Abstract
As the pharmaceutical industry continues to re-strategise and focus on low-risk, relatively short term gains for the sake of survival, we need to re-invigorate the early stages of drug discovery and rebalance efforts towards novel modes of action therapeutics and neglected genetic and tropical diseases. Academic drug discovery is one model which offers the promise of new approaches and an alternative organisational culture for drug discovery as it attempts to apply academic innovation and thought processes to the challenge of discovering drugs to address real unmet need.
Keywords: academia, drug discovery, neglected disease, novel target
2. Introduction
The large international Pharmaceutical and Biotechnology sectors have long been the recognised professionals in the discovery and development of new drugs. Although a number of widely used drugs have their discovery origins in academia (see Table 1), the academic sector’s role was seen more as a background player supporting drug discovery through providing an extended research base to the industry through peer reviewed publication and strategic partnerships. The Biopharma industry is now struggling for survival since increases in R&D expenditure have not translated into new approvals [1]. The resultant mass consolidation of companies and a world-wide dearth of funding for new ventures mean that the sector will have to transform itself and it is unlikely that we can rely solely on the Biopharma sector to deliver our future drugs [2,3]. These challenging times will no doubt inspire new models designed to seek out more cost-effective ways of delivering new drugs. One sector which has, for sometime, been responding to the need for new entrants and new approaches to drug discovery is academia [4-7]. A wave of funding from governmental, charitable and philanthropic agencies which initially stimulated activity to support the less commercially viable diseases such as malaria, tuberculosis and trypanosomiasis [8,9] has recently enhanced both appetites and capabilities for Universities to embark upon drug discovery across a range of therapeutic areas [10]. This article will focus on the concept of drug discovery within an academic setting; highlighting the challenges ahead and suggesting how this sector could live up to its considerable potential.
Table 1. Drugs with Academic Discovery Origins.
| Compound Structure | Compound Name |
Target, Indication | Academic Institute |
Industry Partner |
|---|---|---|---|---|
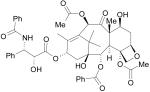
|
Paclitaxel, Taxol, Abraxane |
Microtubule stabilizer, Oncology |
Research Triangle Institute |
Bristol-Myers Squib |
|
|
Vorinostat, SAHA |
Histone deacetylase Inhibitor, Oncology |
Sloan Kettering Cancer Center |
Merck |
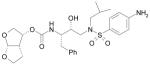
|
Prezista, Darunavir |
HIV protease inhibitor, HIV |
Purdue University |
Tibotech/J&J |
|
|
Tomudex, Raltitrexed |
Thymidylate synthase inhibitor, Oncology |
Institute of Cancer Resreach, UK |
AstraZeneca |
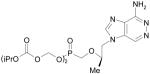
|
Viread, Tenovir disoproxil fumarate |
Reverse transcriptase inhibitor, HIV infection |
Institute of Organic Chemistry and Biochemistry, Czech Republic, Rega Institute, Begium |
Gilead |
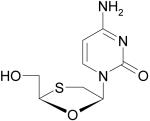
|
Lamivudine, (− )-3TC, Epivir |
Reverse transcriptase inhibitor, HIV infection |
Emory University |
GSK |
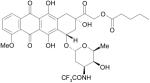
|
Valstar, Valrubicin |
DNA topoisomerase II inhibitor, Oncology |
Dana-Farber Cancer Institute, US. |
Endo Pharmaceuticals |
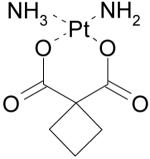
|
Paraplatin, Carboplatin |
DNA alkylating agent, Oncology |
Institute of Cancer Research, UK |
Bristol-Myers Squib |
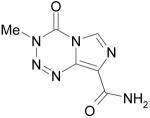
|
Temodar or Temodal, Temozolomide |
DNA methylation, Oncology |
Malcolm Stevens Aston University, UK |
ScheringPlough Corp. |
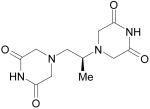
|
Dexrazoxane hydrochloride (Zinecard [Pfizer]; Cardioxane [Novartis]) |
Cardioprotective agent, Oncology |
Cancer Research UK, |
Pfizer for USA & Canada Novartis for EU & ROW |
|
|
Alimta, Pemetrexed |
Dihydrofolate reductase/thymidylate synthase inhibitor, Oncology |
Princeton University |
Eli Lilly |
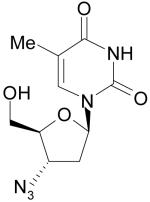
|
Zidovudine, AZT, Azidothymidine |
Reverse transcriptase inhibitor, HIV |
Michigan Cancer Foundation |
GSK |
3. Why drug discovery in academia makes sense
There is a clear need for improvement in how we discover new drugs [11,12]. Without major advances in new medical entities it is possible that the march of diseases such as diabetes and childhood obesity will actually result in a decrease in life expectancy over time [13]. Whilst large Pharma will continue to be the major source of new drugs, there is a compelling argument that academia needs to get more directly involved in translation of fundamental science into therapeutics [14-16] and Universities and government laboratories are increasingly becoming credible, consistent players in early stage drug discovery. These institutions have the appropriate climate for creative and innovative science. They understand the value of individual freedom, intellectual diversity, flexibility, and originality and have expertise in many disciplines in the biological, medical, chemical, computational, engineering, and mathematical sciences. These are the places which, with relatively small amounts of well placed incremental investment, can be transformed into translational ‘hot spots’ bringing enormous added value to institutions, funding agencies and ultimately to healthcare. It stands to reason that increasing the diversity of approach to drug discovery, both in technologies and thought process, will result in greater technological innovation. Furthermore, drug discovery funded through governmental and charitable routes increases our freedom to address unmet needs in small patient populations and diseases of the developing world. A vital and well supported academic drug discovery sector has the possibility of making substantial impacts in at least four major areas:
Partially de-risking novel drug discovery targets; through the parallel running of milestone driven drug discovery projects alongside academically driven development of the underpinning target biology. Such programmes have the promise of delivering high quality data packages with quality leads or pre-clinical candidates, coupled with robust linkage between modulation of the investigated target and the desired clinical response.
Neglected and orphan diseases; through the development of therapies for diseases whose patient base demographics or numbers will not generate a sufficient commercial return to offset the cost of drug development. The early phase of discovery can be resourced by charitable or traditional modes of academic funding. Lead optimisation and/or pre-clinical development opportunities are attractive to dedicated not-for-profit development organisations, which themselves are funded by governmental or charitable donations.
New paradigms for drug discovery; universities are an excellent environment for the development of new paradigms for drug discovery. The broad range of expertise within universities enables the establishment of multidisciplinary collaborations to develop new methods and approaches in areas such as data mining, informatics, computational chemistry, compound library selection and design, synthetic organic chemistry methodology, parallel synthetic and purification technology, in silico pharmacokinetics and toxicity predictions, and animal models for efficacy and toxicity. There are already numerous programmes underway in academia which may innovate future drug discovery process and thinking: the Hopkins laboratory at Dundee is developing predictive adaptive design methods against multiple target profiles to support controlled poly-pharmacology [17]; the Blundell group in Cambridge, UK is developing systematic approaches to fragment–based lead discovery for protein protein interactions; and multiple groups in UK and Canada are making significant strides towards developing the next generation of human cell models for drug discovery using induced pluripotent stem cells [18].
Training young scientists in the practice of drug discovery and educating basic scientists in the requirements for the translation of fundamental research into drug discovery.
The beauty of this sector is that is can create impact though multiple avenues. It can discover leads and, in some cases, pre-clinical candidates, it can thoroughly validate new targets and it can take on projects (both discovery and methodology) with levels of risk simply not possible within the modern-day confines of industry. At the same time these activities will generate information and tools to enable advancement of basic science and result in high impact publications [19,20], allowing these endeavours to sit more comfortably within institutions whose success metrics were once perceived to be at odds with such translational activities.
4. The Emergence of Academic Drug Discovery
Historically many translational opportunities from universities were pursued as spin-out companies, resulting during 1970-80s in the rise of the Biotech industry. Although most floundered and did not deliver sustainable companies, there have been significant exceptions such as Genentech. An alternative route was the early licensing to, or partnering with, the Pharma or Biotech companies. Many such opportunities have delivered high profile drugs to the clinic. Studies have estimated the contribution of publicly funded research to the delivery of FDA approved therapies, both molecular and biological entities, to be from between 16% and 50% [21,22]. In addition within the US there are a number of high profile linkages between academic centres and major companies, allowing the industrial partner first refusal on the whole output from those departments or centres [14]. Latterly with a growing pressure to maximise impact from their research, Universities are increasingly seeking to develop basic biology research by creating core drug discovery capabilities in house, capable of supporting a portfolio of translational projects.
Early examples of such centres were established as far back as the 1980s, such as the University of Florida, Center for Drug Discovery (1985). Since then many such centres have been established worldwide for examples see Table 2. These organisations are operating under very diverse models, from almost virtual set ups with only project management in-house, to groups which run the majority of the pre-development studies in house, and from loose collaborations to fully integrated groups under one management structure (e.g. Universities of Dundee and North Carolina). Whilst this sector was very much stimulated with the large scale adoption of high throughout screening in the US through the NIH molecular libraries initiative (see Table 2), it is the creation of smaller, more focussed groups with more direct drug discovery ambitions that will provide the early impact for this sector. Indeed a number of credible operations have already developed a suite of capabilities which encompass the critical path disciplines including: hit discovery using medium and high throughput screening of appropriately propertied compound libraries; computational chemistry, medicinal chemistry, DMPK and in vivo pharmacology (e.g. Cancer Research UK Centre for Cancer Therapeutics and Universities of Dundee and British Columbia). In addition networks of more virtual organisations are working in collaboration with infrastructure-based groups to broaden the impact of these capabilities across multiple Universities (Imperial, London). It is of note that Emory University in Atlanta, Georgia- historically one of the most successful drug discovery organisations in academia [23] has recently established a new Institute dedicated to drug discovery [24]. These groups all operate on a scale which means they have to be nimble in their decision making and work flexibly across multiple projects but the nature of their funding and success metrics means that they can take risks and approaches not possible within the confines of the typical pharmaceutical company. Recently Yale and Michigan State Universities in the US have taken their commitment to translational science one step further with the acquisition of entire buildings and sites previously occupied by large pharmaceutical corporations [25,26]. The concept of pre-competitive data sharing in drug discovery and development has also resulted in new organisations which operate to tackle the problems experienced by the entire sector. The Critical Path Institute was founded by the FDA to support discoveries which would improve the science of regulatory affairs concentrating on predictive safety testing; patient outcome reporting and major disease standards and models [27]. The SAGE Bionetworks organisation has a mission to establish a pre-competitive position for human disease biology ensuring that the best and most appropriate disease models are available to all [28].
Table 2. Example Academic Drug Discovery Operations.
| Europe and rest of world |
|
|
| Medical Research Council Technology – Centre for Therapeutics Discovery (MRC-CTD), London, UK |
| URL: http://www.mrctechnology.org/CO_DD.htm |
| Remit: To translate MRC-funded scientific discoveries into commercial products for healthcare benefit and to re-invest in future research |
| Focus: Wide target range across broad therapeutic areas |
|
|
| University of Dundee – Drug Discovery Unit (DDU), Dundee, UK |
| URL: http://www.drugdiscovery.dundee.ac.uk/ |
| Remit: Translational drug discovery for unmet medical needs and neglected diseases |
| Focus: Tropical diseases; novel targets and mechanisms; stem cells |
|
|
| Cancer Research Technology Discovery Laboratories (CRT), Cambridge & London, UK |
| URL: http://www.cancertechnology.com/pages/about_devlab_drugdisc.html |
| Remit: To translate CRUK-funded research into novel cancer therapies |
| Focus: Broad target approach to drug discovery and biotherapeutic development in cancer |
|
|
| Cancer Research UK Centre for Cancer Therapeutics (CRUK-CCT), London, UK |
| URL: http://www.icr.ac.uk/research/research_sections/cancer_therapeutics/index.shtml |
| Remit: To identify novel genes and pathways as targets and to implement novel technologies |
| Focus: HTS and HCS including phenotypic screening; target families including kinases, ATPases, protein:protein interactions, signalling pathways |
|
|
| University of Leuven – Centre for Drug Design and Discovery (CD3), Leuven, Belgium |
| URL: http://lrd.kuleuven.be/en/entrepreneur/collaboration/cd3/about.htm |
| Remit: To collaborate with academic research groups and small companies on innovative targets or approaches |
| Focus: Viral, bacterial, fungal diseases; CNS disorders; cancer |
|
|
| University of Liverpool, UK |
| URL: http://www.liv.ac.uk/science/industry/Drug_Discovery.html |
| Remit: medicinal chemsitry and pharmacology |
| Focus: Neglected tropical disease and drug safety |
|
|
| Northern Institute for Cancer research, Newcastle University , UK |
| URL: http://www.ncl.ac.uk/nicr/research/drug |
| Remit: medicinal chemistry and cancer cell biology |
| Focus: adult and paediatric cancer |
|
|
| University of British Columbia – Centre for Drug Research and Development (UBC-CDRD), Vancouver, Canada |
| URL: http://www.cdrd.ca/facilities-expertise/drug-screening |
| Remit: To advance promising discoveries to a commercially attractive stage and to build a collaborative research infrastructure |
| Focus: Cancer, CNS, diabetes, infectious diseases |
|
|
| Eskitis Institute for Cell and Molecular Therapies, Griffith University, Brisbane, Australia |
| URL: http://www.griffith.edu.au/science/eskitis-institute-cell-molecular-therapies |
| Remit: To investigate novel drug-and cell-based therapies for cancer, infection, neglected diseases, neurodegenerative diseases, stem cell biology |
| Focus: The “NatureBank” natural product resource; adult stem cell biology; breast cancer; Parkinson’s disease |
|
|
| Institut Pasteur Korea, Kwanggyo, South Korea |
| URL: http://www.ip-korea.org |
| Remit: To further the Institut Pasteur’s mission by applying Korean technology and competence in areas of neglected diseases |
| Focus: Infectious and chronic diseases; nanotechnology; PhenomicScreen™; high throughput flow cytometry |
|
|
| USA |
|
|
| Broad Institute, Cambridge, MA, USA |
| URL: http://www.broad.mit.edu/ |
| Remit: To capitalise on genome-related science and discoveries |
| Focus: Chemical Biology Platform encompassing chemistry, informatics and screening utilising HTS, HCS, phenotypic screens, RNAi screening |
|
|
| The Scripps Research Institute – Translational Research Institute, Florida, USA |
| URL: http://www.scripps.edu/florida/tri/ |
| Remit: Advanced Technologies and Drug Discovery components to the Translational Research Institute |
| Focus: Broad-based discovery structure including HTS, proteomics, flow cytometry, NMR, med chem., informatics |
|
|
|
St. Jude Children’s Research Hospital – Chemical Biology and Therapeutics Department HTS Core Facility, Memphis, TN, USA |
| URL: http://www.stjude.org/basicscience |
| Remit: To discover and develop novel chemical entities that increase understanding of the pathophysiology of or function as therapeutic leads for the treatment of catastrophic paediatric illnesses |
| Focus: Primarily childhood cancers |
|
|
| Southern Research Institute, Birmingham, AL, USA |
| URL: http://www.southernresearch.org |
| Remit: Drug Discovery Research Team focussing on cancer, infectious disease and neuroscience |
| Focus: HTS using BSL3 pathogens; antiviral, antimicrobial, anticancer screening |
|
|
| Drug Discovery Centre, University of Cincinnati, US |
| URL: http://www.drugdiscovery.uc.edu/capabilities/index.html |
| Remit: HTS, computational biology & chemistry, medicinal chemistry |
| Focus: development commercially viable entities from academic research, some focus on metabolic disease |
|
|
| Centre for Integrative Chemical Biology and Drug Discovery, UNC, US |
| URL: http://pharmacy.unc.edu/labs/center-for-integrative-chemical-biology-and-drug-discovery |
| Remit: HTS and medicinal chemistry aimed at development of leads and probe compounds to support target validation |
|
|
| Small Molecule Discovery Center (SMDC) at University of California, San Francisco |
| URL: http://smdc.ucsf.edu/ |
| Remit: HTS and medicinal chemistry |
| Focus: Cancer and neglected infectious disease |
|
|
| Emory Institute for Drug Discovery, Atlanta, Georgia, US |
| URL: http://www.emory.edu/home/news/releases/2009/05/emory-institute-for-drug-discovery.html |
| Remit: medicinal chemistry, cellular biology, pharmacokinetic profiling and pharmacology and toxicology studies |
| Focus: Commercially neglected diseases –measles, malaria, tuberculosis |
|
|
| Centre for Molecular Innovation and Drug Discovery, Northwestern University, Illinois, US |
| URL: http://www.cmidd.northwestern.edu/ |
| Remit: medicinal chemistry and biology to support translation of basic science |
|
|
| Virginia Commonwealth University Insititute for Structural Biology and Drug Discovery |
| URL: http://www.vcu.edu/structuralbio/index.html |
| Remit: structural biology, medicinal chemistry and biophysics |
|
|
| University of Pittsburgh Chemical Diversity Centre and Drug Discovery Institute, PA, US |
| URLs: http://next.cancer.gov/discoveryResources/cbc_pitt.htm; http://www.upddi.pitt.edu |
| Remit: HTS, pharmacology and medicinal chemistry |
|
|
| University of Florida, Center for Drug Discovery |
| URL: http://www.cop.ufl.edu/centers/cdd/index.htm |
| Remit: Computational design, chemistry, PK and PD |
|
|
| Molecular Libraries Probe Production Centres (MLPCN), 10 locations across USA |
| URL: http://www.ncgc.nih.gov |
| Remit: HTS with follow-up chemistry |
| Focus: To provide a national resource in chemical probe development supporting target validation |
How are these endeavours funded? Perhaps in the long term the sector can support itself to some extent through out-licensing and strategic partnerships but charity, government and private foundation funding will always be the dominant supporter of this translational science. Government-funded bodies, such as the Medical Research Council (MRC) in UK and National Institutes for Health (NIH) in the US, have always invested in leading-edge research activities within universities and research institutes but over the last decade a noticeable focus on small molecule initiatives has been evident: for examples see the MRC’s Translational Research Strategy [29] and the NIH Roadmap Initiative [30]. Charitable and philanthropic funding is also a significant source of support for the translational research sector, including the Wellcome Trust’s Seeding Drug Discovery initiative [10], Cancer Research UK’s drug discovery operations (Institute of Cancer Research Centre for Cancer Therapeutics (ICR-CCT) and Cancer Research Technology (CRT)) and the Broad Institute. Furthermore public-private partnerships also underpin academic centres to push forward their drug discovery portfolios, for example the Drugs for Neglected Diseases Initiative in partnership with the Drug Discovery Unit (DDU) at Dundee [31] and Medicines for Malaria Venture (MMV) currently working with The Broad Institute [8].
5. Challenges Ahead
5.1 Culture, Management and Reward
The real strength of this developing sector will be in the close coupling of academic minds with experienced drug discovery scientists resulting in enormously potent teams. The exchange of knowledge and skills in these operations are ideally placed to occur unhindered by conflicting motivations and lack of trust - so often the curse of more arms length collaborations between the sectors. These enterprises will only work however when populated with academics prepared to ‘let go of full control’ to translate their science effectively and open minded drug discovery professionals prepared to leave behind the culture within which many have been trained - risk and open innovation need to be embraced. To enable such endeavours to fulfil their potential there are significant issues to be addressed. As these groups are situated within Universities it would be seemingly logical to populate these groups with a mixture of PhD students and post docs. However, there are pit falls associated with this approach, first, the need to shift priorities and work flows and to close non-progressing projects to allow the proper management of drug discovery portfolios, does not sit well with the need to have coherent PhD thesis projects and for post docs to publish; second, the lack of experience of PhD and post docs does not match the usual rapid progress required by funders to address the aggressive timelines set. Therefore these groups need an element of both the leadership and core staff to have Biopharma drug discovery experience, recognising that the key driver is the discovery of drugs and not solely publication. With this core team in place, inexperienced post docs can be incorporated and trained by initially contributing through their discipline based experience. PhD students can benefit by carrying out projects which can synergise with the ongoing projects and experienced staff, but which are not part of the critical path, rate limiting aspects of the projects. Such PhD projects could include the development of novel chemistry, informatics and computational programs, novel screening technologies and animal models for efficacy and toxicology; and library design and synthesis.
Although the key driver of drug discovery, the identification of hits, leads and preclinical candidates is alien to the academic measures of success, there are ways the sector can demonstrate its worth within the University environment. Despite the potential delay in the production of publications, which could be caused by the need for confidentiality and to patent key findings, the sector will generate multiple opportunities to publish. Such opportunities include novel methodology, such as assays and computational programs; hit discovery campaigns; medicinal chemistry projects; target validation studies and through their contribution to the basic research of academic collaborators. Indeed, terminated drug discovery projects can be readily returned to the appropriate academic collaborator to enable the necessary studies for publication. The established measures of drug discovery success will deliver funding from bodies administering translational grants, satisfying the financial imperative of Universities. In addition, academic research is under growing pressure to demonstrate its impact on the wider community and economy. Certainly this sector has the ability to impact on global health and to the wealth of regions by tackling crippling diseases such as the neglected diseases of the developing world. Further, the development of potential treatments for diseases creates significant publicity for the host University and a good image for research within the sector.
To ensure a continuing working relationship with academic partners, the University based drug discovery groups will have to devise ways of sharing any income generated from the commercialisation of projects. This will have to take into account the contributions to projects both intellectually and financially and move away from the model of rewarding just those who are named on patents. This will require a split of reward between the target originating laboratory, the drug discovery group, the University and those funding bodies who require also a share in the financial gain.
5.2 Engaging with Biopharma
Academic drug discovery despite all its potential benefits and apparent advantages cannot succeed in isolation. It still needs large pharmaceutical corporations and public private partnerships to make sure it can deliver its successes ultimately to the patient. One obvious mechanism for interplay between the sectors is in the out-licensing or joint venture development of targets and molecules (mostly leads) from academia. As a result of the current unravelling pipelines there are significant in-licensing opportunities at all points of the drug discovery and development process. Universities should however be cautious about being seduced by the potential for large sums paid for in-licensing of promising drug development candidates for two reasons: 1) the inevitable attrition associated with working in areas of innovation means that this will hardly ever cover the total cost of investment and 2) focussing on Biopharma’s needs solely may result in the confounding situation of academic drug discovery acting in competition with, and ultimately just replicating Biopharma in another setting.
Academic drug discovery can also benefit hugely from early intervention and collaboration with industry. Although Pharma is seeking to engage with academic groups [14, 32, 33] industry (as a whole) continues to be highly reluctant to release tools and knowledge, such as proprietary compounds to help advance progress. Biopharma companies can play a major part in kick starting this sector through multiple mechanisms, such as direct funding to allow early phase targets to be progressed into hit discovery, the release of compounds to help validate targets, the release of data sets to allow the development of novel computational and informatics methodology and supporting secondments to aid knowledge transfer. Possible gains from such extended collaborations are being lost due to fear of missing intellectual property rights on compounds which might be screened many years in the future. Building successful and professional academic drug discovery centres with a track record of success should help to redress the balance in favour of the release of proprietary compounds and technology to develop new targets and assets now, rather than holding on for an imaginary future. Fortunately, with the increasing adoption of open innovation activities by many Pharmaceutical companies, [34] the landscape is beginning to change. SAGE Bionetworks previously mentioned in this article [28] was initiated by comprehensive data and analysis tools donated by Merck. In 2009 Eli Lilly announced the Phenotypic Drug Discovery Initiative, which makes their assays and expertise available to academic institutions [35]. In the same year GSK created a patent pool, which aims to remove IP as a barrier to research into treatments for neglected diseases [36] and in 2010 released their malaria screening data to the community [37].
5.3 Sustainable Funding
It is critical to appreciate that philanthropic/charitable and government funding will always be needed to sustain this sector and business plans for these organisations need to be realistic about the level of income likely from down stream out-licensing; particularly when diseases of the developing world and high risk targets are part of the portfolio. In some cases royalties from marketed drugs will provide an endowment to support an academic drug discovery operation e.g. MRC-T [38] but short and medium term strategies need to be in place to enable these eventual successes. It is clear that funding bodies are already stimulating academic drug discovery – it is estimated that approximately £50 million is invested in this sector per year in the UK alone (UKDDC personal communication)-and this will need to be built upon over the next decade to make sure that this sector has the ability to fulfil its obvious potential. Collectively government, charities and philanthropy can deliver a new generation of leads, and in some cases, preclinical candidates across a range of therapeutic areas but realistically industry will still be expected to fund the best projects through to registration. The serious problem of development costs is, of course, more acute for diseases of the developing world where entirely new funding mechanisms are required to get new drugs to patients [39].
6. Final Words
This sector will not solve the enormous scientific, ethical and commercial problems of discovering new drugs on it own but it does has the potential to significantly change how drug discovery might work in the future. Major success in academic discovery research may encourage Pharma corporate management to gradually reduce discovery research such that it may one day largely inhabit pre-competitive space. Pharma could then play to its enormous and unique strengths in development of new formulations of existing drugs and analogues with improved properties, improvements in scale-up methods as well as manufacturing, distribution, marketing, and selling of virtually all products.
7. Expert Opinion
The rationale for creating a 3rd mechanism for drug discovery which works linking Biopharma with fundamental research is thoroughly compelling and, working in this sector for the past 5 years has only served to convince us of its feasibility and enormous potential value. The Drug Discovery Unit at Dundee is a living example of how, with relatively modest investment, a vital and effective organisation can develop and deliver impact in a short period of time. These operations are best kept lean and efficient covering the fundamental skills of quantitative in vitro biology, computational and medicinal chemistry, DMPK, pharmacology and project management, creating an ideal skill set to support a robust portfolio. However, to prosper they need support from senior management of the University and dedicated buy-in from one or two initiating academic groups. They also need to develop and maintain a culture which is distinct from both conventional academia and industry and often through self-selection tend to be populated with ambitious, open-minded drug discovery scientists who want to ‘do things differently’. This difference can come in many forms: developing novel methodologies, using existing approaches to tackle innovative targets or simply addressing targets and diseases not addressed comprehensively by other organisations. For Universities considering developing a drug discovery effort the primary advice is to align these operations with the academic strengths of the organisation in question. In addition, the group has to be properly managed not only to enable hard decisions over project termination but also to take on and embrace high risk targets which struggle to get funding through the established funding routes. Furthermore the traditional discipline based department structure has to be broken down to allow chemistry to be embedded to enable progression of projects in a meaningful and timely manner. It is also important to not restrict academic drug discovery’s ambitions just to critical path progression of targets through discovery but also to allow it to address the challenges the industry continues to face in improvements in compound design, pharmacokinetics, human versus animal toxicology and the search for ever more relevant disease models. Importantly academic drug discovery offers a new, credible career track for both experienced and future discoverers and will help in protecting from the permanent loss of a vital experience base caused by the current rationalisation of the pharmaceutical industry. With the emergence of open innovation models and the recognition by Pharma that they need to change their R&D operating model- the time has never been better for academia to take their place on the drug discovery stage.
Abbreviations
- FDA
Food and Drug Administration
- DMPK
Drug Metabolism and Pharmacokinetics
- MRC
Medical Research Council
- NIH
National Institute for Health
- MMV
Medicines for Malaria Venture
- CRUK-CCT
Cancer Research UK Centre for Cancer Therapeutics
- MRC-T
Medical Research Council Technology
Footnotes
Declaration of Interest:
JF and PW are supported by the University of Dundee as well as grants from the Wellcome Trust (grant number 077705), MRC, Scottish Enterprise, the Medicines for Malaria Venture (MMV) and the Drugs for Neglected diseases initiative (DNDi). PW is also supported by the Wellcome Trust, MMV and DNDi.
References
- 1.Hughes B. Nat. Rev. Drug Discovery. 2008;7:107–109. doi: 10.1038/nrd2514. [DOI] [PubMed] [Google Scholar]
- 2.Who will develop the next generation of medications for mental illness? NIMH Directors Blog. Mar 30, 2010. Available at http://www.nimh.nih.gov/about/director/2010/who-will-develop-the-next-generation-of-medications-for-mental-illness.shtml.
- 3.Cromelli D, Stolk P, Besançon L, et al. Pharmaceutical Sciences in 2020. Nat Rev Drug Discov. 2010;9:99–100. doi: 10.1038/nrd3087. [DOI] [PubMed] [Google Scholar]
- 4.Frantz S. How academia can help drug discovery. Nat. Rev. Drug Discovery. 2004;3:541. doi: 10.1038/nrd1462. [DOI] [PubMed] [Google Scholar]
- 5.Kozikowski AP, Roth B, Tropsha A. Why academic drug discovery makes sense. Science. 2006;313:1235–6. doi: 10.1126/science.313.5791.1235c. [DOI] [PubMed] [Google Scholar]
- 6.Lazo JS, Brady LS, Dingledine R. Building a pharmacological lexicon: small molecule discovery in academia. Mol Pharmacol. 2007;72:1–7. doi: 10.1124/mol.107.035113. [DOI] [PubMed] [Google Scholar]
- 7.Frearson JA, Collie IT. HTS and hit finding in academia--from chemical genomics to drug discovery. Drug Discov Today. 2009;14:1150–8. doi: 10.1016/j.drudis.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medicines for Malaria Venture – Joint Portfolios. Available at http://www.mmv.org/article.php3?id_article=424&recherche=Broad%20Institute.
- 9.Drug discovery at Dundee Wellcome Trust News. 2005. Available at http://www.wellcome.ac.uk/News/Media-office/Press-releases/2005/WTX027342.htm.
- 10.Hughes B. A Wellcome experiment in seeding drug discovery. Nat Rev Drug Discovery. 2010;9:178–180. doi: 10.1038/nrd3130. [DOI] [PubMed] [Google Scholar]
- 11.Cuatrecasas P. Drug discovery in jeopardy. J. Clin. Invest. 2006;116:2837–2842. doi: 10.1172/JCI29999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Reviews Drug Discovery. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- 13.Kimball TR, McCoy CE, Khoury PR, et al. AmericanHeart Association website Obesity, diabetes damage young arteries, could shorten life. 2009. News release. [online] Available at http://americanheart.mediaroom.com/index.php?s=43&item=740)
- 14.Gray NS. Drug discovery through industry-academic partnerships. Nat Chem Biol. 2006;2:649–53. doi: 10.1038/nchembio1206-649. [DOI] [PubMed] [Google Scholar]
- 15.Melese T, Lin SM, Chang JL, Cohen NH. Open innovation networks between academia and industry: an imperative for breakthrough therapies. Nat. Med. 2009;15:502–7. doi: 10.1038/nm0509-502. [DOI] [PubMed] [Google Scholar]
- 16.Vallance P, Williams P, Dollery C. The future is much closer collaboration between the pharmaceutical industry and academic medical centers. Clin Pharmacol Ther. 2010;87:525–7. doi: 10.1038/clpt.2010.29. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682–90. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 18.Kaji K, Norrby K, Paca A, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–5. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frearson JA, Brand S, McElroy SP, et al. N-myristoyltransferase inhibitors as new leads to treat sleeping sickness. Nature. 2010;464:728–32. doi: 10.1038/nature08893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erik De Clercq Highlights in the Discovery of Antiviral Drugs: A Personal Retrospective. J. Med. Chem. 2010;53:1438–1450. doi: 10.1021/jm900932g. [DOI] [PubMed] [Google Scholar]
- 21.Kneller R. National origins of new drugs. Nat. Biotechnol. 2005;23:655–6. doi: 10.1038/nbt0605-655. [DOI] [PubMed] [Google Scholar]
- 22.Maxwell, R Eckhardt, Drug Discovery: a Casebook and Analysis. Humana, Totowa; New Jersey: 1990. [Google Scholar]
- 23.Gilead Sciences and Royalty Pharma Announce $525 Million Agreement with Emory University to Purchase Royalty Interest for Emtricitabine. Gilead Sciences Press; Jul, 2005. Release. Available at http://www.gilead.com/pr_731707. [Google Scholar]
- 24.New Emory Institute for Drug Discovery Combines Research Training, Global Partnerships News Release: Research , University News. May 19, 2009. Available at http://shared.web.emory.edu/emory/news/releases/2009/05/emory-institute-for-drug-discovery.html.
- 25.Yale University To Expand Medical and Scientific Research Programs with Acquisition of Bayer Complex. Yale University Press; Jun, 2007. Release. Available at http://opa.yale.edu/news/article.aspx?id=1587. [Google Scholar]
- 26.U-M to buy Pfizer’s former Ann Arbor property. University of Michigan Press; Jan, 2009. Release. Available at http://www.ur.umich.edu/0809/Dec15_08/29.php. [Google Scholar]
- 27.Woosley RL, Myers RT, Goodsaid F. The Critical Path Institute’s approach to precompetitive sharing and advancing regulatory science. Clin Pharmacol Ther. 2010;87:530–3. doi: 10.1038/clpt.2010.27. [DOI] [PubMed] [Google Scholar]
- 28.Friend SH. The need for precompetitive integrative bionetwork disease model building. Clin Pharmacol Ther. 2010;87:536–9. doi: 10.1038/clpt.2010.40. [DOI] [PubMed] [Google Scholar]
- 29.MRC’s Translational Research Strategy. 2008. http://www.mrc.ac.uk/consumption/groups/public/documents/content/mrc004551.pdf.
- 30.NIH Roadmap for Medical Research. http://nihroadmap.nih.gov/
- 31.Dundee collaboration to identify drug candidates to treat visceral leishmaniasis. University of Dundee press; 2009. release Available at http://www.dundee.ac.uk/pressreleases/2009/prjune09/candidates.htm. [Google Scholar]
- 32.Tralau-Stewart CJ, et al. Drug discovery: new models for industry-academic partnerships. Drug Disc. Today. 2008;14(1/2):95–101. doi: 10.1016/j.drudis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 33.More pharma–academia R&D collaborations News & Analysis. Nat Rev Drug Discovery. 2010 Apr;9:259. [Google Scholar]
- 34.Hunter J, Stephens S. Is open innovation the way forward for big pharma? Nat. Rev. Drug Discovery. 2010;9:87–8. [Google Scholar]
- 35.The Lilly Phenotypic Initiative Connecting compounds to patients. Available at https://pd2.lilly.com/pd2Web.
- 36.Drug patent plan gets mixed reviews. Available at http://www.nature.com/news/2009/090225/full/4571064a.html. [DOI] [PubMed]
- 37.Gamo F-J, Sanz LM, Cristina de Cozar JV, et al. Thousands of chemical starting points for antimalarial lead identification. Nature. 465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 38.Multi-Million Dollar Deal to Benefit Medical Research. The Scripps Research Institute Press; Oct, 2005. Release. Available at http://www.scripps.edu/news/press/102605.html. [Google Scholar]
- 39.Herrling PL. Financing R&D for neglected diseases. Nat Rev Drug Discov. 2009;8:91. doi: 10.1038/nrd2818. [DOI] [PubMed] [Google Scholar]


