Summary
We studied the morphology of four new dileptid ciliates, using standard methods. Dileptus microstoma, which was discovered in Benin (Africa), is outstanding in having a very small oral opening (~4 μm), an interrupted dorsal row of contractile vacuoles, and ampulliform extrusomes. Dileptus semiarmatus, which was discovered in Austria, possesses extrusomes only in the right posterior half of the proboscis and has very widely spaced circumoral and perioral kinetids. Dileptus longitrichus, which was discovered in Japan, is almost unique in having up to 15 μm long brush bristles and a row of contractile vacuoles each in ventral and dorsal side of body. Pseudomonilicaryon brachyproboscis, which was discovered in Greece, differs from the congeners by the narrowly ellipsoidal micronuclei, the dimorphic dorsal brush, the extrusomes, and the contractile vacuole pattern. Four new features are introduced for distinguishing species in dileptids: shape of micronucleus, monomorphic/dimorphic dorsal brush, shape of oral opening, and spacing of circumoral dikinetids. The terrestrial dileptids share several distinct morphological features that are probably adaptations to the soil environment: (1) the body is comparatively slender and small, what is likely related to the narrowness of the habitat; (2) the proboscis is short, which increases the relative volume of the trunk, what might be related to its fragility and/or to the space available for prey digestion; (3) the long dorsal bristles might foster prey recognition; and (4) the pronounced body flexibility in all dileptids likely fosters their high diversity in the narrow and wrinkled soil habitat.
Keywords: Africa, biodiversity, Dileptus, Europe, Japan, Pseudomonilicaryon
INTRODUCTION
Dileptids are rapacious ciliates commonly found in a variety of habitats all over the world. They are characterized by having a proboscis underneath of which is the oral opening (Corliss 1979, Foissner and Foissner 1988). Kahl (1931) and Dragesco (1963) produced the authoritative taxonomic studies and monographs. They used the following features for distinguishing species within the genus Dileptus: body shape and size, ratio of body and proboscis length, and the nuclear and contractile vacuole pattern. Later, Foissner (1984, 1989) and Foissner et al. (2002) added further diagnostic features such as extrusome shape, details of the ciliary pattern, and various morphometrics. The present analysis showed several further characters which are, however, uncommon in most haptorids. Thus, one can expect a considerable number of undescribed species. Only about 20 out of the 50 known species have been studied with modern methods (Golińska 1971; Foissner 1981, 1984, 1989, 1995, 1997, 2000; Foissner et al. 1995, 1999, 2002; Wirnsberger et al. 1984; Song and Wilbert 1989; Song et al. 1988; Vd’ačný and Foissner 2008; Vd’ačný et al. 2006). Thus, many dileptids remain unknown in terms of their ciliature and hence need reinvestigation.
Dileptids belong to the subclass Haptoria and are possibly closely related to the spathidiids, as suggested by morphological, molecular, and conjugation data (Foissner and Foissner 1988, Strüder-Kypke et al. 2006, Vd’ačný and Foissner 2008). Only one attempt has been made to split the large genus Dileptus, using the macronucleus pattern (Jankowski 1967): Dileptus with dispersed nodules; Dimacrocaryon with two nodules and a single micronucleus in between; and Monilicaryon with moniliform macronucleus. Foissner’s (1997) reinvestigation of Dileptus monilatus, type of Monilicaryon, showed that the same nuclear pattern evolved independently in several evolutionary lines of Dileptus s.l. Therefore, Foissner (1984, 1997) and Foissner et al. (1999) redefined Jankowski’s genera by specialities of the ciliary pattern and showed that the typification of Jankowski (1967) required three further genera, viz., Pseudomonilicaryon, Rimaleptus, and Pelagodileptus. However, convincing evidence is lacking for most dileptid genera because the evolutionary significance of the characters is not known and transitions in nuclear pattern exist. Thus, sequence data are urgently needed from the key species.
About 25 out of the 50 Dileptus s.l. species occur in or have been originally described from terrestrial habitats, such as leaf litter, mineral soil, and moss (Foissner 1998, Foissner et al. 2002). This is surprising because dileptids are rather large ciliates most being longer than 150 μm. Possibly, it is the great flexibility of the body which, as a kind of pre-adaptation, fosters the high diversity of dileptids in terrestrial habitats.
MATERIAL AND METHODS
The new species were discovered in soil from various biogeographical regions, using the non-flooded Petri dish method (Foissner et al. 2002). Briefly, this simple technique involves placing 50–500 g of litter and soil in a Petri dish, 10–15 cm in diameter, and slightly over-saturating but not flooding the sample with distilled water. See the occurrence and ecology section in the description of the individual species for details on sites.
Live observation and silver impregnation were performed as described by Foissner (1991). Counts and measurements on silvered specimens were conducted at a magnification of × 1,000. In vivo measurements were done at magnifications of × 40–1,000. Drawings of live specimens were based on free-hand sketches; those of impregnated cells were made with a drawing device. Terminology is according to Corliss (1979) and, especially, Foissner and Xu (2007).
RESULTS
Dileptus microstoma nov. spec. (Figs 1a–w, 3a–c; Tables 1, 2)
Figs 1a–w.
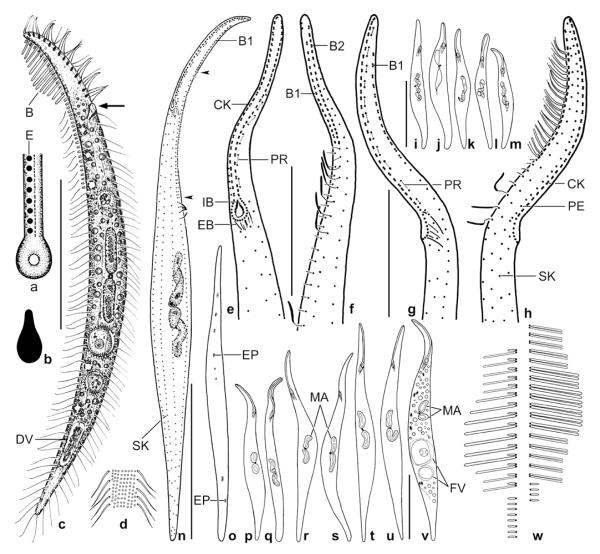
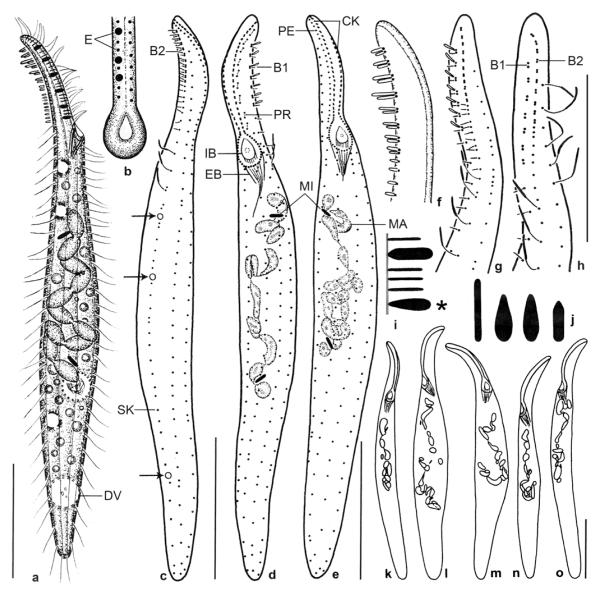
Dileptus microstoma nov. spec., African type population (a–d, g, h, n, o, w) and Singapore voucher population (e, f, i–m, p–v) from life (a–d, w) and after protargol impregnation (e–v). a – frontal view of oral opening; b – extrusomes are ampulliform and 1.5 × 1 μm in size; c – right side view of a representative specimen, length 170 μm. Arrow denotes the very small oral opening, a main feature of this species; d – surface view showing cortical granulation; e, f – ventrolateral and dorsolateral view of ciliary pattern in anterior body portion. Preoral kineties are difficult to recognize because composed of only two widely spaced monokinetids (connected by lines) almost parallel to left half of circumoral kinety; g, h – left and right side view of ciliary pattern in anterior body portion. Basal bodies of preoral kineties are connected by lines; i–m – variability of body shape and size as well as of nuclear apparatus in post-dividers. Drawn to scale; n – ciliary pattern of left side and nuclear apparatus of holotype specimen, length 180 μm. Note that brush row 1 is associated with a monokinetidal bristle tail (arrowheads) extending to second third of body; o – excretory pore pattern; p–v – variability of body shape and size as well as of nuclear apparatus of morphostatic specimens. Drawn to scale; w – structure of dorsal brush. The bristles are up to 10 μm long and gradually decrease to about 6 μm in end regions of rows. B(1, 2) – dorsal brush (rows 1, 2), CK – circumoral kinety, DV – defecation vacuole, E – extrusomes, EB – external basket, EP – excretory pores, FV – food vacuoles, IB – internal basket, MA – macronucleus nodules, PE – perioral kinety, PR – preoral kineties, SK – somatic kinety. Scale bars: 50 μm (c, i–m, n, p–v) and 20 μm (e–h).
Figs 3a–j.
Dileptus microstoma nov. spec. (a–c), D. longitrichus nov. spec. (d, e), and D. semiarmatus nov. spec. (f–j) after protargol impregnation. a, f – lateral views of representative specimens, showing the slender body and the short proboscis above the oral opening (arrows); b – ventrolateral view of ciliary pattern of proboscis. Note the very small oral opening, a main feature of this species; c – dorsal view of ciliary pattern of proboscis. The dorsal brush consists of two staggered rows, that is, row 1 commences slightly more subapically than row 2 (arrowhead). Note the long dorsal bristles (arrow); d – right side view of proboscis; e – left side view of ciliary pattern of proboscis, showing the preoral kineties each composed of 2–4 ordinarily spaced monokinetids forming short rows almost in line with left half of circumoral kinety; g, j – ventral and ventrolateral view of ciliary pattern of proboscis, showing the very widely spaced circumoral and perioral kinetids; h, i – dorsal and dorsolateral view of ciliary pattern of proboscis. The dorsal brush is heteromorphic in posterior third, where bristles are mixed with ordinary cilia (arrowheads). Arrow denotes brush row 1, which commences slightly more subapically than row 2. B1, 2 – dorsal brush rows, CK – circumoral kinety, PE – perioral kinety, PR – preoral kineties, OO – oral opening. Scale bars: 30 μm (a, d, f) and 10 μm (b, c, e, g–j).
Table 1.
Morphometric data on two populations of Dileptus microstoma nov. spec.: type from Africa (1st line); voucher (2nd line) and post--dividers (3rd line) from Singapore. Data based on mounted, protargol-impregnated (Foissner’s method), and randomly selected specimens from non-flooded Petri dish cultures. Measurements in μm. CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of specimens investigated, SD – standard deviation, SE – standard error of mean, – arithmetic mean.
| Characteristics | M | SD | SE | CV | Min | Max | n | |
|---|---|---|---|---|---|---|---|---|
| Body, length | 143.2 | 140.0 | 24.8 | 5.4 | 17.3 | 98.0 | 203.0 | 21 |
| 152.0 | 154.0 | 23.2 | 5.1 | 15.3 | 109.0 | 209.0 | 21 | |
| 91.6 | 96.0 | 14.2 | 4.3 | 15.5 | 64.0 | 108.0 | 11 | |
| Body, width | 13.0 | 13.0 | 1.8 | 0.4 | 14.2 | 10.0 | 17.0 | 21 |
| 11.7 | 12.0 | 2.2 | 0.5 | 18.9 | 8.0 | 15.0 | 21 | |
| 11.4 | 11.0 | 1.8 | 0.5 | 15.6 | 8.0 | 15.0 | 11 | |
| Body length:width, ratio | 11.3 | 10.9 | 2.9 | 0.6 | 25.9 | 6.1 | 20.3 | 21 |
| 13.5 | 13.2 | 3.6 | 0.8 | 26.8 | 9.5 | 21.8 | 21 | |
| 8.2 | 8.3 | 1.5 | 0.4 | 17.9 | 4.6 | 10.3 | 11 | |
| Anterior body end to oral opening, length | 28.5 | 29.0 | 3.1 | 0.7 | 10.8 | 23.0 | 35.0 | 21 |
| 28.4 | 30.0 | 3.5 | 0.8 | 12.2 | 19.0 | 34.0 | 21 | |
| 16.1 | 16.0 | 3.8 | 1.1 | 23.6 | 9.0 | 24.0 | 11 | |
| Proboscis, % of body length | 20.4 | 20.1 | 3.7 | 0.8 | 18.3 | 14.5 | 27.6 | 21 |
| 18.9 | 19.5 | 2.7 | 0.6 | 14.0 | 14.3 | 23.4 | 21 | |
| 17.6 | 18.0 | 2.8 | 0.9 | 16.2 | 12.8 | 22.1 | 11 | |
| Oral opening, lengtha | 4.0 | 4.0 | 0.5 | 12.9 | 0.1 | 3.0 | 6.0 | 20 |
| 4.1 | 4.0 | 0.8 | 0.2 | 20.0 | 3.0 | 5.0 | 21 | |
| 4.4 | 5.0 | 0.8 | 0.3 | 19.3 | 3.0 | 6.0 | 11 | |
| Oral opening, widtha | 2.7 | 3.0 | 0.5 | 0.1 | 17.1 | 2.0 | 4.0 | 11 |
| 2.8 | 3.0 | 0.4 | 0.1 | 14.0 | 2.0 | 4.0 | 13 | |
| 3.3 | 3.0 | – | – | – | 3.0 | 4.0 | 6 | |
| Anterior body end to macronucleus, distance | 65.1 | 62.0 | 10.7 | 2.5 | 16.5 | 47.0 | 82.0 | 19 |
| 64.6 | 65.0 | 9.5 | 2.1 | 14.7 | 48.0 | 85.0 | 21 | |
| 32.4 | 33.0 | 8.3 | 2.5 | 25.6 | 19.0 | 46.0 | 11 | |
| Nuclear figure, length | 26.3 | 26.0 | 6.1 | 1.4 | 23.4 | 15.0 | 38.0 | 19 |
| 24.9 | 25.0 | 4.2 | 0.9 | 16.9 | 17.0 | 33.0 | 21 | |
| 21.3 | 21.0 | 4.5 | 1.3 | 20.9 | 14.0 | 30.0 | 11 | |
| Anterior macronucleus nodule, length | 15.3 | 14.0 | 4.0 | 0.9 | 25.9 | 10.0 | 24.0 | 19 |
| 13.8 | 13.0 | 3.6 | 0.8 | 26.3 | 8.0 | 23.0 | 21 | |
| 11.8 | 12.0 | 1.8 | 0.7 | 15.0 | 9.0 | 15.0 | 7 | |
| Anterior macronucleus nodule, width | 4.9 | 5.0 | 1.3 | 0.3 | 26.7 | 3.0 | 8.0 | 19 |
| 4.3 | 4.0 | 0.7 | 0.1 | 15.6 | 4.0 | 6.0 | 21 | |
| 3.7 | 4.0 | 0.5 | 0.2 | 14.2 | 3.0 | 5.0 | 7 | |
| Posterior macronucleus nodule, length | 15.4 | 14.0 | 4.1 | 1.0 | 26.8 | 10.0 | 24.0 | 19 |
| 14.6 | 15.0 | 3.4 | 0.7 | 23.0 | 8.0 | 21.0 | 21 | |
| 13.2 | 12.0 | 1.9 | 0.7 | 14.1 | 11.0 | 16.0 | 7 | |
| Posterior macronucleus nodule, width | 5.1 | 5.0 | 1.1 | 0.3 | 21.8 | 3.0 | 7.0 | 19 |
| 4.4 | 4.0 | 0.7 | 0.2 | 16.1 | 3.0 | 5.0 | 21 | |
| 3.6 | 4.0 | – | – | – | 3.0 | 4.0 | 7 | |
| Macronucleus nodules, number | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 19 |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 | |
| 2.3 | 2.0 | – | – | – | 1.0 | 8.0 | 11 | |
| Micronucleus, largest diameter | 1.8 | 2.0 | – | – | – | 1.5 | 2.5 | 10 |
| 1.6 | 1.5 | – | – | – | 1.0 | 2.5 | 21 | |
| 2.1 | 2.5 | – | – | – | 1.0 | 2.5 | 9 | |
| Micronucleus, number | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 10 |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 | |
| 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 9 | |
| Ciliary rows, number | 8.8 | 9.0 | 0.9 | 0.2 | 9.9 | 7.0 | 11.0 | 21 |
| 8.8 | 9.0 | 1.1 | 0.2 | 12.2 | 7.0 | 11.0 | 21 | |
| 8.0 | 8.0 | 0.8 | 0.2 | 9.7 | 7.0 | 9.0 | 11 | |
| Cilia, number in mid-body in 10 μm | 5.6 | 5.0 | 1.3 | 0.3 | 22.8 | 3.0 | 8.0 | 21 |
| 3.7 | 3.0 | – | – | – | 3.0 | 6.0 | 21 | |
| 4.7 | 5.0 | 0.9 | 0.3 | 19.1 | 3.0 | 6.0 | 11 | |
| Dorsal brush rows, number | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 | |
| 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 10 | |
| Dikinetids in brush row 1, numberb | 15.2 | 14.0 | 3.5 | 1.0 | 23.2 | 10.0 | 21.0 | 12 |
| Dikinetids in brush row 2, numberb | 18.5 | 19.0 | 3.4 | 1.0 | 18.5 | 13.0 | 24.0 | 12 |
| Anterior body end to last dikinetid of brush row 1, distanceb | 20.2 | 21.0 | 2.9 | 0.8 | 14.5 | 15.0 | 25.0 | 12 |
| Anterior body end to last dikinetid of brush row 2, distanceb | 19.0 | 20.0 | 3.1 | 0.9 | 16.4 | 14.0 | 23.0 | 12 |
Measured as distance between circumoral kinety.
Counted/measured only in morphostatic specimens from Singapore population.
Table 2.
Comparison of macronucleus shape in two populations of Dileptus microstoma nov. spec. and D. semiarmatus nov. spec.
| Species/population | Shape of macronucleus nodules (proportion, %) |
Number of specimens analyzed |
||||
|---|---|---|---|---|---|---|
| Ellipsoidal or ovoidal |
Reniform | Moniliform | Spiralized | Cylindroidal | ||
|
Dileptus microstoma from Benin |
19 | 47 | 4 | 30 | – | 21 |
|
Dileptus microstoma from Singapore |
33 | 52 | 9 | 4 | – | 21 |
|
Dileptus semiarmatus from Austria |
13 | 15 | – | 19 | 53 | 38 |
Diagnosis
Size about 170 × 15 μm in vivo. Shape very narrowly dileptid to rod-like with proboscis about 1/5 of body length and acute posterior body third. Two oblong macronucleus nodules with a micronucleus in between. Contractile vacuoles in dorsal side of trunk, forming a short row each in anterior and posterior third of trunk. Extrusomes attached to right half of oral bulge, ampulliform, 1.5 × 1 μm in size. On average 9 ciliary rows, 2 staggered and differentiated to a conspicuous, isostichad dorsal brush with bristles up to 10 μm long: rows 1 and 2 composed of an average of 14 and 19 dikinetids, respectively; monokinetidal tail of row 1 extending to second third of body and thus longer than that of row 2. Oral opening about 4 × 3 μm in size. Circumoral kinety composed of ordinarily to widely spaced dikinetids. Preoral kineties each usually composed of two ordinarily to widely spaced kinetids, forming minute rows almost parallel to circumoral kinety.
Type locality
Soil from the University Campus in Abomey-Calavi, Benin, Africa, E2°21′ N6°27′.
Type material
One holotype slide and six paratype slides as well as eight voucher slides (Singapore population) with protargol-impregnated specimens have been deposited in the Biology Centre of the Museum of Upper Austria, Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Etymology
Composite of the Greek adjective mikros (small) and the Latin noun stoma (mouth), referring to the very small oral entrance.
Description
This species was studied in two populations, namely from Benin (type) and Singapore (voucher). The populations match very well (Table 1), therefore the diagnosis and description summarize all observations. The living morphology was studied mainly in the type population.
Size 130–250 × 15–20 μm in vivo, usually about 170 × 15 μm, as calculated from some in vivo measurements and the morphometric data (Table 1). Shape very narrowly dileptid to rod-like with proboscis occupying about one fifth of body length; anterior and posterior third gradually narrowing to acute ends, posterior end never tail-like; widest in mid-portion of trunk, anterior quarter of body flattened about 2:1, trunk unflattened; dorsal outline curved to slightly sigmoidal (Figs 1c, p–v, 3a). Nuclear apparatus in mid of trunk, may be dislocated by large food items (Fig. 1v). Macronucleus nodules highly variable in shape, that is, ellipsoidal, reniform, spiralized or, very rarely, moniliform (Figs 1n, p–u; Table 2); nucleoli large, ellipsoidal or lobate, well recognizable both in vivo and in protargol preparations. Micronucleus in between macronucleus nodules, globular to broadly ellipsoidal, about 2 μm in size, usually surrounded by a distinct membrane in protargol preparations (Figs 1c, n, p–v). Contractile vacuoles in dorsal side of trunk, remarkable because lacking in mid-body, thus forming a short row each in anterior and posterior third of trunk (Figs 1c, o). Extrusomes attached only to right half of oral bulge; ampulliform and minute, that is, about 1.5 × 1 μm in size, rather refractive and thus distinct in vivo (Figs 1a, b); developing cytoplasmic extrusomes broadly fusiform, rarely impregnating with protargol. Cortex very flexible, contains about eight oblique granule rows between adjacent kineties; granules narrowly spaced in somatic and oral cortex, about 0.5 × 0.3 μm in size (Fig. 1d). Cytoplasm colourless, hyaline in flattened proboscis and rear body end, opaque throughout trunk due to numerous lipid droplets 1–3 μm across and food vacuoles containing naked amoebae and fungal spores (Fig. 1v); in rear end sometimes a defecation vacuole with crystalline contents. Movement without peculiarities.
Cilia about 8 μm long in vivo, ordinarily spaced; in protargol preparations as typical for dileptids, i.e., with thick and strongly impregnated distal half, except for dorsal and tail bristles; arranged in an average of 9 ordinarily spaced, longitudinal rows leaving a barren area on left side of proboscis (Figs 1e, g, n; Table 1). First row right of circumoral kinety extends as perioral kinety with widely spaced cilia to top of proboscis. Invariably only one ciliary row between perioral kinety and brush row 2 (Fig. 1h). Dorsal brush exactly on dorsal side of proboscis, composed of two staggered, isostichad rows. Dorsal bristles conspicuous because long and thick, that is, up to 10 × 1 μm in size. Brush row 1 commences slightly more subapically than row 2, composed of an average of 15 loosely to ordinarily spaced dikinetids each having an about 1.5 μm long anterior bristle and an up to 10 μm long posterior bristle gradually decreasing to about 6 μm in end regions of row. Brush row 2 begins near top of proboscis, composed of an average of 19 loosely to ordinarily spaced dikinetids each associated with bristles similar to those of row 1, but anterior bristle not shortened. Both rows continue with a monokinetidal tail of about 1.5 μm long bristles, tail of row 1 conspicuously longer than that of row 2 and extending to second third of body; row 2 tail composed of less than 5 bristles (Figs 1a, f, h, n, w, 3c).
Oral apparatus basically as in other dileptids. Oral opening at end of anterior body fifth, broadly ovate but only about 4 × 3 μm in size and thus difficult to recognize in vivo (Figs 1a, c; Table 1). Pharyngeal basket difficult to recognize both in vivo and in protargol preparations because composed of very fine and faintly impregnated fibres. Circumoral kinety composed of ordinarily to widely spaced dikinetids, except of narrowly spaced monokinetids around oral opening. Preoral kineties difficult to recognize because composed of only two (rarely three) ordinarily to widely spaced monokinetids almost in line with left branch of circumoral kinety (Figs 1e, g, n).
Notes on post-dividers from Singapore population
Post-dividers differ from morphostatic cells by the smaller size (90 × 11 μm vs. 150 × 12 μm), the stouter body (8.2:1 vs. 13.5:1), and the shorter (16 μm vs. 30 μm long) and broader proboscis, while the number of ciliary rows (8 vs. 9) and the proportion of body and proboscis length (18% vs. 19%) is quite similar (Table 1). Early post-dividers have a fibre-like elongation of the posterior end of the macronucleus and the dorsal brush dikinetids are very near together, especially in row 2. Further, post-dividers are highly variable in number and pattern of the macronucleus nodules: a moniliform strand composed of about 8 nodules; a single, highly spiralized strand; or, rarely, two ordinary macronucleus nodules with the micronucleus in between (Figs 1i–m; Table 1).
Occurrence and ecology
To date found at type locality and in a very sandy coastal soil (pH 7.0 in water) from Singapore, Asia; possibly occurs also in Kenya and in the Monte Verde National Park of Costa Rica. The sample from type locality consisted of hard, red, circumneutral (ph 7.3 in water) soil mixed with some leaf litter and grass roots. Dileptus microstoma was rather abundant in the non-flooded Petri dish cultures and is well adapted to the soil environment by the very slender, highly flexible body.
Comparison with related species
Dileptus microstoma belongs to the D. breviproboscis group, that is, fairly similar soil dileptids with slender body, rather short proboscis, and two macronucleus nodules with a single micronucleus in between. Among the better known species of this group, D. microstoma is most similar to D. breviproboscis, as redescribed by Foissner et al. (2002); D. semiarmatus (described below); and D. orientalis Song et al., 1988, differing mainly in the shape of the extrusomes (ampulliform vs. oblong, cuneate, or ellipsoidal) and the contractile vacuole pattern (a dorsal row of vacuoles interrupted in mid of trunk vs. a continuous dorsal row). Dileptus alpinus, as described by Kahl (1931) and redescribed by Foissner (1989), has rod-shaped extrusomes and only two contractile vacuoles.
Dileptus semiarmatus nov. spec. (Figs 2a–p, 3f–j; Tables 2, 3)
Figs 2a–p.
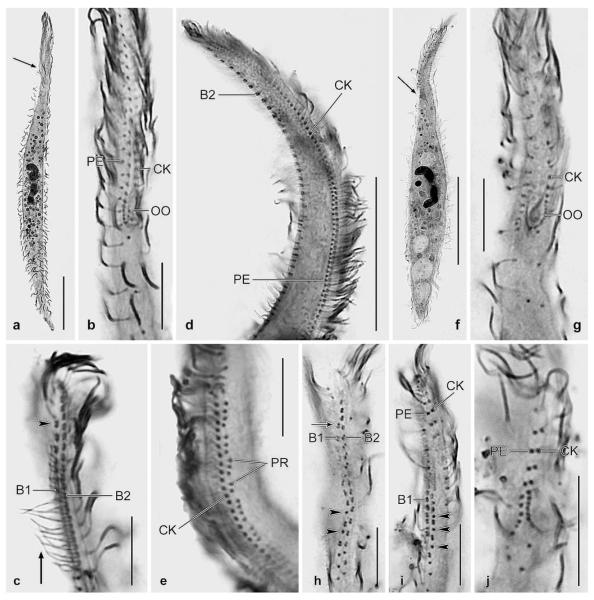
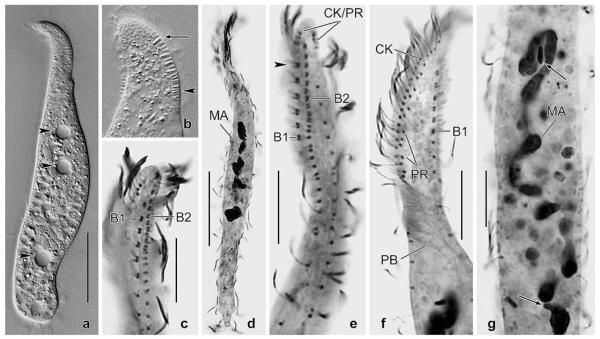
Dileptus semiarmatus nov. spec. from life (a, h–j) and after protargol impregnation (b–g, k–p). a – right side view of a representative specimen, length 180 μm. Note that extrusomes are attached only to the right posterior half of the proboscis (arrows), a main feature of this species; b, c – ventrolateral view of ciliary pattern and nuclear apparatus of holotype specimen, length 200 μm. The preoral kineties are difficult to recognize (b) because they are composed of only two widely spaced monokinetids (connected by lines) almost parallel to left half of circumoral kinety (c); d, e – ventral and dorsal side ciliary pattern of anterior body portion of same specimen. The circumoral kinety is composed of very loosely arranged basal bodies with similar size as those of ordinary cilia; f, g – ventrolateral and dorsolateral view of ciliary pattern in anterior body portion. Basal bodies of preoral kineties are connected by lines; h – frontal view of oral bulge and oral opening. Arrows denote the two types of extrusomes attached to only right posterior half of oral bulge (proboscis); i – oral bulge extrusomes are rod-shaped (3 μm long) and cuneate (2–3 × 1 μm in size); j – dorsal brush bristles are conspicuous because about 10 μm long in central brush region, gradually decreasing in length anteriorly and posteriorly; k–p – variability of body shape and size as well as of nuclear apparatus. Drawn to scale. B(1, 2) – dorsal brush (rows 1, 2), CK – circumoral kinety, E – extrusomes, MA – macronucleus nodule, MI – micronucleus, PB – pharyngeal basket, PE – perioral kinety, PR – preoral kineties, SK – somatic kinety. Scale bars: 50 μm (a, b, k–p) and 20 μm (c–g).
Table 3.
Morphometric data on Dileptus semiarmatus nov. spec. (DS), D. longitrichus nov. spec. (DL), and Pseudomonilicaryon brachyproboscis nov. spec. (PB). Data based on mounted, protargol-impregnated (Foissner’s method), and randomly selected specimens from non-flooded Petri dish cultures. Measurements in μm. CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of specimens investigated, SD – standard deviation, SE – standard error of mean, – arithmetic mean.
| Characteristics | Species | M | SD | SE | CV | Min | Max | n | |
|---|---|---|---|---|---|---|---|---|---|
| Body, length | DS | 160.2 | 160.0 | 23.0 | 5.0 | 14.3 | 119.0 | 203.0 | 21 |
| DL | 187.8 | 188.0 | 24.0 | 5.2 | 12.8 | 145.0 | 223.0 | 21 | |
| PB | 123.6 | 125.0 | 9.1 | 2.0 | 7.4 | 106.0 | 137.0 | 21 | |
| Body, width | DS | 14.7 | 15.0 | 3.8 | 0.8 | 25.7 | 9.0 | 23.0 | 21 |
| DL | 27.7 | 27.0 | 5.4 | 1.2 | 19.6 | 20.0 | 44.0 | 21 | |
| PB | 13.8 | 14.0 | 2.6 | 0.6 | 18.6 | 10.0 | 19.0 | 21 | |
| Body length:width, ratio | DS | 11.6 | 11.3 | 3.4 | 0.8 | 29.8 | 6.6 | 21.1 | 21 |
| DL | 7.0 | 7.0 | 1.3 | 0.3 | 18.3 | 4.3 | 9.6 | 21 | |
| PB | 9.2 | 9.8 | 1.6 | 0.3 | 16.8 | 6.7 | 11.9 | 21 | |
| Anterior body end to oral opening, length |
DS | 30.7 | 30.0 | 4.1 | 0.9 | 13.4 | 24.0 | 39.0 | 21 |
| DL | 69.4 | 67.0 | 11.5 | 2.5 | 16.5 | 49.0 | 101.0 | 21 | |
| PB | 23.5 | 24.0 | 3.0 | 0.7 | 12.7 | 16.0 | 29.0 | 21 | |
| Proboscis, % of body length | DS | 19.3 | 19.0 | 2.4 | 0.5 | 12.5 | 14.5 | 23.7 | 21 |
| DL | 36.9 | 36.8 | 3.6 | 0.8 | 9.7 | 30.9 | 46.1 | 21 | |
| PB | 19.0 | 19.1 | 1.7 | 0.4 | 8.8 | 14.3 | 21.6 | 21 | |
| Oral opening, lengtha | DS | 4.6 | 5.0 | 0.7 | 0.2 | 15.3 | 3.0 | 6.0 | 13 |
| DL | 9.3 | 9.5 | 1.0 | 0.5 | 10.4 | 8.0 | 10.0 | 4 | |
| PB | 7.7 | 8.0 | 0.7 | 0.1 | 8.8 | 7.0 | 9.0 | 21 | |
| Oral opening, widtha | DS | 2.9 | 3.0 | – | – | – | 2.0 | 3.0 | 13 |
| DL | 8.0 | 8.0 | 0.8 | 0.4 | 10.2 | 7.0 | 9.0 | 4 | |
| PB | 5.2 | 5.0 | – | – | – | 5.0 | 6.0 | 19 | |
| Inner oral basket, lengthb | DL | 5.6 | 6.0 | 1.2 | 0.3 | 21.8 | 4.0 | 8.0 | 19 |
| Outer oral basket, lengthb | DL | 9.8 | 10.0 | 2.0 | 0.5 | 20.1 | 8.0 | 14.0 | 17 |
| Anterior body end to macronucleus, distance |
DS | 68.2 | 68.0 | 10.8 | 2.3 | 15.8 | 43.0 | 99.0 | 21 |
| DL | 87.0 | 87.0 | 14.1 | 3.1 | 16.2 | 67.0122.0 | 21 | ||
| PB | 42.9 | 42.0 | 5.4 | 1.2 | 12.7 | 33.0 | 57.0 | 21 | |
| Nuclear figure, length | DS | 31.2 | 31.0 | 4.6 | 1.0 | 14.8 | 24.0 | 38.0 | 21 |
| DL | 53.4 | 52.0 | 6.7 | 1.5 | 12.5 | 43.0 | 65.0 | 21 | |
| PB | 39.3 | 39.0 | 9.4 | 2.1 | 24.0 | 26.0 | 60.0 | 21 | |
| Macronucleus, total length (“uncoiled”) | PB | 68.8 | 69.0 | 11.6 | 2.5 | 16.8 | 44.0 | 92.0 | 21 |
| Anterior or anteriormost (PB) macronucleus nodule, lengthc |
DS | 17.1 | 16.0 | 4.7 | 1.0 | 27.2 | 12.0 | 30.0 | 21 |
| DL | 26.0 | 25.0 | 3.4 | 0.7 | 13.0 | 20.0 | 32.0 | 21 | |
| PB | 8.9 | 9.0 | 2.4 | 0.6 | 27.3 | 6.0 | 16.0 | 19 | |
| Anterior or anteriormost (PB) macronucleus nodule, widthc |
DS | 4.2 | 4.0 | 1.1 | 0.2 | 26.2 | 3.0 | 7.0 | 21 |
| DL | 8.4 | 8.0 | 0.9 | 0.2 | 11.0 | 7.0 | 10.0 | 21 | |
| PB | 3.7 | 4.0 | 0.7 | 0.2 | 19.4 | 3.0 | 5.0 | 21 | |
| Posterior macronucleus nodule, lengthc | DS | 18.4 | 18.0 | 5.3 | 1.2 | 28.9 | 12.0 | 36.0 | 21 |
| DL | 27.6 | 28.0 | 2.9 | 0.6 | 10.6 | 23.0 | 33.0 | 21 | |
| Posterior macronucleus nodule, widthc | DS | 4.1 | 4.0 | 1.2 | 0.3 | 28.8 | 3.0 | 7.0 | 21 |
| DL | 8.2 | 8.0 | 0.9 | 0.2 | 11.3 | 7.0 | 10.0 | 21 | |
| Macronucleus segments, number |
DS | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 |
| DL | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 | |
| PB | 11.8 | 11.0 | 2.7 | 0.6 | 23.3 | 8.0 | 19.0 | 21 | |
| Micronucleus, largest diameter |
DS | 2.0 | 2.0 | 0.4 | 0.1 | 18.2 | 1.5 | 2.5 | 21 |
| DL | 2.8 | 2.5 | 0.5 | 0.1 | 16.1 | 2.5 | 4.0 | 21 | |
| Micronucleus, length | PB | 2.9 | 3.0 | 0.4 | 0.1 | 15.4 | 2.0 | 4.0 | 19 |
| Micronucleus, width | PB | 1.0 | 1.0 | – | – | – | 0.8 | 1.0 | 19 |
| Micronucleus, number | DS | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 |
| DL | 1.5 | 1.0 | – | – | – | 1.0 | 2.0 | 22 | |
| PB | 1.9 | 2.0 | 0.8 | 0.2 | 42.7 | 1.0 | 4.0 | 19 | |
| Ciliary rows, number | DS | 10.3 | 10.0 | 1.1 | 0.3 | 11.1 | 8.0 | 13.0 | 21 |
| DL | 19.5 | 20.0 | 1.5 | 0.3 | 7.9 | 18.0 | 22.0 | 21 | |
| PB | 7.4 | 7.0 | 0.6 | 0.1 | 8.0 | 6.0 | 8.0 | 21 | |
| Cilia in mid-body in 10 μm, number | DS | 4.0 | 4.0 | 0.6 | 0.1 | 14.9 | 3.0 | 5.0 | 21 |
| DL | 5.3 | 5.0 | 0.6 | 0.1 | 10.8 | 4.0 | 6.0 | 21 | |
| PB | 4.8 | 5.0 | 0.9 | 0.2 | 18.1 | 4.0 | 6.0 | 21 | |
| Preoral kineties, number | DL | 26.2 | 25.0 | 3.1 | 1.3 | 11.9 | 23.0 | 30.0 | 6 |
| PB | 8.4 | 8.5 | 0.6 | 0.2 | 7.7 | 7.0 | 9.0 | 14 | |
| Dorsal brush rows, number | DS | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 |
| DL | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 | |
| PB | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 21 | |
| Dikinetids in brush row 1, number | DS | 13.8 | 13.0 | 2.3 | 0.6 | 16.4 | 10.0 | 18.0 | 17 |
| DL | 26.7 | 27.0 | 2.1 | 0.6 | 7.9 | 24.0 | 30.0 | 13 | |
| PB | 6.9 | 7.0 | 0.7 | 0.1 | 9.5 | 6.0 | 9.0 | 21 | |
| Dikinetids in brush row 2, number | DS | 19.5 | 20.0 | 3.2 | 0.8 | 16.5 | 15.0 | 27.0 | 17 |
| DL | 24.0 | 24.0 | 1.7 | 0.5 | 7.2 | 21.0 | 26.0 | 13 | |
| PB | 11.1 | 11.0 | 1.0 | 0.2 | 8.6 | 10.0 | 13.0 | 21 | |
| Anterior body end to last dikinetid of brush row 1, distance |
DS | 23.8 | 24.0 | 2.4 | 0.6 | 10.0 | 18.0 | 27.0 | 18 |
| DL | 37.2 | 37.0 | 5.6 | 1.5 | 15.2 | 30.0 | 54.0 | 15 | |
| PB | 17.3 | 17.5 | 1.6 | 0.4 | 9.3 | 13.0 | 20.0 | 21 | |
| Anterior body end to last dikinetid of brush row 2, distance |
DS | 25.0 | 25.0 | 2.3 | 0.6 | 9.4 | 18.0 | 28.0 | 18 |
| DL | 32.9 | 32.0 | 4.7 | 1.2 | 14.3 | 25.0 | 43.0 | 15 | |
| PB | 13.4 | 13.5 | 1.4 | 0.3 | 10.3 | 11.0 | 16.0 | 21 | |
| Groups of excretory pores, numberd | PB | 3.2 | 3.0 | – | – | – | 3.0 | 4.0 | 21 |
Measured as distance between circumoral kinety.
Proximal end of fibers possibly not impregnated.
Measured in the widest portion, i.e., usually the inflated portion near to the micronucleus.
Usually only a single excretory pore per vacuole, thus the number of pores corresponds with the number of contractile vacuoles.
Diagnosis
Size about 180 × 17 μm in vivo. Shape very narrowly dileptid to rod-like with proboscis about 1/5 of body length and acute posterior body third. Two oblong macronucleus nodules with a micronucleus in between. A dorsal row of contractile vacuoles. Two types of extrusomes attached to only right posterior half of oral bulge: type I cuneate, about 2–3 × 1 μm in size; type II rod-shaped, 3 μm long. On average 10 ciliary rows, 2 staggered and differentiated to a conspicuous, isostichad, posteriorly heteromorphic dorsal brush with bristles up to 10 μm long: rows 1 and 2 composed of an average of 13 and 20 dikinetids, respectively; monokinetidal bristle tail of row 1 extending to second fourth of body and thus much longer than that of row 2. Oral opening about 5 × 3 μm in size, hardly broader than oral bulge. Circumoral kinety in a U-shaped pattern, composed of very widely spaced kinetids. Preoral kineties each composed of two very widely spaced kinetids, forming minute rows almost parallel to circumoral kinety.
Type locality
Soil from a beech forest in the surroundings (Neuhaus area) of the town of Salzburg, Austria, E13° N47°.
Type material
One holotype slide and seven paratype slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Museum of Upper Austria, Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Etymology
Composite of the Latin prefix semi-(half) and the adjective armatus (armed), referring to the extrusomes, which are restricted to the posterior half of the proboscis, a curious feature of this species.
Description
Size 130–230 × 10–25 μm, usually about 180 × 17 μm, as calculated from some in vivo measurements and the morphometric data (Table 3). Shape very narrowly dileptid to rod-like, on average about 11:1 both in vivo and in protargol preparations; proboscis indistinct because hardly set off from body proper and occupying only one fifth of body length; anterior and posterior end acute, i.e., gradually narrowed, posterior end never tail-like; dorsal outline slightly sigmoidal or concave (Figs 2a, k–p, 3f; Table 3). Nuclear apparatus in mid of trunk. Macronucleus nodules highly variable in shape: cylindroidal (53%), spiralized (19%), reniform (15%), ellipsoidal or ovoidal (13%; Table 2); nucleoli small to medium-sized. Micronucleus in between macronucleus nodules, globular to broadly ellipsoidal, about 2 μm in size, usually surrounded by a distinct membrane in protargol preparations (Figs 2a, b, k–p). A dorsal row of contractile vacuoles, first vacuole slightly underneath level of oral opening. Two types of extrusomes attached to right posterior half of proboscis, respectively, oral bulge (Figs 2a, h, arrows), do not impregnate with the protargol method used: type I cuneate to narrowly cuneate, about 2–3 × 1 μm in size; type II rod-shaped with both ends rounded, about 3 μm long (Fig. 2i). Cortical granulation not studied. Cytoplasm colourless and hyaline in flattened proboscis, rather opaque in trunk due to numerous lipid droplets 1–5 μm across and food vacuoles possibly containing flagellates, naked amoebae, and small ciliates; sometimes a defecation vacuole with crystalline contents in posterior body portion. Movement without peculiarities.
Cilia about 8 μm long in vivo, ordinarily spaced, have the same impregnation properties as in congeners; arranged in an average of 10 ordinarily spaced, longitudinal rows leaving a rather narrow, blank stripe left of oral bulge (Figs 2b, c, g; Table 3). First row right of circumoral kinety extends as perioral kinety with very loosely spaced cilia to top of proboscis (Figs 2d, f, 3j). Invariably only one ciliary row between perioral kinety and brush row 2 (Fig. 2f). Dorsal brush as described in D. microstoma, but remarkable because heteromorphic in posterior third, where bristles are mixed with ordinary cilia. Dorsal bristles conspicuous because about 10 μm long in central brush region, gradually decreasing in length anteriorly and posteriorly; posterior bristle of dikinetids slightly shorter than anterior one. Tail of brush row 1 extends to second fourth of body and is composed of up to 10 bristles, tail of row 2 much shorter or lacking (Figs 2e, g, j, 3h, i).
Oral opening at end of anterior body fifth, broadly elliptical, and very small, i.e., about 5 × 3 μm (Fig. 2h). Pharyngeal basket inconspicuous both in vivo and in protargol preparations because composed of very fine and faintly impregnated fibres. Oral apparatus basically dileptid, but with several strange specializations: (1) oral opening hardly broader than oral bulge, thus forming a U-shaped pattern; (2) circumoral kinety probably monokinetidal, that is, composed of basal bodies with similar size as those of somatic cilia; (3) circumoral, perioral, and preoral kinetids very widely spaced; (4) right and left branch of circumoral kinety comparatively widely separated, right branch curves around anterior end of proboscis, while left branch ends subapically; (5) preoral kineties each composed of two (rarely three) monokinetids, forming minute rows almost in line with circumoral kinety (Figs 2b–d, f, g, 3g, j).
Occurrence and ecology
Dileptus semiarmatus was rather abundant in the non-flooded Petri dish culture from the type locality. A second population was found in soil from an oak-hornbeam forest in Vienna, viz., in soil from the Johannser Kogel (see Foissner et al. 2005 for detailed site description; designated as Dileptus n. sp. 1). This species is well adapted to the soil environment by the slender body.
Comparison with related species
The overall appearance of D. semiarmatus is very similar to that of D. microstoma described above. However, D. semiarmatus differs from all described dileptids in that extrusomes occur only in the posterior half of the proboscis, a highly curious and distinct feature which we checked in several specimens from both populations to exclude that it is caused by malformed or wounded specimens. Dileptus semiarmatus has also two other unusual features: the oral opening is hardly broader than the oral bulge, thus forming an U-shaped pattern; and the circumoral, perioral, and preoral kinetids are very widely spaced, a rare feature in haptorids. Further characteristics separating D. semiarmatus from D. microstoma are the extrusomes (cuneate vs. ampulliform) and the contractile vacuole pattern (a dorsal row vs. a dorsal row broken in mid-body).
Dileptus longitrichus nov. spec. (Figs 3d, e, 4a–q; Table 3)
Figs 4a–q.
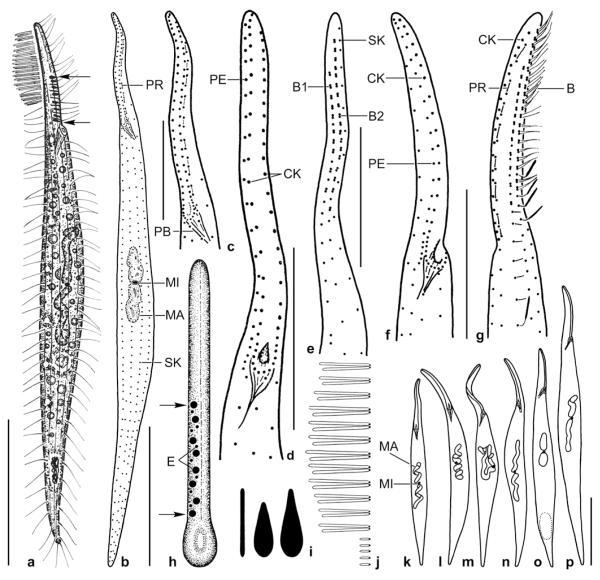
Dileptus longitrichus nov. spec. from life (a, b, j–m) and after protargol impregnation (c–i, n–q). a – right side view of a representative specimen, length 210 μm. Arrowheads denote the two rows of contractile vacuoles, i.e., a main feature of this species; b – frontal view of oral opening; c, d – ciliary pattern of right and left side and nuclear apparatus of holotype specimen, length 206 μm; e, n – left and right side ciliary pattern of proboscis. Asterisks mark gradually shortened right side somatic kineties; f–i – variability of body shape and size as well as of nuclear apparatus. Drawn to scale; j – cortical granules are about 1 × 0.5 μm in size; k – extrusomes are rod-shaped and 2.5–3 μm long; l – surface view showing cortical granulation; m – dorsal brush bristles. The bristles in the anterior region of the brush are up to 15 μm long and are followed by about 4 μm long, clavate bristles; the tail bristles are monokinetidal and only 2 μm long; o, q – dorsal views of proboscis. The dorsal brush consists of two rows both associated with a monokinetidal bristle tail (arrowheads); p – ventrolateral view of ciliary pattern in anterior body third. BA – oral baskets of (microthoracid?) prey, B(1, 2) – dorsal brush (rows 1, 2), CK – circumoral kinety, EB – external basket, G – cortical granules, IB – internal basket, MA – macronucleus nodule, MI – micronuclei, OB – oral bulge, PE – perioral kinety, PR – preoral kineties, SK – somatic kinety. Scale bars: 50 μm (a, c, f–i) and 20 μm (d, e, n–q).
Diagnosis
Size about 210 × 30 μm in vivo. Shape narrowly to cylindroidally dileptid with proboscis about 37% of body length and posterior end narrowly rounded. Two oblong macronucleus nodules with 1–2 micronuclei in between. A row of contractile vacuoles each in ventral and dorsal side of body. Extrusomes rod-shaped, 2.5–3 μm long. On average 20 ciliary rows, 2 anteriorly differentiated to a conspicuous, isostichad dorsal brush with bristles up to 15 μm long: rows 1 and 2 not staggered, composed of an average of 27 and 24 dikinetids, respectively; both rows with a monokinedital bristle tail extending to base of proboscis. Oral opening roundish, about 10 μm across. Circumoral kinety composed of ordinarily spaced dikinetids. On average 25 preoral kineties each composed of 2–5 narrowly to ordinarily spaced kinetids, forming minute rows almost parallel to circumoral kinety.
Type locality
Soil from a mixed forest in the surroundings of the town of Tsukuba, Japan, E140°04′ N36°02′.
Type material
One holotype slide and twelve paratype slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Museum of Upper Austria, Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Etymology
Composite of the Latin adjective longus (long) and the Greek noun thrix (hair ~cilia), referring to the very long dorsal bristles.
Description
Size 160–260 × 20–45 μm in vivo, usually about 210 × 30 μm, as calculated from some in vivo measurements and the morphometric data, assuming 15% preparation shrinkage; length:width ratio rather variable due to slender theronts and broad trophonts, on average 7:1 both in vivo and protargol preparations (Table 3). Shape narrowly to cylindroidally dileptid with flattened proboscis occupying about one third of body length; both body ends narrowly rounded, posterior end never tail-like; dorsal outline straight to sigmoidal (Figs 4a, f–i). Nuclear apparatus slightly above mid of trunk. Two macronucleus nodules with concave proximal end surrounding the micronucleus; individual nodules ellipsoidal to very narrowly ellipsoidal or very narrowly ovoidal, on average 25 × 8 μm in size (Table 3). One to two micronuclei (of 22 specimens investigated, 12 had one and 10 had two micronuclei) in between macronucleus nodules, about 3 μm across, in protargol preparations surrounded by a distinct membrane (Figs 4a, c, f–i; Table 3). A row of contractile vacuoles each in ventral and dorsal side of cell, first dorsal vacuole in base of proboscis. Extrusomes inconspicuous, i.e., rod-shaped with both ends rounded and only 2.5–3 μm long, do not impregnate with protargol (Fig. 4k). Cortex very flexible, contains about four oblique granule rows between adjacent kineties; granules narrowly spaced in somatic and oral cortex, ellipsoidal, ~1 × 0.5 μm in size (Figs 4b, j, l). Cytoplasm colourless, hyaline in proboscis and rear body end, opaque in trunk due to numerous globular and irregular lipid droplets 2–7 μm across and food vacuoles with small ciliates, e.g., Vorticella astyliformis and microthoracid (?) oral baskets (Figs 4a, i). Movement without peculiarities.
Cilia about 8 μm long in vivo, ordinarily spaced; in protargol preparations as typical for dileptids, i.e., with thick, deeply impregnated distal half, except for dorsal bristles; arranged in an average of 20 ordinarily spaced, longitudinal rows anteriorly gradually shortened along right side of oral bulge, except of perioral kinety which extends with ordinarily spaced basal bodies to top of proboscis (Figs 3d, 4c, n; Table 3). Left side of proboscis with conspicuous blank stripe because ciliary rows shortened at level of oral opening, except for 1–2 kineties extending into proximal fourth of proboscis (Figs 4d, e, p). Dorsal brush exactly on dorsal side of proboscis, composed of two isostichad rows with similar number of loosely to ordinarily spaced dikinetids, details difficult to recognize in lateral view due to flattening of proboscis. Both rows begin subapically and continue with a monokinetidal tail to base of proboscis (Figs 4a, o, q). Each brush row composed of three types of bristles (Fig. 4m): (1) anterior portion with rod-shaped bristles obliquely spread backwards in swimming specimens and highly conspicuous because up to 15 μm long and slightly thicker than ordinary somatic cilia, decrease in length anteriorly and posteriorly or, sometimes, gradually from anterior to posterior; (2) followed by some clavate, about 4 μm long bristles; (3) posterior brush portion (tail) composed of 2 μm long, rod-shaped bristles.
Oral opening at beginning of second body third, about 10 μm across, roundish both in vivo and preparations (Figs 4b, p). Pharyngeal basket short, composed of many fine fibres; internal basket about half as long as external one in protargol preparations (Table 3). Circumoral kinety composed of ordinarily spaced dikinetids, except of narrowly spaced monokinetids around oral opening; right branch curves around anterior end of proboscis, while left branch ends subapically almost touching the curved right end. On average 25 preoral kineties each composed of 2–5 narrowly to ordinarily spaced monokinetids forming short rows almost in line with left branch of circumoral kinety and thus difficult to recognize (Figs 3e, 4d, e, p).
Occurrence and ecology
As yet found only at type locality, that is, in a slightly acidic (pH 4.9) mixture of litter, fine plant roots, and red-brown soil from a mixed forest (deciduous and cedar trees) in the surroundings of the town of Tsukuba, Japan.
Comparison with related species
Dileptus longitrichus is almost unique in having a row of contractile vacuoles each in ventral and dorsal side of body. This pattern has been found only in three other species, viz., D. polyvacuolatus Foissner, 1989, D. anatinus Golińska, 1971, and D. gigas (Claparède et Lachmann, 1859) Kahl, 1931. Dileptus polyvacuolatus is distinguished by the body shape (body with vs. without tail), the macronucleus pattern (cylindroidal vs. binucleate), the higher number of ciliary rows (28 vs. 22), and the length of the brush bristles (up to 5 μm vs. 15 μm). Dileptus anatinus differs, inter alia, by body size (900–1200 μm vs. up to 260 μm) and nuclear pattern (many small, scattered nodules vs. binucleate). Dileptus gigas cannot be confused with D. longitrichus because the macronucleus is rod-shaped (vs. binucleate) and the twisted body is up to 1 mm long (vs. 260 μm). The classification of the Japanese population as a new species does not depend on the ventral vacuole row because no other described congener has the combination of features present in D. longitrichus: size about 210 × 30 μm, binucleate, dorsal brush two-rowed and with bristles up to 15 μm long, right side ciliary rows gradually shortened along perioral kinety.
Pseudomonilicaryon brachyproboscis nov. spec. (Figs 5a–w, 6a–g; Table 3)
Figs 5a–o.
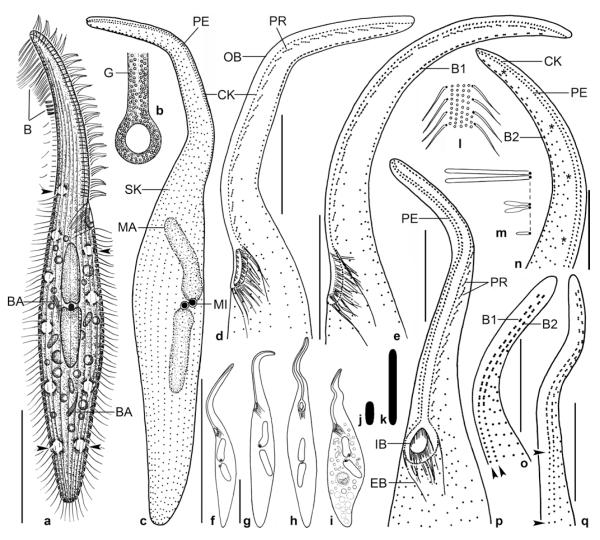
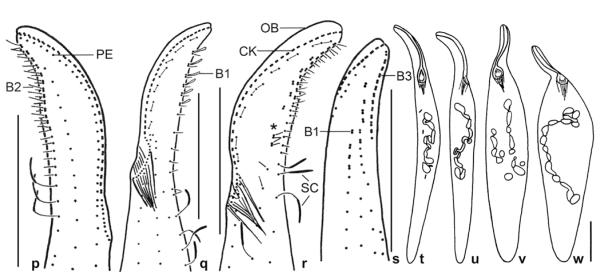
Pseudomonilicaryon brachyproboscis nov. spec. from life (a, b, f, i, j) and after protargol impregnation (c–e, g, h, k–o). a – right side view of a representative specimen, length 140 μm; b – frontal view of oral opening; c, d – dorsolateral and ventrolateral view of ciliary pattern and nuclear apparatus of holotype specimen, length 118 μm. Arrows mark the single excretory pore of the contractile vacuoles; e – ventrolateral view of ciliary pattern and nuclear apparatus of a paratype specimen. Note the narrowly ellipsoidal micronuclei; f – fine structure of dorsal brush; g, h – dorsal ciliary pattern of proboscis. Drawn to scale; i – two types of oral bulge extrusomes. Type I extrusomes are oblong with conical anterior end, 2.5 × 1 μm in size, and appear narrowly ovate when slightly out of focal plane (asterisk). Type II extrusomes are finely rod-shaped and 2 μm long; j – developing cytoplasmic extrusomes are rod-shaped (4 μm long), narrowly ovate to oblong (3 μm long), and oblong with conical anterior end (2.5 × 1 μm); k–o – variability of body shape and size as well as of nuclear apparatus. Drawn to scale. B1, 2 – dorsal brush rows 1, 2, CK – circumoral kinety, DV – defecation vacuole, EB – external basket, E – extrusomes, IB – internal basket, MA – macronucleus, MI – micronucleus, PE – perioral kinety, PR – preoral kinety, SK – somatic kinety. Scale bars: 30 μm (a, c–e, k–o) and 20 μm (g, h).
Figs 5p–w.
Pseudomonilicaryon brachyproboscis nov. spec. after protargol impregnation. p – right side ciliary pattern of anterior body portion; q, r – left side ciliary pattern of anterior body portion. Asterisk marks the break in dorsal brush row 1; s – dorsolateral view of a proboscis with three staggered brush rows; t–w – variability of body shape and size as well as of nuclear apparatus; Drawn to scale. B1–3 – dorsal brush rows 1–3, CK – circumoral kinety, OB – oral bulge, PE – perioral kinety, SC – somatic cilia. Scale bars: 20 μm.
Figs 6a–g.
Pseudomonilicaryon brachyproboscis nov. spec. in vivo (a, b) and after protargol impregnation (c–g). a – right side view of a slightly squeezed specimen. Arrowheads mark the contractile vacuoles; b – there are two types of extrusomes anchored in the proboscis: type I is oblong with a conical anterior end and 2.5 × 1 μm in size (arrow), while type II is rod-shaped and 2 μm long (arrowhead); c, e – dorsolateral views of ciliary pattern of proboscis. The dorsal brush consists of two staggered and dimorphic rows, that is, row 1 commences slightly more subapically than row 2 (arrowhead) and consists of loosely spaced dikentids, while row 2 has ordinarily spaced dikentids; d – ventral view of a representative specimen showing the slender body and moniliform macronucleus; f – left side ciliary pattern of anterior body portion. Note the preoral kineties each composed of 2–3 ordinarily to loosely spaced monokinetids forming short rows almost in a line with left half of circumoral kinety; g – the nuclear apparatus consists of a highly tortuous, moniliform macronucleus strand and two narrowly ellipsoidal micronuclei (arrows) one each near the anterior and posterior end of the macronucleus. B1, 2 – dorsal brush row 1, 2, CK – circumoral kinety, MA – macronucleus, PB – pharyngeal basket, PR – preoral kineties. Scale bars: 30 μm (a, d) and 10 μm (c, e–g).
Diagnosis
Size about 140 × 15 μm in vivo. Shape very narrowly to cylindroidally dileptid with proboscis about 1/5 of body length and posterior end narrowly rounded. Macronucleus moniliform and tortuous, composed of 11 segments on average; usually two narrowly ellipsoidal micronuclei. Contractile vacuoles in dorsal side of trunk: two close together in anterior third of trunk and one vacuole in posterior third. Two types of extrusomes: type I oblong with conical anterior end, 2.5 × 1 μm in size; type II rod-shaped, 2 μm long. On average 7 ciliary rows, 2 staggered and differentiated to a dimorphic, isostichad dorsal brush: brush row 1 composed of an average of 7 widely spaced dikinetids, brush row 2 composed of 11 ordinarily spaced dikinetids; both rows with a monokinetidal bristle tail extending to base of proboscis. Oral opening about 8 × 5 μm in size. Circumoral kinety distinctly narrowed preorally, composed of ordinarily spaced dikinetids. On average 8 preoral kineties each composed of two ordinarily to widely spaced kinetids, forming minute rows almost parallel to circumoral kinety.
Type locality
Soil from a young Pinus forest between the towns of Katarraktis and Vlasia, Peloponnese, Greece, E21°57′ N38°01′.
Type material
One holotype slide and eleven paratype slides with protargol-impregnated specimens have been deposited in the Biology Centre of the Museum of Upper Austria, Linz (LI). Relevant specimens are marked by black ink circles on the coverslip.
Etymology
Composite of the Greek adjective brachy (short) and the Latin noun proboscis, referring to the short proboscis, a main feature of the species.
Description
Size 120–150 × 10–20 μm in vivo, usually about 140 × 15 μm, as calculated from some in vivo measurements and the morphometric data, assuming 15% preparation shrinkage. Shape very narrowly to cylindroidally dileptid, on average near 9:1, trunk distinctly widened in well-fed specimens; anterior end bluntly pointed, posterior narrowly rounded, never tail-like; dorsal outline straight to slightly sigmoidal, ventral margin often rather distinctly convex in mid of trunk; proboscis occupies one fifth of body length on average, slightly curved dorsally, mid-region often inflated in protargol preparations (Figs 5a, k–o, t–w, 6a, d; Table 3). Nuclear apparatus usually in anterior two thirds of trunk. Macronucleus highly variable, moniliform and tortuous, about 70 μm long in “uncoiled” condition, composed of an average of 11 oblong segments; nucleoli medium to large-sized, globular or ellipsoidal. One to four micronuclei, usually one micronucleus each near anterior and posterior end of macronucleus, conspicuous because narrowly ellipsoidal, that is, 3 × 1 μm in size (Figs 5a, d, e, k–o, t–w, 6a, d, g; Table 1). Contractile vacuoles in dorsal side of trunk, arranged in remarkable pattern, viz., two rather close together in anterior third of trunk and one vacuole in posterior third, vacuoles thus lacking in mid-body; rarely a third vacuole occurs in the anterior group and/or a second one in posterior third; usually a single (very rarely two) intrakinetal excretory pore per vacuole (Figs 5a, c, 6a; Table 3). Two types of extrusomes (Figs 5b, i, 6b): type I only in right half of oral bulge, oblong with conical anterior end, appears narrowly ovate (!) when slightly out of focal plane (Fig. 5i, asterisk), about 2.5 × 1 μm in size, very rarely impregnates with protargol; type II in both bulge halves, more numerous than type I, rod-shaped with both ends rounded, 2 μm long, does not stain with the protargol method used; developing cytoplasmic extrusomes sometimes impregnating with protargol, rod-shaped and 4 μm long or narrowly ovate to oblong and 3 μm long, and thus difficult to distinguish from micronuclei (Fig. 5j). Cortex flexible, contains about eight granule rows between adjacent kineties; granule colourless and ~0.5 × 0.2 μm in size. Cytoplasm colourless, packed with lipid droplets 1–3 μm across and some 5 μm-sized food vacuoles with indefinable or crystalline contents; in posterior body end sometimes a defecation vacuole. Movement without peculiarities.
Cilia about 6 μm long in vivo, ordinarily spaced; in protargol preparations as typical for dileptids, i.e., with thick, strongly impregnated distal half, except of dorsal bristles; arranged in an average of 7 longitudinal, ordinarily spaced rows leaving a rather wide, barren area left and right of oral bulge (Figs 5c–e; Table 3). First row right of circumoral kinety extends as perioral kinety with ordinarily to widely spaced cilia to top of proboscis (Figs 5c, e, p). Invariably only one ciliary row between perioral kinety and brush row 2 (Figs 5c, p). Dorsal brush pattern highly constant, that is, two dimorphic, staggered rows in over 700 specimens analyzed; a few, likely distorted cells have either three staggered rows (2 specimens) or some irregularities in row 1 (1 specimen; Figs 5r, s). Brush row 1 commences slightly more subapically than row 2, composed of an average of 7 widely spaced dikinetids each having an about 3 μm long, slightly inflated anterior bristle and an about 2 μm long, rod-shaped posterior bristle in protargol preparations. Brush row 2 begins near top of proboscis, its anterior portion usually slightly curved rightwards, composed of an average of 11 ordinarily spaced dikinetids each associated with bristles similar to those of row 1, but anterior bristle not inflated (Figs 5c, d, g, h, p, q, 6c, e; Table 3). Bristles 4 μm long subapically, decreasing in length anteriorly and posteriorly according to the live observations (Fig. 5f). Both rows continue with a short monokinetidal tail of about 2 μm long bristles (Figs 5a, c, d, g).
Oral opening at end of anterior body fifth, ovate to broadly ovate, in vivo about 6 μm across (Fig. 5b; Table 3). Pharyngeal basket small, internal basket indistinctly bulbous both in vivo and after protargol impregnation, external basket impregnated only in distal portion. Oral ciliary pattern basically as in other dileptids, but with a special feature, viz., a distinct preoral narrowing of the circumoral kinety/oral bulge (Figs 5d, e). Circumoral kinety composed of ordinarily spaced dikinetids, except of narrowly spaced monokinetids around oral opening. On average 8 preoral kineties each composed of only two (rarely three) ordinarily to widely spaced monokinetids almost in line with left part of circumoral kinety and thus difficult to recognize (Figs 5d, q, r, 6f).
Occurrence and ecology
Pseudomonilicaryon brachyproboscis was rather abundant in the non-flooded Petri dish culture from the type locality, where the litter layer was thin, poorly decomposed, but locally penetrated by moss and masses of fungal hyphae. The sample consisted of a mixture of Pinus needles and terrestrial mosses; pH 6.3. A second population was found in slightly saline soil from the margin of the Zicklacke, a saline inland lake in Burgenland, Austria.
Comparison with related species
Considering that the genus Pseudomonilicaryon is weakly supported (Foissner 1997), P. brachyproboscis must be compared not only with the congeners but also with species from the genus Dileptus. Pseudomonilicaryon was created by Foissner (1997) to distinguish Dileptus monilatus, type of the genus Monilicaryon Jankowski, 1967, from other dileptids. Pseudomonilicaryon differs from Monilicaryon by the presence vs. absence of preoral kineties. Dileptus is distinguished from Pseudomonilicaryon by the macronucleus pattern: many nodules vs. a moniliform or vermiform strand.
Pseudomonilicaryon brachyproboscis is almost unique in having narrowly ellipsoidal micronuclei, an unusual shape as yet not found in other dileptids, but present in some spathidiids, e.g., Arcuospathidium namibiense and A. etoschense (Foissner and Xu 2007) and bryophyllids, e.g., Apobryophyllum vermiforme (Foissner et al. 2002). Further, P. brachyproboscis displays a curious contractile vacuole pattern, which is rather constant and is thus possibly a reliable feature of this species. These two basic differences are not included in the following comparison. Pseudomonilicaryon gracilis, as redescribed by Foissner (1989), differs from P. brachyproboscis by the much higher number of ciliary rows (15 vs. 7). Dileptus anguillula, as redescribed by Foissner et al. (2002), is distinguished from P. brachyproboscis by the shape of the extrusomes (finely rod-shaped vs. oblong with conical anterior end). A further similar species is Dileptus breviproboscis Foissner, 1981 which, however, is binucleate.
DISCUSSION
New features for species recognition in dileptids
Our investigations show four new features for species recognition in dileptids. All are important and thus should be noted in future species descriptions and redescriptions.
Shape of micronucleus. As yet, only globular to broadly ellipsoidal micronuclei were known in dileptids (Kahl 1931; Dragesco 1963; Foissner et al. 1995, 1999, 2002). Thus, the narrowly ellipsoidal micronuclei of Pseudomonilicaryon brachyproboscis are an outstanding feature (Figs 5a, d, e, 6g) highly reminiscent of recent observations in spathidiids (Foissner and Xu 2007).
Dorsal brush monomorphic or dimorphic. Some small dileptids are unique in that the spacing of the brush dikinetids is different in the individual brush rows, e.g., Pseudomonilicaryon brachyproboscis (Figs. 5g, h, 6c, e) and Dileptus breviproboscis, where Foissner et al. (2002) figured but did not mention this peculiarity.
Shape of oral opening. Although there are transitions and Foissner et al. (2002) used this feature to distinguish Pseudomonilicaryon angustistoma from P. japonicum, we classify the shape of the oral opening as “new” because Foissner et al. (2002) did not definitely mention it as a new character. The oral opening may be roundish (e.g., Dileptus longitrichus described here; D. tirjakovae Vd’ačný and Foissner, 2008; and P. japonicum Foissner et al., 2002); ovate (e.g., D. microstoma, P. brachyproboscis both described here, and P. massutii as redescribed by Foissner et al. 2002), or elliptical (D. semiarmatus described here; P. angustistoma Foissner et al., 2002; and Pelagodileptus tracheloides as described by Foissner et al. 1999).
Spacing of the circumoral kinetids. As compared to the somatic kinetids, the circumoral kinetids are usually much more narrowly spaced in all haptorids investigated so far, i.e., only one or two kinetids can be inserted between two circumoral kinetids (Figs 4d, e, p, 5d, e; Foissner et al. 1995, 1999, 2002). The sole and conspicuous exception is Dileptus semiarmatus in which both circumoral and perioral kinetids are almost as widely spaced as the somatic kinetids (Figs 2d, g, h, 3g, j).
Morphological adaptations of dileptids to terrestrial habitats
Dileptids occur in limnetic, marine, and terrestrial habitats. The terrestrial species share several distinct features that are probably adaptations to the soil environment. (1) Body shape is usually very slender or even vermiform, and average body length is smaller by about 300 μm than in freshwater species (Foissner 1987). Both peculiarities might be related to the restricted space available in the soil pores, i.e., the narrowness of the habitat. (2) Reduction of the proboscis to one quarter or less of body length, for instance, in Dileptus breviproboscis, D. anguillula, D. armatus, and three of the four species described here. This peculiarity is possibly related to the fragility of the proboscis and the space available for prey digestion. The physical load is likely higher in soil than in free water, e.g., by passive and active transport of soil particles with sharp edges and/or heavy weight. The proboscis is for prey recognition and prey capture. In the small terrestrial dileptids it might be advantageous to increase the space for digestion by shortening of the proboscis because prey cannot escape so easily in the narrow soil pores. However, a short proboscis likely decreases prey recognition; possibly this is compensated by the long dorsal bristles (see next item). Interestingly, a very short proboscis occurs also in Monilicaryon monilatus, typically found in the benthic mud of limnetic habitats (Foissner 1997). (3) The dorsal bristles are usually longer in soil than in limnetic dileptids (up to 15 μm vs. about 5 μm), e.g., in Dileptus alpinus and in three of the four species described here. If a sensory function of the brush bristles is assumed, long bristles may be of advantage in recognizing the prey in a habitat where it is more difficult to notice signals, for instance, prey movement in a water film. The long bristles might compensate for the disadvantage of the short proboscis (see above). (4) Pronounced flexibility of the body which fosters movement in a wrinkled and narrow habitat, i.e., in soil pores. However, distinct body flexibility is not confined to terrestrial species but occurs in all dileptids. This might explain the high diversity of dileptids in terrestrial habitats.
Acknowledgements
This study was supported by a grant of the Austrian Science Foundation (FWF project P-19699-B17). The technical assistance of R. Schörghofer, A. Zankl, and Mag. Gudrun Fuss is greatly acknowledged. Special thanks to Prof. Dr. Jean Dragesco for sending us the soil sample from Benin.
References
- Corliss JO. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. 2nd ed Pergamon Press; Oxford, New York, Toronto, Sydney, Paris, Frankfurt: 1979. [Google Scholar]
- Dragesco J. Révision du genre Dileptus, Dujardin 1871 (Ciliata Holotricha) (systématique, cytologie, biologie) Bull. biol. Fr. Belg. 1963;97:103–145. [Google Scholar]
- Foissner W. Morphologie und Taxonomie einiger neuer und wenig bekannter kinetofragminophorer Ciliaten (Protozoa: Ciliophora) aus alpinen Böden. Zool. Jb. Syst. 1981;108:264–297. [Google Scholar]
- Foissner W. Infraciliatur, Silberliniensystem und Biometrie einiger neuer und wenig bekannter terrestrischer, limnischer und mariner Ciliaten (Protozoa: Ciliophora) aus den Klassen Kinetofragminophora, Colpodea und Polyhymenophora. Stapfia. 1984;12:1–165. [Google Scholar]
- Foissner W. Soil Protozoa: fundamental problems, ecological significance, adaptations in ciliates and testaceans, bioindicators, and guide to the literature. Progr. Protistol. 1987;26:69–212. [Google Scholar]
- Foissner W. Morphologie und Infraciliatur einiger neuer und wenig bekannter terrestrischer und limnischer Ciliaten (Protozoa, Ciliophora) Sber. Akad. Wiss. Wien. 1989;196:173–247. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Europ. J. Protistol. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W. Tropical protozoan diversity: 80 ciliate species (Protozoa, Ciliophora) in a soil sample from a tropical dry forest of Costa Rica, with descriptions of four new genera and seven new species. Arch. Protistenk. 1995;145:37–79. [Google Scholar]
- Foissner W. Faunistic and taxonomic studies on ciliates (Protozoa, Ciliophora) from clean rivers in Bavaria (Germany), with descriptions of new species and ecological notes. Limnologica. 1997;27:179–238. [Google Scholar]
- Foissner W. An updated compilation of world soil ciliates (Protozoa, Ciliophora), with ecological notes, new records, and descriptions of new species. Europ. J. Protistol. 1998;34:195–235. [Google Scholar]
- Foissner W. A compilation of soil and moss ciliates (Protozoa, Ciliophora) from Germany, with new records and descriptions of new and insufficiently known species. Europ. J. Protistol. 2000;36:253–283. [Google Scholar]
- Foissner W, Foissner I. The fine structure of Fuscheria terricola Berger et al., 1983 and a proposed new classification of the subclass Haptoria Corliss, 1974 (Ciliophora, Litostomatea) Arch. Protistenk. 1988;135:213–235. [Google Scholar]
- Foissner W, Xu K. Monograph of the Spathidiida (Ciliophora, Haptoria). Vol. I: Protospathidiidae, Arcuospathidiidae, Apertospathulidae. Springer Verlag; Dordrecht: 2007. [Google Scholar]
- Foissner W, Berger H, Blatterer H, Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobien-systems – Band IV: Gymnostomatea, Loxodes, Suctoria. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1995;1/95:1–540. [Google Scholar]
- Foissner W, Berger H, Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1999;3/99:1–793. [Google Scholar]
- Foissner W, Agatha S, Berger H. Soil Ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa) with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Denisia. 2002;5:1–1459. [Google Scholar]
- Foissner W, Berger H, Xu K, Zechmeister-Boltenstern S. A huge, undescribed soil ciliate (Protozoa: Ciliophora) diversity in natural forest stands of Central Europe. Biodiv. Conserv. 2005;14:617–701. [Google Scholar]
- Golińska K. Comparative studies on the morphology of Dileptus anatinus sp. n. (Holotricha, Gymnostomata) Acta Protozool. 1971;8:367–378. [Google Scholar]
- Jankowski AW. New genera and subgenera of classes Gymnostomatea and Ciliostomea. Materials of the V Conference of young scientists of Moldavia. Akad. Sci. Moldav. SSSR, Kishinev. 1967:36. year 1967. (in Russian) [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 2. Holotricha außer den im 1. Teil behandelten Prostomata. Tierwelt Dtl. 1931;21:181–398. [Google Scholar]
- Song W, Wilbert N. Taxonomische Untersuchungen an Aufwuchsciliaten (Protozoa, Ciliophora) im Poppelsdorfer Weiher, Bonn. Lauterbornia. 1989;3:2–221. [Google Scholar]
- Song W, Packroff G, Wilbert N. Morphologie und Infraciliatur von Dileptus orientalis sp. n., einem Bodenciliaten aus Qingdao, China. Acta Protozool. 1988;27:271–277. [Google Scholar]
- Strüder-Kypke MC, Wright A-DG, Foissner W, Chatzinotas A, Lynn DH. Molecular phylogeny of litostome ciliates (Ciliophora, Litostomatea) with emphasis on free-living haptorian genera. Protist. 2006;157:261–278. doi: 10.1016/j.protis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Vd’ačný P, Foissner W. Morphology, conjugation, and post-conjugational reorganization of Dileptus tirjakovae n. sp. (Ciliophora, Haptoria) J. Eukaryotic. Microbiol. 2008 doi: 10.1111/j.1550-7408.2008.00343.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vd’ačný P, Hlúbiková D, Tirjaková E. Spathidium seppelti foissneri nov. subspec., Spathidium simplinucleatum nov. stat., and Dileptus americanus Kahl, 1931, one new and two poorly known gymnostome ciliates from soils of Slovakia. Europ. J. Protistol. 2006;42:175–189. doi: 10.1016/j.ejop.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Wirnsberger E, Foissner W, Adam H. Morphologie und Infraciliatur von Perispira pyriformis nov. spec., Cranotheridium foliosus (Foissner, 1983) nov. comb. und Dileptus anser (O. F. Müller, 1786) (Protozoa, Ciliophora) Arch. Protistenk. 1984;128:305–317. [Google Scholar]