Abstract
The progressive degradation of resin-dentin bonds is due, in part, to the slow degradation of collagen fibrils in the hybrid layer by endogenous matrix metalloproteinases (MMPs) of the dentin matrix. In in vitro durability studies, the storage medium composition might be important because the optimum activity of MMPs requires both zinc and calcium.
Objective
This study evaluated the effect of different storage media on changes in matrix stiffness, loss of dry weight or solubilization of collagen from demineralized dentin beams incubated in vitro for up to 60 days.
Methods
Dentin beams (1×2×6mm) were completely demineralized in 10% phosphoric acid. After baseline measurements of dry mass and elastic modulus (E) (3-point bending, 15% strain) the beams were divided into 5 groups (n=11/group) and incubated at 37°C in either media containing both zinc and calcium designated as complete medium(CM), calcium-free medium, zinc-free medium, a doubled-zinc medium or water. Beams were retested at 3, 7, 14, 30, and 60 days of incubation. The incubation media was hydrolyzed with HCl for the quantitation of hydroxyproline (HOP) as an index of solubilization of collagen by MMPs. Data were analyzed using repeated measures of ANOVA.
Results
Both the storage medium and storage time showed significant effects on E, mass loss and HOP release (p<0.05). The incubation in CM resulted in relatively rapid and significant (p<0.05) decreases in stiffness, and increasing amounts of mass loss. The HOP content of the experimental media also increased with incubation time but was significantly lower (p<0.05) than in the control CM medium, the recommended storage medium.
Conclusions
The storage solutions used to age resin-dentin bonds should be buffered solutions that contain both calcium and zinc. The common use of water as an aging medium may underestimate the hydrolytic activity of endogenous dentin MMPs.
Keywords: Dentin, Matrix metalloproteinases, storage solution, elastic modulus, zinc, calcium
1. Introduction
When the durability of resin-dentin bonds are tested in vitro, the commonly used method is to soak the bonded teeth in 37°C water for 24 hrs and then section the teeth in the x and y planes into 1 mm2 beams. This preparation is considered a form of accelerated aging because water needs only to diffuse 0.5 mm from each cut surface to reach the center of the beam [1]. Water also diffuses into the adhesive and causes water sorption, plasticization of the polymers and solubilizes unreacted monomers [2]. The other degradative mechanism in resin-dentin bonds is caused by the acid-etching step in bonding that uncovers and activates endogenous dentin matrix metalloproteinases (MMPs) [3-5] These enzymes are neutral proteases that add water across specific peptide linkages in collagen peptides [6]. Metalloproteinases require calcium and zinc ions to maintain their proper tertiary structure and functional active sites, respectively [6]. These ions, that are normally constituents of body fluids [7,8] may be lost during acid-etching and water rinsing. If the resin-bonded dentin beams are incubated in water rather than calcium and zinc containing buffers, the influence of MMPs on matrix degradation in vitro may be underestimated. Another important variable in the durability of resin-dentin bonds is how well the adhesive monomers infiltrate the acid-etched matrix which is regarded as a technique-sensitive procedure [9]. Recently, a simple model to study the rate of degradation of demineralized dentin matrices independent of resin, was developed by making 6×2×1 mm beams of coronal dentin, and then completely demineralizing them in 10% phosphoric acid [10]. By using demineralized beams devoid of resin, the decrease in their modulus of elasticity by 3-point flexure before and after 30 days of incubation in experimental media at 37°C can be measured. By not infiltrating them with resin, uncertainty about how well the collagen fibrils were encapsulated could be eliminated [10]. When the beams are incubated in experimental media containing calcium and zinc at 37°C for 30-60 days, if the MMPs are active, they will slowly solubilize collagen peptide fragments that will accumulate in the media and can be collected and quantitated for collagen release.
The purpose of this in vitro study was to test the null hypothesis that there are no differences in matrix stiffness, loss of dry weight or solubilization of collagen from demineralized dentin matrices when incubated in water, in complete media or media deficient in calcium or zinc. The activity of endogenous dentin matrix MMPs was assessed indirectly by measuring the modulus of elasticity of demineralized beams before and after incubation for up to 60 days at 37°C. Solubilization of collagen was quantitated gravimetrically by measuring the loss of dry mass over time and by measuring the amount of the hydroxyproline that was released from the solubilized collagen into the incubation media.
2. Materials and methods
2.1 Preparation of dentin disks
Fifty-five extracted human third molars were obtained with patient's informed consent under a protocol approved by the Human Assurance Committee of the Medical College of Georgia. The teeth were stored at 4°C in 0.9% NaCl supplemented with 0.02% sodium azide for no more than one month before use. The enamel and superficial dentin of each tooth were removed from the crown using a diamond-encrusted copper disk (Isomet saw, Buehler Ltd., Lake Bluff, IL, USA), by a horizontal section 1 mm below the deepest central groove. This removes the high concentration of MMP-2 reported to reside at the dentinoenamel junction [11]. One millimeter thick dentin disks of mid-coronal dentin were then prepared by moving the blade slightly more than 1 mm apical to the first section. This second section removed the deep dentin/predentin source of MMP-2 [11] and yielded mid-coronal dentin that has been shown to have a relatively constant distribution of MMPs [11,12]. Dentin beams 6 × 2 × 1 mm were sectioned from each dentin disk to obtain fifty-five beams.
For demineralization, the beams were submerged in 10 wt % phosphoric acid for 18 hrs at 25 °C. Digital radiography was used to document the absence of residual minerals. The initial modulus of elasticity of the beams was measured by 3-point flexure to 15% strain. Previous work showed no plastic deformation of the beams at 15% strain [10]. The initial modulus values also confirmed complete demineralization, since mineralized beams were previously shown to have moduli of elasticity of 16-19 GPa. Beams accepted as demineralized had moduli of elasticity <5 MPa [10]. After initial modulus of elasticity measurements, the beams were distributed to five balanced groups (n=11/group) so that the mean initial elastic modulus of each group was statistically similar in all groups.
2.2 Experimental design
Five incubation media were used: Group 1) complete media (CM) containing 5 mM HEPES, 2.5 mM CaCl2.H2O, 0.05 mM ZnCl2, and 0.3 mM NaN3, pH 7.4 (Table 1); Group 2) a calcium- free modified media (Calcium-free) containing all the other compounds except for CaCl2; Group 3) a zinc-free modified media (Zinc-free) containing all the other compounds except ZnCl2; Group 4) a double-zinc media (Double-zinc) containing all the other compounds, except that the concentration of ZnCl2 which was doubled (0.1 mM); Group 5) distilled water containing 0.3 mM NaN3 (Water).
Table 1.
Electrolyte composition of unsimulated whole saliva vs. simulated oral fluid vs. plasma.
| Whole salivaa | Complete medium (simulated oral fluid)b |
Plasmac | |
|---|---|---|---|
| Sodium | 4-6 | 0 | 145 |
| Potassium | 14 | 0 | 4 |
| Calcium | 1.5-4 | 2.5 | 2.5 |
| Magnesium | 0.07 | 0 | 1.5 |
| Chloride | 11.9 | 0 | 10.5 |
| Phosphorus | *3.6 | 0 | 1.5 |
| Bicarbonate | 1.0 | 5 mM HEPES | 25 |
| Fluoride μg% | 8-25 μg/100 mL | 0 | 10-20 |
| Iodide μg% | 4-24 μg/100 mL | 0 | 3-8 |
| Zn | ? | 0.05 | 0.02 |
| NaN3 (antimicrobial) | 0 | 0.3 mM | 0 |
3.6 mM phosphate should be added to simulated oral fluids if mineralized tissues are to be incubated in the solution for more than 24 h;
from Dawes (1974;
current paper;
Each beam was separately incubated in one of the five incubation media, in individually labeled propylene tubes with threaded caps containing 1 mL of incubation media. The tubes were placed in a shaking water bath (Reciprocal Shaking Bath, Thermo Scientific, Marietta, OH, USA) (60 cycles/min) at 37°C for 3, 7, 14, 30, 60 days of incubation.
2.3 Indirect assessment of the activity of the endogenous MMP activity in demineralized matrices
2.3.1 Loss of modulus of elasticity
Demineralized dentin beams were placed on the 3-point flexure jig with a 2.5 mm separation between supports, immersed under water. Using a 1N load cell (Transducer Techniques, Temecula, CA, USA) mounted on a Vitrodyne universal tester (Model V1000, John Chatillon & Sons, Greensboro, NC, USA), the beams were deformed to a 15% strain at a displacement rate of 0.5 mm min−1. After maximum displacement, the load was returned immediately to 0, to prevent creep of the demineralized collagen matrix [13,14]. After initial testing, the beams were arranged into five balanced groups so that their mean values were not statistically different. They were then incubated in the control or experimental media in designated polypropylene tubes, and then retested after each incubation period. At every time point, the beams were rinsed free of salts for 10 min with running distilled water, and then retested in 3-point flexure to the same degree of strain.
The modulus of elasticity (E; in MPa) of each specimen was calculated as the steepest slope of the linear portion of the stress-strain curve using the following formula:
where m is the steepest slope of the linear portion of the load-displacement curve (N/mm), L is the span length (2.5 mm), b is the width of the test specimen (2.0 ± 0.1 mm) and h is the beam thickness (1.0 ± 0.05 mm).
2.3.2 Loss of dry mass over time
After the measurements of the initial modulus of elasticity, the beams were transferred to individually labeled polypropylene tubes and placed in a sealed plastic box containing anhydrous calcium sulfate (Drierite, W.A. Hammond Drierite Company, Xenio, OH, USA). With the vial caps off, the beams were desiccated to a constant weight within 8 hrs. The initial dry mass was measured to the nearest 0.001 mg on an analytical balance (Mettler XP6 Microbalance, Mettler Toledo, Hightstown, NJ, USA). After dry mass measurement, dried dentin beams were reimmersed in water and placed back in its corresponding polypropylene tube containing the original incubation medium. After each incubation period, determination of the dry mass was repeated under the same conditions following the modulus of elasticity measurements.
2.3.3 Analysis of incubation media for solubilized collagen peptides
The collagen in demineralized dentin is insoluble type I collagen. Demineralization of the matrix uncovers the endogenous MMPs and activates them even though they remain bound to collagen [15]. If the MMPs are incubated in media that contain calcium and zinc [3], the enzymes can slowly solubilize collagen peptide fragments that accumulate in the 1 mL of test media over the 30-60 day experiment [10]. At the end of each incubation period, 400 μL of the media was collected from each vial to an individually labelled ampule, diluted with an equal volume of 12 N HCl to give a final concentration of 6 N HCl. Ampules were sealed using an ampule sealer (Ampulmatic, Biosciences Inc, PA, USA). The sealed ampules were hydrolyzed at 120 °C in an oil bath for 18 hrs. After hydrolysis, the ampules were opened and placed in large glass desiccators containing anhydrous calcium sulfate and trays of sodium hydroxide pellets used to trap the HCl vapor that was released as the hydrolyzate evaporated to dryness in a mild vacuum. When dry, the hydroxyproline content of the hydrolyzate was analyzed using the method of Jamall et al. [16] in a spectrophotometer (Model UV-A180, Shimadzu, Tokyo, Japan) at 558 nm. The measured amount of hydroxyproline was used to estimate the percent of solubilized (i.e. degraded) collagen assuming that 90% of the dry mass of the demineralized dentin beams consisted of type I collagen [17] and that the dentin collagen contains 9.6 mass % of hydroxyproline [17]. For each specimen, the solubilized collagen from demineralized dentin beam was expressed as μg of hydroxyproline/mg of the dry mass of the demineralized dentin before the incubation for each incubation period.
2.4. Statistical analysis
Modulus of elasticity, dry mass loss and HOP data were analyzed separately. Repeated measures analysis with “storage media” as the group variable and “timepoint” (3 d, 7 d, 14 d, etc.) as the repeated factor was used to analyze the data. Repeated measures analysis of variance (ANOVA) was used to test for interaction between the group and timepoint factors and, if no significant interaction was found, to test the main effects for group and timepoint. If significant interaction was found, then the five groups were compared separately at each timepoint and timepoints were compared separately for each group. If either the normality or equal-variance assumptions underlying the traditional repeated measures ANOVA tests were violated, rank-based ANOVA tests were used. The Tukey-Kramer method of pair-wise comparisons for repeated measures designs was used to evaluate the significance of the difference between each pair of groups and between each pair of timepoints. A significance level of 0.05 was used for all other statistical tests. All analyses were carried out using SAS 9.2 for Windows (SAS Institute Inc., Cary, NC, 2008).
3. Results
For repeated measures analysis, comparison of several possible covariance patterns for the repeated factor “timepoint” indicated that “unstructured” provided the best fit to the data, according to Schwartz Bayesian Information Criterion. This covariance pattern was assumed throughout all remaining analyses.
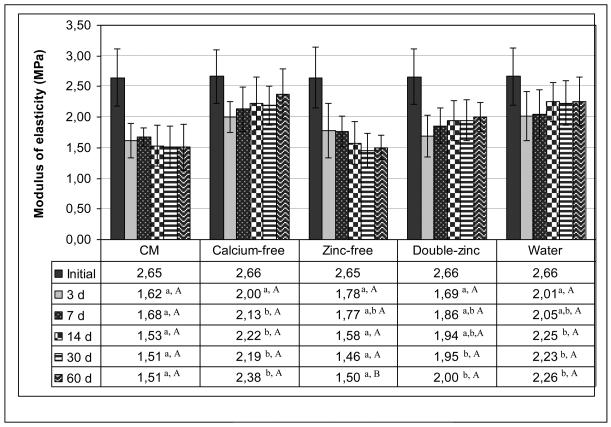
The changes in the elastic moduli (E) of the beams in time are shown in Fig. 1. All groups initially exhibited a mean E of 2.66 (±0.01) MPa. Since the data were not normally distributed, rank-based methods were used. Analysis of covariance was used to adjust all analyses for the initial modulus values. Both the storage media and incubation time showed significant effects on E (p<0.001) and also the interaction between the factors were significant (p<0.001), so each storage medium was compared to each other (Fig. 1) and each time point was compared to each other (Fig. 1). After 3 days of incubation, the control CM group showed the highest loss in E (39%). After this reduction in E, the stiffness of this group remained relatively constant over the rest of the incubation period. In terms of comparison of individual groups at each time point, at 3 days none of the groups were statistically different from each other, and at 7 days, only CM group and calcium-free group were statistically different (Fig. 1). At 14 days, 30 days and 60 days, CM and zinc-free groups each differed significantly from calcium-free, doubled-zinc and water groups. However, CM and zinc-free groups were not statistically different from each other, nor were the calcium-free, doubled-zinc and water groups different from each other (Fig. 1).
Fig. 1.
Changes in the modulus of elasticity of control (CM) vs calcium-free, zinc-free, double-zinc media and water incubated beams over 60 d. In each row of the table different lowercase letters are significantly different groups at each time point (p<0.05, Tukey-Kramer method). In each column, the capital letters show the comparison of time points of each group. The different capital letters in each column are significantly different time points at each group (p<0.05, Tukey-Kramer method). N = 11 per group. Height of bar is mean value. Brackets indicate ± SD.
In the zinc-free modified group, the E modulus fell slowly over time, ending up at the same level of the CM control group. In the double-zinc group, the mean E modulus was similar to the calcium-free and water groups. In the water group, the 3-day E-modulus was lower than the initial value, but there was no significant differences (p>0.05) detected when the specimens were measured at subsequent incubation times (Fig. 1).
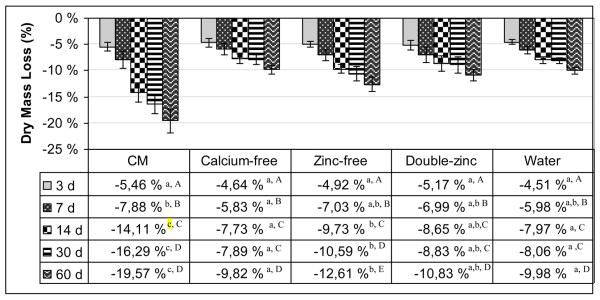
The changes in the dry mass loss over time are shown in Fig. 2. Since the data for several combinations of group and time were not normally distributed, rank-based methods for repeated measures analysis were used. Both the storage media and the time showed significant effect on mass loss (p<0.001) and also the interaction between the factors were significant (p<0.001), so each storage medium was compared to each other and each of the groups compared separately for each timepoint.
Fig. 2.
The loss of dry mass (%) of control (CM) vs calcium-free, zinc-free, double-zinc media and water incubated beams over 60 d. In each row of the table different lowercase letters are significantly different groups at each time point (p<0.05, Tukey-Kramer method). In each column, the capital letters show the comparison of time points of each group. The different capital letters in each column are significantly different time points at each group (p<0.05, Tukey-Kramer method). N = 11 per group. Length of bar is mean value. Brackets indicate ± SD.
The control CM group showed a gradual, increasing loss of dry mass of 5.5 wt% after 3 days, 7.9% after 7 days, 14% after 14 days, 16.3% after 30 days and 19.6% after 60 days. Smaller mass losses (ca 8%) were seen in the calcium-free group than in the zinc-free group after 30 days. The mass loss in the calcium-free and double-zinc and water groups were about the same and were significantly lower than the CM control group (p<0.05) after 60 days (Fig. 2). In terms of comparisons of individual groups at each timepoint, at 3 days, none of the groups were significantly different from each other (Fig. 2). At 7 days, none of the groups were significantly different from each other except CM and calcium-free groups. At 14, 30 and 60 days, CM group differed significantly from each of the other groups. In addition, the zinc-free group differed significantly from each of the other groups except double-zinc group. The calcium-free, doubled-zinc and water groups did not differ significantly from each other.
In terms of comparisons of the time points in each group, in control CM group, each of the timepoints differed significantly from each other, with the exception of 30 and 60 days (Fig. 2). In calcium-free, doubled-zinc and water groups, all the timepoints significantly differed from each other, with the exception of 14 and 30 days. In zinc-free group also all the time points differed significantly from each other.
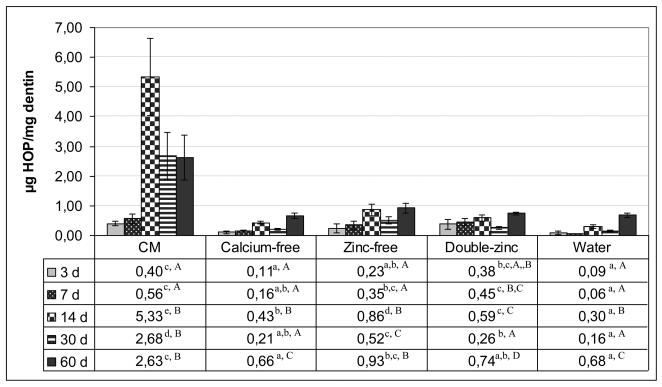
The release of HOP over time is shown in Fig. 3. Since the data of several combinations of group and timepoint were not normally distributed, rank-based methods for repeated measures analysis were used. Both the storage media and incubation time showed significant effects on HOP release (p<0.001) and also the interaction between the factors were significant (p<0.001), each storage medium was compared to each other, and each of the groups compared separately for the various time points. When the incubation medium was hydrolyzed to release all of the amino acids from any solubilized collagen peptides, the CM control group gradually released more and more HOP for the first two weeks reaching a maximum of about 5.3 μg of HOP/ mg dry weight of the matrix after 14 days. The rate of release of solubilized collagen then fell in half for the next 30-60 days. In terms of comparison of the timepoints in CM group, the 3 and 7 day values did not differ significantly from each other, but were significantly lower than the 14, 30 and 60 day values. Similarly, the 14, 30 and 60 day values did not differ from each other (Fig. 3). All other incubation groups (i.e. calcium free, zinc-free, double zinc and water) released less hydroxyproline than the CM group, indicating that the intrinsic MMP activity of dentin matrix was not operating at their optimal level. All these groups librated between 0.5-0.9 μg hyroxyproline/mg of dentin matrix at each time point. The cumulative release of HOP from CM group over 60 days period (11.6 μg hydroxyproline/ mg of dentin) was 9 times higher than the cumulative release (1.29 μg hydroxyproline/ mg dentin) of water stored group.
Fig. 3.
The μg of hydroxyproline released per mg of dentin at each time point over 60 days incubation. In each row of the table different lowercase letters are significantly different groups at each time point (p<0.05, Tukey-Kramer method). In each column, the capital letters show the comparison of time points of each group. The different capital letters in each column are significantly different time points at group (p<0.05, Tukey-Kramer method). N = 11 per group. Height of bar is mean value. Brackets indicate ± SD.
4. Discussion
This in vitro investigation evaluated the effect of the composition of storage medium on the matrix stiffness, dry mass and release of hydroxyproline from collagen solubilized from the demineralized dentin matrices over a 60 day period.
When the changes in the stiffness of the matrix of various groups were compared (Fig. 1), the highest stiffness was obtained in the calcium–free Group 2, followed by the water group (Group 5). This suggests that the MMPs in the matrix specimens of these groups were least active. This result indicates that medium calcium is more important for MMP activity than medium zinc. We speculate that zinc is more firmly bound to the matrix than is calcium. The fact that the media containing no zinc and media containing twice normal zinc gave similar stiffness over time suggests that the collagen matrix retained enough zinc to maintain MMP activity regardless of the medium zinc concentration.
The significant decrease in stiffness of dentin matrices in all incubation groups except calcium-free group indicates that endogenous MMPs were cleaving collagen peptides over time in all groups albeit in different rates. Previous work using an identical dentin matrix model showed that incubation of demineralized beams in 2% chlorhexidine, a known MMP-inhibitor [18], prevented the decrease in E modulus and solubilization of collagen over 30 days [10]. That positive control revealed that matrix-bound endogenous MMPs are responsible for slow degradation of dentin matrices over time.
True collagenases such as MMP-8, cleave collagen peptides into ¼ and ¾ fragments [19,20]. When the media in the control groups in the current study were subjected to SDS-PAGE analysis, no ¼ or ¾ fragments could be detected (data not shown). Dentin collagen is highly crosslinked between peptide chains so that these peptide fragments may not solubilize as much as expected because they remain tethered by interpeptide cross-links which are not susceptible to collagenase activity. The significant decrease in E-modulus of most groups within 3 days suggests that the MMP activity is initially relatively rapid. Peptide chain scission within 3 days significantly lowered the stiffness of matrix (p<0.05). The slower subsequent decreases in stiffness are interpreted as being due to cleaveage of less readily accessible peptide bonds.
Solubilization of dentin matrix over time was measured indirectly as a loss of dry weight. The loss of dry weight over time in all groups was similar over the first 3-7 days but then accelerated in the control group (CM group) by 14 days to become significantly greater (p<0.05). All other groups lost only around half as much dry weight as the control group. Since 90% of the dentin matrix is type I collagen [21], most of this weight loss must have been due to the solubilization of previously insoluble collagen by MMPs.
Removal of calcium or zinc or all ions from the media (i.e. water) did not prevent the loss of dry mass from the matrix. We speculate that zinc remains firmly bound to the demineralized matrix regardless of the composition of the media. Even though a larger volume of incubation medium could have been used to dilute any residual calcium or zinc ion concentrations from acid-etched beams that would have also diluted the concentration of solubilized collagen in the medium.
When solubilized collagen was hydrolyzed to amino acids, hydroxyproline analyses clearly showed that specimens incubated in the control CM released significantly more collagen than any other group (p<0.05, Fig. 3). It is likely that MMP-8 bound to the matrix cleaved adjacent collagen peptides into ¼, ¾ fragments and that MMP-2 and 9 attacked the nonhelical fragments and reduced them to even smaller peptide fragments. Those collagen molecules closest to the any free surface would have the highest probability of releasing fragment of collagen peptides into the media. Collagen molecules located more toward the middle of the 1 mm thick beams might not be able to release their fragments to the surface. A recent report published about the size exclusion characteristics of type I collagen fibrils concluded that unmineralized type I collagen fibrils could exclude molecules larger than 40 KDa but were freely permeable to molecules <6000 Da [22]. Thus, when MMPs are degradating collagen peptides, those peptide fragments that are smaller than 6000 Da should be able to diffuse into the incubation medium, while those larger than 40 KDa will be restricted in their diffusion.
The results of this study demonstrated that demineralized dentin beams incubated in a complete medium lost more dry mass and solubilized collagen over time, than did similar beams incubated in calcium-free media, zinc-free media, doubled-zinc media or water. These results require the rejection of the null hypothesis that the composition of incubation media has no effect on matrix stiffness, loss of dry weight or solubilization of collagen.
The collagen-binding sites of MMPs are very close to the catalytic site of these enzymes [6]. We speculate that the endogenous MMPs in the dentin matrix are bound to collagen peptides via their hemopexin-like domains (MMP-1, -8) and/ or their fibronectin like domains (MMPs-2, -9). The presence of a propeptide in the MMPs contains a cysteine residue, which forms the coordination between the cysteine thiol with the zinc atom in the catalytic domain, and prevents MMPs from being active. Additionally, many MMPs are inactive due to the presence of tissue inhibitors of MMPs (TIMPs) that also inactivate these proteases. It is not clear if all endogenous dentin MMPs in dentin are inactivated by TIMPs. In mineralized collagen, the presence of apatite crystallites in and on collagen fibrils and their bound noncollagenous proteins (eg. growth factors, MMPs) probably sterically blocks access of collagen to the catalytic site of MMPs.
However, during the acid-etching step of dentin bonding, the apatite crystallites are removed from collagen and noncollagenous proteins. Acid also disrupts the cysteine-zinc coordination that is lost when the propeptide is treated with acid. This seems to activate MMP-2 [23]. MMP-2, in turn, may activate MMP-8 and -9 [19]. The stiffness of the collagen matrix falls from 18,000 MPa in the mineralized state to 1-3 MPa in demineralized state. This low modulus of elasticity permits much more rotational and lateral movement of adjacent collagen peptides, bringing them within reach of the active site of the MMPs. This permits MMP-8 to cleave the peptide bond immediately before a residue with a hydrophobic side chain, such as Leu, Ile, Met, Phe or Tyr. As the collagen hydrolysis continues, one would expect the number of unhydrolyzed nearby peptide bonds to become steadily depleted in the 3-dimesional space around each matrix-bound collagenase. This may be why the rate of solubilization of collagen peptides detected in the medium fell over time. Further, as collagen was cleaved, it may have increased the molecular mobility of the microfibrils bringing additional hydrolyzible peptide bonds within reach of the active site.
Alternatively, the hydrolytic activity of peptide bonds may cause MMPs to become solubilized from the collagen matrix, allowing the enzyme much more molecular mobility. However, the activated MMP-8 has a molecular weight of about 70 KDa. This relatively large size may trap it within water-filled space between microfibrils [15] until the collagen matrix begins to loosen up to the point that it can no longer exclude large molecular weight fragments. As helical portions of collagen are cleaved they may begin to unravel (i.e denature). This makes them susceptible to attack by gelatinase A and B (i.e. MMP-2 and -9).
A previous study reported the stability of EDTA-demineralized dentin matrices over 2 years of storage in buffer [24]. When 0.5 moles/L ethylenediaminetetricacid (EDTA) pH 7.4 is used to demineralize dentin, it does so by chelating the calcium ions from apatite crystallites. As calcium is lost, the apatite crystalline lattice dissolves because the ion product of calcium phosphate falls below the solubility product constant for apatite. Chealators such as EDTA preferentially chealate divalent cations. Thus, EDTA is also effective in chealating zinc. That is, EDTA inhibits MMPs by removing the calcium ions from three-dimensional structure of collagen and noncollagenous proteins, and by removing zinc from the catalytic side of the enzyme. Without zinc, MMPs are inactive. Therefore, the 2 year stability of EDTA-demineralized dentin matrix reported previously [24] was most probably due to the removal of both calcium and zinc from the matrix. When the demineralized specimens were incubated in a medium containing phosphate-buffered saline (PBS), there was sufficient calcium to maintain the proper 3-D configuration of MMPs bound to the matrix, but there was no zinc in PBS to allow the catalytic site of the MMP s to be active.
The durability of amalgam restorations has been attributed, in part, to the release of zinc ions from zinc-containing amalgams [25,26]. Those authors found that 15 μM ZnSO4 inhibited MMP-2 by 52 %. Their results also showed that 0.5 mM 1,10-phenanthroline, a divalent metal chealator, could completely inhibit MMP-2 activity. These observations seem paradoxical; although metalloproteinases require zinc, 15 μM zinc, a physiological concentration inhibited soluble MMP-2 by 52% and MMP-9 by 40% [25]. However, doubling the zinc concentration to 30 μM only increased the inhibition from 52 to 64.3%. When the authors doubled the zinc concentration again to 60 μM, the amount of zinc only increased the % inhibition by 13.1%. Obviously the inhibitory activity of zinc on soluble MMPs is not linear [26] and may depend on the calcium concetration of the medium. These results were obtained using purified rhMMPs. The dose-response between zinc concentrations and % inhibition of MMPs may be different for soluble MMPs versus matrix-bound MMPs in demineralized dentin because of the ability of collagen to bind zinc. The normal serum range of zinc is reported to be between 11.5-19.1μM [8]. Although it is possible that, complete demineralization of dentin beams in 10% phosphoric acid may have removed some of the endogenous zinc in dentin, those beams incubated in water for 30-60 days lost about the same dry mass and solubilized about the same amount of collagen as the zinc-free or double-zinc groups, arguing that the completely demineralized dentin beams may contain enough residual zinc to be partially active.
An alternative explanation for the presence of residual collagenolytic activity in the demineralized dentin beams in the various groups is the recent discovery of the presence of cysteine cathepsins in human dentin [27]. This class of proteases is known to be capable of hydrolyzing collagen. This speculation will be tested in future experiments by including E-64, specific inhibitor of cysteine proteinase, in the incubation medium.
Clearly, the results of this work indicate that demineralized dentin matrices degraded more rapidly in a medium containing 2.5 mM Ca and 0.05 mM Zn when the matrix was in direct contact with the media. Thus, the storage solutions used to age demineralized matrices and/or sticks of resin-dentin bonds should be incubated in buffered solutions that contain 2.5 mM calcium ions and 20 μM soluble zinc salts such as ZnCl2. Such a storage medium more closely simulates fluids than does water. The common use of water as an aging medium may underestimate the hydrolytic activity of endogenous dentin MMPs and should be discouraged because it would promote the loss of calcium and zinc ions from dentin matrices, rather than restore them.
Most simulated body fluids (SBF) attempt to approximate the composition of plasma (Table 1) [28]. The major ingredient of plasma is 0.14-0.15 M NaCl, giving such solutions an osmolarity of 280-300 mOsm/Kg that is isotonic with plasma and cells. That much sodium and chloride is sufficient to displace cationic and anionic ions, including Zn++ that electrostatically bind to ligands [29,30]. Even after adding glucose, buffer, calcium and phosphate ions, such solutions provide poor simulations of oral-fluids that are composed primarily of mixed unstimulated saliva. Mixed saliva has a low sodium (5 mM) and chloride (12 mM) concentration [31], giving it an osmolarity of 36 mOsm/kg. Its calcium concentration is 1.5-4 mM calcium. Saliva is almost glucose-free. The composition of an ideal simulated oral fluid depends upon its purpose. If it is to prevent demineralization or promote remineralization, it should contain between 1.5-2.5 mM CaCl2 and 1.5-3.6 mM inorganic phosphate, pH 7.4. No glucose is required unless it is used as a cellular medium. However, oral fluids are hypotonic (i.e. 36-50 mOsm/kg) rather than isotonic and would injure cells and dividing bacteria. In resin-enamel or resin-dentin bond durability studies, simulated oral fluids should also include at least 20 μM (0.02 mM ZnCl2). This value is based on normal plasma values, not unstimulated mixed salivary levels that are unknown. We used 50 μM zinc chloride because we anticipated collagen binding of zinc that might lower the medium concentration of half. Clearly, more research is required on the zinc content of mineralized acid-demineralised and EDTA-demineralized, normal and caries-affected dentin, as well as whole unstimulated versus stimulated saliva. Known amounts of these substances should be ashed in a muffle furnace and the ash analyzed for zinc by atomic absorption spectrophometry.
Future experiments on the durability of resin dentin bonds should include calcium and zinc free media as well as calcium and zinc containing media.
Acknowledgements
Supported, in part, by R01 DE015306-06 from the NIDCR to (P.I David H. Pashley) and by grant# 8126472 from the Academy of Finland to (PI. Arzu Tezvergil-Mutluay).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pashley DH, Carvalho RM, Sano H, Nakajima M, Yoshiyama M, Shono Y, Fernandez CA, Tay FR. The microtensile bond test: A review. J Adhes Dent. 1999;1:299–309. [PubMed] [Google Scholar]
- 2.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–22. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 4.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007;86:529–33. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S-C, Kern M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesive. Int J Oral Sci. 2009;1:163–76. doi: 10.4248/IJOS.09044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases. Structure, function and biochemistry. Cir Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert RJ, Ingram GS. The oral disposition of zinc following the use of an anticalculus toothpaste containing 0.5% zinc citrate. J Pharm Pharmacol. 1988;40:399–402. doi: 10.1111/j.2042-7158.1988.tb06303.x. [DOI] [PubMed] [Google Scholar]
- 8.Hedera P, Peltier A, Fink JK, Wilcock S, London Z, Brewer GJ. Myelopolyneuropathy and pancytopenia due to copper deficiency and high zinc levels of unknown origin: II. The denture cream is a primary source of excessive zinc. Neurotoxicity. 2009;30:996–99. doi: 10.1016/j.neuro.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto M, Tay FR, Svizero NR, DeGee A, Feilzer AJ, Sano H, Kaga M, Pashley DH. The effects of common errors on sealing ability of total etch adhesives. Dent Mater. 2006;22:560–68. doi: 10.1016/j.dental.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Carrilho MRO, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin stiffness associated with solubilization of collagen. J Biomed Mater Res Part B: Appl Biomater. 2009;90B:373–80. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boushell LW, Kaku M, Mochida Y, Bagnell R, Yamachi M. Immunohistochemical localization of matrix metalloproteinase-2 in human coronal dentin. Arch Oral Biol. 2008;53:109–16. doi: 10.1016/j.archoralbio.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzoni A, Pashley DH, Tay FR, Gobbi P, Orsini G, Ruggeri A, Jr, et al. Immunohistochemical identification of MMP-2 and MMP-9 in human dentin. Correlative FEI-SEM/TEM analysis. J Biomed Mater Res. 2009;88A:697–703. doi: 10.1002/jbm.a.31920. [DOI] [PubMed] [Google Scholar]
- 13.Balooch M, Wu-Magidi IC, Balazs A, Lundkvist AS, Marshall SJ, Marshall GW, et al. Viscoelastic properties of demineralized human dentin measured in water with atomic force microscope (AFM)-based indentation. J Biomed Mater Res. 1998;40:539–44. doi: 10.1002/(sici)1097-4636(19980615)40:4<539::aid-jbm4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Pashley DH, Agee KA, Wataha JC, Rueggeberg F, Ceballos L, Itou K, et al. Viscoelastic properties of demineralized dentin matrix. Dent Mater. 2003;19:700–06. doi: 10.1016/s0109-5641(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Delas Heras S, Valenzuela A, Overall CM. The matrix methalloprotaine gelatinase A in human dentin. Arch Oral Biol. 2000;45:757–65. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 16.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–5. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 17.Butler WT. Dentin collagen: Chemical structure and role in mineralization, Chap 8. In: Linde A, editor. Dentin and Dentinogenesis. II. CRC Press; Boca Raton: 1984. p. 40. [Google Scholar]
- 18.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8 and 9 by chlorhexidine. Clin Diag Lab Immunol. 1999;6:437–39. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massova I, Kotra L, Fridman R, Mobashery Matrix metalloproteinases, structure, evolution and diversification. FASEB J. 1998;12:1075–95. [PubMed] [Google Scholar]
- 20.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects of Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindhe A. Collagenous proteins and proteoglycans in dentinogenesis, Chapter 9. In: Lindhe A, editor. Dentin and Dentinogenesis. II. CRC press; Boca Raton: 1984. p. 56. [Google Scholar]
- 22.Toroian D, Lim JE, Price PA. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. J Biol Chem. 2007;282:22437–47. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- 23.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjäderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–27. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho RM, Tay FR, Sano H, Yoshiyama M, Pashley DH. Long-term mechanical properties of EDTA-demineralized dentin matrix. J Adhes Dent. 2000;2:193–99. [PubMed] [Google Scholar]
- 25.Souza AP, Gerlach RF, Line SRP. Inhibition of human gingival gelatinases (MMP-2 and – MMP-9)by metal salts. Dent Mater. 2000;16:103–08. doi: 10.1016/s0109-5641(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 26.Souza AP, Gerlach RF, Line SRP. Inhibition of human gingival gelatinases by metals released from dental amalgam. Biomater. 2001;22:2025–30. doi: 10.1016/s0142-9612(00)00388-4. [DOI] [PubMed] [Google Scholar]
- 27.Tersariol IL, Gerardeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins M, et al. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–81. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Suddick RP, Hyde RJ, Feller RP. Menaker, The Biologic Basis of Dental Caries. Harger & Row; New York: 1980. Salivary water and electrolytes in oral health; p. 132. [Google Scholar]
- 29.Blackburn RS, Harvey A, Kettle LL, Manian AP, Payne JD, Russell SL. Sorption of chlorhexidine on cellulose: mechanism of binding and molecular recognition. J Phy Chem B. 2007;111:8775–8784. doi: 10.1021/jp070856r. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, Carvalho RM, Tjäderhane L, Tezvergil-Mutluay A, Tay FR, Pashley DH. Chlorhexidine binding to human dentin. 2010 doi: 10.1016/j.dental.2010.04.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawes C. The effects of flow rate and duration of stimulation on the concentrations of protein and the main electrolytes in human submandibular saliva. Arch Oral Biol. 1974;19:887–895. doi: 10.1016/0003-9969(74)90051-x. [DOI] [PubMed] [Google Scholar]