row 2, composed of 7 and 5 dikinetids, respectively; brush row 2 about 16 μm long and composed of an average of 14 dikinetids (Figs 2h, p); all rows with an anterior tail usually comprising a single monokinetid sometimes slightly out of line (Figs 2h, j, l, 3o); rarely, the anterior tail is lacking; row 3 continues to second third of body with a monokinetidal tail of 1.5 μm long bristles (Figs 2p, 3g, i, p). Bristles of rows 1 and 2 cylindroidal and gradually shortened from about 3 μm anteriorly to 2 μm posteriorly both in vivo and in protargol preparations; row 3 bristles similar to those of rows 1 and 2, but posterior bristle of last dikinetids shorted by about 50% (Figs 2p, 3m, p).
Oral bulge conspicuous in vivo, even at low magnification (× 100), because distinctly set off from body proper and about 4–6 μm across; in protargol preparations hemispherical to slightly discoidal, i.e., 5 × 3 μm on average (Figs 2a–c, e–h, j–l; 3a–p; Table 5). Circumoral kinety at base of oral bulge, composed of slightly oblique dikinetids with basal bodies side by side; left basal body with a fibre extending sigmoidally to centre of oral bulge, right one associated with a weakly impregnating nematodesma contributing to the inconspicuous, conical pharyngeal basket (Figs 2j–l, 3j, m–p).
Occurrence and ecology
As yet found only at type locality, i.e., highly saline (21‰, pH 6.7), red sand soil grown with some halophilous vegetation. Sand and soil from 0–15 cm were sieved for plant residues and used to set up, together with about 50% sand, a non-flooded Petri dish culture. Enchelyodon alqasabi and Spathidium alqasabi were numerous 48 hours after rewetting the sample. The slender shape and the contents of the food vacuoles indicate that they are euedaphic, rapacious species.
Generic assignment and comparison with related species
The generic home of this species is difficult to find because there are similarities with several families. An enchelyodonid (family Enchelyodontidae Foissner et al., 2002; Fig. 2i) relationship is suggested by the hemispherical oral bulge, the circular circumoral kinety, the meridionally extending ciliary rows, and the three-rowed dorsal brush. However, the basal bodies of the circumoral kinety pairs are arranged side by side (Figs 2j, 3j), similar to most members of the families Fuscheriidae Foissner et al., 2002 and Acropisthiidae Foissner and Foissner, 1988. Thus, we checked carefully the absence of oralized somatic monokinetids, the main feature of these families. There are also similarities with the Trachelophyllidae Kent, 1881, especially in body shape and contractility and the slight subapical widening of the neck (Figs 2e, k, 3a, b, n). However, E. alqasabi lacks epicortical scales, the main feature of the trachelophyllids (Foissner et al. 2002). Last but not least, E. alqasabi resembles the genus Semispathidium Foissner et al., 2002 because, in some specimens, the ciliary rows are slightly curved anteriorly (Figs 2k–m, 3n). However, this curvature might be caused by the contraction of the cell during fixation. Thus, we assign the Saudi Arabian population to Enchelyodon, the genus to which it fits best at the present state of knowledge (Fig. 2i).
We did not find any species in the literature that could be identical with E. alqasabi. However, size, shape, and contractility of E. alqasabi resemble E. kenyaensis and E. armatides, both described in Foissner et al. (2002), while the macronucleus pattern is distinctly different: many nodules in the former (Figs 2h, 3a, k) and a tortuous strand in the latter (Fig. 2i); the same applies to Trachelophyllum s.l. species, which have a great overall similarity to E. alqasabi, but usually possess only two large macronucleus nodules (Foissner et al. 2002). In vivo, E. alqasabi is easily identified by the slender shape, the conspicuous length, the hemispherical oral bulge, and the scattered macronucleus nodules.
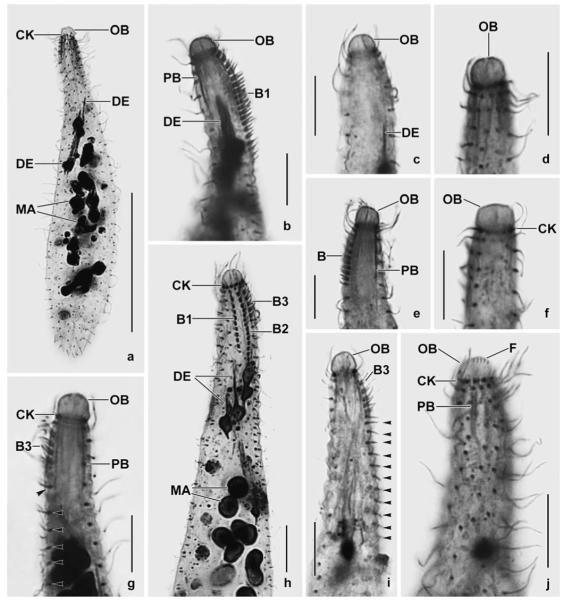
Enchelyodon nodosus Berger et al., 1984 (Figs 4a–q; Table 6)
Figs 4.
a–j. Enchelyodon nodosus from life (a–f, i, j) and after protargol impregnation (g, h). a – right side view of a representative specimen, length 150 μm; b, i – swimming specimens; c – frontal view of oral bulge. Note lack of extrusomes in bulge centre; d – two size types of extrusomes (40 μm, 20 μm); e – part of dorsal brush row 3. Note the monokinetidal tail of flame–shaped bristles; f, j – surface view and optical section showing the conspicuous cortical granulation; g – ciliary pattern of dorsal side; h – anterior body portion showing oral structures and the thick cortex separated from the cytoplasm by the darkly impregnated tela corticalis. B – dorsal brush, C – cortex, CK – circumoral kinety, CV – contractile vacuole, E – extrusomes, FV – food vacuole, G – cortical granules, LD – lipid droplets, MA – macronucleus, OB – oral bulge, PB – pharyngeal basket, T – tela corticalis. Scale bars: 50 μm (a, g) and 10 μm (h).
k–q. Enchelyodon nodosus from life (k, m, n, p, q) and after silver carbonate impregnation (l, o). k, n – freely motile specimens shown in interference contrast and bright field; l, o – overview and detail, showing the dorsal brush (B) and the long, deeply impregnated extrusomes; m, q – optical sections showing the thick cortex (opposed arrowheads) and the cytoplasm studded with food vacuoles, lipid droplets, and granules; p – a long extrusome. B – dorsal brush, C – cortex, CK – circumoral kinety, CV – contractile vacuole, E – extrusomes, FV – food vacuoles, LD – lipid droplets, MA – macronucleus, OB – oral bulge. Scale bars: 80 μm (k, l, n), 40 μm (m, o, p), 5 μm (q).
Table 6.
Morphometric data from Enchelyodon nodosus. Data based on mounted, protargol-impregnated (Foissner 1991, protocol A), and randomly selected specimens from a non-fooded Petri dish culture. Measurements in μm. CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of specimens investigated, SD – standard deviation, SE – standard error of arithmetic mean, x̄ – arithmetic mean
| Characteristics | x̄ | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|
| Body, length | 131.7 | 130.0 | 16.4 | 4.5 | 12.4 | 103.0 | 172.0 | 13 |
| Body, width | 64.5 | 60.0 | 12.6 | 3.5 | 19.6 | 46.0 | 87.0 | 13 |
| Macronucleus, lengtha | 170.8 | 160.0 | - | - | - | 120.0 | 260.0 | 13 |
| Macronucleus, width | 9.8 | 10.0 | 1.2 | 0.3 | 12.6 | 8.0 | 12.0 | 13 |
| Brush row 1, lengthb | 24.8 | 25.0 | 6.7 | 3.4 | 27.1 | 18.0 | 31.0 | 4 |
| Brush row 1, number of dikinetids | 19.7 | 20.5 | 6.9 | 3.4 | 34.9 | 11.0 | 27.0 | 4 |
| Brush row 2, lengthb | 32.6 | 30.0 | 6.5 | 2.9 | 20.1 | 25.0 | 42.0 | 5 |
| Brush row 2, number of dikinetids | 29.8 | 29.0 | 7.9 | 3.5 | 26.6 | 19.0 | 38.0 | 5 |
| Brush row 3, lengthb | 14.4 | 13.0 | 2.9 | 1.3 | 20.0 | 12.0 | 18.0 | 5 |
| Brush row 3, number of dikinetids | 12.2 | 12.0 | 2.6 | 1.2 | 21.2 | 9.0 | 16.0 | 5 |
| Number of ciliary rows (including brush) | 42.9 | 43.0 | 1.8 | 0.5 | 4.3 | 40.0 | 45.0 | 12 |
| Number of brush rows | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 7 |
| Number of cilia in 20 μmc | 8.1 | 7.5 | 2.6 | 0.8 | 32.7 | 6.0 | 15.0 | 12 |
Rough values because usually strongly curved.
Measured as distance from circumoral kinety to last dikinetid of row.
Counted in 10 μm each in anterior and posterior third of specimens.
Voucher material
Eight slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Upper Austrian Museum in Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Description of a Saudi Arabian population
Size 120–200 × 50–90 μm in vivo, usually about 150 × 70 μm; length–width ratio rather variable, on average near 2.2:1 in vivo and protargol preparations (Table 6). Shape elongate ovoidal with distinct oral bulge, usually slightly flattened ventrally and thus not fully symmetrical, rarely unflattened; swimming cells often distinctly curved in anterior third; in preparations frequently ellipsoidal or nearly ellipsoidal; not contractile (Figs. 4a, b, g, i, k, n). Macronucleus in central quarters of cell, stands out as a bright strand from the more refractive cytoplasm, usually strongly curved or wrinkled, on average 10 μm width and slightly longer than cell; rarely in two parts; numerous globular to irregular nucleoli (Figs 4a, g, k, l, n). Possibly, several globular micronuclei along and near macronucleus, but not definitely recognizable due to many similar-sized cytoplasmic inclusions. Contractile vacuole in posterior body end, surrounded by smaller adventiv blisters; several excretory pores in pole centre (Figs 4a, g, n). Two size types of rod-shaped, slightly curved, fine extrusomes scattered in oral bulge, except for bulge centre; many rod-shaped developmental stages in cytoplasm. Type I extrusomes about 40 × 0.2 μm (35–50 × 0.2–0.3 μm) in size; type II like type I but only about 20 μm long, thus possibly a developmental stage of type I; both types do not impregnate with protargol, but stain deeply with silver carbonate (Figs 4a, d, l, o, p). Somatic and oral cortex very flexible, conspicuous in vivo because about 1.5 μm thick due to narrowly spaced rows of ellipsoidal, colourless granules about 1 × 0.4 μm in size; conspicuous also in protargol-prepared specimens because 1.5 μm thick and separated from cytoplasm by a very thin (<0.5 μm) but deeply stained sheet, likely the lamina (tela) corticalis (Figs 4a, f, h, j, m, q). Cytoplasm colourless, studded with lipid droplets 0.2–10 μm across, specimens thus dark at ≤ ×100 magnification; contains many granular food vacuoles up to 20 μm across, likely feeds on small ciliates because a Halteria grandinella was observed in a food vacuole (Figs 1a, m). Swims rather rapidly by rotation about main body axis.
Cilia about 12 μm long in vivo, ordinarily spaced, i.e., on average of 8 cilia in 20 μm (Table 6); arranged in an average of 43 meridional, ordinarily spaced rows abutting to circumoral kinety at right angles; rather loosely spaced underneath circumoral kinety in about half of specimens (Figs 4a, g). Dorsal brush heterostichad, inconspicuous because bristles only up to 4 μm long and largest row extending merely 24% of body length; row 1 slightly shorter than longest row 2, row 3 two thirds shorter than row 2, but with a monokinetidal bristle tail extending to last quarter of cell. Bristles similar in all rows, anterior bristle of pairs about 4 μm long and slightly inflated distally, posterior bristle stump-like because only 1.5 μm long; bristles of monokinetidal tail narrowly cuneate and about 4 μm long (Figs 4a, e, g, o; Table 6).
Oral bulge massive, on average 18 × 7 μm both in vivo and protargol preparations; circular in frontal view, studded with extrusomes except of concave centre. Circumoral kinety circular, dikinetids comparatively widely spaced, give rise to rods forming a conical basket 40–60 μm long (Figs 4a, c, g, h, k, n; Table 6). Oral bulge not recognizable in two specimens and minute (~6 μm across) in a third one; possibly, these are post-dividers because two of the 13 specimens investigated commenced division.
Occurrence and ecology
Found only at site 1, where it was rare in the non-flooded Petri dish culture three days after rewetting the air-dried sample. As the sample was from an irrigated vegetable field and contained a mixture of soil and freshwater species, e.g., Monodinium perrieri (redescribed from this locality by Foissner et al. 1999), Stylonychia bifaria and Urostyla grandis, we could not decide which habitat E. nodosus prefers. The same problem adheres to the type population, which was discovered in soil from the margin of a lake.
Comparison with other populations
This population was difficult to impregnate and few specimens were available. Thus, the morphometric data are incomplete. The same applies to the Austrian type population, where Berger et al. (1984) could not count the ciliary rows, but estimated their number by multiplying kinety distance with body diameter. This, of course, provides rough values. Thus, we reinvestigated the type slide series of E. nodosus and could count the ciliary rows in six specimens: 60, 64, 60, 62, 62, 62; this gives an average of 62, in contrast to the 76 kineties calculated by Berger et al. (1984). Further, Berger et al. (1984; their Figures 14, 15) figured a very small specimen because it showed the ciliary pattern more clearly. Obviously, this specimen has only 45–50 ciliary rows and thus looks highly similar to the Arabian population in this respect. The slides also show that the Austrian specimens have as distinct cortical granules as the Arabian ones.
Berger et al. (1984) studied a second population of E. nodosus from soil of the Austrian Central Alps. These specimens have, like the Arabian ones, 42 ciliary rows, but possess only one type of rather short (~20 μm) extrusomes. Unfortunately, the data are not very detailed, i.e., no information is given on the attachment of the extrusomes and the possible occurrence of a second type of very short extrusomes. Thus, this population will not be discussed further.
The Arabian specimens of E. nodosus differs from the Austrian type population by three main features: extrusomes lacking in bulge centre vs. present; two long (40 μm and 20 μm) types of extrusomes vs. a long (~30 μm) and a very short (~2.5 μm) type; 43 vs. 62 ciliary rows. Further, the macronucleus is longer by about 60% in the Arabian than Austrian specimens. Except of the ciliary rows, these are rather sophisticated features, although we could confirm the differences by a reinvestigation of specimens from the type locality (Burgenland, eastern Austria) and from Namibia, Southwest Africa. However, little is known on the variability and key features of large Enchelyodon species. Thus, we prefer to assign the Arabian population to the Austrian one, waiting for detailed data from further populations.
Metauroleptus nov. gen
Diagnosis
Slightly twisted hypotrichs with 1 right and 1 left row of marginal cirri as well as 2 long and 2–3 short ventral rows and a row of buccal cirri. With transverse and caudal cirri, the latter originating exclusively from dorsal kinety 1 and thus forming a short row. All dorsal kineties generated intrakinetally.
Type species
Metauroleptus arabicus nov. spec.
Etymology
Composite of the Greek prefix meta (associated with, changed, substituted for) and the generic name Uroleptus, referring to the supposed affinity to the Kahliellidae. Masculine gender.
Species assignable
Metauroleptus arabicus nov. spec. (type); Metauroleptus procerus (Berger and Foissner, 1987) nov. comb. (basionym – Pseudouroleptus procerus Berger and Foissner, 1987); Metauroleptus terrestris (Hemberger, 1985) nov. comb. (basionym: Pseudouroleptus terrestris Hemberger, 1985). For distinguishing these species, see Table 8 and the description of M. arabicus.
Table 8.
Comparison of main characteristics in Metauroleptus arabicus (present paper), M. procerus (from Berger and Foissner 1987), and M. terrestris (from Hemberger 1985). Data are arithmetic means based on protargol-impregnated specimens
| Characteristics | M. arabicus | M. procerus | M. terrestris |
|---|---|---|---|
| Body length: width, ratio | 4:1 | 4.3:1 (4–6:1) | 3 or 4:1 |
| Body length: AZM length, ratio | 3.8:1 | 3.8:1 | 4–5:1 |
| Geographic location | Arabia | Austria | Germany |
| Adoral membranelles, number | 38 | 33 | 30–33 |
| Ventral cirral rows, number | 4 (rarely 5) | 4 | 4 |
| Buccal cirri, number | 6 | 4 | 4 |
| Transverse cirri, number | 2 | 2 | 2–3 |
| Right marginal cirri, number | 53 | 48 | 31–35 |
| Left marginal cirri, number | 51 | 46 | 32–37 |
| Dorsal kineties, number | 3 ordinary rows | 3 rows with row 1 reduced to a few kinetids |
4 ordinary rows |
Two other species have been assigned to Pseudouroleptus (Berger, 2001). They do not meet the diagnosis of Metauroleptus: Uroleptus humicola Géllert (transverse and caudal cirri lacking) and Paraurostyla buitkampi Foissner (lacks caudal cirri).
Comparison with related genera and systematic affinity
The cirral pattern of Metauroleptus resembles that of Pseudouroleptus Hemberger, 1985. However, Pseudouroleptus lacks the short frontal cirral rows so typical and important (see ontogenesis!) for Metauroleptus. On the other hand, Metauroleptus lacks a posteristomial cirrus which has a complex ontogenesis (Hemberger 1985, Berger 1999). Likely, ontogenesis of the ventral cirral rows is also different and should be included in the diagnosis of Metauroleptus when more detailed data become available: in Metauroleptus, the two long ventral rows are likely silent (Fig. 5j), while the left row is ontogenetically active, producing both ventral rows in Pseudouroleptus (Hemberger 1985, Berger 1999). Another main difference concerns the dorsal ontogenesis: in Pseudouroleptus, kinety 3 splits, producing a fourth row, while all dorsal kineties of Metauroleptus are produced intrakinetally (Fig. 5k); further, Pseudouroleptus produces a caudal cirrus each in kineties 1, 2 and 4, while Metauroleptus produces caudal cirri only in kinety 1, a highly unusual feature possibly occurring also in some urostylids, for instance, in Keronella gracilis, Caudiholosticha sylvatica, and Bicoronella costaricana (for reviews, see Berger 1999, 2006).
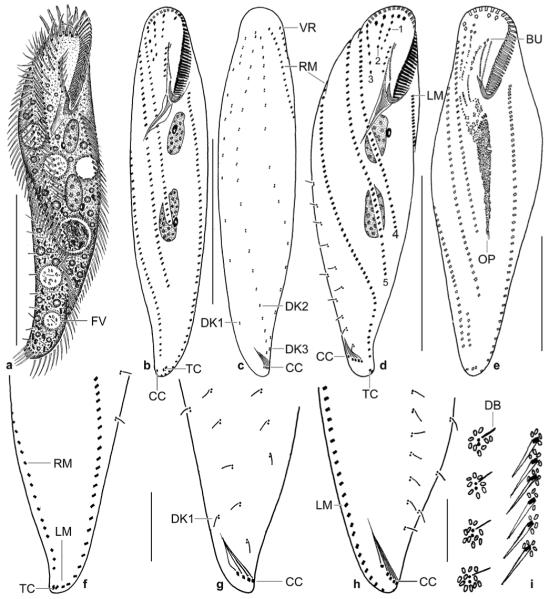
Figs 5.
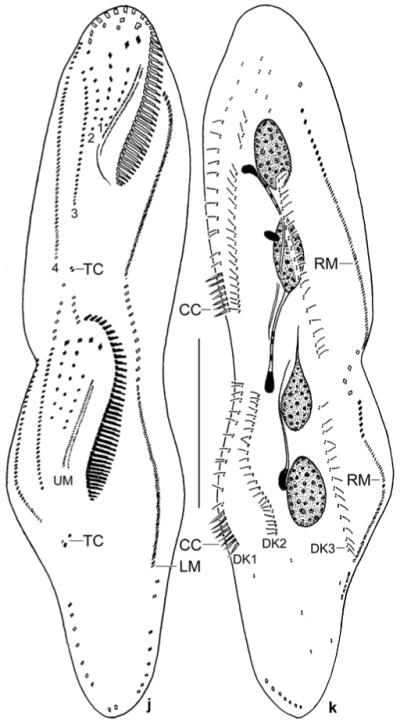
a–i. Metauroleptus arabicus from life (a, i) and after protargol impregnation (b–h). a – ventral view of a representative, slightly twisted specimen, length 180 μm; b–d – ventral and dorsal view of holotype specimen (180 μm) and ventral view of a distinctly twisted paratype specimen. Both specimens have three (1–3) short and two (4, 5) long ventral cirral rows; e – ventral view of an early divider; f, g – ventral and dorsal view of posterior body region, showing the location of the transverse and caudal cirri; h – dorsal view of a distinctly twisted specimen with seven caudal cirri, of which five are recognizable; i – cortical granulation around dorsal bristles and cirri. BU – buccal cirral row, CC – caudal cirri, DB – dorsal bristles, DK 1–3 – dorsal kineties, FV – food vacuole, LM – left row of marginal cirri, OP – oral primordium, RM – right row of marginal cirri, TC – transverse cirri, VR – ventral cirral row. Scale bars: 80 μm (a–d), 50 μm (e), and 20 μm (f–h).
j, k. Metauroleptus arabicus, ventral and dorsal view of a late divider after protargol impregnation. Parental structures shown by contour, newly formed structures shaded black. The four (1–4) ventral cirral rows likely originated from extensions of the oral primordium, the buccal cirral row, and the two short ventral rows (cp. Fig. 5e). Note that caudal cirri are formed only in dorsal kinety 1. CC – new caudal cirri, DK 1–3 – newly formed dorsal kineties, LM, RM – newly formed left and right row of marginal cirri, TC – newly formed transverse cirri, UM – newly formed undulating membranes. Scale bar: 50 μm.
We cannot assign Metauroleptus to a certain family because family classification is highly controversial in hypotrichs (for reviews see, Berger 1999, 2006; Lynn and Small 2002; Foissner and Stoeck 2008). If Berger’s system is applied, it can be excluded that Metauroleptus belongs to the Dorsomarginalia (Oxytrichidae s.l.) because it lacks dorsomarginal kineties. Likewise, an urostylid relationship can be excluded due to the lack of paired ventral cirri in zigzag pattern. The Amphisiellidae can also be excluded because Metauroleptus lacks an “amphisiellid median cirral row” (Eigner and Foissner 1994). Possibly, Metauroleptus belongs to the kahliellids, keronopsids (Figs 6a, c), or to a not yet defined group.
Figs 6.
a–c. Metauroleptus arabicus (a, b) and Keronopsis dieckmanni (c) after protargol impregnation. a, b – ventral and dorsal view showing main features, such as the four ventral cirral rows (1–4), the transverse and caudal cirri, and the two macronucleus nodules. The arrowhead marks the last kinetid in dorsal kinety 1; c – ventral view showing a main feature of K. dieckmanni, viz., the four macronucleus nodules. The cirral pattern is highly similar to that of M. arabicus, but caudal cirri are absent from K. dieckmanni. AZM – adoral zone of membranelles, BC – buccal cavity, CC – caudal cirri, DK 1–3 – dorsal bristle rows, FV – food vacuoles, LM – left row of marginal cirri, MA – macronucleus nodules, MI – micronucleus, RM – right row of marginal cirri, TC – transverse cirri. Scale bars: 80 μm.
Metauroleptus arabicus nov. spec. (Figs 5a–k, 6a, b; Table 7)
Table 7.
Morphometric data on Pseudohemisincirra arabica (PA) and Metauroleptus arabicus (MA). Data based on mounted, protargol-impregnated (Foissner 1991, protocol A), and randomly selected specimens from a non-flooded Petri dish culture. Measurements in μm. AZM – adoral zone of membranelles, CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of individuals investigated, SD – standard deviation, SE – standard error of arithmetic mean, VR – ventral cirral row, x̄ – arithmetic mean
| Characteristics | Species | x̄ | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|---|
| Body, length | PA | 86.7 | 82.0 | 13.4 | 3.1 | 15.4 | 68.0 | 110.0 | 19 |
| MA | 159.6 | 160.0 | 18.2 | 4.2 | 11.4 | 135.0 | 194.0 | 19 | |
| Body, width | PA | 15.4 | 16.0 | 2.7 | 0.6 | 17.3 | 11.0 | 19.0 | 19 |
| MA | 40.6 | 41.0 | 5.3 | 1.2 | 13.1 | 32.0 | 50.0 | 19 | |
| Body length: width, ratio | PA | 5.7 | 5.7 | 1.0 | 0.2 | 17.9 | 4.0 | 8.4 | 19 |
| MA | 4.0 | 4.0 | 0.5 | 0.1 | 12.0 | 3.0 | 4.9 | 19 | |
| Anterior body end to proximal end of adoral zone of membranelles (AZM), distance |
PA | 19.1 | 19.0 | 1.9 | 0.4 | 10.0 | 14.0 | 22.0 | 19 |
| MA | 42.1 | 42.0 | 3.0 | 0.7 | 7.1 | 37.0 | 48.0 | 19 | |
| Body length: AZM length, ratio | PA | 4.6 | 4.7 | 0.6 | 0.1 | 13.4 | 3.5 | 5.4 | 19 |
| MA | 3.8 | 3.7 | 0.5 | 0.1 | 11.9 | 3.2 | 5.1 | 19 | |
| Anterior body end to right marginal row, distance | PA | 13.7 | 14.0 | 2.2 | 0.5 | 15.7 | 10.0 | 17.0 | 19 |
| MA | 11.5 | 11.0 | 4.1 | 1.0 | 36.0 | 5.0 | 19.0 | 19 | |
| Anterior body end to paroral membrane, distance | PA | 7.0 | 7.0 | 1.2 | 0.3 | 17.6 | 5.0 | 9.0 | 19 |
| MA | 12.0 | 12.0 | 1.4 | 0.3 | 11.8 | 9.0 | 15.0 | 19 | |
| Anterior body end to frontoventral row/buccal row, distance | PA | 6.6 | 6.0 | 1.0 | 0.2 | 15.5 | 5.0 | 9.0 | 19 |
| MA | 14.5 | 15.0 | 1.9 | 0.4 | 13.1 | 11.0 | 18.0 | 19 | |
| Anterior body end to end of frontoventral row/ventral row 1, distance |
PA | 20.3 | 20.0 | 3.4 | 0.8 | 16.6 | 15.0 | 27.0 | 19 |
| MA | 16.8 | 18.0 | 4.6 | 1.1 | 27.4 | 6.0 | 24.0 | 19 | |
| Anterior body end to first macronucleus nodule, distance | PA | 17.3 | 17.0 | 2.7 | 0.6 | 15.8 | 10.0 | 21.0 | 19 |
| MA | 44.1 | 43.0 | 4.2 | 1.0 | 9.6 | 38.0 | 52.0 | 19 | |
| Anterior body end to end of VR 2, distance | MA | 27.0 | 27.0 | 4.6 | 1.1 | 17.3 | 19.0 | 36.0 | 19 |
| Anterior body end to end of VR 3, distance | MA | 82.5 | 82.0 | 14.6 | 3.3 | 17.7 | 60.0 | 111.0 | 19 |
| Anterior body end to end of VR 4, distance | MA | 110.7 | 110.0 | 14.8 | 3.4 | 13.4 | 87.0 | 138.0 | 19 |
| Posterior body end to lowermost transverse cirrus, distance | PA | 1.6 | 2.0 | - | - | - | 1.0 | 2.0 | 19 |
| MA | 2.1 | 2.0 | 1.0 | 0.2 | 47.2 | 1.0 | 5.0 | 19 | |
| Posterior body end to right marginal row, distance | PA | 7.4 | 7.0 | 3.0 | 0.7 | 40.0 | 4.0 | 16.0 | 19 |
| Posterior body end to left marginal row, distance | PA | 6.8 | 6.0 | 4.1 | 0.9 | 60.3 | 2.0 | 17.0 | 19 |
| Length of nuclear figure/distance between macronucleus nodules |
PA | 52.6 | 51.0 | 10.2 | 2.3 | 19.3 | 37.0 | 73.0 | 19 |
| MA | 12.4 | 10.0 | 6.5 | 1.5 | 51.9 | 5.0 | 26.0 | 19 | |
| Macronucleus nodules/anterior nodule, length | PA | 6.2 | 6.0 | 1.2 | 0.3 | 19.7 | 4.0 | 8.0 | 19 |
| MA | 20.1 | 20.0 | 1.9 | 0.4 | 9.4 | 16.0 | 24.0 | 19 | |
| Macronucleus nodules/anterior nodule, width | PA | 3.2 | 3.0 | 0.7 | 0.2 | 20.2 | 2.0 | 4.0 | 19 |
| MA | 8.3 | 8.0 | 1.2 | 0.3 | 13.9 | 6.0 | 10.0 | 19 | |
| Posterior macronucleus nodule, length | MA | 21.2 | 21.0 | 2.5 | 0.6 | 11.6 | 16.0 | 26.0 | 19 |
| Posterior macronucleus nodule, width | MA | 8.8 | 9.0 | 1.0 | 0.2 | 11.1 | 7.0 | 10.0 | 19 |
| Macronucleus nodules, number |
PA | 15.6 | 16.0 | 0.9 | 0.2 | 5.7 | 14.0 | 17.0 | 19 |
| MA | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 19 | |
| Micronuclei, length | PA | 2.8 | 3.0 | 0.5 | 0.1 | 17.0 | 2.0 | 4.0 | 19 |
| MA | 4.7 | 5.0 | 0.6 | 0.1 | 11.9 | 4.0 | 6.0 | 19 | |
| Micronuclei, width | PA | 1.7 | 1.5 | - | - | - | 1.0 | 2.0 | 19 |
| MA | 3.1 | 3.0 | - | - | - | 3.0 | 4.0 | 19 | |
| Micronuclei, number | PA | 2.2 | 2.0 | - | - | - | 2.0 | 3.0 | 19 |
| MA | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 19 | |
| Adoral membranelles, number |
PA | 15.1 | 15.0 | 1.2 | 0.3 | 7.8 | 12.0 | 17.0 | 19 |
| MA | 38.5 | 38.0 | 2.2 | 0.5 | 5.6 | 34.0 | 43.0 | 19 | |
| Longest membranellar base, length | PA | 4.4 | 4.0 | 0.6 | 0.1 | 13.7 | 3.0 | 5.0 | 19 |
| MA | 6.4 | 6.0 | 0.8 | 0.2 | 13.0 | 5.0 | 8.0 | 19 | |
| Cirri in right marginal row, number | PA | 17.8 | 17.0 | 2.7 | 0.6 | 15.0 | 13.0 | 23.0 | 19 |
| MA | 53.4 | 54.0 | 6.5 | 1.5 | 12.1 | 41.0 | 63.0 | 19 | |
| Cirri in left marginal row, number | PA | 19.1 | 19.0 | 2.6 | 0.6 | 13.4 | 15.0 | 26.0 | 19 |
| MA | 50.9 | 51.0 | 6.8 | 1.6 | 13.4 | 41.0 | 65.0 | 19 | |
| Frontoventral cirral rows, number | PA | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 19 |
| MA | 4.2 | 4.0 | - | - | - | 4.0 | 5.0 | 19 | |
| Frontoventral cirral row/ventral row 1, number of cirri | PA | 7.1 | 7.0 | 1.7 | 0.4 | 23.9 | 5.0 | 11.0 | 19 |
| MA | 4.1 | 4.0 | 1.2 | 0.3 | 30.2 | 2.0 | 6.0 | 19 | |
| Ventral row 2, number of cirri | MA | 5.1 | 5.0 | 1.1 | 0.3 | 21.4 | 3.0 | 7.0 | 19 |
| Ventral row 3, number of cirri | MA | 30.0 | 31.0 | 4.4 | 1.0 | 14.8 | 22.0 | 36.0 | 19 |
| Ventral row 4, number of cirri | MA | 39.6 | 41.0 | 5.3 | 1.2 | 13.3 | 30.0 | 52.0 | 19 |
| Frontoterminal cirri, number |
PA | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 19 |
| MA | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 19 | |
| Frontal cirri, number | PA | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 19 |
| MA | 4.1 | 4.0 | - | - | - | 3.0 | 5.0 | 19 | |
| Buccal cirri, number | PA | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 19 |
| MA | 6.2 | 6.0 | 0.8 | 0.2 | 12.7 | 5.0 | 8.0 | 19 | |
| Transverse cirri, number | PA | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 19 |
| MA | 1.8 | 2.0 | - | - | - | 1.0 | 2.0 | 19 | |
| Caudal cirri, number | PA | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 19 |
| MA | 4.6 | 4.0 | 1.3 | 0.3 | 27.5 | 3.0 | 7.0 | 19 | |
| Dorsal kineties, number | PA | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 19 |
| MA | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 19 | |
| Dorsal kinety 1, number of kinetids | MA | 11.8 | 12.0 | 2.4 | 0.5 | 20.1 | 8.0 | 18.0 | 19 |
| Dorsal kinety 2, number of kinetids | MA | 21.0 | 22.0 | 3.1 | 0.7 | 14.6 | 15.0 | 29.0 | 19 |
Diagnosis
Differs from the congeners in having 3 complete dorsal kineties.
Type locality
Very sandy pasture soil in the surroundings of the village of Al-Qasab, about 150 km NW of the town of Riyadh, Saudi Arabia, 45°30′ E 26° N.
Etymology
Named after the region the species was discovered.
Type material
1 holotype and 4 paratype slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Upper Austrian Museum in Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Description
Size 150–220 × 35–50 μm in vivo, usually near 180 × 40 μm; length–width ratio fairly constant, i.e., 3–5:1, on average 4.5:1 both in vivo and protargol preparations (Table 7); flattened up to 2:1 dorsoventrally; not contractile. Outline pisciform with posterior half gradually narrowed; slightly to distinctly sigmoidal and usually more or less twisted about main body axis, especially when just collected from soil; anterior end broadly rounded, posterior narrowly rounded, never acute, directed to the right or to the left when cells are distinctly twisted (Figs 5a, b, d, f, h, 6a). Nuclear apparatus slightly above mid-body left of midline. Macronucleus nodules on average only 12 μm apart, broadly to slenderly ellipsoidal, on average near 2.5:1, contain many small, globular nucleoli. A micronucleus each attached in variable positions to macronucleus nodules; individual micronuclei globular to ellipsoidal, on average 5 × 3 μm in protargol preparations (Figs 5a, b, d, k, 6a; Table 7). Contractile vacuole slightly above midbody near left margin of cell. Cortex thin and flexible. Cortical granules difficult to recognize because colourless and only 0.5 × 0.25 μm in size, form small clusters around bases of cirri and dorsal bristles, do not impregnate with protargol (Fig. 5i). Cytoplasm colourless, usually studded with lipid droplets up to 7 μm across, ordinary crystals in posterior third, and food vacuoles up to 30 μm across. Feeds on filamentous bacteria, flagellates (Polytomella) and middle-sized ciliates, such as Colpoda inflata and Halteria grandinella (Fig. 5a). Glides slowly on microscope slide and between soil particles, showing great flexibility.
Cirri short (12–15 μm in vivo) and fine compared to body size; cirral pattern of usual variability, rows extend more or less obliquely, depending on body twist (Figs 5a, b, d, e, 6a, b; Table 7). Marginal rows almost as long as body, both rows extend onto dorsal side in anterior (right row) or posterior (left row) third of cell. Two to three, usually two short ventral cirral rows (rows 1 and 2) in oral area right of buccal row and two long ventral rows extending slightly (row 3) to distinctly (row 4) above mid-body. One or two minute transverse cirri near posterior body end. Frontoterminal and postperistomial cirri lacking. Dorsal bristles about 4 μm long, arranged in three rows slightly shortened anteriorly and posteriorly. Row 1 consisting of an average of 12 kinetids with kinetid distances gradually increasing posteriorly (Figs 5c, g; Table 7). Three to seven, on average four caudal cirri in an oblique row originating from dorsal kinety 1 (see ontogenesis) and associated with rather conspicuous fibres (Figs 5c, d, g, h, 6a, b; Table 7).
Oral apparatus of ordinary structure. Adoral zone short, occupies only 26% of body length, composed of an average of 38 membranelles with largest bases about 8 μm wide in vivo (Table 7). Buccal cavity rather narrow and flat, buccal lip inconspicuous. Undulating membranes short, almost straight, optically intersect in anterior third; paroral cilia about 7 μm long in vivo. Pharyngeal fibres of ordinary distinctness (Figs 5a, b, d, 6a; Table 7).
Ontogenesis
Some key ontogenetic stages are contained in the slides deposited. They show: (i) the oral primordium develops apokinetally and forms a long streak of disordered basal bodies between the left long ventral row and the left marginal row (not shown); (ii) cirral row anlagen originate from the anterior end of the oral primordium, the buccal cirral row, and from the short ventral rows 1 and 2, i.e., the long ventral rows are likely not involved (Figs 5e, j); (iii) two transverse cirri are generated (Fig. 5j); and (iv) caudal cirri are produced only by dorsal kinety 1 (Fig. 5k).
Occurrence and ecology
As yet found only at type locality, i.e., a rather fertile site in a semi-desert. The European P. procerus and P. terrestris were discovered also in fertile soils, viz., a pasture in Salzburg and a forest near Bonn, Germany, respectively.
Comparison with congeners
The data compiled in Table 8 show that the three Metauroleptus species differ only in one reliable feature, viz., the number of dorsal kineties: 4 ordinary rows in M. terrestris, 3 ordinary rows in M. arabicus, and 3 rows in M. procerus with row 1 reduced to a single kinetid. Further, M. arabicus is slightly larger in many features, e.g., has more adoral membranelles and buccal cirri on average (Table 8). The number of dorsal kineties is considered a “strong” species feature (Berger 1999, 2006). None the less, subspecies rank would be probably more appropriate for these three species because the number of dorsal kineties is a sophisticated feature well recognizable only in protargol preparations.
No cortical granules have been mentioned in the original descriptions of M. procerus and M. terrestris. However, a Namibian population of M. procerus has cortical granules like M. arabicus, but 1 × 0.5 μm in size, indicating that they were overlooked in the type population. Further, the Namibian specimens have composed dorsal kinety 1 of 2–3 widely spaced kinetids.
Keronopsis dieckmanni Foissner, 1998 (Figs 6c, 7g, h)
Figs 7.
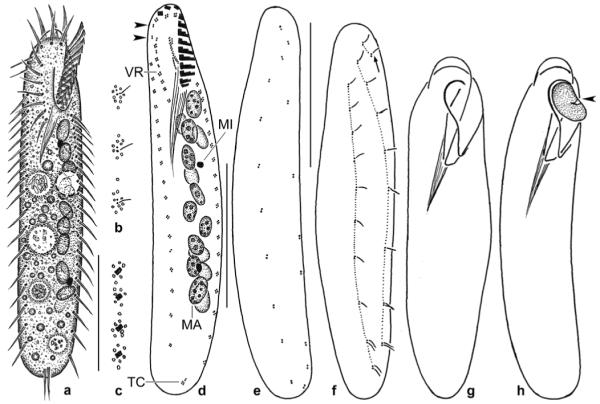
a–h. Pseudohemisincirra arabica (a–f) and Keronopsis dieckmanni (g, h) from life (a, g, h) and after protargol impregnation (b–f). a – ventral view of a representative specimen, length 90 μm; b, c – cortical granulation around dorsal bristles and cirri; d, e – infraciliature of ventral and dorsal side and nuclear apparatus of holotype specimen, length 80 μm. Note lack of buccal and caudal cirri as well as the minute cirri each composed of only 2–4 cilia. Arrowheads mark frontoterminal cirri; f – dorsal view of a paratype specimen with kinetids connected by dotted lines. Arrow marks row 3 consisting of only two kinetids; g – outline of an ordinary specimen, length 250 μm; h – outline of a slender specimen just ingesting a Colpoda maupasi (arrowhead), length 220 μm. MA – macronucleus nodules in two rows one upon the other, MI – micronucleus, VR – ventral cirral row. Scale bars: 30 μm (a, d–f).
The Saudi Arabian specimens of this species, which is probably restricted to the African continent, match the original description, both in morphology (Fig. 6c) and habitat (saline oasis soil; saline soil from shore of Lake Baringo, Kenya). Thus, a redescription is not necessary but three voucher slides have been deposited and some in vivo observations are added: (i) specimens more slender (about 230 × 50 μm vs. 220 × 70 μm) and frequently rather distinctly narrowed posteriorly (Figs 7g, h); (ii) cortex without specific granules, confirming the original description; (iii) paroral and endoral membrane optically intersecting in mid-buccal cavity; (iv) feeds an Colpoda maupasi ingested whole (Fig. 7h).
Pseudohemisincirra nov. gen
Diagnosis
Oblong hypotrichs with 1 right and left row of marginal cirri, a short row of frontoventral cirri, and transverse cirri. Buccal cirri and caudal cirri absent.
Type species
Pseudohemisincirra arabica nov. spec.
Etymology
The name is a composite of pseudo (false, i.e., resembling but not equalling) and the generic name Hemisincirra.
Comparison with similar genera
Hypotrichs of this type are difficult to classify. Thus, we compiled main characteristics of similar genera in Table 9, showing the unique combination of features in Pseudohemisincirra. Holostichides terricola Foissner, 1988 likely belongs to Periholosticha which, however, lacks frontoterminal cirri according to the ontogenetic data of Hemberger (1982); however, the investigations of Foissner et al. (2002) indicate the presence of such cirri. Generally, the frontoterminal cirri are insufficiently defined and thus a difficult feature. This is why we did not include them in Table 9. Likewise, several Hemisincirra species have been described that likely belong to other or new genera.
Table 9.
Comparison of Pseudohemisincirra nov. gen. with similar generaa
| Characteristics |
Pseudo- hemisincirra |
Hemisincirra | Circinella | Periholosticha | Paragastrostyla | Holostichides |
Holostichides
terricola |
|---|---|---|---|---|---|---|---|
| Body acut posteriorly | no | most species | yes | yes | yes | no | yes |
| Buccal cirri | absent | present | present | absent | absent | present | absent |
| Transverse cirri | present | present | absent | absent | absent | absent | absent |
| Caudal cirri | absent | absent | absent | present | present | present | present |
| Midventral pattern in anterior half of ventral row |
absent | present | absent | present | absent | present | present |
Based on data from Hemberger 1982, 1985 (Hemisincirra, Periholosticha, Paragastrostyla); Foissner 1982, 1987a, 1988, 1994 (Hemisincirra, Circinella, Holostichides, H. terricola), and Foissner et al. 2002 (Hemisincirra, Periholosticha).
Pseudohemisincirra arabica nov. spec. (Figs 7a–f; Tables 7, 9)
Diagnosis
Size about 90 × 15 μm in vivo; narrowly oblong. Cortical granules <1 μm in size, colourless, form clusters around bases of cirri and dorsal kinetids. On average 16 macronucleus nodules and 2 micronuclei in a series left of midline, 15 adoral membranelles, 17 cirri in right and 19 cirri in left marginal row, 7 cirri in frontoventral row, 2 frontoterminal cirri, 2 transverse cirri, and 3 dorsal kineties with kinety 3 composed of only two kinetids. All cirri comprising 2–4 cilia.
Type locality
Very sandy pasture soil in the surroundings of the village of Al-Qasab, about 150 km NW of the town of Riyadh, Saudi Arabia, 45°30′ E 26° N.
Etymology
Named after the region the species was discovered.
Type material
1 holotype and 2 paratype slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Upper Austrian Museum in Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Description
Size in vivo 70–120 × 10–20 μm, usually near 90 × 15 μm; length–width ratio 4–8:1, on average about 6:1 both in vivo and protargol preparations (Tab. 7); flattened about 2:1 dorsoventrally. Outline oblong (elongate rectangular) because both ends broadly rounded and margins straight or only slightly convex (Figs 7a, d, f). Macronucleus nodules invariably in two rows on top of each other between adoral zone of membranelles and posterior body end left of cell’s midline; individual nodules globular to ellipsoidal, on average about 6 × 3 μm, contain many small nucleoli. Usually a broadly ellipsoidal, compact micronucleus each near ends of macronucleus row (Figs 7a, d). Contractile vacuole slightly above midbody near left margin of cell. Cortex thin and very flexible. Cortical granules about 0.5 μm across, colourless to yellowish, form small clusters around bases of cirri and dorsal kinetids, impregnate faintly with protargol (Figs 7b, c). Cytoplasm colourless, contains some lipid droplets 1–3 μm across and food vacuoles with bacterial remnants. Glides rather fast on slide surface and between soil particles showing great flexibility.
All cirri about 10 μm long in vivo and composed of 2–4 cilia only; pattern of usual variability (Figs 7a, d; Table 7). Frontal cirri in transverse row subapically; frontoterminal cirri in gap between frontal cirri and ventral cirral row at right margin of cell, each consisting of only two cilia; buccal cirrus lacking. Ventral cirral row usually ends at or underneath buccal vertex, slightly oblique, cirri not zigzagging and composed of 2–4 cilia. Transverse cirri near posterior margin of cell and thus distinctly projecting from body proper, one cirrus usually composed of four cilia, the other of only two. Both marginal rows end subterminally, right row commences far subapically, i.e., at level of mid-buccal cavity, most cirri composed of four cilia. Dorsal bristles about 3 μm long, widely spaced, arranged in three rows in and right of cell’s midline, row 3 consists only of two kinetids (Figs 7e, f). Caudal cirri absent.
Adoral zone composed of an average of 15 membranelles, occupies about 22% of body length, longest bases ca 5 μm wide in vivo; three, rarely four frontal membranelles separated from ventral membranelles by an indistinct gap at left anterior body end. Buccal cavity narrow and flat. Paroral and endoral membrane inconspicuous, slightly curved, optically intersect at level of mid-buccal cavity, paroral composed of oblique dikinetids. Pharyngeal fibres conspicuous, compared to size of oral apparatus (Figs 7a, d; Table 7).
Ontogenesis commences at proximal end of ventral cirral row.
Comparison with similar species
Small, slender hypotrichs are numerous in soil habitats and difficult to identify. In vivo, P. arabica highly resembles Hemisincirra gellerti verrucosa Foissner and Schade, 2000 in Foissner (2000) due to the specific arrangement of the cortical granules. The features separating these species are recognizable only on very careful live observation or in protargol preparations: the buccal cirrus (absent vs. present) and the number of dorsal kineties (3 vs. 4). There are also several other morphometric differences, for instance, the number of macronucleus nodules (16 vs. 8) and transverse cirri (2 vs. 4), but none is sufficiently distinct to make P. arabica easily identifiable. The same applies to several other small soil hypotrichs, especially H. inquieta (for a brief review, see Foissner et al. 2002). Thus, protargol impregnation and the character combination given in Table 9 are indispensable for a reliable identification of P. arabica.
Oxytricha arabica nov. spec. (Figs 8a–g; Table 10)
Figs 8.
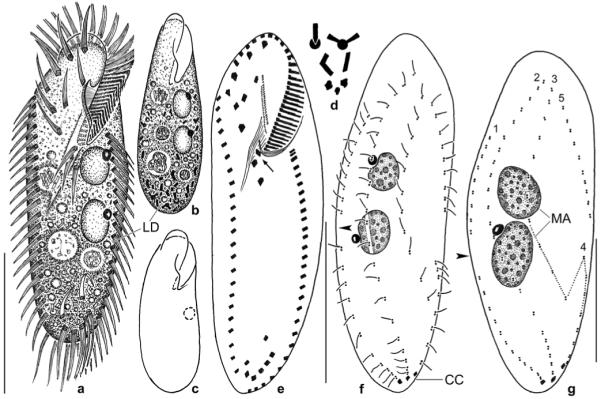
a–g. Oxytricha arabica from life (a–d) and after protargol impregnation (e–g). a, d – ventral view of a representative specimen, length 110 μm. The posterior half is highly refractive and thus dark due to masses of crystals (d) and lipid droplets; b, c – when touched by the coverslip, specimens become elongate ovoid (b) and contract ellipsoidally (c) when touching an obstacle. Drawn to scale; e, f – infraciliature of ventral and dorsal side and nuclear apparatus of holotype specimen, length 110 μm. Arrow marks first postoral cirrus very close to the buccal vertex, arrowhead denotes gap in dorsal kinety 1; g – ciliary pattern of dorsal side of a paratype specimen with only one micronucleus. The split kinety 3 is connected by a dotted line; arrowhead marks gap in kinety 1. Note condensation of kinetids in end region of kineties 1 and 2. CC – caudal cirri, LD – lipid droplets, MA – macronucleus nodules, 1–5 – kinety numbers. Scale bars: 50 μm (a, e, f) and 30 μm (g).
Table 10.
Morphometric data on Oxytricha arabica (OA) and Erimophrya monostyla (EM). Data based on mounted, protargol-impregnated (Foissner 1991, protocol A), and randomly selected specimens from non-flooded Petri dish cultures. Measurements in μm. CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of individuals investigated, SD – standard deviation, SE – standard error of arithmetic mean, x̄ – arithmetic mean
| Characteristics | Species | x̄ | M | SD | SE | CV | Min | Max | n |
|---|---|---|---|---|---|---|---|---|---|
| Body, length | OA | 98.1 | 97.0 | 9.2 | 2.0 | 9.4 | 82.0 | 116.0 | 21 |
| EM | 60.8 | 60.0 | 8.6 | 2.5 | 14.1 | 50.0 | 74.0 | 12 | |
| Body, width | OA | 35.4 | 35.0 | 4.7 | 1.0 | 13.3 | 30.0 | 45.0 | 21 |
| EM | 10.0 | 10.0 | 0.7 | 0.2 | 7.4 | 9.0 | 11.0 | 12 | |
| Body length: width, ratio | OA | 2.8 | 2.8 | 0.3 | 0.1 | 9.9 | 2.2 | 3.2 | 21 |
| EM | 6.1 | 6.0 | 1.1 | 0.3 | 17.6 | 5.0 | 8.2 | 12 | |
| Adoral zone of membranelles, lengtha | EM | 13.1 | 13.0 | 0.8 | 0.2 | 6.1 | 12.0 | 14.0 | 12 |
| OA | 33.6 | 34.0 | 2.4 | 0.5 | 7.2 | 29.0 | 38.0 | 21 | |
| Anterior body end to paroral membrane, distance | OA | 10.7 | 11.0 | 1.3 | 0.3 | 12.2 | 8.0 | 13.0 | 21 |
| Anterior body end to endoral membrane, distance | OA | 15.5 | 15.0 | 1.6 | 0.3 | 10.1 | 14.0 | 19.0 | 21 |
| Anterior body end to last frontoventral cirrus, distance | OA | 25.5 | 26.0 | 2.5 | 0.5 | 9.7 | 21.0 | 30.0 | 21 |
| Anterior body end to first postoral cirrus, distance | OA | 34.3 | 34.0 | 3.0 | 0.7 | 8.8 | 29.0 | 40.0 | 21 |
| Anterior body end to third postoral cirrus, distance | OA | 42.1 | 42.0 | 3.6 | 0.8 | 8.5 | 36.0 | 48.0 | 21 |
| Anterior body end to right marginal row, distance | OA | 16.6 | 17.0 | 2.9 | 0.6 | 17.6 | 10.0 | 20.0 | 21 |
| Anterior end to first macronucleus nodule, distance | OA | 32.6 | 33.0 | 3.8 | 0.8 | 11.7 | 21.0 | 38.0 | 21 |
| Posterior end to uppermost transverse cirrus, distance | OA | 10.5 | 11.0 | 1.3 | 0.3 | 11.9 | 8.0 | 12.0 | 21 |
| Macronucleus nodules, distance in between | OA | 3.2 | 3.0 | 1.7 | 0.4 | 53.7 | 1.0 | 7.0 | 21 |
| EM | 2.3 | 2.0 | 1.7 | 0.5 | 73.7 | 0.0 | 5.0 | 12 | |
| Anterior macronucleus nodule, length | OA | 14.0 | 14.0 | 2.1 | 0.5 | 14.8 | 11.0 | 19.0 | 21 |
| EM | 11.0 | 10.0 | 1.9 | 0.6 | 17.4 | 9.0 | 15.0 | 12 | |
| Anterior macronucleus nodule, width | OA | 11.1 | 11.0 | 1.3 | 0.3 | 11.5 | 9.0 | 13.0 | 21 |
| EM | 4.0 | 4.0 | 0.7 | 0.2 | 18.5 | 3.0 | 5.0 | 12 | |
| Posterior macronucleus nodule, length | OA | 15.0 | 16.0 | 1.8 | 0.4 | 11.4 | 13.0 | 20.0 | 21 |
| Posterior macronucleus nodule, width | OA | 10.8 | 10.0 | 1.3 | 0.3 | 11.7 | 9.0 | 14.0 | 21 |
| Micronuclei, maximum length |
OA | 3.3 | 3.0 | - | - | - | 3.0 | 4.0 | 21 |
| EM | 2.2 | 2.1 | 0.4 | 0.1 | 18.8 | 1.6 | 3.0 | 12 | |
| Micronuclei, width | EM | 1.5 | 1.5 | - | - | - | 1.2 | 2.0 | 12 |
| Largest membranellar base, length | OA | 7.5 | 8.0 | - | - | - | 7.0 | 8.0 | 21 |
| Adoral membranelles, number |
OA | 29.8 | 30.0 | 1.3 | 0.3 | 4.2 | 27.0 | 32.0 | 21 |
| EM | 14.9 | 15.0 | 0.5 | 0.2 | 3.5 | 14.0 | 16.0 | 12 | |
| Macronucleus nodules, number |
OA | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 |
| EM | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 12 | |
| Micronuclei, number | OA | 1.8 | 2.0 | 0.5 | 0.1 | 28.3 | 1.0 | 3.0 | 21 |
| EM | 1.8 | 2.0 | - | - | - | 1.0 | 2.0 | 12 | |
| Right marginal cirri, number | OA | 20.6 | 20.0 | 1.9 | 0.4 | 9.4 | 17.0 | 25.0 | 21 |
| EM | 18.0 | 18.0 | 1.4 | 0.4 | 7.5 | 16.0 | 21.0 | 12 | |
| Left marginal cirri, number | OA | 22.9 | 23.0 | 1.5 | 0.3 | 6.7 | 21.0 | 27.0 | 21 |
| EM | 17.3 | 18.0 | 1.8 | 0.5 | 10.5 | 14.0 | 20.0 | 12 | |
| Frontal cirri, number | OA | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 |
| EM | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 12 | |
| Frontoventral cirri, number | OA | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 21 |
| EM | 4.0 | 4.0 | 0.4 | 0.1 | 10.7 | 3.0 | 5.0 | 12 | |
| Buccal cirri, number | OA | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 |
| EM | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 12 | |
| Postoral ventral cirri, number | OA | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 |
| EM | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 12 | |
| Pretransverse cirri, number | OA | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 |
| EM | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 12 | |
| Transverse cirri, number | OA | 5.0 | 5.0 | 0.0 | 0.0 | 0.0 | 5.0 | 5.0 | 21 |
| EM | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 12 | |
| Caudal cirri, number | OA | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 21 |
| EM | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 12 | |
| Dorsal ciliary rows, number | OA | 5.0 | 5.0 | 0.0 | 0.0 | 0.0 | 5.0 | 5.0 | 21 |
| EM | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 12 | |
| Kinetids in dorsal kinety 1, number | OA | 19.9 | 20.0 | 1.8 | 0.4 | 9.1 | 16.0 | 24.0 | 21 |
| EM | 5.0 | 5.0 | 0.0 | 0.0 | 0.0 | 5.0 | 5.0 | 6 | |
| Kinetids in dorsal kinety 4, number | OA | 7.4 | 7.0 | 1.3 | 0.3 | 16.8 | 5.0 | 10.0 | 21 |
| EM | 4.0 | 4.0 | 0.0 | 0.0. | 0.0 | 4.0 | 4.0 | 10 | |
| Kinetids in dorsal kinety 5, number | OA | 11.6 | 12.0 | 1.5 | 0.3 | 12.7 | 9.0 | 14.0 | 21 |
Measured as distance from anterior body end to proximal end of zone.
Diagnosis
Size, cytology, and ventral cirral pattern very similar to that of O. lanceolata. Dorsal kinety 1 with distinct gap underneath mid-body; kinety 3 split subequatorially, producing a classical Oxytricha pattern; kinetids very narrowly spaced in posterior region of kineties 1 and 2.
Type locality
Sand dune soil from the Khurays area, NE of the town of Riyadh, Saudi Arabia, 48° E 25° N.
Etymology
Named after the region the species was discovered.
Type material
1 holotype and 4 paratype slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Upper Austrian Museum in Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Description
Size 80–125 × 30–40 μm in vivo, usually near 110 × 35 μm, length–width ratio 2.2–3.2, on average 2.8:1 (Table 10); flattened about 2:1 dorsoventrally. Shape fairly constant, ellipsoidal to elongate ellipsoidal with both ends broadly rounded, thus appearing very similar to large-sized specimens of O. longa and Urosomoida agiliformis (Fig. 8a).Very flexible but not contractile, oral area may swing slightly right and left; shows, however, distinct contractility in anterior body half under mild coverslip pressure, i.e., becomes elongate ovoidal and contracts ellipsoidally when touching an obstacle (Figs 8b, c). Nuclear apparatus in mid-body left of midline, conspicuous because nodules only 3 μm apart on average (Table 10). Macronucleus nodules globular to broadly ellipsoidal, about 15 × 12 μm in vivo and protargol preparations, posterior nodule longer by 2 μm than anterior one; contain many small, globular nucleoli. Usually, a globular to broadly ellipsoidal micronucleus 4 × 3 μm in size attached to each macronucleus nodule; rarely specimens with 1 or 3 micronuclei (Figs 8a, f, g). Contractile vacuole in mid-body near left margin of cell, with short collecting canals. Cortex flexible, without specific granules. Cytoplasm colourless, but specimens appear black-white-spotted in posterior half due to masses of highly refractive crystals up to 7 μm in size among the bright food vacuoles and many lipid droplets up to 5 μm across (Figs 8a, b, d). Food vacuoles up to 15 μm in size, contain bacteria, naked amoebae, flagellates (Polytomella), and fungal spores (Fig. 8a). Glides rather rapidly in small arcs or straight ahead when touched by the coverslip.
Cirral pattern and number of cirri constant, pattern exactly as in Oxytricha. Cirri conspicuous because thick and forming distinct fringe terminally (Figs 8a, e; Table 10). Marginal cirri about 15 μm long, each composed of three ciliary rows, slightly decrease in size posteriorly, right row ends subterminally, left extends to body midline and thus frequently difficult to distinguish from caudal cirri. Frontoventral cirri thick, about 15 μm long, buccal cirrus at right anterior end of paroral membrane; postoral ventral cirri close together underneath buccal vertex. Transverse cirri slightly thinner than frontoventral cirri, proximal end frayed, about 20 μm long in vivo near body end and thus distinctly projecting.
Adoral zone of ordinary shape and distinctness, occupies about 34% of body length, composed of 30 membranelles on average, largest bases about 8 μm wide in vivo and protargol preparations (Figs 8a, e; Table 10). Buccal cavity narrow, short, and flat; buccal lip angular (for terminology, see Foissner and Al-Rasheid 2006), narrow, proximal margin thickened. Paroral and endoral membrane short and staggered with paroral slightly projecting anteriorly; side by side, rarely optically intersecting at level of mid-buccal cavity; paroral cilia gradually decreasing in length from 10 μm anteriorly to 6 μm posteriorly. Pharyngeal fibres of ordinary location and length (Figs 8a, e; Table 10).
Dorsal kinety (bristle) pattern as in O. granulifera type of the genus, that is, composed of five rows with rows 3 and 4 originating by a subequatorial split of row 3 (Figs 8f, g); bristles about 5 μm long in vivo Kinety 1 usually with a distinct gap, one to three kinetids wide, underneath mid-body; kinetids more narrowly spaced posteriorly than anteriorly in kineties 1 and 2, an unusual feature hardly found in any other hypotrich. Caudal cirri in gap left by marginal rows, beat rapidly, comparatively thick and about 20 μm long in vivo.
Comparison with related species
The species mentioned in the following discussion have been monographed by Berger (1999). Thus, only the more recent literature is cited.
Oxytricha arabica has several distinct features fostering identification, at least to group level. These are the comparatively thick and long cirri, producing a conspicuous cirral fringe posteriorly; the narrowly spaced macronucleus nodules; the tight spacing of the postoral ventral cirri underneath the buccal vertex; the 4–5 μm long dorsal bristles (3 μm in most congeners); and the moderate size (~100 μm). However, these features are found also in the following species (main distinguishing characteristics in parentheses): O. longa (4 vs. 5 transverse cirri, 2 vs. 3 caudal cirri), O. quadricirrata (special cortical granules present vs. absent; 4 vs. 5 transverse cirri), O. minor (poorly described; length–width ratio 4–6:1 vs. about 3:1), O. similis (2 vs. 3 caudal cirri) and O. lanceolata. The last mentioned species matches O. arabica in almost all features, except for the dorsal bristle pattern (Figs 8f, g): kinety 3 continuous vs. split subequatorially, as in the type species of Oxytricha; kinety 1 continuous vs. with subequatorial gap; kinetids of rows 1 and 2 ordinarily vs. narrowly spaced in posterior region. Of these characteristics, the split of kinety 3 is the most distinct and important feature because it is ontogenetically fixed and thus used to distinguish species. To be sure, we checked the protargol slides of the three O. lanceolata populations described by Foissner and Berger (1987, 1989) and Foissner (1996). We confirm that the descriptions are correct, i.e., that the three specific features of O. arabica are absent from O. lanceolata.
The species mentioned above are difficult to distinguish in vivo, and thus identifications should be checked in protargol preparations. Possibly, the biogeographic pattern might be useful: while O. arabica is rare and probably restricted to a certain area (North Africa?), O. lanceolata is a cosmopolitan occurring, inter alia, in Austria, Japan, Madeira and Antarctica (Foissner 1998, Berger 1999).
Erimophrya monostyla nov. spec. (Figs 9a–f; Table 10)
Figs 9.
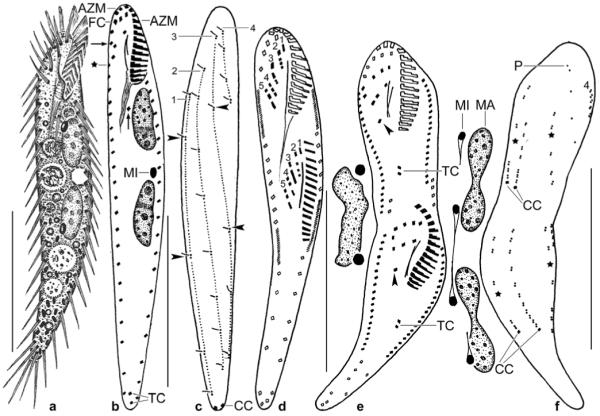
a–f. Erimophrya monostyla from life (a) and after protargol impregnation (b–f). a – ventral view of a representative specimen, length 70 μm; b, c – ventral and dorsal view of holotype specimen, length 72 μm. Arrows mark the single postoral cirrus and frontoventral cirri III/2 and IV/3, which are close together. Asterisk denotes begin of right marginal row. Arrowheads delimit gaps in dorsal bristle rows 1 and 3. Note that this specimen has only one micronucleus; d – cirral pattern and nuclear apparatus of an early divider, showing that only five (numerals) fronto-ventral- -transverse cirral anlagen streaks are produced; e, f – ventral and dorsal view of a late divider, showing that two transverse and two caudal cirri are generated. Arrowheads mark the postoral cirrus, which is migrating posteriorly. Asterisks mark gaps in dorsal kineties 1 and 3 (cp. Fig. 9c). Dorsal bristle row 4 originates close to the right row of marginal cirri. The macronucleus nodules divide a second time. Parental structures shown by contour, newly formed shaded black. AZM – adoral zone of membranelles, CC – caudal cirri, FC – frontal cirri, MA – macronucleus nodule, MI – micronucleus, P – parental dorsal bristles, TC – transverse cirri, 1–4 – dorsal bristle rows, 1–5 – cirral anlagen streaks. Scale bars: 30 μm.
Diagnosis
Size about 70 × 12 μm in vivo; slenderly pisciform. On average 2 almost abutting, elongate ellipsoidal macronucleus nodules, 15 adoral membranelles, 18 cirri each in right and left marginal row, 1 postoral ventral cirrus, 2 transverse and caudal cirri, and 4 dorsal kineties with an average of 5 almost equidistantly spaced kinetids in kinety 1.
Type locality
Soil from Tenerife, southwest coast of Candelaria, 28° N 17° W.
Etymology
Composite of the Greek numeral mono (single) and the Latin noun stylo (style, process), referring to the single postoral ventral cirrus, a main feature of the species.
Type material
1 holotype and 2 paratype slides with protargol-impregnated morphostatic and dividing specimens have been deposited in the Biology Centre of the Museum of Upper Austria, Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Description
Size 55–80 × 10–14 μm in vivo, usually near 70 × 12 μm; length–width ratio comparatively constant, i.e., 5–8:1, on average 6:1 in protargol preparations; very flexible but not contractile (Table 10). Shape fairly constant, usually slenderly oblanceolate and slightly sigmoidal, that is, pisciform and widest in mid-body, gradually narrowing posteriorly with narrowly rounded or acute end; hardly flattened dorsoventrally, but sometimes slightly twisted about main body axis (Figs 9a, b). Nuclear apparatus slightly above middle body third between midline and left body margin. Macronucleus nodules ellipsoidal (2:1) to very elongate ellipsoidal (5:1), on average near 3:1 and only 2 μm apart; contain many small and some middle-sized nucleoli. On average two broadly ellipsoidal micronuclei, one each attached to macronucleus nodules in variable positions (Figs 9a, b). Contractile vacuole slightly above mid-body at left cell margin. No specific cortical granules. Cytoplasm colourless, with some lipid droplets and many 1–2 μm long, cylindroid crystals in posterior body third, which thus appears dark under low magnification (≤ × 100). Food vacuoles 4–8 μm across, contain bacteria and heterotrophic flagellates. Moves rather slowly on microscope slide and between soil particles, showing great flexibility.
Cirral pattern rather constant, number of cirri of usual variability (Figs 9a, b; Table 10). Most cirri about 8 μm long in vivo and rather fine; transverse and caudal cirri about 10 μm long and V-like spread in vivo. Right marginal row begins underneath last frontoventral cirrus and ends subterminally; distances between individual cirri increase slightly from anterior to posterior in both right and left marginal row. Frontal cirri of about same length as other cirri, form an oblique, subapical row. Buccal cirrus right of anterior end of paroral membrane. Frontoventral cirri near right body margin, first cirrus at level of buccal cirrus, cirrus IV/3 close to cirrus III/2, a special pattern found also in E. quadrinucleata. A single postoral cirrus far underneath buccal vertex. Two transverse cirri one after the other near body end and thus difficult to distinguish from marginal and caudal cirri, both in vivo and protargol preparations (Figs 9a, b). Dorsal bristles about 2 μm long in vivo and widely spaced, arranged in four rows (Fig. 9c; Table 10): row 1 distinctly shortened anteriorly commencing at level of buccal vertex, with conspicuous, one-kinetid-wide gap in mid-body; row 2 commences far subapically and ends subterminally; row 3 commences subapically and ends far subterminally, with conspicuous, one-kinetid-wide gap above mid-body; row 4 composed of only four kinetids extending in anterior body fifth close to right body margin. Two caudal cirri at posterior body end.
Oral apparatus inconspicuous because adoral zone occupies only 20–23% of body length, of usual shape and structure; consists of an average of 15 membranelles, bases of largest membranelles about 4 μm wide in vivo. Buccal cavity narrow and flat; buccal lip angularly projecting and thus prominent partially covering proximal third of adoral zone, bears paroral membrane. Both undulating membranes slightly curved and almost in parallel, paroral begins about 2 μm in front of endoral at level of buccal cirrus; exact structure of membranes not clearly recognizable. Pharyngeal fibres distinct in vivo and protargol preparations, of ordinary length and structure, extend obliquely backwards (Figs 9a, b; Table 10).
Ontogenesis
Some key ontogenetic stages were found, showing (i) five fronto-ventral-transverse cirral anlagen streaks (Fig. 9d); (ii) two transverse cirri (Fig. 9e); and (iii) two caudal cirri one each developing in dorsal kineties 1 and 2 (Fig. 9f).
Occurrence and ecology
At Tenerife, E. monostyla occurred in a sandy, greyish, meagre, non-saline coastal soil with pH 8.2 in water (sample kindly collected by Dr. B. Krassnigg, Salzburg University). In Saudi Arabia, this species was found at sites 1, 2 and 6, that is, in a wide variety of habitats, including saline oasis soil (see site descriptions). Thus, E. monostyla has a wide ecological range, possibly preferring sandy, meagre soils in hot areas. With its slender and flexible body, E. monostyla is perfectly adapted to the soil habitat.
Comparison with related species
The genus Erimophrya was established by Foissner et al. (2202) for Urosomoida – like oxytrichids with only five frontoventral-transverse cirral anlagen streaks. The Saudi Arabian and Tenerife populations match this specific feature (Figs 9d, e).
Of the four described Erimophrya species, E. monostyla is most similar to E. arenicola which has also only one postoral cirrus (Figs 9b, e). However, E. arenicola has only a single (vs. two) transverse cirrus, only 3 (vs. 4) dorsal kineties, and the kinetid pattern in dorsal kinety 1 and the arrangement of the frontoventral cirri are different; the latter is rather similar to that found in E. quadrinucleata. As mentioned above, Erimophrya species are difficult to identify in vivo; thus, identifications should be checked in protargol preparations.
Acknowledgements
Financial support was provided by the Austrian Science Foundation (FWF project P-19699-B17) and the King Saud University. The technical assistance of Mag. Barbara Harl, Mag. Gudrun Fuss, Robert Schörghofer, and Andreas Zankl is greatly acknowledged.
REFERENCES
- Al-Rasheid KAS. A review of marine and brackish water interstitial ciliates from the Arabian Gulf, its offshore islands and Al--Hassa oasis with notes on their ecological status and recovery after the 1991 gulf war oil spill. Arab Gulf J. Scientific Res. 1999;17:336–368. [Google Scholar]
- Al-Rasheid KAS. New records of interstitial ciliates (Protozoa Ciliophora) from the Saudi coasts of the Red Sea. Trop. Zool. 2001;14:133–156. [Google Scholar]
- Berger H. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia) Monogr. Biol. 1999;78:1–1080. [Google Scholar]
- Berger H. Catalogue of ciliate names 1. Hypotrichs. Verlag Helmut Berger; Salzburg: 2001. pp. i–vii.pp. 206 [Google Scholar]
- Berger H. Monograph of the Urostyloidea (Ciliophora, Hypotricha) Monogr. Biol. 2006;85:1–1304. [Google Scholar]
- Berger H, Foissner W. Morphology and biometry of some soil hypotrichs (Protozoa: Ciliophora) Zool. Jb. Syst. 1987;114:193–239. [Google Scholar]
- Berger H, Foissner W, Adam H. Taxonomie, Biometrie und Morphogenese einiger terricoler Ciliaten (Protozoa: Ciliophora) Zool. Jb. Syst. 1984;111:339–367. [Google Scholar]
- Berger H, Al-Rasheid KAS, Foissner W. Morphology and cell division of Saudithrix terricola n. gen., n. sp., a large, stichotrich ciliate from Saudi Arabia. J. Eukaryot. Microbiol. 2006;53:260–268. doi: 10.1111/j.1550-7408.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- Blatterer H, Foissner W. Beitrag zur terricolen Ciliatenfauna (Protozoa: Ciliophora) Australiens. Stapfia. 1988;17:1–84. [Google Scholar]
- Bodenheimer FS. Animal life in Palestine. L. Mayer; Jerusalem: 1935. p. 506. [Google Scholar]
- Eigner P, Foissner W. Divisional morphogenesis in Amphisiellides illuvialis n. sp., Paramphisiella caudata (Hemberger) and Hemiamphisiella terricola Foissner, and redefinition of the Amphisiellidae (Ciliophora, Hypotrichida) J. Eukaryot. Microbiol. 1994;41:243–261. [Google Scholar]
- Esteban GF, Clarke KJ, Olmo JL, Finlay BJ. Soil protozoa – an intensive study of population dynamics and community structure in an upland grassland. Appl. Soil Ecol. 2006;33:137–151. [Google Scholar]
- Finlay BJ. Protozoa. Encyclopedia of Biodiversity. 2001;4:901–915. [Google Scholar]
- Foissner W. Ökologie und Taxonomie der Hypotrichida (Protozoa: Ciliophora) einiger österreichischer Böden. Arch. Protistenk. 1982;126:19–143. [Google Scholar]
- Foissner W. Neue und wenig bekannte hypotriche und colpodide Ciliaten (Protozoa: Ciliophora) aus Böden und Moosen. Zool. Beitr. N. F. 1987a;31:187–282. [Google Scholar]
- Foissner W. Soil protozoa: fundamental problems, ecological significance, adaptations in ciliates and testaceans, bioindicators, and guide to the literature. Progr. Protistol. 1987b;2:69–212. [Google Scholar]
- Foissner W. Gemeinsame Arten in der terricolen Ciliatenfauna (Protozoa: Ciliophora) von Australien und Afrika. Stapfia. 1988;17:85–133. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Eur. J. Protistol. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W. Colpodea (Ciliophora) Protozoenfauna. 1993;4:i–x. 798. [Google Scholar]
- Foissner W. Morphology and morphogenesis of Circinella arenicola nov. gen., nov. spec., a cephalized hypotrich (Ciliophora, Hypotrichida) from sand dunes in Utah, USA. Eur. J. Protistol. 1994;30:156–170. [Google Scholar]
- Foissner W. Tropical protozoan diversity: 80 ciliate species (Protozoa, Ciliophora) in a soil sample from a tropical dry forest of Costa Rica, with descriptions of four new genera and seven new species. Arch. Protistenk. 1995;145:37–79. [Google Scholar]
- Foissner W. Faunistics, taxonomy and ecology of moss and soil ciliates (Protozoa, Ciliophora) from Antarctica, with description of new species, including Pleuroplitoides smithi gen. n., sp. n. Acta Protozool. 1996;35:95–123. [Google Scholar]
- Foissner W. An updated compilation of world soil ciliates (Protozoa, Ciliophora), with ecological notes, new records, and descriptions of new species. Eur. J. Protistol. 1998;34:195–235. [Google Scholar]
- Foissner W. Notes on the soil ciliate biota (Protozoa, Ciliophora) from the Shimba Hills in Kenya (Africa): diversity and description of three new genera and ten new species. Biodivers. Conserv. 1999;8:319–389. [Google Scholar]
- Foissner W. A compilation of soil and moss ciliates (Protozoa, Ciliophora) from Germany, with new records and descriptions of new and insufficiently known species. Eur. J. Protistol. 2000;36:253–283. [Google Scholar]
- Foissner W. Biogeography and dispersal of micro-organisms: a review emphasizing protists. Acta Protozool. 2006;45:111–136. [Google Scholar]
- Foissner W. Dispersal and biogeography of protists: recent advances. Jpn. J. Protozool. 2007;40:1–16. [Google Scholar]
- Foissner W. Protist diversity and distribution: some basic considerations. Biodivers. Conserv. 2008a;17:235–242. [Google Scholar]
- Foissner W. Notes on soil ciliates from Singapore, with description of Suturothrix monoarmata nov. gen., nov. spec. (Protozoa, Ciliophora) Soil Organisms. 2008b;80:81–97. [PMC free article] [PubMed] [Google Scholar]
- Foissner W, Foissner I. The fine structure of Fuscheria terricola Berger et al., 1983 and a proposed new classification of the subclass Haptoria Corliss, 1974 (Ciliophora, Litostomatea) Arch. Protistenk. 1988;135:213–235. [Google Scholar]
- Foissner W, Al-Rasheid K. A unified organization of the stichotrichine oral apparatus, including a description of the buccal seal (Ciliophora: Spirotrichea) Acta Protozool. 2006;45:1–16. [Google Scholar]
- Foissner W, Al-Rasheid K. Notes on soil ciliates (Protozoa, Ciliophora) from The Netherlands, with description of Keronopsis schminkei nov. spec. and Apobryophyllum schmidingeri nov. spec. Acta Protozool. 2007;46:201–220. [PMC free article] [PubMed] [Google Scholar]
- Foissner W, Xu K. Monograph of the Spathidiida (Ciliophora, Haptoria). Vol. I: Protospathidiidae, Arcuospathidiidae, Apertospathulidae. Monogr. Biol. 2007;81:1–485. [Google Scholar]
- Foissner W, Stoeck T. Morphology, ontogenesis and molecular phylogeny of Neokeronopsis (Afrokeronopsis) aurea nov. subgen., nov. spec. (Ciliophora: Hypotricha), a new African flagship ciliate confirms the CEUU hypothesis. Acta Protozool. 2008;47:1–33. [PMC free article] [PubMed] [Google Scholar]
- Foissner W, Berger H, Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1999;3:793. [Google Scholar]
- Foissner W, Agatha S, Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Denisia. 2002;5:1–1459. [Google Scholar]
- Foissner W, Berger H, Xu K, Zechmeister-Boltenstern S. A huge, undescribed soil ciliate (Protozoa: Ciliophora) diversity in natural forest stands of Central Europe. Biodivers. Conserv. 2005;14:617–701. [Google Scholar]
- Fried J, Foissner W. Differentiation of two very similar glaucomid ciliate morphospecies (Ciliophora, Tetrahymenida) by fluorescence in situ hybridization with 18S rRNA targeted oligonucleotide probes. J. Eukaryot. Microbiol. 2007;54:381–387. doi: 10.1111/j.1550-7408.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- Hemberger H. Revision der Ordnung Hypotrichida Stein (Ciliophora, Protozoa) an Hand von Protargolpräparaten und Morphogenesedarstellungen. Diss. Univ. Bonn. 1982:i–iv. 296. [Google Scholar]
- Hemberger H. Neue Gattungen und Arten hypotricher Ciliaten. Arch. Protistenk. 1985;130:397–417. [Google Scholar]
- Kent WS. A manual of the infusoria: including a description of all known flagellate, ciliate, and tentaculiferous protozoa, British and foreign, and an account of the organization and affinities of the sponges. Volumes I–III. David Bogue; London: 1880–1882. p. 913. Vol. I 1880: 1–432; Vol. II 1881: 433–720; Vol. II 1882: 721–913; Vol. III 1882: Plates I–LI. [Google Scholar]
- Lüftenegger G, Foissner W, Adam H. r- and K-selection in soil ciliates: a field and experimental approach. Oecologia. 1985;66:574–579. doi: 10.1007/BF00379352. [DOI] [PubMed] [Google Scholar]
- Lynn DH, Small EB. In: Phylum Ciliophora. In: An Illustrated Guide to the Protozoa. 2nd Lee JJ, Leedale GF, Bradbury P, editors. Vol I. Society of Protozoologists; Lawrence: 2002. pp. 371–656. [Google Scholar]
- Müller P. Arealsysteme und Biogeographie. Ulmer; Stuttgart: 1981. p. 704. [Google Scholar]
- Petz W, Foissner W. Morphology and infraciliature of some soil ciliates (Protozoa, Ciliophora) from continental Antarctica, with notes on the morphogenesis of Sterkiella histriomuscorum. Polar Rec. 1997;33:307–326. [Google Scholar]
- Schmidt SL, Foissner W, Schlegel M, Bernhard D. Molecular phylogeny of the Heterotrichea (Ciliophora; Postciliodesmatophora) based on small subunit rRNA gene sequences. J. Eukaryot. Microbiol. 2007;54:358–363. doi: 10.1111/j.1550-7408.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- Varga E. Études sur la faune des protozoaires de quelques sols du Sahara et des hauts plateaux Algériens. Annls Inst. Pasteur. 1936;56:101–123. [Google Scholar]
- Vd’ačný P, Hlúbiková D, Tirjaková E. Spathidium seppelti foissneri nov. subspec., Spathidium simplinucleatum nov. stat., and Dileptus americanus Kahl, 1931, one new and two poorly known soil gymnostome ciliates from soils of Slovakia. Eur. J. Protistol. 2006;42:175–189. doi: 10.1016/j.ejop.2006.02.002. [DOI] [PubMed] [Google Scholar]