Summary
Fifty-six ciliate taxa, including three new species, were found in two soil samples from the Hoge Veluwe National Park in The Netherlands. A literature search showed that The Netherlands, Belgium and Denmark are terra incognita with respect to soil ciliates: only about 100 species have been recorded. Likely, a much greater number, including many undescribed species, can be found on more detailed investigations. Two of the three new species are described in detail. Keronopsis schminkei nov. spec. differs from the congeners by the nuclear apparatus and cirral pattern. Keronopsis wetzeli Wenzel, 1953 is neotypified with the Austrian population described by Berger and Foissner (1987). Apobryophyllum schmidingeri differs from the congeners by body shape, the macronucleus pattern (many nodules), the arrangement of the extrusomes, and various morphometric features. Scanning electron micrographs and protargol preparations of Enchelys polynucleata confirm earlier transmission electron microscopic data on the occurrence of oralized somatic monokinetids and the lack of a circumoral kinety. Extrusome length and morphology of the resting cysts are rather different in various populations of E. polynucleata, indicating high genetic diversity (subspecies?).
Keywords: Austria, Biodiversity, Enchelys polynucleata, Hawaii, Hoge Veluwe National Park, inland sand dunes, resting cysts
INTRODUCTION
About 800 soil ciliate species are known (Foissner 1998; Foissner et al. 2002). Evidence is accumulating that this is only half or less of the actual soil ciliate diversity: Foissner et al. (2002) described 128 new species from terrestrial habitats of Namibia, and Foissner et al. (2005) found 30 new species in soil from twelve natural forest stands of Austria, showing our ignorance of even the Central European soil ciliate diversity. This is emphasized by the present study which discovered three new species in two soil samples from the Hoge Veluwe National Park in The Netherlands. This is not very surprising because The Netherlands, Belgium and Denmark are terra incognita with respect to soil ciliates. Only Chardez (1967) and Brunberg Nielsen (1968) provided some data.
MATERIAL AND METHODS
The material is from the Hoge Veluwe National Park in The Netherlands, that is, about 15 km NE of the town of Arnheim, E5°43′N52°4′. Samples were collected on 7.3.2004, air-dried, and investigated during May 2004. Sampling, sample processing, and taxonomic methods followed Foissner (1991) and Foissner et al. (2002). Briefly, the samples were processed with the non-flooded Petri dish method and species identified in vivo; identification of difficult taxa was checked in protargol preparations.
Site 1: Inland sand dune area between the villages of Ede and Otterloo, about 50 m aside the rod to the Kröller-Müller museum (see site 2). This area has very sandy soils formed from postglacial ground moraines. Originally, it was covered by oak, birch and alder. During the Middle Ages and also more recently, the region was overgrazed, the soil degraded, a heath vegetation developed, and eolian sand formed a dune landscape. Presently, the heath is maintained by grazing and fire, otherwise forest vegetation would develop. Accordingly, the sampling area is a man-made and still influenced ecosystem. The sample was taken from an area where only part of the sand is covered by a mossy grass carpet. Carpet litter and humic sand were collected to a depth of up to 10 cm from several sites and mixed to a composite sample, which had pH 5.1 in water.
Site 2: Mixed deciduous forest in the centre of the National Park, that is, around the Kröller-Müller museum. Here, an about 5 cm thick litter layer covers the humic, darkbrown, acidic (pH 5.1 in water) soil. Several samples were taken from the upper 10 cm and mixed to a composite used for the investigation.
RESULTS AND DISCUSSION
Faunistics
Very few data are available on soil ciliates from The Netherlands, Belgium, and Denmark. Thus, the species list is meagre (Table 1). Certainly, more detailed studies will easily double the number.
Table 1.
Ciliate species found in soil of a dune (site 1) and a mixed forest (site 2) from The Netherlands (original data); of sand dunes from Norderney, Germany (site 3; from Verhoeven 1999, 2001); and in various sites of Belgium and Denmark (site 4; from Brunberg Nielsen 1968 and Chardez 1967). + = found, − = not found.
| Speciesa | Sites |
|||
|---|---|---|---|---|
| 1 | 2 | 3b | 4c | |
| Amphisiella magnigranulosa Foissner | + | + | − | − |
| Amphisiella terricola Gellért | + | − | − | − |
| Arcuospathidium namibiense namibiense Foissner, Agatha & Berger | + | − | − | − |
| Apobryophyllum schmidingeri nov. spec. | + | − | − | − |
| Aspidisca marsupialis Penard | − | − | − | + |
| Blepharisma hyalinum Perty | − | + | + | − |
| Blepharisma steini Kahl | − | − | − | + |
| Bresslaua vorax Kahl | − | + | − | − |
| Bryometopus pseudochilodon Kahl | + | + | − | − |
| Bryometopus sphagni (Penard) | + | − | − | + |
| Chilodonella capucina Penard | − | − | − | + |
| Cinetochilum impatiens Penard | − | − | − | + |
| Cinetochilum margaritaceum (Ehrenberg) | − | − | + | + |
| Circinella filiformis (Foissner) | − | − | + | − |
| Clavoplites australiensis Foissner, Agatha & Berger | − | + | − | − |
| Colpidium colpoda (Losana) | − | − | − | + |
| Colpoda aspera Kahl | + | + | + | − |
| Colpoda cucullus (Müller) | + | + | − | + |
| Colpoda edaphoni Foissner | − | + | − | − |
| Colpoda henneguyi Fabre-Domergue | + | − | + | − |
| Colpoda inflata (Stokes) | + | + | + | + |
| Colpoda lucida Greeff | + | + | − | − |
| Colpoda maupasi Enriques | + | + | + | + |
| Colpoda steinii Maupas | + | + | + | + |
| Cyclidium glaucoma Müller | − | − | − | + |
| Cyrtolophosis muscicola Stokes | + | + | + | − |
| Dileptus alpinus Kahl | + | − | + | − |
| Dileptus anguillula Kahl | + | − | − | + |
| Dileptus margaritifer (Ehrenberg) | − | − | − | + |
| Dileptus sp. | + | − | − | − |
| Dileptus tenuis Penard | − | − | − | + |
| Dimacrocaryon amphileptoides (Kahl) | − | + | − | − |
| Drepanomonas exigua exigua Penard | − | + | − | − |
| Drepanomonas pauciciliata Foissner | + | + | − | − |
| Drepanomonas revoluta Penard | − | − | + | − |
| Enchelyodon lagenula (Kahl) | + | − | − | − |
| Enchelys polynucleata (Foissner) | + | − | − | − |
| Epenardia myriophylli (Penard) | − | − | − | + |
| Epispathidium terricola Foissner | + | + | − | − |
| Epistylis alpestris Foissner | + | − | − | − |
| Euplotes gracilis Kahl | − | − | − | + |
| Euplotes terricola Penard | − | − | − | + |
| Euplotopsis muscicola (Kahl) | − | − | − | + |
| Frontonia depressa (Stokes) | + | + | − | + |
| Fuscheria terricola Berger, Foissner and Adam | − | + | − | − |
| Gonostomum affine (Stein) | + | + | + | + |
| Grossglockneria acuta Foissner | + | + | + | − |
| Halteria grandinella (Müller) | − | + | − | + |
| Hausmanniella discoidea (Gellért) | − | + | − | − |
| Hemisincirra gracilis (Foissner) | − | − | + | − |
| Hemisincirra inquieta Hemberger | + | + | − | − |
| Holosticha adami Foissner | − | − | + | − |
| Holosticha navicularum Kahl | − | − | − | + |
| Holosticha tetracirrata Buitkamp and Wilbert | + | + | − | − |
| Homalogastra setosa Kahl | − | − | + | − |
| Kahliella acrobates (Horváth) | − | − | − | + |
| Kahlilembus attenuatus (Smith) | − | − | − | + |
| Keronopsis schminkei nov. spec. | + | − | − | − |
| Kreyella muscicola Kahl | − | − | + | − |
| Lamtostyla australis (Blatterer and Foissner) | − | + | − | − |
| Leptopharynx costatus Mermod | + | + | − | + |
| Litonotus lamella (Müller) | − | − | − | + |
| Metopus setifer Kahl | − | − | − | + |
| Microdiaphanosoma arcuatum (Grandori and Grandori) | + | − | + | − |
| Microthorax simulans (Kahl) | − | − | + | − |
| Mykophagophrys terricola (Foissner) | + | + | − | − |
| Nassulides pictus (Greeff) | − | − | − | + |
| Nivaliella plana Foissner | + | + | + | − |
| Ottowphrya dragescoi (Foissner) | + | + | − | − |
| Oxytricha fallax Stein | − | − | − | + |
| Oxytricha longigranulosa Berger and Foissner | − | + | − | − |
| Oxytricha proximata (?) | − | + | − | − |
| Oxytricha setigera Stokes | − | − | + | − |
| Paraholosticha muscicola Kahl | − | − | − | + |
| Phacodinium metchnicoffi (Certes) | − | − | − | + |
| Phialina vertens (Stokes) | − | − | + | − |
| Platyophrya spumacola Kahl | − | − | + | − |
| Platyophrya vorax Kahl | + | − | + | − |
| Plesiocaryon elongata (Schewiakoff) | + | − | + | − |
| Podophrya tristriata Foissner, Agatha and Berger | − | + | − | − |
| Protocyclidium muscicola (Kahl) | + | + | − | − |
| Pseudochilodonopsis mutabilis Foissner | − | − | + | − |
| Pseudocyrtolophosis alpestris Foissner | + | + | − | − |
| Pseudoplatyophrya saltans Foissner | − | + | − | − |
| Pseudoplatyophrya nana (Kahl) | + | + | + | − |
| Sathrophilus muscorum (Kahl) | + | + | + | + |
| Sikorops namibiensis Foissner, Agatha and Berger | + | − | − | − |
| Spathidium procerum Kahl | − | − | + | − |
| Sterkiella histriomuscorum (Foissner, Blatterer, Berger and Kohmann) | − | + | − | − |
| Stylonychia mytilus (Müller) | − | − | − | + |
| Stylonychia stylomuscorum (Foissner, Blatterer, Berger and Kohmann) | − | − | − | + |
| Tetrahymena pyriformis − complex | − | − | − | + |
| Uroleptus paranotabilis Foissner, Agatha and Berger | + | + | − | − |
| Urozona buetschli Schewiakoff | − | − | − | + |
| Vorticella lichenicola Greeff | − | − | − | + |
| Vorticella microstoma Ehrenberg | − | − | − | + |
| Vorticella muralis Penard | − | − | − | + |
| Vorticella nebulifera Müller | − | − | − | + |
| Number of species | 41 | 39 | 29 | 41 |
See Foissner (1998) and Foissner et al. (2002) for dates, combining authors, and nomenclature of species. Names used by Brunberg Nielsen (1968), Chardez (1967), and Verhoeven (1999, 2001, 2002) were adapted to Foissner (1998) and Foissner et al. (2002).
Plus at least 10 unidentified species not included in the present list.
Exclusive Chardez’s site M which is a limnetic biotope (Pressière en fagnes). Data of Chardez (1967) doubtful because most of the soil ciliate fauna was unknown at that time. Most data are from forest soils (beech, oak, mixed forests), including those of Brunberg Nielsen (1968) who investigated a Danish beech forest five times during a period of two years; she did not determine members of the genera Spathidium and Dileptus.
The two samples investigated each contain about 40 species (Table 1). Such figures are typical for single soil samples. Usually, they provide only one third of the species actually present at a certain site because part of the resting cysts cannot be reactivated with the methods available and/or many species do not grow to detectable numbers (Foissner et al. 2002). When the two samples are pooled, only 56 species are obtained, showing that the communities are rather similar, which is not surprising because the samples are from the same area. However, 98 species are reached, if the sites studied by Brunberg Nielsen (1968), Chardez (1967) and Verhoeven (1999, 2001, 2002) are added; further, Foissner (1987) described two new species (Holosticha bergeri, Hemisincirra wenzeli) and Paraholosticha muscicola from Bornholm, a small Danish island. The Verhoeven sites are natural and fertilized coastal dunes on Norderney, a small island at the NW coast of Germany. As this is near and similar to The Netherlands site 1, Verhoeven’s data are included in Table 1. Coastal dunes are a widespread and interesting ecosystem in Europe (Bakker et al. 1990). In spite of this, their ciliate biota are poorly known, except of the mainly ecological studies of Verhoeven (1999, 2001, 2002). We are convinced that detailed taxonomic studies will reveal many new ciliates, as in other sandy regions of the world (Blatterer and Foissner 1988; Foissner 1993, 1994; Foissner et al. 2002). This applies also to the ecological study of Esteban et al. (2006), who did not find any undescribed ciliate species in 150 (!) samples from 1 ha of upland grassland in Southern Scotland. We suppose that the rather low total number of species (~ 100) and the lack of new taxa is related to the method used, viz., 5 g samples which were investigated only twice during a period of 10 days. In contrast, the non-flooded Petri dish method uses 100–1000 g samples and investigates the culture five times over a month period (Foissner et al. 2002). Usually, 100 or more ciliate species are found, including 5–25% undescribed ones, when a single (!) site is investigated four of five times during a year (Foissner 1999, 2004; Foissner et al. 2005); if a larger area and many samples are investigated, hundreds of species are found, of which one third is undescribed (Foissner 1997; Foissner et al. 2002).
So, we arrive at roughly 100 soil ciliate species in the area under investigation. This is about one eight of the described global soil ciliate diversity (Foissner 1998; Foissner et al. 2002). However, this does not mean cosmopolitan distribution and low diversity of soil ciliates (Esteban et al. 2006)! Foissner (1997) and Chao et al. (2006) showed that at least half of the soil ciliate diversity is still undescribed and the methods available reveal mainly the more common, euryoecious species. This becomes evident also when communities are compared. Foissner et al. (2002) described 128 new ciliate species from 73 terrestrial habitats of Namibia. Several species, which are quite common in Europe and were present also in The Netherlands (Table 1) and in Scotland (Esteban et al. 2006), were not found in the extensive Namibian study, viz., Bryometopus sphagni, Dileptus alpinus, Dimacrocaryon amphileptoides, Enchelyodon lagenula, and Paraholosticha muscicola. Further, the two The Netherlands samples contained at least three undescribed species (Table 1) which were not found in Namibia, but occurred in other regions of the world (see descriptive section). Thus, at least 10 out of the 56 species present in the two The Netherlands samples could not be found in the 73 Namibian samples; further, the two new species (Holosticha bergeri, Hemisincirra wenzeli) described by Foissner (1987) from Denmark did not occur in the Namibian material. All these ob servations cast strong shades on the proposed ubiquity and cosmopolitan distribution of protists (for a detailed discussion, see Foissner 2006).
Description of species
Family Keronopsidae Jankowski, 1979 Keronopsis schminkei nov. spec. (Figs 1–7; Table 2)
Figs 1-7.
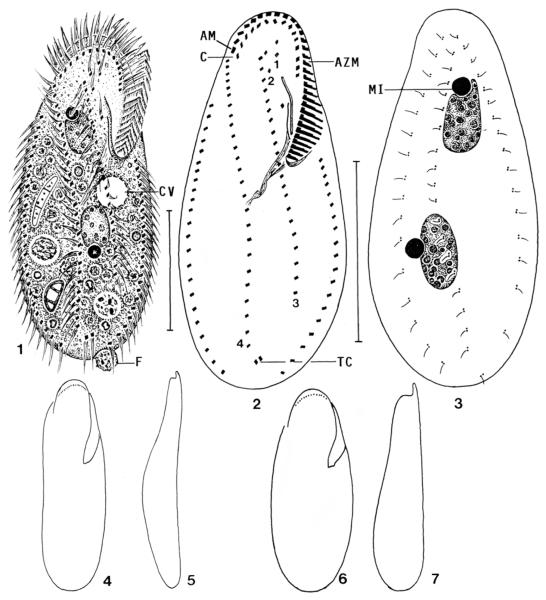
Keronopsis schminkei, Hawaiian specimens from life (1, 4–7) and after protargol impregnation (2, 3). 1 – ventral view of a representative specimen packed with minute (3–4 μm) food vacuoles containing bacterial rods; 2, 3 – infraciliature of ventral and dorsal side and nuclear apparatus of holotype specimen. Note the transverse cirri, which are the sole difference to the genus Paraholosticha; 4, 5– ventral and lateral outline of a slender specimen; 6, 7 – ventral and lateral outline of a well-nourished specimen. AM – distalmost adoral membranelle, AZM – adoral zone of membranelles, C – coronar cirri, CV – contractile vacuole, F – faecal mass, MI – micronucleus, TC – transverse cirri, 1–4 – ventral rows. Scale bars: 40 μm.
Table 2.
Morphometric data on Keronopsis schminkei. Data based on mounted, protargol-impregnated (Foissner 1991, protocol A; Stieve’s fixative strengthened by some ml 2% osmic acid because specimens are fragile), and randomly selected specimens from a non-flooded Petri dish culture. Measurements in μm. CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of individuals investigated, SD – standard deviation, SE – standard error of arithmetic mean, – arithmetic mean.
| Characteristics | M | SD | SE | CV | Min | Max | n | |
|---|---|---|---|---|---|---|---|---|
| Body, length | 88.8 | 90.0 | 9.2 | 2.1 | 10.3 | 73.0 | 110.0 | 19 |
| Body, width | 38.9 | 39.0 | 4.3 | 1.0 | 11.2 | 29.0 | 45.0 | 19 |
| Body length: width, ratio | 2.3 | 2.3 | 0.3 | 0.1 | 11.5 | 2.0 | 2.9 | 19 |
| Anterior end to proximal end of adoral zone, distance | 33.3 | 33.0 | 2.4 | 0.6 | 7.3 | 29.0 | 38.0 | 19 |
| Body length: length of adoral zone, ratio | 2.7 | 2.7 | 0.2 | 0.1 | 8.1 | 2.3 | 3.0 | 19 |
| Anterior end to right marginal row, distance | 19.5 | 20.0 | 2.2 | 0.5 | 11.4 | 13.0 | 22.0 | 19 |
| Anterior end to ventral row 3, distance | 9.7 | 10.0 | 1.4 | 0.3 | 14.5 | 7.0 | 13.0 | 19 |
| Anterior end to ventral row 4, distance | 11.6 | 12.0 | 1.4 | 0.3 | 12.3 | 7.0 | 14.0 | 19 |
| Anterior end to right undulating membrane, distance | 18.1 | 19.0 | 2.5 | 0.6 | 13.9 | 13.0 | 22.0 | 19 |
| Right undulating membrane, length | 10.7 | 11.0 | 0.9 | 0.2 | 8.1 | 10.0 | 13.0 | 19 |
| Anterior end to left undulating membrane, distance | 15.0 | 15.0 | 3.1 | 0.7 | 20.6 | 11.0 | 22.0 | 19 |
| Left undulating membrane, length | 16.0 | 16.0 | 1.2 | 0.3 | 7.4 | 14.0 | 18.0 | 19 |
| Anterior end to first macronucleus nodule, distance | 18.1 | 18.0 | 2.7 | 0.6 | 14.8 | 12.0 | 22.0 | 19 |
| Posterior end to ventral row 3, distance | 22.3 | 21.0 | 4.6 | 1.1 | 20.8 | 13.0 | 31.0 | 19 |
| Posterior end to ventral row 4, distance | 9.3 | 10.0 | 3.7 | 0.8 | 39.4 | 4.0 | 19.0 | 19 |
| Posterior end to transverse cirri, distance | 5.3 | 5.0 | 2.3 | 0.5 | 42.6 | 2.0 | 10.0 | 19 |
| Macronucleus nodules, length of anterior nodule | 14.3 | 14.0 | 2.2 | 0.5 | 15.3 | 11.0 | 20.0 | 19 |
| Macronucleus nodules, width of anterior nodule | 10.0 | 10.0 | 1.0 | 0.2 | 9.8 | 8.0 | 12.0 | 19 |
| Macronucleus nodules, number | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 19 |
| Macronucleus nodules, distance in between | 13.8 | 14.0 | 3.5 | 0.8 | 25.3 | 9.0 | 21.0 | 19 |
| Micronuclei, length of anteriormost | 4.6 | 4.5 | 0.8 | 0.2 | 16.4 | 3.5 | 7.0 | 19 |
| Micronuclei, width of anteriormost | 4.5 | 4.5 | 0.5 | 0.1 | 11.2 | 3.5 | 5.0 | 19 |
| Micronuclei, number | 2.1 | 2.0 | 0.7 | 0.2 | 31.3 | 1.0 | 4.0 | 19 |
| Adoral membranelles, number | 33.2 | 33.0 | 1.8 | 0.4 | 5.5 | 29.0 | 37.0 | 19 |
| Coronar cirri, number | 15.4 | 15.0 | 1.5 | 0.3 | 9.5 | 13.0 | 19.0 | 19 |
| Ventral cirral rows, number | 4.0 | 4.0 | 0.0 | 0.0 | 0.0 | 4.0 | 4.0 | 19 |
| Ventral cirral row 1, number of cirri | 2.2 | 2.0 | 0.6 | 0.1 | 28.5 | 1.0 | 3.0 | 19 |
| Ventral cirral row 2, number of cirri | 2.0 | 2.0 | 0.5 | 0.1 | 23.2 | 1.0 | 3.0 | 19 |
| Ventral cirral row 3, number of cirri | 22.4 | 21.0 | 3.0 | 0.7 | 13.2 | 19.0 | 31.0 | 19 |
| Ventral cirral row 4, number of cirri | 26.4 | 26.0 | 2.9 | 0.7 | 11.1 | 22.0 | 33.0 | 19 |
| Right marginal cirri, number | 24.6 | 24.0 | 2.3 | 0.5 | 9.4 | 22.0 | 31.0 | 19 |
| Left marginal cirri, number | 23.3 | 23.0 | 2.3 | 0.5 | 9.9 | 19.0 | 28.0 | 19 |
| Buccal cirri, number | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 19 |
| Transverse cirri, number | 2.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 2.0 | 19 |
| Dorsal kineties, number | 3.0 | 3.0 | 0.0 | 0.0 | 0.0 | 3.0 | 3.0 | 19 |
| Kinetids in dorsal kinety 1, number | 16.1 | 16.0 | 2.3 | 0.5 | 14.0 | 12.0 | 23.0 | 19 |
Diagnosis
Size about 110 × 50 μm in vivo; ellipsoidal to slightly ovate. Two macronucleus nodules with a large micronucleus each. Two short and two long ventral cirral rows. On average 33 adoral membranelles, about 24 cirri each in right and left marginal row, 15 coronar cirri, 1 buccal cirrus, 2 transverse cirri, and 3 dorsal kineties; ventral rows one and two each composed of an average of 2 cirri, row three of 22 cirri, and row four of 26 cirri.
Type locality
Mossy bark from Koa trees in the Bird Park of the Volcano National Park on Big Island, Hawaiian Archipelago, W155°20′N19°25′.
Type material
One holotype and one paratype slide have been deposited in the Oberösterreichische Landesmuseum in Linz (LI), Biologiezentrum. Relevant specimens are marked by black ink circles on the coverslip.
Dedication
I name this species in honour of Prof. Dr. Horst Kurt Schminke (Oldenburg University), acknowledging his great efforts to promote taxonomic research.
Description
Size 80–130 × 35–60 μm in vivo, usually near 110 × 50 μm, length:width ratio about 2.3:1 both in vivo and protargol preparations (Table 2). Outline slightly ovate or elliptical to broadly elliptical, both ends broadly rounded; dorsoventrally flattened about 2:1, ventral side flat or slightly concave, dorsal convex in or underneath mid-body (Figs 1–7). Nuclear apparatus in body midline and middle quarters of cell. Macronucleus nodules globular to ellipsoidal, about 15 × 10 μm in vivo, anterior nodule invariably in oral portion, as in congeners; contain many globular nucleoli. Usually, a micronucleus each in distinct indentation of macronucleus nodules; individual micronuclei in variable position, globular to broadly ellipsoidal, conspicuous both in vivo and protargol preparations because about 5 μm across (Figs 1, 3; Table 2). Contractile vacuole slightly above mid-body between cell’s midline and left margin, that is, underneath proximal end of adoral zone of membranelles. Cytopyge in posterior end left of midline, faecal mass slimy and about 7 μm across. Cortex colourless and flexible, but swimming specimens do not bend; no specific cortical granules. Cytoplasm usually packed with lipid droplets 1 μm across and minute, 3–4 μm-sized vacuoles containing bacterial rods; feeds also on colourless (Fusarium) and brown fungal spores, coccal cyanobacteria 3 μm across, and heterotrophic flagellates digested in food vacuoles up to 12 μm across (Fig. 1). Movement without peculiarities, glides rather rapidly on microscope slides and debris showing distinct flexibility.
Cirral pattern and number of cirri of usual variability, except for the highly variable length of the ventral rows (Figs 1, 2; Table 2). All cirri 10–12 μm long and composed of two, rarely three rows with four to six cilia each. Coronar cirri close to adoral membranelles, right end of corona at same level as distal end of adoral zone. Ventral rows 1 and 2 short, each consisting of only one to three cirri right and above undulating membranes; rows 3 and 4 commence subapically and extend slightly obliquely to posterior body quarter, row 3 invariably shorter than row 4, which ends above the two inconspicuous transverse cirri slightly projecting above body margin. Buccal cirrus right of anterior end of right undulating membrane. Marginal rows widely open posteriorly, right row commences far subapically at level of buccal cirrus. Dorsal bristles about 3 μm long in vivo, arranged in three bipolar rows, leaving blank a fusiform area right of body midline because rows 1 and 2 extend in left body half and row 3 runs along right cell margin. Caudal cirri lacking (Fig. 3; Table 2).
Adoral zone slightly gonostomoid (Berger 1999), conspicuous because occupying an average of 38% of body length, distal half semicircular and thus ending far subapically; composed of an average of 33 membranelles of usual size and structure (Figs 1, 2; Table 2). Buccal cavity short, narrow and flat, matching the minuteness of the food vacuoles (see above). Two undulating membranes in highly characteristic pattern at right margin of buccal cavity: right membrane (paroral?) with 5 μm long cilia almost straight and distinctly shorter anteriorly than convex left membrane (endoral?) bearing 10 μm long cilia. Both membranes extend side by side and at nearly same focal plane. Thus, it is impossible to decide which one is the paroral and endoral. Pharyngeal fibres of usual structure, length and location.
Occurrence and ecology
As yet found in mossy tree bark from Hawaii and mossy soil from The Netherlands (Table 1). In Hawaii, moderate numbers of K. schminkei occurred six days after rewetting the sample (pH 4.9) and remained for another week. In The Netherlands sample (pH 5.1), K. schminkei was rare and observed only two days after rewetting, as typical for this group of r-selected hypotrichs preferring moss and leave litter habitats.
Generic classification and comparison with related species
Both Keronopsis and Paraholosticha have a similar general organisation and a conspicuous frontal cirral corona. Thus, they were synonymized by several authors. However, we agree with Dieckmann (1988) that the presence (Keronopsis), respectively, absence (Paraholosticha) of transverse cirri is sufficient to maintain both genera. There is, indeed, indication that Keronopsis needs further splitting because it comprises species with rather different oral apparatus and cirral corona (Blatterer and Foissner 1988; Foissner 1998). This is sustained by K. schminkei which highly resembles Paraholosticha muscicola Kahl (redescribed by Foissner 1987) and P. sterki Garnjobst (redescribed by Dieckmann 1988), even in the curious pattern of the undulating membranes. However, split of the genus should await further data, especially on the type species.
Presently, six Keronopsis species are recognized. They differ from K. schminkei by the following features: K. helluo Penard, 1922, type of the genus, is 250–300 μm long and has five to six macronucleus nodules and eight transverse cirri; K. alpestris (Kahl 1932) and K. wetzeli Wenzel, 1953 possess two macronucleus nodules with a single micronucleus in between; K. polychaeta (Borror, 1966) is a brackwater species with eight transverse cirri in rectangular pattern; K. tasmaniensis Blatterer and Foissner, 1988 has the cirral corona composed of only seven cirri and its undulating membranes are distinctly curved; and K. dieckmanni Foissner, 1998 has four macronucleus nodules and only five cirri in the frontal corona.
A note is necessary on K. wetzeli, which Wenzel (1953) described with two micronuclei, while the illustration shows a single, large micronucleus in between the two macronucleus nodules. Likely, Wenzel (1953) confused observations from K. wetzeli and K. schminkei! No type material is available from K. wetzeli. Thus, we designate the Austrian population described by Berger and Foissner (1987) as a neotype, which is deposited at the same locality as the type slides of K. schminkei. The neotype population has a single micronucleus in between the two macronucleus nodules.
Enchelys polynucleata (Foissner 1984) Foissner et al. 2002 (Figs 8–15; Table 3)
Figs 8-11.
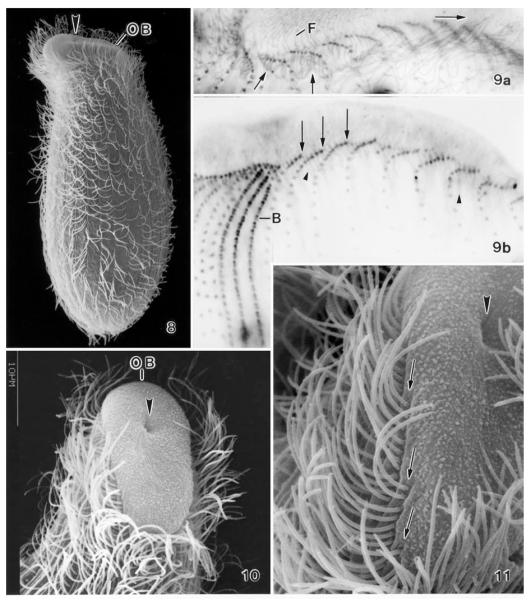
Enchelys polynucleata, The Netherlands specimens in the scanning electron microscope (8, 10, 11) and after protargol impregnation (9). Large arrowheads mark the cytopharyngeal entrance near the dorsal end of the oral bulge. 8 – right side overview, length about 150 μm in vivo; 9 – ciliary pattern of left and dorsal side. Arrows denote cilia (9a) and basal bodies (9b) at anterior end of ciliary rows. The pharyngeal basket is made of nematodesmata (small arrowheads) originating from the anterior basal bodies, that is, from oralized somatic monokinetids; 10, 11 – ventrolateral views showing the elliptical oral bulge and the anterior end of the ciliary rows merging into the oral bulge. B – dorsal brush, F – fibres in oral bulge, OB – oral bulge.
Figs 12-15.
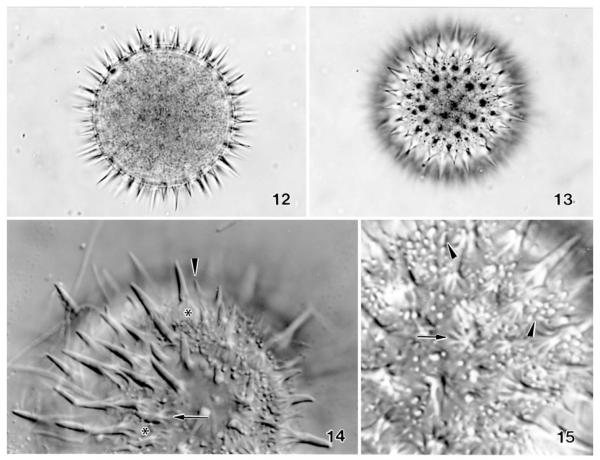
Enchelys polynucleata, resting cysts of The Netherlands specimens from life. 12, 13 – bright field micrographs of a cyst in optical section and surface view showing the spiny and punctate (by small spines) surface. Diameter 55 μm without spines; 14, 15 – surface views in the interference contrast microscope. The long spines originate from bulbs (asterisks) made of minute pillars (arrows). Many minute spines (arrowheads), which appear as bright dots in surface view (15), are scattered between the long ones.
Table 3.
Morphometric data on one-week-old cysts of The Netherlands Enchelys polynucleata. In vivo measurements of well-formed cysts from specimens transferred from the non-flooded Petri dish culture to Eau de Volvic (French table water); malformed cysts discarded. Measurements in μm. CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of cysts investigated, SD – standard deviation, SE – standard error of arithmetic mean, – arithmetic mean.
| Characteristics | M | SD | SE | CV | Min | Max | n | |
|---|---|---|---|---|---|---|---|---|
| Length with spines | 71.5 | 70.0 | 4.8 | 1.3 | 6.7 | 65.0 | 84.0 | 13 |
| Width with spines | 70.6 | 70.0 | 4.4 | 1.2 | 6.3 | 63.0 | 82.0 | 13 |
| Length without spines | 54.8 | 54.0 | 4.6 | 1.3 | 8.4 | 50.0 | 67.0 | 13 |
| Width without spines | 54.2 | 53.0 | 4.6 | 1.3 | 8.4 | 48.0 | 66.0 | 13 |
| Wall, thickness | 2.2 | 2.0 | 0.5 | 0.1 | 23.8 | 1.5 | 3.0 | 13 |
Foissner and Foissner (1985) showed by transmission electron microscopy that E. polynucleata lacks a circumoral kinety and the oral basket is made of nematodesmata originating from the anterior basal bodies of the somatic ciliary rows. They named this type of ciliature “oralized somatic monokinetids.” Foissner and Foissner (1985) suggested that ciliates of this type could be the free-living ancestors of the archistomatids, which have the same type of oral apparatus, but live endocommensally in various mammals (Corliss 1979).
The scanning electron microscopic investigations on The Netherlands specimens confirm the data by Foissner (1984) and Foissner and Foissner (1985), that is, show the elliptical shape of the oral bulge, the eccentric location of the cytopharyngeal entrance, and the lack of a circumoral kinety (Figs 8, 10, 11). Foissner and Foissner (1985) found that the anteriormost, nematodesmata-bearing basal bodies are unciliated. The protargol preparations and SEM micrographs of The Netherlands specimens show only ciliated basal bodies at the anterior end of the ciliary rows (Figs 9a, 11). However, the occurrence of unciliated and thus unstained or invisible basal bodies ahead of the ciliated ones cannot be excluded.
The Netherlands specimens of E. polynucleata match the Austrian type population, except of the following differences: extrusomes are straight to inconspicuously curved, 8–10 μm long rods in the former and slightly curved, about 14 μm long rods in the latter; two vs. one shape and size types of cyst spines (Figs 12–15). The latter difference was confirmed by a reevaluation of the original notes and micrographs. The 4–7 μm long spines are very similar in both populations, that is, have a proximal inflation made of minute pillars (Figs 12–15). The 1–4 μm long small spines, which give The Netherlands cysts a punctate appearance (Figs 13, 15), likely lack the bulb. Small spines occur also in the Austrian specimens, but they are rare and have a bulb, suggesting that they are small long spines. Cyst size is very similar in the Austrian ( 56 μm) and The Netherlands specimens (Table 3), while the wall is colourless in the former and distinctly yellowish in the later. Taken together, these are rather conspicuous differences, indicating that the Austrian and The Netherlands populations could represent distinct subspecies.
Family Bryophyllidae Foissner in Foissner and Lei, 2004
Apobryophyllum schmidingeri nov. spec. (Figs 16–51; Table 4)
Figs 16-29.
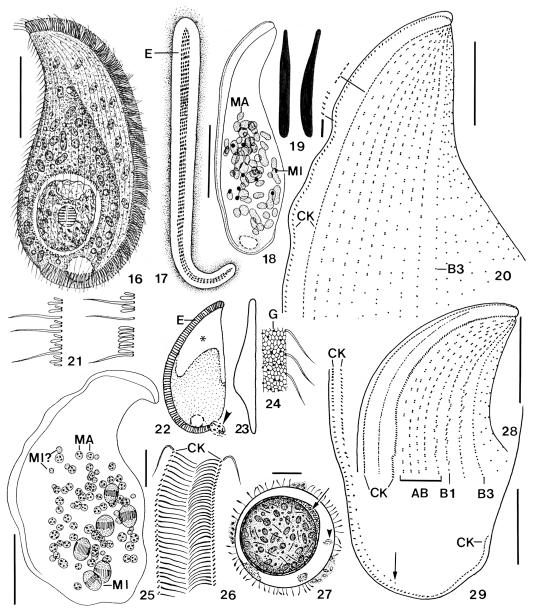
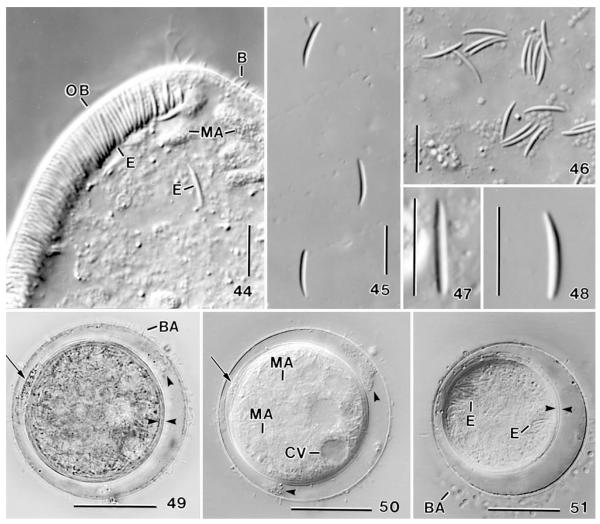
Apobryophyllum schmidingeri, Austrian specimens from life (16, 17, 19, 21–24, 27) and after protargol impregnation (18, 20, 25, 26, 28, 29). 16 – right side view of a representative specimen with an ingested rotifer; 17 – frontal view of oral bulge, showing short rows of extrusomes (E); 18 – nuclear apparatus; 19 – the long extrusomes are asymmetric and conspicuous because they are 9 × 1 μm in size, while the short extrusomes (right) are only 2 μm long; 20, 21, 28, 29 – ciliary pattern of left anterior (20, 28) and posterior (29) portion, showing the genus-specific, heteromorphic dorsal brush (21) and the shortened left branch of the circumoral kinety (29, arrow); 22, 23 – left side and dorsal view, showing the brush area (asterisk) and a faecal mass leaving the cell (arrowhead); 24 – surface view showing cortical alveoli and sparse cortical granules; 25 – possibly a post-conjugate with some dividing micronuclei; 26 – frontal view of oral bulge in anterior body half; 27 – resting cyst with extruded material between the inner cyst wall (arrow) and fuzzy material between ecto- and endocyst (arrowhead). The cyst is covered with various bacteria. AB –accessory brush rows, B1, B3 – dorsal brush rows, CK – circumoral kinety, E – extrusomes, G – cortical granules, MA – macronucleus nodules, MI – micronuclei. Scale bars: 50 μm (Figs 16, 18, 25), 20 μm (20, 27–29), 5 μm (26).
Figs 44-51.
Apobryophyllum schmidingeri, Austrian specimens from life. 44 – anterior body region showing the oral bulge studded with 8–10 μm long extrusomes (cp. Figs 45–48); 45–48 – long (type I)oral bulge extrusomes at various magnifications, showing the asymmetric shape (47, 48); 49, 50 – resting cyst in bright field and interference contrast. Arrows mark extruded material between the bipartite inner wall; opposed arrowheads denote the inner cyst wall; arrowheads mark fuzzy material between the outer and inner wall; 51 – resting cyst showing the bipartite inner wall (opposed arrowheads) and various cytoplasmic inclusions. B – dorsal brush bristles, BA – bacteria attached to the cyst wall, CV – contractile vacuole, E – extrusomes, MA – macronucleus nodules, OB – oral bulge. Scale bars: 30 μm (49–51), 10 μm (44–48).
Table 4.
Morphometric data on Apobryophyllum schmidingeri. Data based on mounted, protargol-impregnated (Foissner 1991, protocol A), and randomly selected specimens from a non-flooded Petri dish culture. Measurements in μm. CV – coefficient of variation in %, M – median, Max – maximum, Min – minimum, n – number of individuals investigated, SD – standard deviation, SE – standard error of arithmetic mean, – arithmetic mean.
| Characteristics | M | SD | SE | CV | Min | Max | n | |
|---|---|---|---|---|---|---|---|---|
| Body, length | 166.9 | 157.0 | 32.5 | 7.5 | 19.5 | 107.0 | 230.0 | 19 |
| Body, width | 74.0 | 72.0 | 22.0 | 5.0 | 29.7 | 42.0 | 118.0 | 19 |
| Body length: width, ratio | 2.4 | 2.0 | 0.6 | 0.1 | 26.1 | 1.7 | 3.4 | 19 |
| Anterior end to first macronucleus nodule | 57.3 | 60.0 | 13.3 | 3.0 | 23.2 | 34.0 | 82.0 | 19 |
| Anterior end to end of dorsal brusha | 52.5 | 50.0 | 7.7 | 2.4 | 14.7 | 42.0 | 65.0 | 10 |
| Anterior end to circumoral kinety, distance | 12.3 | 12.0 | 5.9 | 1.3 | 47.6 | 0.0 | 20.0 | 19 |
| Circumoral kinety, distance of branches in anterior third | 8.2 | 8.0 | 1.5 | 0.4 | 17.9 | 7.0 | 12.0 | 18 |
| Somatic kineties, number on right side | 19.3 | 20.0 | 2.3 | 0.7 | 11.7 | 15.0 | 23.0 | 10 |
| Somatic kineties, number on left side | 16.0 | 14.0 | 4.8 | 1.3 | 30.3 | 12.0 | 27.0 | 15 |
| Somatic kineties, total numer | 32.9 | 32.5 | 3.4 | 1.2 | 10.2 | 29.0 | 38.0 | 8 |
| Dorsal brush rows, total number | 9.1 | 9.0 | 1.2 | 0.3 | 12.8 | 7.0 | 11.0 | 17 |
| Dikineties in brush row 2, number | 29.3 | 25.0 | 9.3 | 2.8 | 31.9 | 17.0 | 50.0 | 11 |
| Dikineties and monokineties in brush row 2, number | 31.7 | 30.0 | 12.4 | 3.8 | 39.2 | 10.0 | 58.0 | 11 |
| Macronucleus nodules, number | 55.2 | 54.0 | 15.5 | 3.6 | 28.0 | 33.0 | 83.0 | 19 |
| Macronucleus nodules, length | 8.6 | 9.0 | 2.9 | 0.7 | 33.7 | 5.0 | 15.0 | 19 |
| Macronucleus nodules, width | 5.9 | 5.0 | 1.7 | 0.4 | 28.2 | 4.0 | 10.0 | 19 |
| Micronuclei, number | 9.3 | 8.5 | 2.1 | 0.5 | 22.8 | 6.0 | 13.0 | 16 |
| Micronuclei, length | 3.8 | 3.5 | 0.6 | 0.2 | 16.2 | 3.0 | 5.0 | 16 |
| Micronuclei, width | 3.1 | 3.0 | – | – | – | 3.0 | 4.0 | 16 |
Row 3 excluded. Rough values because the end of the brush is difficult to recognize due to the occurrence of monokinetids.
Diagnosis
Size about 170 × 75 μm in vivo; lanceolate with an average length: width ratio of 2.4:1. On average 55 scattered, ellipsoidal macronucleus nodules and 9 globular micronuclei. Type I extrusomes asymmetrical, that is, slightly curved rods with conical anterior region and a size of 8–10 × 1 μm; type II rodshaped, fine, about 2 μm long. On average 33 ciliary rows, 9 dorsal and left side kineties modified to conspicuous brush in anterior third.
Type locality
Litter and surface soil of a spruce-firbeech forest (Neuwald, site N in Foissner et al. 2005) in Styria (Austria), 47°46′N 15°32′E.
Type material
One holotype and seven paratype slides have been deposited in the Oberösterreichische Landesmuseum in Linz (LI), Biologiezentrum. Relevant specimens are marked by black ink circles on the coverslip.
Dedication
We dedicate this new species to Prof. Dr. Heinrich Schmidinger, Rector of Salzburg University, who greatly supported protozoological research by granting our group a technican.
Description
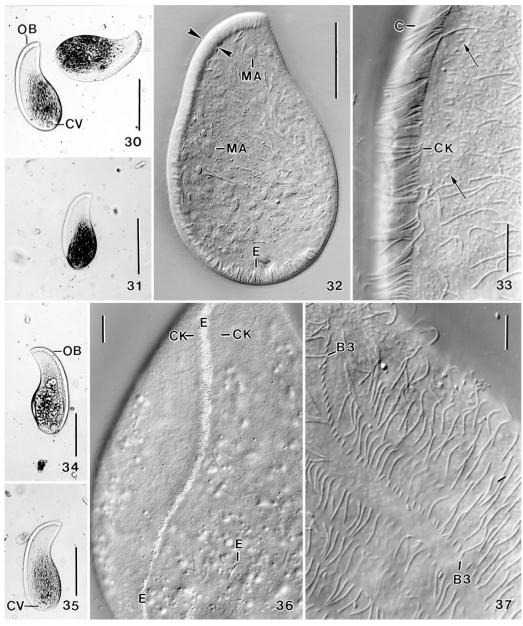
Size 110–230 × 45–100 μm in vivo, usually near 170 × 75 μm with an average length: width ratio of 2.4:1 both in vivo and protargol preparations; highly flexible, but not contractile (Fig. 43), rarely with slightly undulating ventral margin (Fig. 25). Shape rather constant, lanceolate to broadly lanceolate, i.e., ventral margin convex, dorsal sigmoidal with conspicuous concavity between first and second third of body (Figs 16, 30, 31, 34, 35). Right side flat, left usually distinctly vaulted and dark at low magnification (≤ ×100) due to food inclusions in posterior left quadrant of cell; anterior third and ventral margin leaflike flattened and thus hyaline (Figs 22, 23, 30, 31, 34, 35). About 10% of specimens malformed, i.e., doublets (Figs 38, 40) or with sharp indentations (Figs 39, 41), possibly injuries caused by rotifer prey. Nuclear apparatus in middle and posterior third of cell (Figs 16, 18, 32, 44). Macronucleus nodules globular to elongate ellipsoidal, on average ellipsoidal, that is, 2:1 with a range of 1–4:1. Many globular to slightly ellipsoidal micronuclei among macronucleus nodules. 30% of the specimen investigated have dividing micronuclei and small, globular macronucleus nodules, indicating post-conjugational reorganisation processes (Fig. 25). Contractile vacuole in midline of posterior body end, rarely slightly neighbouring ventral side (Figs 16, 18, 30, 34, 35). Cytopyge near dorsal end of cell, faecal mass globular and slimy (Fig. 22). Two types of extrusomes in oral bulge and cytoplasm, do not impregnate with protargol (Figs 16, 17, 19, 22, 32, 36, 44–48): type I conspicuous because 8–10 × 1 μm in size, compact and highly refractive, and studded in middle third of oral bulge forming oblique rows each consisting of 4–7 extrusomes in anterior body half and of 2–4 extrusomes in posterior half (Figs 17, 36); individual type I extrusomes asymmetrical, that is, slightly curved rods with conical anterior portion and rounded posterior (Figs 19, 44–48). Type II extrusomes also rod-shaped, but inconspicuous and difficult to recognize because only 2× < 0.3 μm in size (Fig. 19). Cortex very flexible, composed of minute (~1 μm), polygonal alveoli well recognizable in fortunate live preparations (Fig. 24); cortical granules sparse and loosely spaced. Cytoplasm colourless, posterior left quadrant usually packed with globular and irregular fat inclusions up to 7 μm across and, in 60% of specimens, with a large food vacuole containing an ingested bdelloid rotifer (Figs 16, 30, 34, 39, 41). Glides slowly on microscope slide and soil particles showing great flexibility and, in large specimens, a slightly undulating ventral margin (Fig. 25).
Figs 38-43.
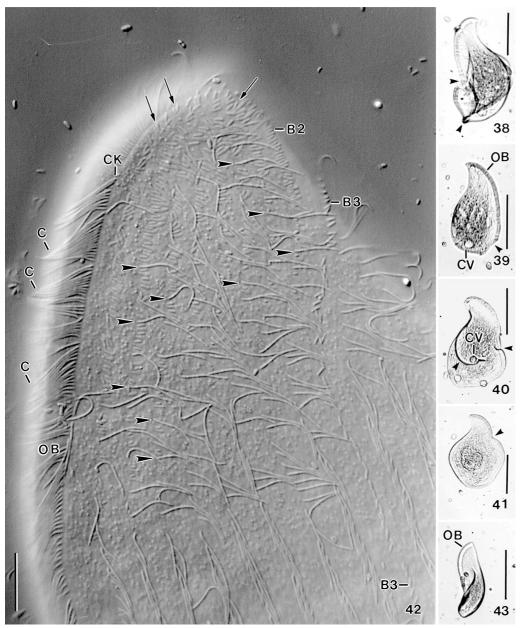
Apobryophyllum schmidingeri, Austrian specimens from life. 38–41 – malformed specimens (arrowheads) were frequent in the non-flooded Petri dish culture; 42 – high magnification of anterior region of left side, showing the genus-specific, heteromorphic dorsal brush, i.e., ordinary cilia (arrowheads) among the minute dorsal bristles. Brush row 3 has a long monokinetidal tail (cp. Fig. 37). Arrows mark V-like spread bristles in anterior region of rows. The narrowly spaced cilia (C) of the circumoral kinety form metachronal waves; 43– a twisted specimen. B2, 3 – dorsal brush rows, C – cilia of circumoral kinety, CK – circumoral kinety, CV – contractile vacuole, OB – oral bulge. Scale bars: 100 μm (38–41, 43), 10 μm (42).
Figs 30-37.
Apobryophyllum schmidingeri, Austrian specimen from life. 30, 31, 34, 35 – overviews showing body shape and oral bulge, which is distinct due to the many extrusomes contained. The blackish areas are convex and contain food inclusions; 32 – a slightly flattened (by coverslip) specimen, showing the massive oral bulge (opposed arrowheads) and the many macronucleus nodules; 33 – mid-region of oral area, showing the densely ciliated circumoral kinety and some minute dorsal bristles (arrows); 36 – ventral view showing transverse extrusome rows in oral bulge; 37 – brush row 3 has a monokinetidal tail composed of 1.5 μm long bristles. B3 – dorsal brush row 3, C – circumoral cilia, CK – circumoral kinety, CV – contractile vacuole, E – extrusomes, MA – macronucleus nodules, OB – oral bulge. Scale bars: 100 μm (30, 31, 34, 35), 50 μm (32), 10 μm (33, 36, 37).
Cilia 8–10 μm long in vivo, arranged in an average of 33 rather evenly spaced rows anteriorly usually abutting to dorsal portion of circumoral kinety (partially laterally abutting in typical Bryophyllum species), except of some slightly shortened rows in cell’s midline (Figs 20, 28; Table 4); kineties more loosely ciliated in posterior third and abutting to circumoral kinety extending along posterior end of cell. Anterior third of about nine dorsal and left lateral ciliary rows modified to conspicuous dorsal brush; three of the nine kineties form “ordinary” brush rows on dorsal side of cell, while six become accessory brush rows occupying anterior third of left side. Anterior portion of brush rows usually composed of dikinetids with V-like spread bristles, middle and posterior region heteromorphic, that is, composed of a mixture of bristles and ordinary cilia. “Ordinary” brush rows 1 and 2 very similar to accessory rows; row 3 isomorphic and composed of 4–8 dikinetids anteriorly, followed by narrowly spaced monokinetids with 1.5 μm long bristles, forming a tail extending to second third of body (Figs 20, 28, 37, 42). Brush bristles about as thick as ordinary cilia and slightly inflated distally, gradually decrease in length from up to 3.5 μm anteriorly to 1.5 μm posteriorly; anterior bristle of dikinetids about half as long as posterior one in anterior portion of brush, followed by dikinetids with anterior and posterior bristle of similar length or with an ordinary cilium anteriorly and a 1.5 μm long bristle posteriorly (Figs 21, 42).
Oral apparatus occupies entire body length and curves around posterior body end. Height and width of oral bulge gradually decreasing from 8–10 μm anteriorly to 2–4 μm posteriorly, rear bulge end thus hardly recognizable; anterior half of bulge very conspicuous in vivo because glossy due to the many extrusomes contained (see above and Figs 16, 17, 30, 32, 34, 36, 42, 44); bulge surface smooth and without depression in midline. Circumoral kinety composed of dikinetids so narrowly spaced in anterior half that cilia form metachronal waves (Figs 16, 33, 42); left branch shortened and composed of monokinetids posteriorly (Fig. 29), although extrusome fringe extends to dorsal edge of cell (Figs 16, 32); dikinetids each associated with a 10 μm long cilium and a distinct fibre extending to bulge midline (Fig. 26).
Of 10 specimens isolated in a moist chamber, six formed colourless, globular resting cysts with an average diameter of 66 μm (extremes: 60–77 μm). Outer cyst wall (ectocyst?) about 1 μm thick and colonized by various bacteria in older cysts, separated from inner wall by an about 4 μm wide, hyaline space, containing accumulations of fuzzy materials. Inner wall about 2 μm thick and bipartite (mesoand endocyst?), sometimes containing an accumulation of granular material between the two layers. Cytoplasm finely granular, packed with macronucleus nodules and extrusomes, even in one-week-old cysts (Figs 27, 49–51).
Occurrence and ecology
See Tables 1 and 4 in Foissner et al. (2005) for detailed site data (pH, organic C, etc.) on the Austrian type population. Of the 12 forest stands investigated in this study, A. schmidingeri occurred only at one site (see type locality), indicating that it is a rare species. Likewise, it occurred only in the mossy dune soil in The Netherlands, suggesting that it is a moss and litter species. On the other hand, we cannot exclude that some of our previous records of Bryophyllum loxophylliforme are misidentified A. schmidingeri (see below).
In The Netherlands sample, A. schmidingeri was rare, while it became rather numerous in the Austrian sample seven weeks after rewetting, that is, when there were many rotifers, the preferred food. Apobryophyllum schmidingeri attaches firmly to soil particles. Thus, the sample must be irrigated with distilled water and /or tilted many times to get sufficient specimens for preparations. Soon after these procedures, the specimen attach again to soil.
Comparison with related species
Foissner (1984, 1988), Foissner et al. (2002), and Foissner and Lei (2004) studied several bryophyllids and classified them into three genera: Bryophyllum (3 isomorphic brush rows), Neobryophyllum (more than 3 isomorphic brush rows), and Apobryophyllum (3 or more heteromorphic brush rows with brush bristles irregularly alternating with ordinary cilia). Thus, the population described here doubtlessly belongs to the genus Apobryophyllum, differing from the four described congeners (Foissner 1998; Foissner et al. 2002; Foissner and Lei 2004) by body shape (lanceolate to broadly lanceolate vs. knifeshaped to linear), the nuclear apparatus (many macronucleus nodules vs. a single strand), and the number of ciliary rows (33 vs. 16 or less). Thus, A. schmidingeri is a very distinct species within the genus.
However, Kahl (1931) described Bryophyllum loxophylliforme which is highly similar to A. schmidingeri, except of the dorsal brush, which Kahl (1931) described as follows: “Dorsal brush distinct; the right row extends on the left side near to posterior body end, while the two other rows are shorter and near to the dorsal margin of the cell.” Thus, B. loxophylliforme has an ordinary, three-rowed dorsal brush. At first glance, it appears reasonable to assume that Kahl (1931) overlooked details of the dorsal brush, and our population thus should be identified as B. loxophylliforme. However, there are indeed species which have only three ordinary brush rows, viz., B. tegularum Kahl, 1931 (redescribed from protargol-impregnated specimens by Foissner 1984) and B. longisetum Foissner and Lei, 2004. Thus, it is likely that a species exists as described by Kahl (1931) and which is highly similar to A. schmidingeri, except of the dorsal brush. Accordingly, it would be unwise to declare Kahl’s data as incorrect because, as B. loxophylliforme is the typus generis, this would create many taxonomical and nomenclatural problems more difficult to solve than the description of the present population as a new species.
Dileptus sp.
Likely, this is also a new species because it is not contained in the revision of Kahl (1931). It will be described from Venezuelan material, where I discovered it.
Arcuospathidium namibiense namibiense Foissner et al., 2002
This is a remarkable record because this species was known from only a single site in Namibia, viz., the very sandy soil of an Aloe dichotoma forest. The extrusomes, which distinguish it from the more common subspecies tristicha, match perfectly, that is, they are oblong and have a size of about 1.5 × 0.8 μm.
Podophrya tristriata Foissner et al., 2002
The Netherlands population matches the original description and confirms the stability of the three ridges of the resting cyst.
Uroleptus paranotabilis Foissner et al., 2002
The Netherlands populations differ from the Namibian type specimens by the cortical granules which are 0.5–1 × 0.3–0.4 μm in size and colourless (vs. ≤ 0.5 μm and yellowish to citrine), like in the Antarctic specimens. Possibly, such populations represent a distinct subspecies, as already suggested by Foissner et al. (2002).
Oxytricha proximata (?)
Only a few, about 150 μm long specimens were found two days after rewetting of the sample. Likely, it was O. proximata Shibuya, a rare species discovered in Japanese field soil. Later, it was reported from moss and soil in Hungary (for a review, see Berger 1999; unfortunately, Berger mentions that it has been recorded also from Belgium, although Chardez 1967 listed it only as a member of the global soil ciliate fauna).
Amphisiella magnigranulosa Foissner, 1988
This species has the same type of buccal lip as A. multinucleata Foissner et al. 2002, indicating that these two species comprise a distinct genus or subgenus.
Acknowledgements
Thanks to Prof. Dr. Johannes Hackstein for organizing the trip to the Hoge Veluwe National Park and the background data to site 1. We also acknowledge the technical assistance of Mag. Birgit Peukert, and Andreas Zankl. The study was supported by the Austrian Science Foundation (FWF), P-15017 and P-19699-B17 as well as by the King Saud University.
REFERENCES
- Bakker Th. W., Jungerius PD, Klijn JA., editors. Dunes of the European coasts. Catena, Suppl. 1990;18:1–227. [Google Scholar]
- Berger H. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia) Monographia boil. 1999;78:i–xii. 1–1080. [Google Scholar]
- Berger H, Foissner W. Morphology and biometry of some soil hypotrichs (Protozoa: Ciliophora) Zool. Jb. Syst. 1987;114:193–239. [Google Scholar]
- Blatterer H, Foissner W. Beitrag zur terricolen Ciliatenfauna (Protozoa: Ciliophora) Australiens. Stapfia, Linz. 1988;17:1–84. [Google Scholar]
- Borror AC. Paraholosticha polychaeta n. sp. (Ciliata, Hypotrichida) from a New Hampshire tidal marsh. J. Protozool. 1966;13:418–421. doi: 10.1111/j.1550-7408.1966.tb01932.x. [DOI] [PubMed] [Google Scholar]
- Brunberg Nielsen L. Investigations on the microfauna of leaf litter in a Danish beech forest. Natura Jutlandica. 1968;14:79–87. [Google Scholar]
- Chao A, Li PC, Agatha S, Foissner W. A statistical approach to estimate soil ciliate diversity and distribution based on data from five continents. Oikos. 2006;114:479–493. [Google Scholar]
- Chardez D. Infusoires ciliés terricoles (Protozoa, Infusoria Ciliata) Revue Ecol. Biol. Sol. 1967;4:289–298. [Google Scholar]
- Corliss JO. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. 2nd ed Pergamon Press; Oxford, New York, Toronto, Sydney, Paris, Frankfurt: 1979. [Google Scholar]
- Dieckmann J. Infraciliature and morphogenesis of Paraholosticha sterkii (Garnjobst, 1934) n. comb. (Ciliophora, Hypotrichida) Europ. J. Protistol. 1988;23:218–228. doi: 10.1016/S0932-4739(88)80038-5. [DOI] [PubMed] [Google Scholar]
- Esteban GF, Clarke KJ, Olmo JL, Finlay BJ. Soil protozoa – an intensive study of population dynamics and community structure in an upland grassland. Appl. Soil Ecol. 2006;33:137–151. [Google Scholar]
- Foissner W. Infraciliatur, Silberliniensystem und Biometrie einiger neuer und wenig bekannter terrestrischer, limnischer und mariner Ciliaten (Protozoa: Ciliophora) aus den Klassen Kinetofragminophora, Colpodea und Polyhymenophora. Stapfia, Linz. 1984;12:1–165. [Google Scholar]
- Foissner W. Neue und wenig bekannte hypotriche und colpodide Ciliaten (Protozoa: Ciliophora) aus Böden und Moosen. Zool. Beitr. N. F. 1987;31:187–282. [Google Scholar]
- Foissner W. Gemeinsame Arten in der terricolen Ciliatenfauna (Protozoa: Ciliophora) von Australien und Afrika. Stapfia, Linz. 1988;17:85–133. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Europ. J. Protistol. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W. Colpodea (Ciliophora) Fischer; Stuttgart: 1993. pp. i–x.pp. 1–798. (Protozoenfauna 4). [Google Scholar]
- Foissner W. Morphology and morphogenesis of Circinella arenicola nov. gen., nov. spec., a cephalized hypotrich (Ciliophora, Hypotrichida) from sand dunes in Utah, USA. Europ. J. Protistol. 1994;30:156–170. [Google Scholar]
- Foissner W. Global soil ciliate (Protozoa, Ciliophora) diversity: a probability-based approach using large sample collections from Africa, Australia and Antarctica. Biodiv. Conserv. 1997;6:1627–1638. [Google Scholar]
- Foissner W. An updated compilation of world soil ciliates (Protozoa, Ciliophora), with ecological notes, new records, and descriptions of new species. Europ. J. Protistol. 1998;34:195–235. [Google Scholar]
- Foissner W. Protist diversity: estimates of the near-imponderable. Protist. 1999;150:363–368. doi: 10.1016/S1434-4610(99)70037-4. [DOI] [PubMed] [Google Scholar]
- Foissner W. Some new ciliates (Protozoa, Ciliophora) from an Austrian floodplain soil, including a giant, red “flagship”, Cyrtohymena (Cyrtohymenides) aspoecki nov. subgen., nov. spec. Denisia. 2004;13:369–382. [Google Scholar]
- Foissner W. Biogeography and dispersal of micro-organisms: a review emphasizing protists. Acta Protozool. 2006;45:111–136. [Google Scholar]
- Foissner W, Foissner I. Oral monokinetids in the free-living haptorid ciliate Enchelydium polynucleatum (Ciliophora, Enchelyidae): ultrastructural evidence and phylogenetic implications. J. Protozool. 1985;32:712–722. [Google Scholar]
- Foissner W, Agatha S, Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha Region and the Namib Desert. Denisia. 2002;5:1–1459. [Google Scholar]
- Foissner W, Lei YL. Morphology and ontogenesis of some soil spathidiids (Ciliophora, Haptoria) Linzer biol. Beitr. 2004;36:159–199. [Google Scholar]
- Foissner W, Berger H, Xu K, Zechmeister-Boltenstern S. A huge, undescribed soil ciliate (Protozoa: Ciliophora) diversity in natural forest stands of Central Europe. Biodiv. Conserv. 2005;14:617–701. [Google Scholar]
- Jankowski AW. Revision of the order Hypotrichida Stein, 1859. Generic catalogue, phylogeny, taxonomy. Trudy zool. Inst. Leningr. 1979;86:48–85. (in Russian with English summary) [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 2. Holotricha außer den im 1. Teil behandelten Prostomata. Tierwelt Dtl. 1931;21:181–398. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 3. Spirotricha. Tierwelt Dtl. 1932;25:399–650. [Google Scholar]
- Penard E. Études sur les Infusiores d’Eau Douce. Georg & Cie; Genève: 1922. [Google Scholar]
- Verhoeven R. Ciliaten (Protozoa) in Primärdünen. Faun.-Ökol. Mitt. Kiel, Suppl. 1999;26:61–67. [Google Scholar]
- Verhoeven R. Response of soil microfauna to organic fertilisation in sandy virgin soils of coastal dunes. Biol. Fertil. Soils. 2001;34:390–396. [Google Scholar]
- Verhoeven R. The structure of the microtrophic system in a development series of dune soils. Pedobiologia. 2002;46:75–89. [Google Scholar]
- Wenzel F. Die Ciliaten der Moosrasen trockner Standorte. Arch. Protistenk. 1953;99:70–141. [Google Scholar]