Abstract
Rationale
The heterotypic interactions of endothelial cells and mural cells (smooth muscle cells or pericytes) are crucial for assembly, maturation and subsequent function of blood vessels. Yet, the molecular mechanisms underlying their association have not been fully defined.
Objective
Our previous in vitro studies indicated that Notch3, which is expressed in mural cells, mediates these cell-cell interactions. To assess the significance of Notch3 on blood vessel formation in vivo, we investigated its role in retinal angiogenesis.
Methods and Results
We show that Notch3-deficient mice exhibit reduced retinal vascularization, with diminished sprouting and vascular branching. Moreover, Notch3 deletion impairs mural cell investment, resulting in progressive loss of vessel coverage. In an oxygen-induced retinopathy (OIR) model, we demonstrate that Notch3 is induced in hypoxia and interestingly, pathological neovascularization is decreased in retinas of Notch3 null mice. Analysis of OIR mediators revealed that Angiopoietin-2 expression is significantly reduced in the absence of Notch3. Further, in vitro experiments showed that Notch3 is sufficient for Angiopoietin-2 induction, and this expression is additionally enhanced in the presence of HIF1α.
Conclusions
These results provide compelling evidence that Notch3 is important for the investment of mural cells and is a critical regulator of developmental and pathological blood vessel formation.
Keywords: Notch3, retina, angiogenesis, smooth muscle cell, pericytes, blood vessel
Introduction
Blood vessel formation is a dynamic and complex process that serves a vital role in both health and disease. At its onset, endothelial cells coalesce into tube-like structures, which become stabilized by the recruitment of mural cells such as pericytes and vascular smooth muscle cells (VSMCs) that encase the nascent vessel.1, 2 A host of reports have implied that endothelial cells and mural cells are closely associated, and the interactions between them are required for the regulation of vessel formation, stabilization, remodeling and function in vivo and in vitro.2-4 However, the way in which endothelial and mural cells communicate with each other remains poorly understood. Several different ligand-receptor systems have been implicated in heterotypic cell interactions to regulate the development and maintenance of the vasculature. Endothelial cell-secreted PDGF-B is known to be necessary for the recruitment of pericytes to newly formed vessels through PDGFR-β.5-7 Angiopoietin-1 and Tie2 signaling has been shown to be critical for vessel maturation and stabilization,3, 8 while, the differentiation of VSMCs surrounding blood vessels depends on endothelial-derived TGF-β.3, 9 In addition to these signaling mediators, it is believed that additional receptor-ligand pairs regulate vascular cell-cell communication. For example, High et al., demonstrated that Notch signaling between endothelial cells and VSMCs governs smooth muscle differentiation.10
Notch genes encode cell surface receptors that transduce signals between neighboring cells to regulate developmental processes such as cell fate decisions.11, 12 Mammals express four Notch receptors (Notch 1-4) and five membrane-bound ligands (Jagged (Jag) 1, Jag2, Delta-like (Dll)1, Dll3, and Dll4). Upon binding, Notch receptors undergo proteolytic cleavages by tumor necrosis factor-β converting enzyme and the γ-secretase complex to release the Notch intracellular domain (NICD). NICD then translocates to the nucleus where it binds with the transcription factor CSL (CBF-1/RBP-Jk, Su(H), and Lag-1) and coactivator Mastermind-like (MAML) to trigger downstream gene expression. In the vasculature, alterations in Notch signaling result in abnormalities in vessel patterning and maturation.13-15 In fact, recent findings of several research groups revealed a vital role for endothelial-dependent Notch signaling in tip cell formation and sprouting.16-18 Yet, it is unclear to what extent Notch-regulated vascular patterning events are consequences of homotypic or heterotypic signaling.
In contrast to Notch1, which is widely expressed,19 Notch3 is exclusively expressed in mural cells in the vasculature.20-24 In humans, Notch3 gene mutations give rise to CADASIL, an inherited early stroke syndrome leading to dementia due to systemic vascular degeneration and eventual loss of VSMCs within the arterial wall.25 In the mouse, genetic studies have implicated Notch3 in the regulation of smooth muscle maturation, modulation of vascular physiology and response to ischemia.26-28 Although these studies shed light on the importance of Notch3 in blood vessel structure and function, they did not investigate the direct role of Notch3 in governing blood vessel formation. In this study, we explored the contribution of Notch3 in developmental and pathological angiogenesis using the mouse retina as a model. Our findings imply that Notch3 facilitates heterotypic interaction between endothelial and mural cells that in turn dictates blood vessel patterning. Furthermore, Notch3 appears to play a role in response to hypoxia by regulating the expression of Angiopoietin-2.
Methods
Animals
Notch3-/- mice were a generous gift from Dr. Tom Gridley.29 Notch3-/-, Notch3+/- and wild type littermates were obtained by crossing Notch3+/- and Notch3+/-mice. All experimental procedures on mice were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Committee for the Use of Animals in Research.
Cell culture
Human aortic smooth muscle cells from Lonza were grown in DMEM supplemented with 10% FBS, 100I.U./ml penicillin-streptomycin. Cells between passages 6 and 8 were used for experiments. Mouse aortic smooth muscle cells were isolated from aortas of Notch3+/- and Notch3-/- mice. Cells between passages 3 and 8 were used for experiments. Lentiviral and adenoviral transduction was performed as described previously.30, 31
Immunostaining on whole-mount retinas
The immunostaining in retinas was performed as previously described.30 Briefly, eyes were isolated from Notch3-/- and Notch3+/- mice at indicated time points and fixed in 4% paraformaldehyde for 30 minutes. The cornea, sclera, lens, vitreous, and hyaloid vessels were removed to isolate retinas and radial incisions were made at equal intervals along the retinal edge. Subsequently retinas were placed in cold methanol for 20 minutes, blocked and permeabilized in PBS containing 5% donkey serum and 0.3% Triton-X-100 for 1 hour. Primary antibody, Notch3 (1:100) (Santa Cruz), sm α-actin (1:1000) (Sigma), NG2 (1:200) (Millipore), or Ang-2 (1:200) (Zymed), Collagen IV (1:200) (Millipore), or phospho-histone H3 (1:200) (Millipore) was costained with 10μg/ml TRITC labeled-isolectin B4 (Griffonia simplicifolia) (Invitrogen). Retinas were flat mounted in Vectashield (Vector Laboratories). Confocal images were captured and vessels were quantified with NIH ImageJ software by a blind observer.
Cell death detection ELISA
For the detection of apoptosis, a cell death detection ELISAPLUS kit (Roche) was used. Isolated retinas were homogenized in 100μl lysis buffer, and 20μl of the supernatant of each retina were assayed. For cells, aortic smooth muscle cells were serum starved in DMEM with 0.25% FBS. An equal number of cells were utilized for cell death detection.
Aortic ring assay
Aortic sprouting assays were performed as previously described with modification.32 Briefly, aortas from Notch3+/- and Notch3-/- littermates at P11 were dissected, cut into 1-mm-thick rings, and incubated overnight in complete EBM-2 media (Lonza) containing 10% FBS before being placed into a rat tail collagen gel (1.5mg/ml). 48 hours after embedding, rings were stained with 10μg/ml TRITC labeled-isolectin B4 and NG2, and imaged by confocal microscopy. Vessels were quantified with ImageJ software.
Oxygen-induced retinopathy (OIR)
OIR was induced as previously described, with minor modifications.33 In brief, at postnatal day (P)7 pups, along with nursing mothers, were placed in 70% oxygen. At P12, they were returned to 21% oxygen for 5 days. From P12 to P17, the animals were anesthetized, their retinas were collected and iso-lectinB4 immunostaining on whole-mount retinas were performed. Avascular area was quantified as percentage of whole retinal area. Neovascularization was quantified using ImageJ software by calculating the area of iso-lectinB4 staining of neovascular tufts as reported.34
RNA isolation and quantitative reverse transcriptase-polymerase chain reaction (qPCR)
Total RNA was extracted from mouse retinas using RNAqueous-4PCR kit (Invitrogen), and reverse transcribed with M-MLV reverse transcriptase (Invitrogen) to generate cDNA. Quantitative PCR was performed using a StepOne PCR system (Applied Biosystems) with Power SYBR Green.
Statistical analysis
Data analyses were performed using PrismGraph and comparisons between data sets were made using Student's t test. Differences were considered significant if P < 0.05, and data are presented as mean ± standard error of the mean (SEM). Data shown are representative of at least three independent experiments.
Results
Notch3 protein is expressed in retinal mural cells
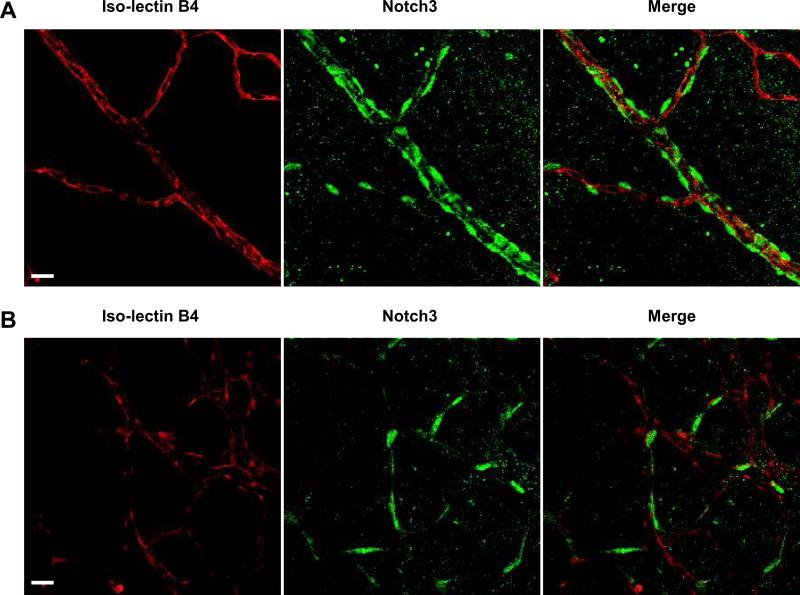
Recently, using an in vitro angiogenesis assay we demonstrated that Notch3 mediates mural cell-enhanced blood vessel formation.30 From these studies, we predicted that Notch3 would have a similar effect in vivo, and would act to modulate blood vessel formation in a mural cell-dependent fashion. To address this premise, we examined angiogenesis in retinas of mice deficient for Notch3. Earlier reports had shown that Notch3 mRNA is expressed in the retinal vasculature and appeared localized to mural cells.35 We confirmed expression of Notch3 protein in the retinal blood vessels by immunostaining. Whole-mount retinas stained with a Notch3 antibody in conjunction with endothelial-specific iso-lectin B4 demonstrated that Notch3 protein is specifically expressed in mural cells and is absent from endothelial cells within the retinal vascular network (Figure 1A). Furthermore, Notch3 protein is observed in mural cells at the angiogenic front, where endothelial sprouting activity is abundant (Figure 1B).
Figure 1. Notch3 expression in the retina.
Retinas isolated from wild type mice at postnatal day (P) 5 were immunostained to detect Notch3 expression (green). An endothelial-specific iso-lectin B4 (red) was used to highlight the vasculature. Confocal images of retinal arteries (A) and vessels at the angiogenic front (B) were taken at 400X magnification. Bar, 20μm.
Notch3 deletion influences development of the retinal vasculature
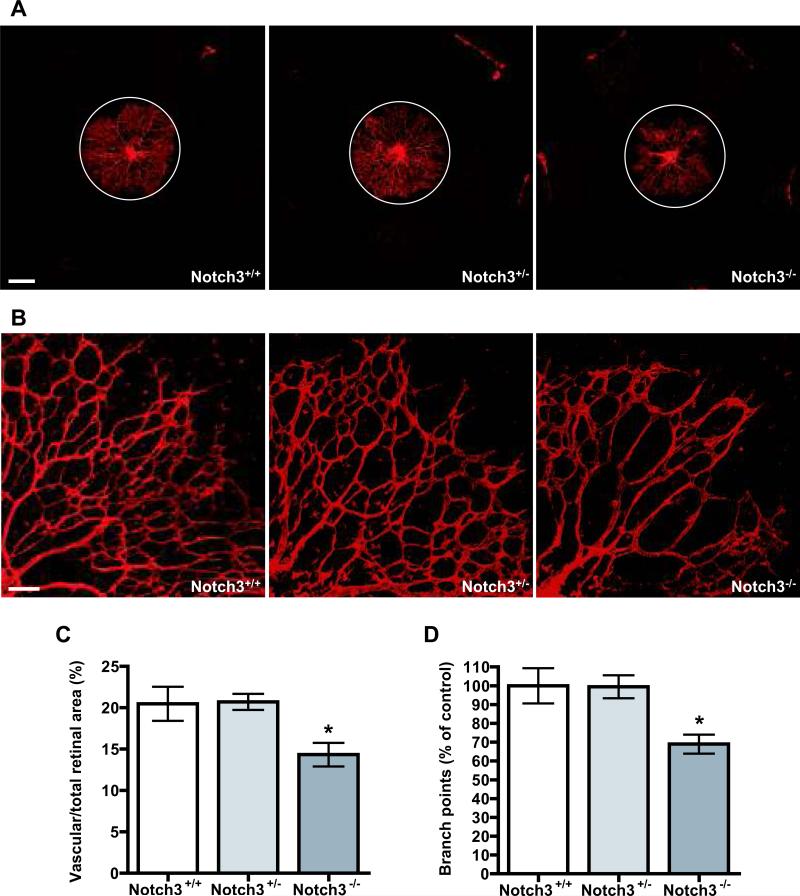
Notch3 null mice were previously shown to be viable and fertile, but adult mice exhibit structural defects in the distal arteries, including enlargement of the vessels and defective smooth muscle maturation.28 To determine whether the expression of Notch3 in retinal mural cells regulates the process of blood vessel formation, we performed whole-mount iso-lectin B4 staining of mouse retinas and evaluated the pattern of the vasculature from Notch3-/-, Notch3+/-, and wild type littermates at different developmental stages. Retinal vessels begin to sprout from the optic disc right after birth and extend out to the periphery at postnatal day (P) 8. At P3, we found that retinas from Notch3-/- mice displayed diminished vascularization. Coverage of the superficial retinal plexus was decreased by 30% in Notch3-/- mice compared with Notch3+/- and Notch3+/+ retinas (Figure 2A and C). Examination of vessel patterning revealed a 30% reduction in vessel branch points (Figure 2B and D), leading to an overall decline in vascular density. No difference was apparent in comparison of Notch3 heterozygote and wild-type animals. At P5 and P7, similar defects were observed in Notch3 null retinas, albeit to a lesser extent (Online Figure I). Formation of the deep capillary network at the P10 and P14 stages revealed similar Notch3-dependent defects (Online Figure I). In adult mice (P43), the vascular patterning defects associated with the absence of Notch3 were only slight (data not shown). These findings indicate that Notch3 acts as a regulator of vessel formation in vivo, and may have a more pronounced role in early angiogenesis.
Figure 2. Notch3 deletion compromises retinal angiogenesis in vivo.
(A) Retinal vasculature from Notch3+/+ (left), Notch3+/- (middle) and Notch3-/- (right) littermates were stained with iso-lectin B4 at P3. White circles depict the size of vascular outgrowth from a representative Notch3+/+ retina for comparison. Bar, 500μm. (B) High magnification images of retinal vasculature were taken at 100X by confocal microscopy to highlight defects in branching. Bar, 100μm. (C) Graph represents the ratio of the vascularized to total retinal area at P3 (n=10 mice per group). (D) Quantification of branch points in retinas of Notch3+/+, Notch3+/- and Notch3-/- at P3. * P < 0.05 compared to Notch3+/+ and Notch3+/-.
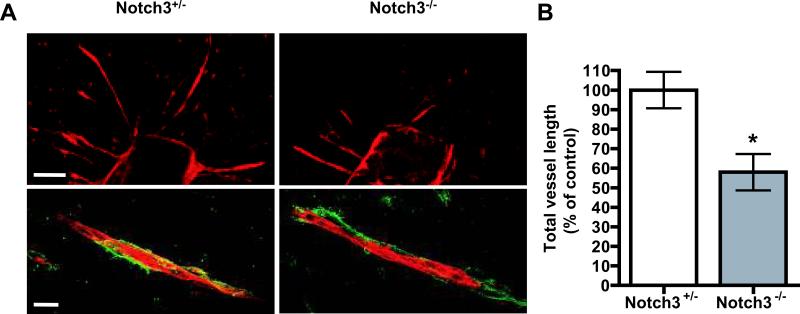
The blood vessel-forming phenotype in Notch3 null retinas indicated a defect in vessel sprouting, which resulted in an overall decrease in vascular density. To determine if the angiogenic defects observed in the Notch3 null mice might be due to an absence of mural cells at the angiogenic front, we performed colocalization studies with iso-lectin B4 and the mural cell marker NG236. These results showed that the mural cells in the Notch3 null retinas were normally distributed and appeared to associate with the developing vessels in a similar fashion to wild type and heterozygous littermates (Online Figure II). Furthermore, to exclude the possibility that reduced vessel density was a result of increased vessel regression; we stained for Collagen IV to detect empty collagen sleeves. 37 We observed no differences between the Notch3 null mice and controls (Online Figure III). To directly evaluate the influence of Notch3 on vessel sprouting, we preformed ex vivo sprouting assays with isolated aortic rings. Thoracic aortas from Notch3 null mice exhibited reduced vessel sprouts when cultured for two days compared to heterozygous controls, while mural cell recruitment was not affected (Figure 3). Taken together, these results demonstrate that mural cell-expressed Notch3 can regulate vessel sprouting, not only in developing retinal capillaries, but also in large elastic arteries prompted to undergo angiogenesis.
Figure 3. Aortic ring assays reveal sprouting defects in Notch3 null mice ex vivo.
(A) Representative images showing microvascular sprouting of aortic explants from Notch3+/-(left) and Notch3-/- mice (right). Aortic sprouts were stained with iso-lectin B4 (red) and mural cell marker NG2 antibody (green). Images were taken at 100X (upper panel; bar, 100μm) or 400X (lower panel; bar, 20μm) by confocal microscopy. (B) Quantification of vessels from aortic rings after 48 hours (n=9 per group from three litters). * P < 0.05 compared to relevant control.
Notch3 deletion impairs the investment of mural cells
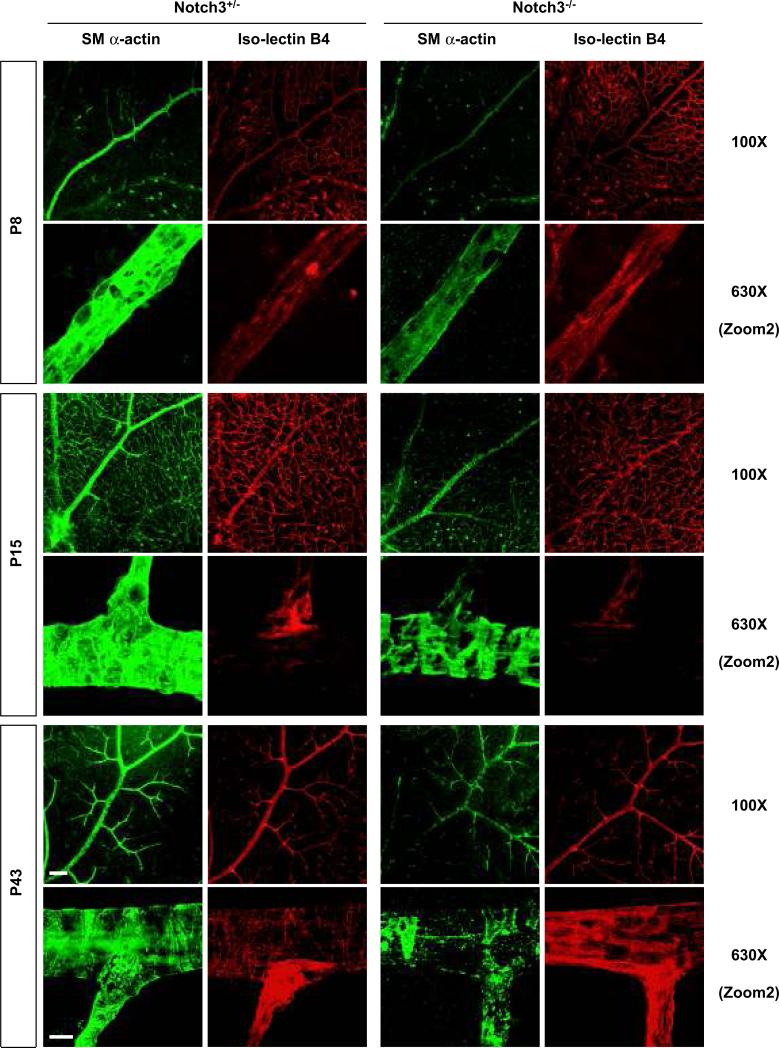
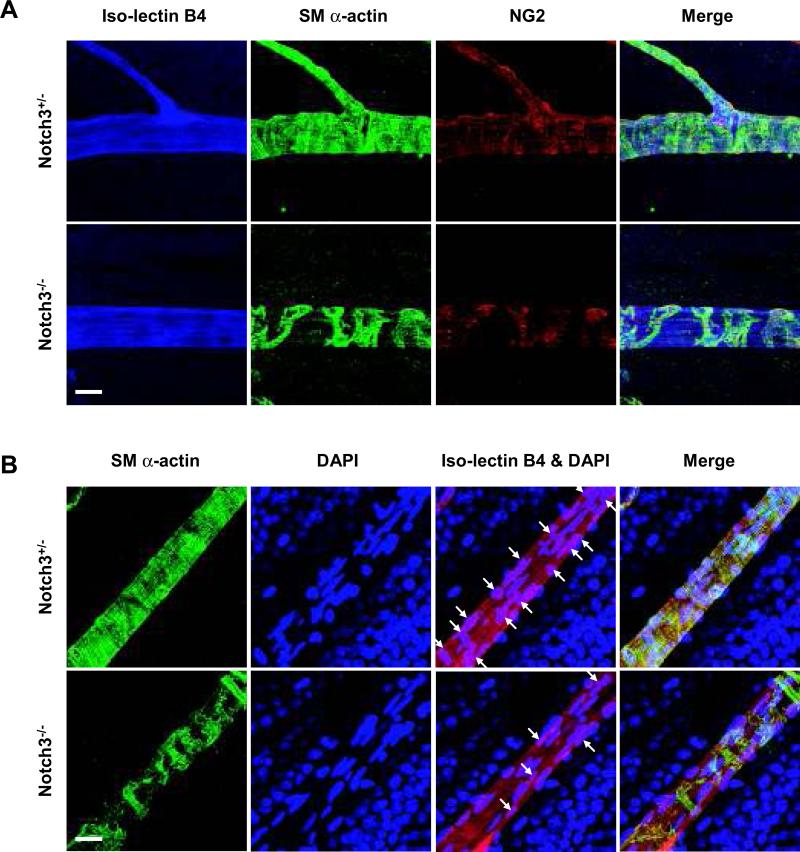
Given that prior analysis of Notch3-deficient mice uncovered defects in smooth muscle maturation,28 we sought to investigate how pericytes and smooth muscle cells of the retina were invested in the vascular network in the absence of Notch3. To do so, we undertook smooth muscle (sm) α–actin and NG2 staining to highlight retinal pericytes and smooth muscle cells, in conjunction with iso-lectin B4. In early developmental stages, mural cells appeared to be normally distributed in the vascular plexus of Notch3-/- mice (Online Figure II). However, the null animals showed significant reduction in sm α–actin staining at P8 and P15 (Figure 4). Additionally, at P15, it was evident that the density of surrounding mural cells in arteries was dramatically decreased in Notch3-/- mice, with the formation of visible gaps between adjacent cells (Figure 4). The decrease in sm α-actin staining was even more pronounced in P43 adults, where mural cells were found sparsely surrounding the arteries (Figure 4, and Online Figure IV). These results indicate that the absence of Notch3 progressively impacts the investment of pericytes and smooth muscle cells over time. Because Notch3 is known to affect smooth muscle differentiation and particularly the expression of sm α-actin,28 we utilized an additional mural cell marker, NG2 to highlight these cells. Consistent with the expression of sm α-actin, we observed a similar pattern of NG2 staining in null mice (Figure 5A). Thus, these findings show that the lack of sm α-actin staining was not due to an absence of marker expression, but rather, was due to the progressive loss of mural cells. To further verify the absence of pericyte and smooth muscle cell coverage in adult retinas lacking Notch3, we costained with DAPI to mark nuclei within individual blood vessels. As predicted, the Notch3 null vessels had an average of 58% fewer mural cell nuclei compared with wild type and heterozygous littermates (Figure 5B, and Online Figure IV).
Figure 4. Notch3 deletion impairs mural cell investment in retinal vasculature.
Retinas were isolated from Notch3+/- (left) and Notch3-/- (right) littermates at P8, P15 and adults (P43). At indicated time points, retinas were costained with mural cell marker sm α-actin (green) and iso-lectin B4 (red) to highlight the vasculature. Confocal images were taken at 100X (bar, 100μm) and 630X (bar, 10μm) magnification.
Figure 5. Notch3 deletion decreases the coverage of mural cells in retinal vasculature.
Retinas were isolated from Notch3+/- and Notch3-/- littermates at P43. (A) Retinas were immunostained with mural cell markers sm α-actin (green) and NG2 (red). An iso-lectin B4 (blue) was used to visualize the vasculature. Confocal images were taken at 630X magnification. Bar, 20μm. (B) Retinas were immunostained with sm α-actin (green), DAPI (blue, for nuclei) as well as iso-lectin B4 (red). Arrows indicate the nuclei of mural cells. Confocal images were taken at 630X magnification. Bar, 20μm.
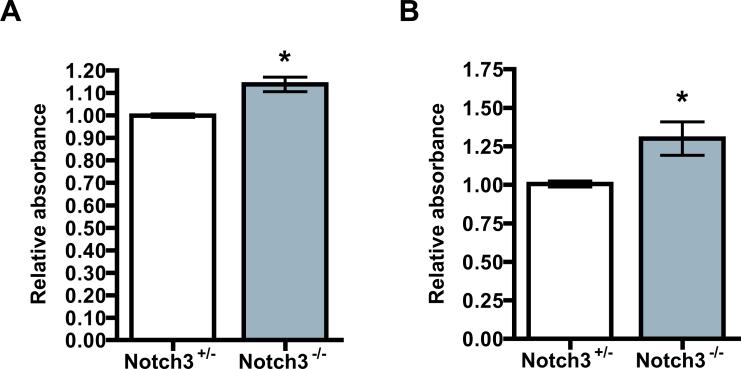
We reasoned that the loss of mural cells from the retina had to occur by one of two processes: by mural cells becoming disassociated from the vessels and migrating away,7, 38 or by apoptosis. From careful analysis, we did not observe migrating mural cells in retinas of Notch3 null mice. Cells that remained were closely attached to the endothelial abluminal surface as in control retinas (unpublished observation, H.L., B.L.). Prior evidence linking Notch3 to cell survival led us to pursue apoptosis as a mechanism for cell loss in the retinas.39, 40 Apoptosis was assessed by a cell death ELISA kit using whole retinas from mice at P10. We observed that Notch3-/- mice exhibited a statistically significant increase in apoptosis (Figure 6A). Assessment of proliferation in the Notch3 null and control retinas did not reveal any obvious differences (Online Figure V). Although the difference in apoptosis was small, the progressive loss of mural cells suggests that Notch3 acts to suppress cell death, and in its absence over time, cells gradually succumb resulting in considerable deficiency of mural cells. To further confirm this, we performed in vitro apoptosis assays using mouse aortic smooth muscle cells isolated from heterozygous and homozygous animals. Consistent with our in vivo data, Notch3-deficient smooth muscle cells exhibited an increase in apoptosis compared with control cells (Figure 6B).
Figure 6. Notch3 deficiency increases apoptosis in mural cells.
(A) Retinas were isolated from Notch3+/- and Notch3-/- littermates at P10, homogenized and the supernatant of each retina was subjected to cell death detection ELISAPLUS kit to measure histone-associated DNA fragmentation (n=6 mice for each group). (B) Aortic smooth muscle cells from Notch3+/- and Notch3-/- mice were serum starved in DMEM with 0.25% FBS for 72 hours and cell apoptosis was assessed by cell death detection ELISAPLUS kit (n=3). * P < 0.05 compared to relevant control.
Notch3 modulates pathological angiogenesis
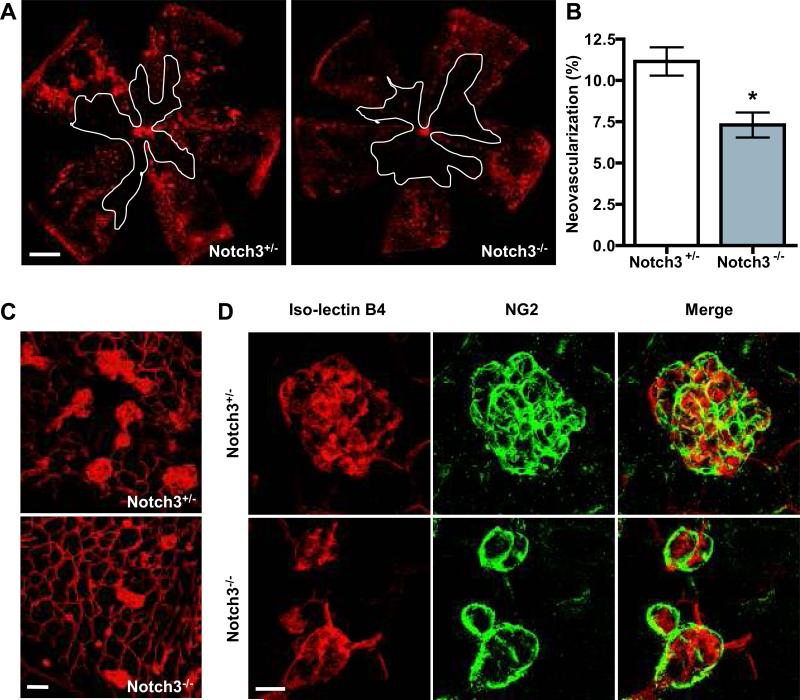
In order to investigate the role of Notch3 in pathological angiogenesis, we used a mouse model of oxygen-induced retinopathy (OIR).33 In this model, P7 pups were exposed to 70% oxygen (hyperoxia) for five days (P7–P12) to induce vascular regression and obliteration in the central retina. At P12, mice were returned to room air for five days, during which time period relative hypoxia initiates rapid vessel growth and pathological neovascularization. We examined the vascular phenotypes of mice subjected to OIR. At the height of vascular regression in P12 retinas, Notch3-/- mice and Notch3+/- littermates had a comparable vascular obliteration, which quantitatively occupied approximately 35% of the total retinal surface (Online Figure VI). These data show that Notch3 deficiency does not affect the susceptibility of retinal vasculature to oxygen-induced vessel regression. In contrast, during the neovascularization phase, Notch3–/– retinas exhibited a diminished number of new blood vessels, compared to Notch3+/– littermates (Figure 7A-C). In the null mice, there was a 35% decrease in neovascularization. Moreover, the number and the size of vascular tufts were reduced in Notch3-/- animals. To characterize the vascular tufts in more detail, retinas were costained for iso-lectin B4 and NG2.41 These images revealed that NG2-positive staining was seen in both the heterozygous and null tufts at similar intensities (Figure 7D). Thus, under pathological conditions, Notch3 deletion has a negligible effect on vascular regression, but substantially impairs the process of neovascularization, as evidenced by a decrease in the size and number of vascular tufts.
Figure 7. Notch3 deletion results in decreased retinal neovascularization in OIR.
(A) Representative retinal vasculature from Notch3+/- (left) and Notch3-/- (right) littermates at P17 subjected to OIR. White lines outline the area of vaso-obliteration. Bar, 500μm. (B) Graph represents neovascularization in Notch3+/- and Notch3-/- mice at P17 (n=10 mice for each group). * P < 0.05 compared to relevant control. (C) High magnification images of retinal vasculature at P17 were taken at 200X by confocal microscopy to highlight the neovascular tufts. Bar, 50μm. (D) Retinas at P17 from OIR-treated Notch3+/- and Notch3-/- mice were double-labeled with mural cell marker NG2 (green) and endothelial-specific iso-lectin B4 (red). Images were taken at 630X by confocal microscopy. Bar, 20μm. Yellow color reflects overlay of endothelial and mural cells.
Notch3 expression is increased during oxygen-induced neovascularization and regulates Angiopoietin-2
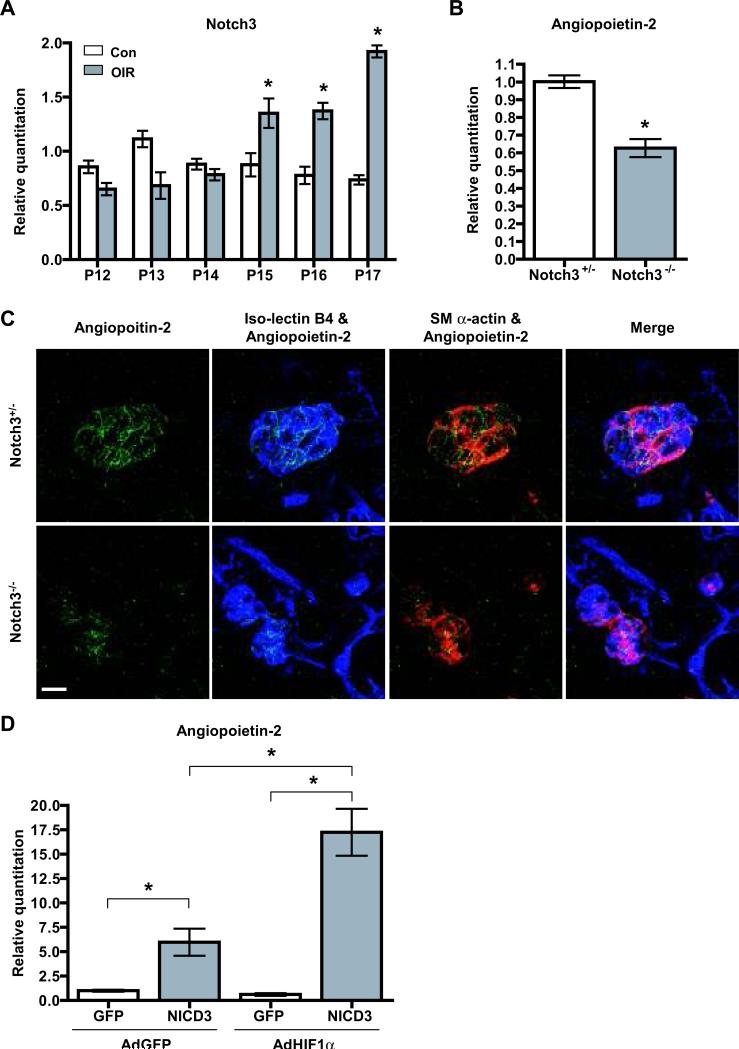
To further explore the role of Notch3 in retinal neovascularization, we examined its expression in retinas of wild-type mice subjected to OIR. Compared to mice in normoxia, Notch3 mRNA was increased in relative hypoxia as assessed by quantitative PCR (Figure 8A). At P12, just after hyperoxia treatment, Notch3 expression was slightly lower than that of normoxia controls, probably due to an overall decrease in blood vessels. However by P15, Notch3 mRNA surpassed control levels and at P17 exceeded control expression by more than 2-fold. These observations signify that Notch3 might have a unique role in modulating vascular development under pathological conditions.
Figure 8. Notch3 regulates Angiopoietin-2.
(A) Quantitative PCR analysis of Notch3 mRNA expression in the retinas of wild type mice at indicated time points (P12-P17) in oxygen-induced retinopathy (OIR) or normal condition (Con). (B) mRNA expression of Angiopoietin-2 in P17 retinas of Notch3+/- and Notch3-/- mice subjected to OIR. (C) Retinas at P17 from OIR-treated Notch3+/- and Notch3-/- mice were stained with an Angiopoietin-2 antibody (green), iso-lectin B4 (blue) and mural cell marker sm α-actin (red). Images were taken at 630X by confocal microscopy. Bar, 20μm. (D) Induction of Angiopoietin-2 expression by Notch3 and HIF1α in vitro. Human aortic smooth muscle cells were transduced with lentivirus expressing the human Notch3 intracellular domain (NICD3) or GFP. 72 hours after transduction, cells were infected with adenovirus expressing GFP (AdGFP) or HIF1α (AdHIF1α) as indicated for 48 hours. Cells were then collected and analyzed by qPCR to evaluate Angiopoietin-2 expression. * P < 0.05 compared to relevant control.
It is known that pathological angiogenesis is stimulated by an increase in angiogenic factors such as VEGF and the Angiopoietins.42 Given that Notch proteins function as transcription coactivators, and Notch3 is increased in hypoxia, we theorized that it might act to regulate the expression of certain other factors during hypoxia. Examination of known Notch target genes43 in hypoxia-treated retinas at P17, revealed a significant decrease in Hrt3 mRNA expression in Notch3 null mice (Online Figure VII). We next evaluated mRNA levels of angiogenic growth factors and their receptors in these same hypoxia-treated retinas. Expression of VEGF-A, VEGF-C, Angiopoietin-1 and Tie-2 all exhibited similar expression levels in Notch3+/– and Notch3-/– littermates (Online Figure VII). Conversely, Angiopoietin-2 (Ang-2) mRNA expression was decreased by approximately 40% in the null retinas compared to control (Figure 8B). The expression of Ang-2 was additionally assessed by immunostaining, which showed the Ang-2 protein was significantly reduced in Notch3 deficient mice (Figure 8C). In the retina, Ang-2 expression is upregulated during both physiological and pathological neovascularization (Online Figure VIII, and 44). Mice that are deficient for Ang-2 exhibit reduced hypoxia-driven angiogenesis,45, 46 similar to that observed in our Notch3 null animals. These combined results suggest that one mechanism in which Notch3 governs pathological angiogenesis is by regulation of Ang-2. To evaluate if the Notch3-dependent reduction in Ang-2 is unique to the pathological conditions produced by hypoxia, we examined the expression of Ang-2 in Notch3 null and heterozygous mice under normoxia. In P17 retinas isolated from mice exposed to room air, the Notch3 deletion had no effect on Ang-2, Ang-1 or Tie-2 expression, or that of Hes1, Hrt2, and Hrt3 (Online Figure IX). These experiments imply that Ang-2 is a target of Notch3 only during oxygen-induced neovascularization, and suggest that crosstalk between Notch3 and hypoxic signaling triggers this distinct expression.
To determine whether Notch3 could directly induce Ang-2 expression, we overexpressed a constitutively active form of Notch3 (NICD3) and measured Ang-2 mRNA. Lentiviral transduction of the NICD3 in primary cultures of human aortic smooth muscle cells caused Ang-2 mRNA expression to be significantly increased compared with control cells transduced with a GFP-expressing lentivirus (Figure 8D). Hypoxia-inducible factor-1α (HIF1α is a key regulator of hypoxia-induced signaling, and was previously shown to directly interact with Notch1 to activate transcription.42, 47 To determine if HIF1α and Notch3 cooperate to regulate the expression of Ang-2, we cotransduced the NICD3 with a HIF1α cDNA and measured Ang-2 transcript levels. Indeed, while Ang-2 expression was augmented by NICD3, addition of HIF1α with NICD3 produced a greater increase in Ang-2 (Figure 8D). Interestingly, HIF1α transduction in aortic smooth muscle cells did not invoke an increase in Ang-2 expression on its own, and required the NICD3. Thus, as suggested by previous reports,48, 49 the regulation of Ang-2 expression in mural cells differs from that in endothelial cells, and likely depends on Notch3 in hypoxia. Overall, these results indicate that Ang-2 is a direct target of Notch3 in mural cells, and Notch3 specifically regulates its expression under pathological conditions, possibly in conjunction with HIF1α. In summary, our data support the notion that Notch3 modulates oxygen-induced neovascularization by regulating the expression of Ang-2 from mural cells, which then acts on adjacent Tie-2-expressing endothelial cells to modulate blood vessel assembly.
Discussion
Notch signaling can regulate cell-to-cell communication and control cell fate decisions in a variety of cell types. In the vascular endothelium, signaling between Dll4 and Notch1 in tip and stalk cells, respectively, prevents stalk cell sprouting.16 This Notch-dependent homotypic interaction helps to pattern blood vessels. In this study, we asked how Notch signaling might facilitate heterotypic interactions between mural cells and endothelial cells that help shape the vasculature. Previous work has suggested an important role for pericytes in modulating tube formation,50, 51 and an in vitro study by our lab demonstrated that mural cell-expressed Notch3 affects angiogenesis.30 Here, we show that under physiological conditions, Notch3 deletion decreases vascularization in the mouse retina at early developmental stages. These angiogenic defects are not a result of enhanced regression, which points to growth retardation as the likely cause. The decline in angiogenesis is associated with a reduction in branching, and accordingly, these data reveal that Notch activity in mural cells can modulate vascular patterning. Our results show that in the absence of Notch3, mural cells are still present at leading edge of growing vessels. Thus, the observed angiogenic defects must be due to improper signaling from these cells. The way in which Notch3 receptor signaling from mural cells instructs endothelial cells is not entirely clear. Though, previous studies have offered insight into a potential mechanism. One study showed that an endothelial-specific knockout of Jagged1 has reduced retinal angiogenesis, including branching defects52 that resemble those we observed in Notch3-deficient mice. Another demonstrated that mice lacking endothelial-expressed Jagged1 have severe mural cell defects.10 In addition, previous data from our lab showed that Jagged1 on endothelial cells promotes Notch activity and mural cell differentiation.30 In light of these previously published findings, one possible model to explain the Notch3-dependent angiogenic phenotype is that endothelial-expressed Jagged1 signals to Notch3 on mural cells to promote mural cell investment. The invested mural cells then influence endothelial growth and branching by producing secreted factors. Indeed, additional studies will be required to elucidate the exact mechanism through which Notch3 influences vessel patterning.
Adult Notch3 null mice exhibit impaired arterial differentiation, enlargement of vessels, and defective maturation of VSMCs.28 In addition, Notch3 deficiency impacts vascular tone in resistance arteries, indicative of functional defects in smooth muscle cells.27 To investigate more thoroughly the vascular smooth muscle defects associated with loss of Notch3, we examined them in the retinal vasculature. This vascular bed is formed postnatally and is conducive to stepwise analysis of mural cell recruitment and differentiation. From our experiments, mural cells initially appeared to be recruited normally in Notch3 null mice, albeit the expression of sm α-actin was slightly decreased. However, over time mural cells surrounding the vessels were progressively lost. Apoptosis assays revealed a small but significant increase in cell death, consistent with previous reports that linked Notch3 to cell viability.39, 40 These results are in contrast to those published by Domenga et al., which reported no detectable apoptosis in the tail arteries of Notch3-/- adult mice.28 This discrepancy could be due to differences in the vascular beds that were analyzed. Yet, in vitro apoptosis assays that we performed on aortic smooth muscle cells from Notch3 heterozygous and null mice were consistent with our in vivo results done on retinal tissue. We believe that in the absence of Notch3, the small increase in apoptosis leads to an incremental loss of mural cells over time. Thus, Notch3 not only functions to regulate arterial differentiation and maturation,28 but also controls the investment and stability of mural cells contributing to functional blood vessels.
Our data indicate that the loss of pericytes has little effect on retinal angiogenesis, as mural cells begin to be lost after P8 in Notch3 null mice, yet the angiogenic phenotypes we observed were most pronounced prior to this stage. The loss of Notch3 appears to have no effect on recruitment. Our data show that mural cells are present and distributed normally at the angiogenic front. These would imply it is not the absence of Notch3-deficient mural cells that affects vessel growth, but a defect in their function. Currently, we do not know the exact mechanism through which Notch3 conveys angiogenic signals. Possibilities include, heterotypic signaling to endothelial cells or Notch-regulated gene expression of angiogenic factors in mural cells. At later stages after the vessels are formed, mural cell loss does not seriously impact their stability or regression. We did observe an overall enlargement of arteries in Notch3 deficient retinas at later stages (data not shown), similar to the Domenga et al., study.28 Taken together, these findings imply that pericyte-expressed Notch3 impinges on endothelial cells vessel-forming capabilities early on to regulate assembly, and once the vessel network is established, mural cells have little effect on modulating blood vessel structure. Importantly however, we cannot exclude the possibility that compensatory mechanisms are activated in the null mice, which mask additional roles of Notch3 in mural cells. Perhaps other Notch receptors, such as Notch1, take over in the absence of Notch3.
Notch3 function has been linked to ischemic diseases. Arboleda-Velasquez et al., showed that Notch3-deficient mice were more susceptible to ischemic stroke,26 whereas, Li et al., revealed a protective effect of Notch3 deficiency for the development of pulmonary hypertension.53 In this study, using an ischemia-induced retinopathy model, we demonstrate that Notch3 plays a direct role in proliferative neovascularization. In the absence of Notch3, vascular tufts are decreased, suggesting that in ischemia, Notch3 acts to promote blood vessel formation, possibly as a maladaptive response to hypoxia. Similar to physiological conditions, Notch3 does not appear to be required for recruitment of mural cells in hypoxic conditions. Our experiments did not address if Notch3 is required for mural cell stability and investment in this disease state, as it is during development. The Notch pathway is known to intersect with hypoxic signaling, and Notch1 and HIF1α can directly interact to regulate transcription.47, 54 Consequently, it is not surprising that Notch3 affects blood vessel formation in disease states, though the transcriptional targets of Notch signaling in ischemia have not been previously described. Here, our results show for the first time that Ang-2 is a target of Notch3 under ischemic conditions. Moreover, we show that Notch3 cooperates with HIF1α to regulate Ang-2. Indicative of a mutual pathway, Ang-2-deficient mice share phenotypic similarities with Notch3 null mice subjected to OIR.45, 46 Therefore, the convergence of Notch3 and HIF1α onto Ang-2 likely serves to precisely control this vital signaling factor exclusively in ischemia.
In summary, our data conclusively show that mural cell-expressed Notch3 is important for modulating distinct aspects of developmental and pathological blood vessel formation. Notch3 signaling to endothelial cells regulates vessel branching and its expression on mural cells is critical for their differentiation and viability. Furthermore, in pathological conditions, Notch3 regulates Ang-2 expression under hypoxia, demonstrating a role for this Notch family member in governing neovascularization. Our results suggest that Notch3 utilizes multiple mechanisms to control different aspects of blood vessel formation and function, and they differ under normal and aberrant states. Thus, therapeutic targeting of Notch3 might be a novel approach for disrupting angiogenesis in certain vascular related diseases.
Summary.
Angiogenesis is a stepwise process governed by intrinsic and extrinsic cell signaling. Understanding how signals from adjacent mural cells affect vascular patterning is critical for defining mechanisms that regulate blood vessel formation in normal and disease states. Here, we show that Notch3, which is expressed in mural cells, is involved in blood vessel patterning and acts to maintain vascular structure by regulating mural cell investment. Our data also demonstrate a role for Notch3 in the regulation of pathological angiogenesis, likely by regulating downstream gene expression. These findings advance our understanding of how Notch signaling in mural cells influences angiogenesis. Our studies suggest that Notch3 could serve as a target for therapeutic intervention in the treatment of vascular diseases.
Supplementary Material
SUPPLEMENTAL MATERIAL
Detailed Methods
Animals. Notch3-/- mice in a C57BL/6 background were a generous gift from Dr. Tom Gridley.1 Notch3-/-, Notch3+/- and wild type littermates were obtained by crossing Notch3+/- and Notch3+/- mice. All experimental procedures on mice were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Committee for the Use of Animals in Research.
Cell culture. Human aortic smooth muscle cells from Lonza were grown in DMEM supplemented with 10% FBS, 100I.U./ml penicillin-streptomycin. Cells between passages 6 and 8 were used for experiments. Mouse aortic smooth muscle cells were isolated from aortas of Notch3+/- and Notch3-/- mice. Cells between passages 3 and 8 were used for experiments. All cultures were maintained in humidified 5% CO2 at 37 °C. Lentiviral and adenoviral transduction was performed as described previously.2, 3 Briefly, recombinant lentiviruses were produced by transient transfection of TN-293 cells (Clontech). Subconfluent TN-293 cells were transiently co-transfected with pCDF1-MCS2-EF1-copGFP-NICD3 or the control plasmid without insert, and the lentiviral packaging plasmids pFIV34N and pVSV-G overnight. 48 hours after transfection, viral supernatant was harvested and used for infection. The human HIF1α cDNA was cloned into the pAdTrack shuttle vector in front of the CMV promoter using KpnI and EcoRI restriction sites. The adenovirus plasmids containing GFP alone (AdGFP) and GFP together with HIF1α (AdHIF1α) were transfected into HEK293 cells, and the viral particles were amplified and purified. For infection, human aortic smooth muscle cells were seeded in a 12-well plate at a density of 2×104 cells per well 24 hours prior to viral infection. To each well, 0.5ml of lentivirus suspension diluted in 0.5ml DMEM with 10% FBS was added. Polybrene was supplemented at a final concentration of 6μg/ml. 24 hours later, cells were transferred to fresh DMEM containing 10% FBS. 72 hours after infection, cells were infected with adenoviral particles, which were diluted in 400μl Opti-MEM (Invitrogen). 2 hours after incubation, 600μl normal growth media was supplemented. The following day, the virus-containing media was replaced with fresh media. Efficiency of transduction was monitored by GFP expression.
Immunostaining on whole-mount retinas. The immunostaining in retinas was performed as previously described.3 Briefly, eyes were isolated from Notch3-/- and Notch3+/- mice at indicated time points and fixed in 4% paraformaldehyde for 30 minutes. The cornea, sclera, lens, vitreous, and hyaloid vessels were removed to isolate retinas and radial incisions were made at equal intervals along the retinal edge. Subsequently retinas were placed in cold methanol for 20 minutes, blocked and permeabilized in PBS containing 5% donkey serum and 0.3% Triton-X-100 for 1 hour. Primary antibody, Notch3 (1:100) (Santa Cruz), sm α-actin (1:1000) (Sigma), NG2 (1:200) (Millipore), Ang-2 (1:200) (Zymed), Collagen IV (1:200) (Millipore), or phospho-histone H3 (1:200) , was costained with 10μg/ml TRITC labeled-isolectin B4 (Griffonia simplicifolia) (Invitrogen) for 2 hours at 37°C. Incubations with fluorescently tagged secondary antibodies were performed at 4°C overnight, including Alexa-Fluor 488 donkey anti-mouse (1:250), Alexa-Fluor 594 goat anti-rabbit and Alexa-Fluor 488 goat anti-rabbit (Invitrogen). Retinas were flat mounted in Vectashield (Vector Laboratories). Confocal images were captured and vessels were quantified with NIH ImageJ software by a blind observer.
Cell death detection ELISA. For the detection of apoptosis, a cell death detection ELISAPLUS kit (Roche) was used to quantify DNA fragmentation according to the manufacturer's instructions. Briefly, isolated retinas were homogenized in 100μl lysis buffer, incubated for 30 minutes at room temperature, and centrifuged for 10 minutes at 2000rpm. 20μl of the supernatant of each retina were assayed. For cells, aortic smooth muscle cells from Notch3+/- and Notch3-/- mice were plated at a density of 5×104 cell in a 12-well plate, starved in DMEM with 0.25% FBS for 72 hours. An equal number of cells were lysed in 100μl lysis buffer and were utilized for cell death detection.
Aortic ring assay. Aortic sprouting assays were performed as previously described with modification.4 Briefly, aortas from Notch3+/- and Notch3-/- littermates at P11 were dissected, cut into 1-mm-thick rings, and incubated overnight in complete EBM-2 media (Lonza) containing 10% FBS before being placed into a rat tail collagen gel (1.5mg/ml). 48 hours after embedding, rings were stained with 10μg/ml TRITC labeled-isolectin B4 and NG2, and imaged by confocal microscopy. Vessels were quantified with ImageJ software.
Oxygen-induced retinopathy (OIR). OIR was induced as previously described, with minor modifications.5 In brief, at postnatal day (P)7 pups, along with nursing mothers, were placed in 70% oxygen. At P12, they were returned to 21% oxygen for 5 days. From P12 to P17, the animals were anesthetized, their retinas were collected and iso-lectinB4 immunostaining on whole-mount retinas were performed. Avascular area was quantified as percentage of whole retinal area. Neovascularization was quantified using ImageJ software by calculating the area of iso-lectinB4 staining of neovascular tufts as reported.6
RNA isolation and quantitative reverse transcriptase-polymerase chain reaction (qPCR). Total RNA was extracted from mouse retinas using RNAqueous-4PCR kit (Invitrogen) according to the manufacturer's instruction, and reverse transcribed with M-MLV reverse transcriptase (Invitrogen) to generate cDNA. Quantitative PCR was performed using a StepOne PCR system (Applied Biosystems) with Power SYBR Green. The fold difference in various transcripts was calculated by the ΔΔCT method using 18S as the internal control. After PCR, a melting curve was constructed in the range of 60°C to 95°C to evaluate the specificity of the amplification products. Primer sequences for mouse transcripts were as follows: 18S For-5’-GTT GGT TTT CGG AAC TGA GGC-3’; 18S Rev-5’-GTC GGC ATC GTT TAT GGT CG-3’; Notch3 For-5’-TTG TCT GGA TGG AAG CCC ATG T-3’; Notch3 Rev-5’-ACT GAA CTC TGG CAA ACG CCT-3’; Angiopoietin-2 For-5’-ACA CCG AGA AGA TGG CAG TGT-3’; Angiopoietin-2 Rev-5’-CTC CCG AAG CCC TCT TTG TA-3’; Angiopoietin-1 For-5’-GGG CTG GAA GGA GTA TAA AAT GG-3’; Angiopoietin-1 Rev-5’-GAA CTC GTT CCC AAG CCA ATA T-3’; Tie-2 For-5’-CAA TCA GGC CTG GAA ATA CAT TG-3’; Tie-2 Rev-5’-TCC GCG GCT CCA AGT AGT T-3’. For human Angiopoietin-2 For-5’-AAC AGG AGG CTG GTG GTT TG-3’; Rev-5’-TGT GGA TAG TAC ATT CCG TTC AAG TT-3’. Hrt3 For-5’-CGC AGA GGG ATC ATA GAG AAA CG-3’; Hrt3 Rev-5’-GCC AGG GCT CGG GCA TCA AAG AA-3’; Hes1 For-5’-CCC CAG CCA GTG TCA ACA C-3’; Hes1 Rev-5’-TGT GCT CAG AGG CCG TCT T-3’; Hrt2 For-5’-CAC ATC AGA GTC AAC CCC ATG T -3’; Hrt2 Rev-5’-GCC ATG AGC AGA AGG CAC TT-3’.
Statistical analysis. Data analyses were performed using PrismGraph and comparisons between data sets were made using Student's t test. Differences were considered significant if P < 0.05, and data are presented as mean ± standard error of the mean (SEM). Data shown are representative of at least three independent experiments.
Supplemental References
1. Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis. 2003;37:139-143.
2. Corley KM, Taylor CJ, Lilly B. Hypoxia-inducible factor 1alpha modulates adhesion, migration, and FAK phosphorylation in vascular smooth muscle cells. J Cell Biochem. 2005;96:971-985.
3. Liu H, Kennard S, Lilly B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res. 2009;104:466-475.
4. Masson VV, Devy L, Grignet-Debrus C, Bernt S, Bajou K, Blacher S, Roland G, Chang Y, Fong T, Carmeliet P, Foidart JM, Noel A. Mouse Aortic Ring Assay: A New Approach of the Molecular Genetics of Angiogenesis. Biol Proced Online. 2002;4:24-31.
5. Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101-111.
6. Stahl A, Connor KM, Sapieha P, Willett KL, Krah NM, Dennison RJ, Chen J, Guerin KI, Smith LE. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297-301.
Online Figure I. Notch3 deletion compromises retinal angiogenesis in vivo. (A, B) Retinal vasculature from Notch3+/+, Notch3+/- and Notch3-/- littermates were stained with iso-lectin B4 at P5 and P7. Graphs show quantification of vascularized area and branch points in retinas. * P < 0.05 compared to heterozygous and wild type. Images were taken at 50X by confocal microscopy. Bar, 25μm. (C, D) Superficial and deep vascular plexus from Notch3+/+ and Notch3-/- littermates were stained with iso-lectin B4 at P10 and P14. Images were taken at 100X by confocal microscopy. Bar, 100μm.
Online Figure II. Mural cells in the angiogenic front of the retina. Retinas isolated from Notch3+/+, Notch3+/- and Notch3-/- at P3 (A) and P5 (B) were stained with iso-lectin B4 (red) and mural cell marker NG2 (green). Confocal images were taken at 200X magnification. Bar, 50μm.
Online Figure III. Notch3 deletion does not increase vessel regression. Retinas were isolated from Notch3+/- and Notch3-/- littermates at P3, and stained with iso-lectin B4 (red) and anti-collagen IV (green). Confocal images were taken at 250X magnification. Bar, 25μm. Arrows indicate the empty sleeves (collagen IV+ iso-lectin B4−) resulting from vessel regression.
Online Figure IV. Notch3 deletion decreases the coverage of mural cells in retinal vasculature. Retinas were isolated from Notch3+/+, Notch3+/- and Notch3-/- littermates at P43, and stained with mural cell marker sm α-actin (green) and iso-lectin B4 (red) (A). Confocal images were taken at 100X magnification. Bar, 100μm. Retinas were stained with sm α-actin (green), DAPI (blue, for nuclei) as well as iso-lectin B4 (red) (B). Arrows indicate the nuclei of mural cells. Confocal images were taken at 630X magnification. Bar, 20μm. (C) Quantification of mural cell nuclei in retinal arteries of Notch3+/+, Notch3+/- and Notch3-/- at P43. * P < 0.05 compared to relevant controls.
Online Figure V. Notch3 deletion does not affect proliferation of mural cells in retinal arteries. (A) Retinas were isolated from Notch3+/+, Notch3+/- and Notch3-/- littermates at P10, and stained with iso-lectin B4 (red), anti-phospho-histone H3 (pH-H3, green, for proliferation) and DAPI (blue, for nuclei). Confocal images were taken at 400X magnification. Bar, 20μm. (B) Quantification of proliferating mural cells in retinal arteries.
Online Figure VI. Notch3 deletion does not affect vessel obliteration at P12 in the OIR model. Retinas from Notch3+/- and Notch3-/- littermates were examined at P12 by microscopy after oxygen-induced retinopathy. (A) Representative retinal vasculature from Notch3+/- (left) and Notch3-/- (right) littermates at P12. White lines outline the area of vaso-obliteration. Bar, 500μm. (B) Graph represents vaso-obliteration in Notch3+/- and Notch3-/- mice at P12 (n=6 per group); ns, not significant.
Online Figure VII. Gene expression comparison in P17 retinas of Notch3+/- and Notch3-/- mice subjected to OIR. Quantitative PCR analysis of Hes1, Hrt2, Hrt3, Angiopoietin-1, Tie-2, VEGF-A and VEGF-C mRNA expression in the retinas of Notch3+/- and Notch3-/- mice at P17 after oxygen-induced retinopathy (n=3); ns, not significant; * P < 0.05 compared to relevant control.
Online Figure VIII. Gene expression in the course of OIR and normal conditions. Quantitative PCR analysis of Angiopoietin-1, Angiopoietin-2 and Tie-2 mRNA expression in the retinas of wild type mice at indicated time points (P12-P17) in OIR model and normal condition (Con) (n=3).
Online Figure IX. Gene expression comparison in P17 retinas of Notch3+/- and Notch3-/- mice under normal conditions. Quantitative PCR analysis of Hes1, Hrt2, Hrt3, Angiopoietin-1, Angiopoietin-2 and Tie-2 in P17 retinas of Notch3+/- and Notch3-/- mice under normal conditionS (n=3); ns, not significant.
Acknowledgments
The support from the Metabolic Vascular Disease Group of the Vascular Biology Center is greatly appreciated.
Source of Funding
This work was supported by NIH grant R01 HL076428 to BL and American Heart Association predoctoral fellowship 09PRE2220351.
Non-standard Abbreviations and Acronyms
- NICD
Notch intracellular domain
- OIR
oxygen induced retinopathy
- P
postnatal
- sm
smooth muscle
- VSMCs
vascular smooth muscle cells
Footnotes
Disclosures
None
Novelty and Significance
-
-Blood vessel formation or angiogenesis is a complex process that relies on cell-cell interaction.
-
-Mural cells are known to regulate angiogenesis by modulating the activity of endothelial cells.
-
-Notch signaling has been implicated in the regulation of angiogenesis.
-
-The expression of Notch3 in mural cells influences blood vessel patterning in the retina.
-
-Notch3 acts to maintain vascular integrity by controlling mural cell investment.
-
-Notch3 regulates pathological angiogenesis and regulates Angiopoietin-2 expression.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 3.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 4.Hughes CC. Endothelial-stromal interactions in angiogenesis. Curr Opin Hematol. 2008;15:204–209. doi: 10.1097/MOH.0b013e3282f97dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjarnegard M, Enge M, Norlin J, Gustafsdottir S, Fredriksson S, Abramsson A, Takemoto M, Gustafsson E, Fassler R, Betsholtz C. Endothelium-specific ablation of pdgfb leads to pericyte loss and glomerular, cardiac and placental abnormalities. Development. 2004;131:1847–1857. doi: 10.1242/dev.01080. [DOI] [PubMed] [Google Scholar]
- 7.Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial pdgf-b retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17:1835–1840. doi: 10.1101/gad.266803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 9.Bertolino P, Deckers M, Lebrin F, ten Dijke P. Transforming growth factor-beta signal transduction in angiogenesis and vascular disorders. Chest. 2005;128:585S–590S. doi: 10.1378/chest.128.6_suppl.585S. [DOI] [PubMed] [Google Scholar]
- 10.High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the notch ligand jagged1 is required for vascular smooth muscle development. Proc Natl Acad Sci U S A. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi S, Dotti MT, Federico A. Physiology and pathology of notch signalling system. J Cell Physiol. 2006;207:300–308. doi: 10.1002/jcp.20542. [DOI] [PubMed] [Google Scholar]
- 12.Bray SJ. Notch signalling: A simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 13.Gridley T. Notch signaling in vascular development and physiology. Development. 2007;134:2709–2718. doi: 10.1242/dev.004184. [DOI] [PubMed] [Google Scholar]
- 14.Roca C, Adams RH. Regulation of vascular morphogenesis by notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann JJ, Iruela-Arispe ML. Notch signaling in blood vessels: Who is talking to whom about what? Circ Res. 2007;100:1556–1568. doi: 10.1161/01.RES.0000266408.42939.e4. [DOI] [PubMed] [Google Scholar]
- 16.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 17.Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. Faseb J. 2005;19:1027–1029. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- 18.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (dll4) is induced by vegf as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Amo FF, Smith DE, Swiatek PJ, Gendron-Maguire M, Greenspan RJ, McMahon AP, Gridley T. Expression pattern of motch, a mouse homolog of drosophila notch, suggests an important role in early postimplantation mouse development. Development. 1992;115:737–744. doi: 10.1242/dev.115.3.737. [DOI] [PubMed] [Google Scholar]
- 20.Wang T, Baron M, Trump D. An overview of notch3 function in vascular smooth muscle cells. Prog Biophys Mol Biol. 2008;96:499–509. doi: 10.1016/j.pbiomolbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E. The ectodomain of the notch3 receptor accumulates within the cerebrovasculature of cadasil patients. J Clin Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindsell CE, Boulter J, diSibio G, Gossler A, Weinmaster G. Expression patterns of jagged, delta1, notch1, notch2, and notch3 genes identify ligand-receptor pairs that may function in neural development. Mol Cell Neurosci. 1996;8:14–27. doi: 10.1006/mcne.1996.0040. [DOI] [PubMed] [Google Scholar]
- 23.Loomes KM, Taichman DB, Glover CL, Williams PT, Markowitz JE, Piccoli DA, Baldwin HS, Oakey RJ. Characterization of notch receptor expression in the developing mammalian heart and liver. Am J Med Genet. 2002;112:181–189. doi: 10.1002/ajmg.10592. [DOI] [PubMed] [Google Scholar]
- 24.Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161–164. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 25.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in cadasil, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 26.Arboleda-Velasquez JF, Zhou Z, Shin HK, Louvi A, Kim HH, Savitz SI, Liao JK, Salomone S, Ayata C, Moskowitz MA, Artavanis-Tsakonas S. Linking notch signaling to ischemic stroke. Proc Natl Acad Sci U S A. 2008;105:4856–4861. doi: 10.1073/pnas.0709867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Chantemele EJ, Retailleau K, Pinaud F, Vessieres E, Bocquet A, Guihot AL, Lemaire B, Domenga V, Baufreton C, Loufrani L, Joutel A, Henrion D. Notch3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler Thromb Vasc Biol. 2008;28:2216–2224. doi: 10.1161/ATVBAHA.108.171751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, Klonjkowski B, Berrou E, Mericskay M, Li Z, Tournier-Lasserve E, Gridley T, Joutel A. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T. Characterization of notch3-deficient mice: Normal embryonic development and absence of genetic interactions with a notch1 mutation. Genesis. 2003;37:139–143. doi: 10.1002/gene.10241. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Kennard S, Lilly B. Notch3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed jagged1. Circ Res. 2009;104:466–475. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corley KM, Taylor CJ, Lilly B. Hypoxia-inducible factor 1alpha modulates adhesion, migration, and fak phosphorylation in vascular smooth muscle cells. J Cell Biochem. 2005;96:971–985. doi: 10.1002/jcb.20559. [DOI] [PubMed] [Google Scholar]
- 32.Masson VV, Devy L, Grignet-Debrus C, Bernt S, Bajou K, Blacher S, Roland G, Chang Y, Fong T, Carmeliet P, Foidart JM, Noel A. Mouse aortic ring assay: A new approach of the molecular genetics of angiogenesis. Biol Proced Online. 2002;4:24–31. doi: 10.1251/bpo30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 34.Stahl A, Connor KM, Sapieha P, Willett KL, Krah NM, Dennison RJ, Chen J, Guerin KI, Smith LE. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297–301. doi: 10.1007/s10456-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claxton S, Fruttiger M. Periodic delta-like 4 expression in developing retinal arteries. Gene Expr Patterns. 2004;5:123–127. doi: 10.1016/j.modgep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. Ng2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 37.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-b2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 39.Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, Cummins PM, Walls D, Redmond EM, Cahill PA. Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a cbf-1/rbp-jk dependent pathway. Faseb J. 2004;18:1421–1423. doi: 10.1096/fj.04-1700fje. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Prince CZ, Mou Y, Pollman MJ. Notch3 signaling in vascular smooth muscle cells induces c-flip expression via erk/mapk activation. Resistance to fas ligand-induced apoptosis. J Biol Chem. 2002;277:21723–21729. doi: 10.1074/jbc.M202224200. [DOI] [PubMed] [Google Scholar]
- 41.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marti HH. Angiogenesis--a self-adapting principle in hypoxia. Exs. 2005:163–180. doi: 10.1007/3-7643-7311-3_12. [DOI] [PubMed] [Google Scholar]
- 43.Iso T, Kedes L, Hamamori Y. Hes and herp families: Multiple effectors of the notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 44.Hackett SF, Ozaki H, Strauss RW, Wahlin K, Suri C, Maisonpierre P, Yancopoulos G, Campochiaro PA. Angiopoietin 2 expression in the retina: Upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184:275–284. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Feng Y, Wang Y, Pfister F, Hillebrands JL, Deutsch U, Hammes HP. Decreased hypoxia-induced neovascularization in angiopoietin-2 heterozygous knockout mouse through reduced mmp activity. Cell Physiol Biochem. 2009;23:277–284. doi: 10.1159/000218174. [DOI] [PubMed] [Google Scholar]
- 46.Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 47.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Phelps ED, Updike DL, Bullen EC, Grammas P, Howard EW. Transcriptional and posttranscriptional regulation of angiopoietin-2 expression mediated by igf and pdgf in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290:C352–361. doi: 10.1152/ajpcell.00050.2005. [DOI] [PubMed] [Google Scholar]
- 49.Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, Hano H, Endou H, Kanai Y. Localization of ang-1, -2, tie-2, and vegf expression at endothelialpericyte interdigitation in rat angiogenesis. Lab Invest. 2006;86:1172–1184. doi: 10.1038/labinvest.3700476. [DOI] [PubMed] [Google Scholar]
- 50.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by pdgf-b and vegf. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 51.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands dll4 and jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med. 2009;15:1289–1297. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poellinger L, Lendahl U. Modulating notch signaling by pathway-intrinsic and pathway-extrinsic mechanisms. Curr Opin Genet Dev. 2008;18:449–454. doi: 10.1016/j.gde.2008.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL MATERIAL
Detailed Methods
Animals. Notch3-/- mice in a C57BL/6 background were a generous gift from Dr. Tom Gridley.1 Notch3-/-, Notch3+/- and wild type littermates were obtained by crossing Notch3+/- and Notch3+/- mice. All experimental procedures on mice were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Committee for the Use of Animals in Research.
Cell culture. Human aortic smooth muscle cells from Lonza were grown in DMEM supplemented with 10% FBS, 100I.U./ml penicillin-streptomycin. Cells between passages 6 and 8 were used for experiments. Mouse aortic smooth muscle cells were isolated from aortas of Notch3+/- and Notch3-/- mice. Cells between passages 3 and 8 were used for experiments. All cultures were maintained in humidified 5% CO2 at 37 °C. Lentiviral and adenoviral transduction was performed as described previously.2, 3 Briefly, recombinant lentiviruses were produced by transient transfection of TN-293 cells (Clontech). Subconfluent TN-293 cells were transiently co-transfected with pCDF1-MCS2-EF1-copGFP-NICD3 or the control plasmid without insert, and the lentiviral packaging plasmids pFIV34N and pVSV-G overnight. 48 hours after transfection, viral supernatant was harvested and used for infection. The human HIF1α cDNA was cloned into the pAdTrack shuttle vector in front of the CMV promoter using KpnI and EcoRI restriction sites. The adenovirus plasmids containing GFP alone (AdGFP) and GFP together with HIF1α (AdHIF1α) were transfected into HEK293 cells, and the viral particles were amplified and purified. For infection, human aortic smooth muscle cells were seeded in a 12-well plate at a density of 2×104 cells per well 24 hours prior to viral infection. To each well, 0.5ml of lentivirus suspension diluted in 0.5ml DMEM with 10% FBS was added. Polybrene was supplemented at a final concentration of 6μg/ml. 24 hours later, cells were transferred to fresh DMEM containing 10% FBS. 72 hours after infection, cells were infected with adenoviral particles, which were diluted in 400μl Opti-MEM (Invitrogen). 2 hours after incubation, 600μl normal growth media was supplemented. The following day, the virus-containing media was replaced with fresh media. Efficiency of transduction was monitored by GFP expression.
Immunostaining on whole-mount retinas. The immunostaining in retinas was performed as previously described.3 Briefly, eyes were isolated from Notch3-/- and Notch3+/- mice at indicated time points and fixed in 4% paraformaldehyde for 30 minutes. The cornea, sclera, lens, vitreous, and hyaloid vessels were removed to isolate retinas and radial incisions were made at equal intervals along the retinal edge. Subsequently retinas were placed in cold methanol for 20 minutes, blocked and permeabilized in PBS containing 5% donkey serum and 0.3% Triton-X-100 for 1 hour. Primary antibody, Notch3 (1:100) (Santa Cruz), sm α-actin (1:1000) (Sigma), NG2 (1:200) (Millipore), Ang-2 (1:200) (Zymed), Collagen IV (1:200) (Millipore), or phospho-histone H3 (1:200) , was costained with 10μg/ml TRITC labeled-isolectin B4 (Griffonia simplicifolia) (Invitrogen) for 2 hours at 37°C. Incubations with fluorescently tagged secondary antibodies were performed at 4°C overnight, including Alexa-Fluor 488 donkey anti-mouse (1:250), Alexa-Fluor 594 goat anti-rabbit and Alexa-Fluor 488 goat anti-rabbit (Invitrogen). Retinas were flat mounted in Vectashield (Vector Laboratories). Confocal images were captured and vessels were quantified with NIH ImageJ software by a blind observer.
Cell death detection ELISA. For the detection of apoptosis, a cell death detection ELISAPLUS kit (Roche) was used to quantify DNA fragmentation according to the manufacturer's instructions. Briefly, isolated retinas were homogenized in 100μl lysis buffer, incubated for 30 minutes at room temperature, and centrifuged for 10 minutes at 2000rpm. 20μl of the supernatant of each retina were assayed. For cells, aortic smooth muscle cells from Notch3+/- and Notch3-/- mice were plated at a density of 5×104 cell in a 12-well plate, starved in DMEM with 0.25% FBS for 72 hours. An equal number of cells were lysed in 100μl lysis buffer and were utilized for cell death detection.
Aortic ring assay. Aortic sprouting assays were performed as previously described with modification.4 Briefly, aortas from Notch3+/- and Notch3-/- littermates at P11 were dissected, cut into 1-mm-thick rings, and incubated overnight in complete EBM-2 media (Lonza) containing 10% FBS before being placed into a rat tail collagen gel (1.5mg/ml). 48 hours after embedding, rings were stained with 10μg/ml TRITC labeled-isolectin B4 and NG2, and imaged by confocal microscopy. Vessels were quantified with ImageJ software.
Oxygen-induced retinopathy (OIR). OIR was induced as previously described, with minor modifications.5 In brief, at postnatal day (P)7 pups, along with nursing mothers, were placed in 70% oxygen. At P12, they were returned to 21% oxygen for 5 days. From P12 to P17, the animals were anesthetized, their retinas were collected and iso-lectinB4 immunostaining on whole-mount retinas were performed. Avascular area was quantified as percentage of whole retinal area. Neovascularization was quantified using ImageJ software by calculating the area of iso-lectinB4 staining of neovascular tufts as reported.6
RNA isolation and quantitative reverse transcriptase-polymerase chain reaction (qPCR). Total RNA was extracted from mouse retinas using RNAqueous-4PCR kit (Invitrogen) according to the manufacturer's instruction, and reverse transcribed with M-MLV reverse transcriptase (Invitrogen) to generate cDNA. Quantitative PCR was performed using a StepOne PCR system (Applied Biosystems) with Power SYBR Green. The fold difference in various transcripts was calculated by the ΔΔCT method using 18S as the internal control. After PCR, a melting curve was constructed in the range of 60°C to 95°C to evaluate the specificity of the amplification products. Primer sequences for mouse transcripts were as follows: 18S For-5’-GTT GGT TTT CGG AAC TGA GGC-3’; 18S Rev-5’-GTC GGC ATC GTT TAT GGT CG-3’; Notch3 For-5’-TTG TCT GGA TGG AAG CCC ATG T-3’; Notch3 Rev-5’-ACT GAA CTC TGG CAA ACG CCT-3’; Angiopoietin-2 For-5’-ACA CCG AGA AGA TGG CAG TGT-3’; Angiopoietin-2 Rev-5’-CTC CCG AAG CCC TCT TTG TA-3’; Angiopoietin-1 For-5’-GGG CTG GAA GGA GTA TAA AAT GG-3’; Angiopoietin-1 Rev-5’-GAA CTC GTT CCC AAG CCA ATA T-3’; Tie-2 For-5’-CAA TCA GGC CTG GAA ATA CAT TG-3’; Tie-2 Rev-5’-TCC GCG GCT CCA AGT AGT T-3’. For human Angiopoietin-2 For-5’-AAC AGG AGG CTG GTG GTT TG-3’; Rev-5’-TGT GGA TAG TAC ATT CCG TTC AAG TT-3’. Hrt3 For-5’-CGC AGA GGG ATC ATA GAG AAA CG-3’; Hrt3 Rev-5’-GCC AGG GCT CGG GCA TCA AAG AA-3’; Hes1 For-5’-CCC CAG CCA GTG TCA ACA C-3’; Hes1 Rev-5’-TGT GCT CAG AGG CCG TCT T-3’; Hrt2 For-5’-CAC ATC AGA GTC AAC CCC ATG T -3’; Hrt2 Rev-5’-GCC ATG AGC AGA AGG CAC TT-3’.
Statistical analysis. Data analyses were performed using PrismGraph and comparisons between data sets were made using Student's t test. Differences were considered significant if P < 0.05, and data are presented as mean ± standard error of the mean (SEM). Data shown are representative of at least three independent experiments.
Supplemental References
1. Krebs LT, Xue Y, Norton CR, Sundberg JP, Beatus P, Lendahl U, Joutel A, Gridley T. Characterization of Notch3-deficient mice: normal embryonic development and absence of genetic interactions with a Notch1 mutation. Genesis. 2003;37:139-143.
2. Corley KM, Taylor CJ, Lilly B. Hypoxia-inducible factor 1alpha modulates adhesion, migration, and FAK phosphorylation in vascular smooth muscle cells. J Cell Biochem. 2005;96:971-985.
3. Liu H, Kennard S, Lilly B. NOTCH3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res. 2009;104:466-475.
4. Masson VV, Devy L, Grignet-Debrus C, Bernt S, Bajou K, Blacher S, Roland G, Chang Y, Fong T, Carmeliet P, Foidart JM, Noel A. Mouse Aortic Ring Assay: A New Approach of the Molecular Genetics of Angiogenesis. Biol Proced Online. 2002;4:24-31.
5. Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101-111.
6. Stahl A, Connor KM, Sapieha P, Willett KL, Krah NM, Dennison RJ, Chen J, Guerin KI, Smith LE. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297-301.
Online Figure I. Notch3 deletion compromises retinal angiogenesis in vivo. (A, B) Retinal vasculature from Notch3+/+, Notch3+/- and Notch3-/- littermates were stained with iso-lectin B4 at P5 and P7. Graphs show quantification of vascularized area and branch points in retinas. * P < 0.05 compared to heterozygous and wild type. Images were taken at 50X by confocal microscopy. Bar, 25μm. (C, D) Superficial and deep vascular plexus from Notch3+/+ and Notch3-/- littermates were stained with iso-lectin B4 at P10 and P14. Images were taken at 100X by confocal microscopy. Bar, 100μm.
Online Figure II. Mural cells in the angiogenic front of the retina. Retinas isolated from Notch3+/+, Notch3+/- and Notch3-/- at P3 (A) and P5 (B) were stained with iso-lectin B4 (red) and mural cell marker NG2 (green). Confocal images were taken at 200X magnification. Bar, 50μm.
Online Figure III. Notch3 deletion does not increase vessel regression. Retinas were isolated from Notch3+/- and Notch3-/- littermates at P3, and stained with iso-lectin B4 (red) and anti-collagen IV (green). Confocal images were taken at 250X magnification. Bar, 25μm. Arrows indicate the empty sleeves (collagen IV+ iso-lectin B4−) resulting from vessel regression.
Online Figure IV. Notch3 deletion decreases the coverage of mural cells in retinal vasculature. Retinas were isolated from Notch3+/+, Notch3+/- and Notch3-/- littermates at P43, and stained with mural cell marker sm α-actin (green) and iso-lectin B4 (red) (A). Confocal images were taken at 100X magnification. Bar, 100μm. Retinas were stained with sm α-actin (green), DAPI (blue, for nuclei) as well as iso-lectin B4 (red) (B). Arrows indicate the nuclei of mural cells. Confocal images were taken at 630X magnification. Bar, 20μm. (C) Quantification of mural cell nuclei in retinal arteries of Notch3+/+, Notch3+/- and Notch3-/- at P43. * P < 0.05 compared to relevant controls.
Online Figure V. Notch3 deletion does not affect proliferation of mural cells in retinal arteries. (A) Retinas were isolated from Notch3+/+, Notch3+/- and Notch3-/- littermates at P10, and stained with iso-lectin B4 (red), anti-phospho-histone H3 (pH-H3, green, for proliferation) and DAPI (blue, for nuclei). Confocal images were taken at 400X magnification. Bar, 20μm. (B) Quantification of proliferating mural cells in retinal arteries.
Online Figure VI. Notch3 deletion does not affect vessel obliteration at P12 in the OIR model. Retinas from Notch3+/- and Notch3-/- littermates were examined at P12 by microscopy after oxygen-induced retinopathy. (A) Representative retinal vasculature from Notch3+/- (left) and Notch3-/- (right) littermates at P12. White lines outline the area of vaso-obliteration. Bar, 500μm. (B) Graph represents vaso-obliteration in Notch3+/- and Notch3-/- mice at P12 (n=6 per group); ns, not significant.
Online Figure VII. Gene expression comparison in P17 retinas of Notch3+/- and Notch3-/- mice subjected to OIR. Quantitative PCR analysis of Hes1, Hrt2, Hrt3, Angiopoietin-1, Tie-2, VEGF-A and VEGF-C mRNA expression in the retinas of Notch3+/- and Notch3-/- mice at P17 after oxygen-induced retinopathy (n=3); ns, not significant; * P < 0.05 compared to relevant control.
Online Figure VIII. Gene expression in the course of OIR and normal conditions. Quantitative PCR analysis of Angiopoietin-1, Angiopoietin-2 and Tie-2 mRNA expression in the retinas of wild type mice at indicated time points (P12-P17) in OIR model and normal condition (Con) (n=3).
Online Figure IX. Gene expression comparison in P17 retinas of Notch3+/- and Notch3-/- mice under normal conditions. Quantitative PCR analysis of Hes1, Hrt2, Hrt3, Angiopoietin-1, Angiopoietin-2 and Tie-2 in P17 retinas of Notch3+/- and Notch3-/- mice under normal conditionS (n=3); ns, not significant.