Abstract
Polychlorinated biphenyls (PCBs) are an important group of environmental pollutants that have been associated with adverse human health effects. Despite recent advances in their synthesis, the availability of PCB congeners in sufficient quantity and purity still represents one obstacle in the investigation of their biological effects. We report herein improved syntheses of PCB congeners (and their metabolites) containing two or more ortho-chlorine substituents. The Suzuki coupling reaction at 110 °C yielded PCB congeners with a 2,2′-substitution pattern in good yields (78–99%), but failed to give PCB congeners with 3 or 4 ortho chlorine substituents. Symmetrically substituted PCB congeners with multiple ortho chlorine substituents were obtained in 20–52% yields using a modified Ullmann coupling reaction. The yield of the coupling increased with increasing degree of chlorination of the starting material. The modified Ullmann coupling reaction employed much milder reaction conditions (copper-bronze/CuCl in N-methylpyrrolidinone, 110°C) and, therefore, appears to be advantageous compared to the classical Ullmann coupling reaction (copper-bronze, no solvent, 230°C). PCBs 136 and 155 prepared via the modified Ullmann coupling reaction were nitrated and reduced with Na2S2O4 to the corresponding amino-PCBs. Subsequently, the amino-PCBs were converted into sulfur-containing PCB metabolites or PCB 184 via the corresponding diazonium salt. These modified reaction conditions allow the synthesis of large quantities of pure, non-dioxin-like PCB congeners and their sulfur-containing metabolites for environmental and toxicological studies by overcoming problems associated with classical PCB synthesis strategies.
Keywords: Suzuki coupling, Ullmann coupling, dihedral angle, crystal structure, methylsulfonyl PCB, PCB 47, PCB 184
1. Introduction
Polychlorinated biphenyls (PCBs) are ubiquitous environmental contaminants that represent a significant environmental and human health concerns (Hansen, 1999; Robertson and Hansen, 2001). PCBs were manufactured by batch chlorination of biphenyl and have been released into the environment as complex mixtures containing a large number of structurally different compounds with the general structure C12HnCl10-n, with n = 0 to 9. The biological effects and toxicity of individual PCB congeners greatly depend on their structure, which is largely determined by the number of chlorines atoms and their substitution pattern (Lehmler et al., 2002; Shaikh et al., 2008). For example, PCB congeners with zero or one ortho chlorine substituent are frequently referred to a dioxin-like PCB congeners because they have a high affinity for the aryl hydrocarbon receptor (Bandiera et al., 1982) and, like 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), induce cytochrome P450 (CYP) 1A enzymes. Non-dioxin-like PCB congeners have multiple ortho chlorine substituents and can activate the constitutive androstane (Denomme et al., 1983) and/or the pregnane X receptor (PXR) (Schuetz et al., 1998). In recent years, non-dioxin-like PCB congeners have been of interest because of health effects, such as adverse (neuro-)developmental effects (Kodavanti, 2004). Therefore, there is considerable need for synthetic methods for the preparation of large quantities of pure non-dioxin-like PCB congeners and their metabolites for environmental and toxicological studies.
Different approaches can be employed to synthesize PCB derivatives. We reported that the Suzuki coupling reaction can be used for the synthesis of individual PCB congeners and their metabolites (Bauer et al., 1995; Kania-Korwel et al., 2004; Lehmler and Robertson, 2001a). However, this approach does not yield PCB congeners with multiple ortho chlorines. The Ullmann coupling reaction, another valuable tool for the synthesis of PCBs (Bolgar et al., 1995), can be used to synthesize PCB derivatives with three or four ortho chlorine substituents. This approach also suffers from several drawbacks, such its limitation to symmetrical coupling products and the formation of undesired and potentially toxic byproducts (Goldstein et al., 1978; Moron et al., 1973; Shaikh et al., 2006).
Here we build on advances in the Suzuki and the Ullmann coupling reactions and describe improved approaches for the synthesis of non-dioxin-like PCB congeners and their sulfur containing PCB metabolites. In addition, the three-dimensional structure of PCB 47 and 184 was characterized by x-ray crystallography.
2. Results and discussion
2.1. Suzuki coupling reaction
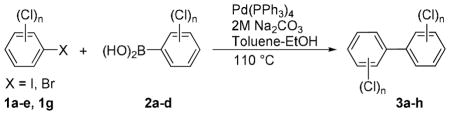
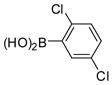
The Pd(PPh3)4 catalyzed Suzuki coupling reaction is a powerful reaction for the synthesis of individual PCB congeners (Kania-Korwel et al., 2004; Lehmler and Robertson, 2001b) and their mono- and dihydroxy metabolites (Bauer et al., 1995; Kania-Korwel et al., 2004; Lehmler and Robertson, 2001a) in good-to-excellent yields and high purity. In our hands, one major disadvantage of the Suzuki coupling reaction is its limitation to afford only PCB congeners with chlorine or methoxy substituents in 2- and 2,6-positions. Based on earlier studies of the synthesis of sterically hindered biphenyls (Suzuki, 1999), we revisited the synthesis of PCB congeners with multiple ortho chlorine substituents using the Pd(PPh3)4-catalyzed Suzuki coupling reaction in toluene/ethanol/water as solvent. Conducting the coupling reaction of iodo/bromobenzenes 1a–e with chlorinated benzene boronic acids 2a–d at higher temperature (110°C instead of 80°C) gave PCB congeners 3a–h with the desired 2,2′-dichloro substitution pattern in good-to-excellent yields (Table 1), whereas no Suzuki coupling products were isolated when the reaction was performed at 80°C. However, coupling of 2,3,6-trichloroiodobenzene (1g) with 2,5-dichlorophenyl boronic acid (2d) using the same reaction conditions did not give the desired PCB congener.
Table 1.
PCB congeners synthesized by modified Suzuki coupling
 | |||||
|---|---|---|---|---|---|
| Entry | Bromo/iodobenzene | Boronic acid | Pcb congener | Yield (%) | Mpa,b [°C] |
| 1 |
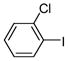 1a |
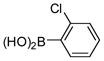 2a |
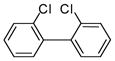 3a (PCB 4) |
95 | 57–58.5 (60–61) |
| 2 |
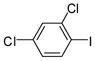 1b |
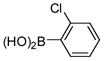 2a |
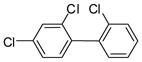 3b (PCB 17) |
99 | Oil |
| 3 |
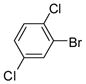 1c |
 2a |
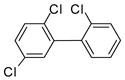 3c (PCB 18) |
87 | 40–42 (44–46) |
| 4 |
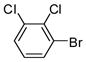 1d |
2b |
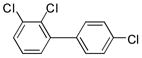 3d (PCB 22) |
88 | 70.5–71.5 (72–73) |
| 5 |
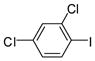 1b |
 2c |
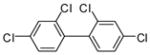 3e (PCB 47) |
99 | 39–41 (41–42) |
| 6 |
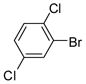 1c |
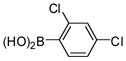 2c |
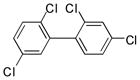 3f (PCB 49) |
89 | 63–64 (66–67) |
| 7 |
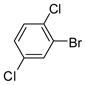 1c |
 2d |
 3g (PCB 52) |
88 | 84–86 (85–86.5) |
| 8 |
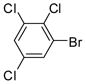 1e |
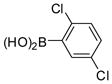 2d |
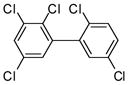 3h (PCB 92) |
78 | 58.5–60 (60–61) |
| 9 |
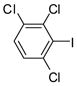 1g |
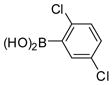 2d |
No PCB 149 isolated | ||
Values in parenthesis are literature values (Bolgar et al., 1995);
recrystallized from methanol.
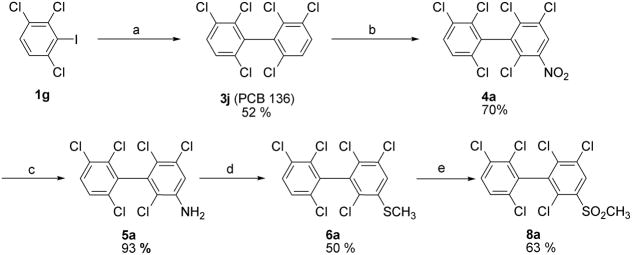
2.2. Symmetrical Ullmann coupling reaction in solution
The Ullmann coupling is frequently used to synthesize PCB congeners with three or four ortho-chlorine substituents. Typically, PCB congeners are synthesized using the classical Ullmann coupling conditions, i.e., a chlorinated iodobenzene is reacted for several days in the presence of copper bronze at approximately 230°C (Shaikh et al., 2006). Although the Ullmann coupling reaction yields many PCB congeners in good yields, it only allows the straightforward synthesis of symmetrical PCB congeners. Although it can be used to synthesize unsymmetrical PCB congeners, the resulting products are mixtures of three PCB congeners that are often difficult to separate. In addition, the formation of undesired (by-) products, such as tetraarylstannanes (Shaikh et al., 2006) or toxic dibenzofuran by-products (Goldstein et al., 1978), limits the usefulness of the Ullmann coupling reaction. Therefore, PCB congeners derived from the Ullmann coupling reaction need to be subjected to a rigorous charcoal clean-up to remove the dibenzofuran impurities, especially for in vivo studies.
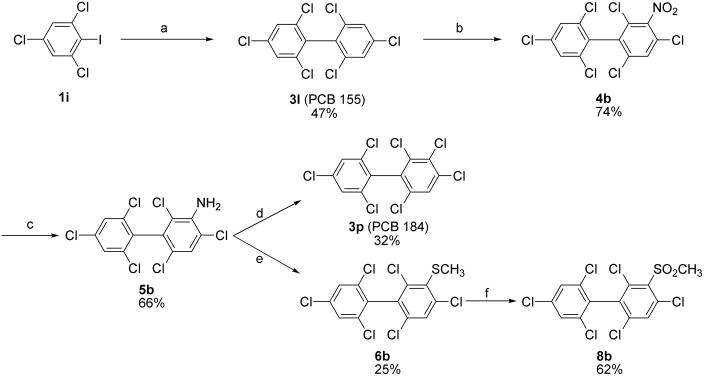
Several modified coupling conditions have been reported to overcome problems associated with the classical Ullmann coupling reaction. These modified reactions are typically performed at reduced temperatures in solvents like N-methylpyrrolidinone (NMP) (Zhang et al., 1997) with Copper-bronze and catalytic amounts of Cu(I)Cl (Newman and Logue, 1971); however, these conditions have not been applied to the synthesis of individual PCB congeners. As shown in Table 2, symmetrical Ullmann coupling reactions with copper-bronze/CuCl in NMP at 110°C gave good yields in the case of PCBs with ≥ 6 chlorine substituents (3j–l) and moderate yields in the case of PCBs with ≤ 4 chlorines (3e and 3i). The coupling reaction is typically complete within 17–48 hours; however, small quantities of dechlorination products were observed in addition to the desired PCB congener. For example, the reaction of 2,4,6-trichloroiodobenzene (1i) in the presence of copper-bronze/CuCl in NMP at 110°C gave the desired 3l in 47% yield and the undesired dechlorination product 2,2′,4,6,6′-pentachlorobiphenyl, PCB 104 (3m), in 5% yield.
Table 2.
PCB congeners prepared by the modified symmetrical Ullmann coupling reaction
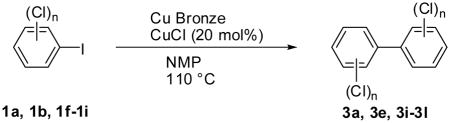 | ||||
|---|---|---|---|---|
| Entry | Iodobenzene | PCB congener | Yield (%) | mpa,b [°C] |
| 1 |
 1a |
No PCB 4 isolated | ||
| 2 |
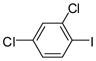 1b |
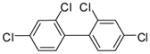 3e (PCB 47) |
24 | 37–39 (41–42) |
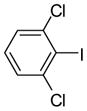
| 3 |
 1f |
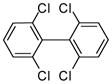 3i (PCB 54) |
20 | 197–199.5 (197–198) |
| 4 |
 1g |
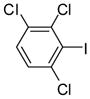
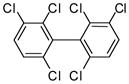 3j (PCB 136) |
52 | 109–110 (112–113.5) |
| 5 |
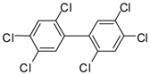
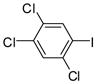 1h |
 3k (PCB 153) |
47 | 100–102 (102–103) |
| 6 |
 1i |
 3l (PCB 155) |
47 | 111–112 (111–113) |
Values in parenthesis are literature values (Bolgar et al., 1995);
recrystallized from methanol.
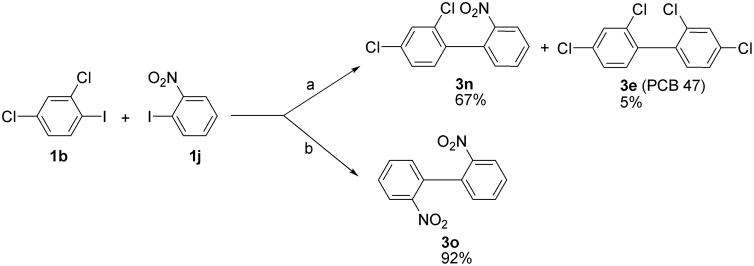
The coupling of 2-chloro-1-iodobenzene (1a) did not yield the desired 2,2′-dichlorobiphenyl (3a), whereas 2-nitro-1-iodobenzene (1j) gave 2,2′ dinitrobiphenyl (3o) in excellent yield (92%). These observations are not surprising because electron withdrawing groups generally increase the ease of the formation of organocopper intermediates and, thus, facilitate the Ullmann coupling reaction (Fanta, 1974).
2.3. Modified unsymmetrical Ullmann coupling reaction in solution
In general, acceptable yields of unsymmetrical biaryls can be obtained via the Ullmann coupling reaction when the two starting materials differ significantly in the reactivity (Fanta, 1974) and the reaction is performed in NMP at 150°C to minimize homo coupling of the starting materials (Suzuki et al., 1997). Based on these earlier observations and our results with symmetrical copper-bronze/CuCl-catalyzed Ullmann coupling reactions, we investigated the coupling reaction of 2,4-dichloro-1-iodobenzene (1b) and 2-nitro-1-iodobenzene (1j) with copper-bronze in NMP at 150 °C (Scheme 1). Under these conditions, the desired 2,4-dichloro-2′-nitrobiphenyl (3n) was obtained in 67% yield. At the same time, only 5% of PCB 47 (3e), the homocoupling product of 2,4-dichloro-1-iodobenzene (1b), were isolated from the reaction mixture. In contrast, the reaction of 2,4-dichloro-1-iodobenzene (1b) and 2-nitro-1-iodobenzene (1j) using copper-bronze/CuCl at 110°C gave exclusively 2,2′-dinitrobiphenyl (3o) (Scheme 1).
Scheme 1.
Modified symmetrical and unsymmetrical Ullmann coupling reaction. (a) Cu-bronze, N-methyl-2-pyrrolidinone (NMP), 150 °C, Argon; 4 h; (b) Cu-bronze, CuCl (20 mol%), N-methyl-2-pyrrolidinone (NMP), 110 °C, Argon.
Although we did not explore this further, the unsymmetrical Ullmann coupling reaction of chlorinated iodobenzenes with nitro-1-iodobenzenes in the presence of copper-bronze/NMP may be particularly useful for the synthesis of unsymmetrical PCB metabolites. As mentioned above, the nitro group not only activates the iodobenzene for unsymmetrical coupling, but also facilitates the isolation of the desired unsymmetrical biaryl by column chromatography. Furthermore, the nitro group can be converted into -Cl, -OH, and -SO2CH3 groups (see below), thus making PCBs and PCB metabolites available for toxicological studies.
2.4. Synthesis of PCBs and PCB metabolites with multiple ortho chlorine substituents
Because PCB congeners with multiple ortho chlorine substituents (e.g., 3a–l) are available in large (gram) quantities using the modified Suzuki or the Ullmann coupling reactions described above, we employed PCBs 136 (3j) and 155 (3l) as intermediates for the synthesis of methyl sulfonyl PCBs and other PCB derivatives (Schemes 2 and 3). As shown in Scheme 2, several putative sulfur-containing metabolites of PCB 136 were synthesized from PCB 136 (3j). The nitration of PCB 136 (3j) and the subsequent reduction of the nitro group were performed as described previously (Waller et al., 1999). The methyl sulfide derivative 6a was prepared using a Sandmeyer-type reaction (Mizutani et al., 1978), followed by oxidation of the methyl sulfide 6a at 70 °C in H2O2-acetic acid (Bergman and Wachtmeister, 1978). In an initial experiment this oxidation reaction yielded the desired PCB methyl sulfone 8a as minor product (20% yield) and PCB sulfoxide 7 as major product (48% yield). In a subsequent experiment with fresh H2O2, PCB sulfone 8a was isolated as single product in 63 % yield.
Scheme 2.
Synthesis of PCB 136 methyl sulfide and sulfone derivatives 6a and 8a. (a) Cu-bronze, N-methyl-2-pyrrolidinone (NMP), 110 °C, Argon; (b) HNO3-H2SO4, CH2Cl2, 0 °C to rt, 16 h; (c) Na2S2O4, EtOH-H2O, 80 °C, 4 h; (d) NaNO2/HCl, Cu, NaOH, CH3SNa, 0 °C to rt, 50°C for 0.5 h; (e) AcOH-H2O2, 70 °C, 4h.
Scheme 3.
Synthesis of PCB 184 (3p) via PCB 155 (3l) using the modified Ullmann coupling reaction. (a) Cu-bronze, N-methyl-2-pyrrolidinone (NMP), 110 °C, Argon; (b) HNO3-H2SO4, CH2Cl2, 0 °C to rt, 12 h; (c) Na2S2O4, EtOH-H2O, 80 °C, 4 h; (d) NaNO2/HCl, CuCl, 0 °C to rt, 50°C for 0.5 h; (e) NaNO2/HCl, Cu, NaOH, CH3SNa, 0 °C to rt, 50°C for 0.5 h; (f) AcOH-H2O2, 70 °C, 4h.
A similar reaction sequence was used to synthesize methyl sulfide 6b and methyl sulfone 8b, two putative metabolites of PCB 155, as well as PCB 184 (3p) from PCB 155 (3l) (Scheme 3). In short, amino-PCB 155 5b was synthesized by nitration of PCB 155 (3l) with HNO3/H2SO4, followed by reduction of nitro-PCB 155 (4b) with Na2S2O4. The structure of both intermediates was characterized by crystal structure analysis (see Figures SM1 and SM2 in the Supplementary Material). The methyl sulfide derivative 6b was prepared analogous to 6a in 25% yield (Mizutani et al., 1978), followed by oxidation to 8b with H2O2-acetic acid in 62% yield (Bergman and Wachtmeister, 1978). Similarly, PCB 184 (3p) was synthesized from amino-PCB 155 5b in 32% yield and >98% purity (Scheme 3). Overall, the low yields of both reactions with amino-PCB 155 5b are probably due to its poor solubility in hydrochloric acid.
2.5. Molecular structure of PCB derivatives with multiple ortho chlorine substituents
Several PCB congeners with more than two ortho chlorine substituents, such as PCBs 47, 155 and 184, have been shown to activate PXR in vitro and in vivo (Schuetz et al., 1986). With the exception of PCB 155 (Singh and McKinney, 1979), only limited information is available about the structure of PCB congeners that are (putative) agonists for PXR. To add to the number of available structures, we herein describe the molecular structure of two additional PXR agonists, PCB 47 and PCB 184, in the solid state (Figure 1). Of particular interest is the dihedral angle as an important determinant of the biological activities of PCBs (Lehmler et al., 2002; Shaikh et al., 2008). PCB 47 (3e) had a solid state dihedral angles of 61.0°, whereas the dihedral angle for PCB 184 (3p) was significantly larger with 86.05(16)° and 86.64(16)° for the two molecules in the asymmetric unit. Overall, these dihedral angles are comparable to other PCB congeners with the same number of ortho chlorine substituents (Vyas et al., 2006).
Figure 1.
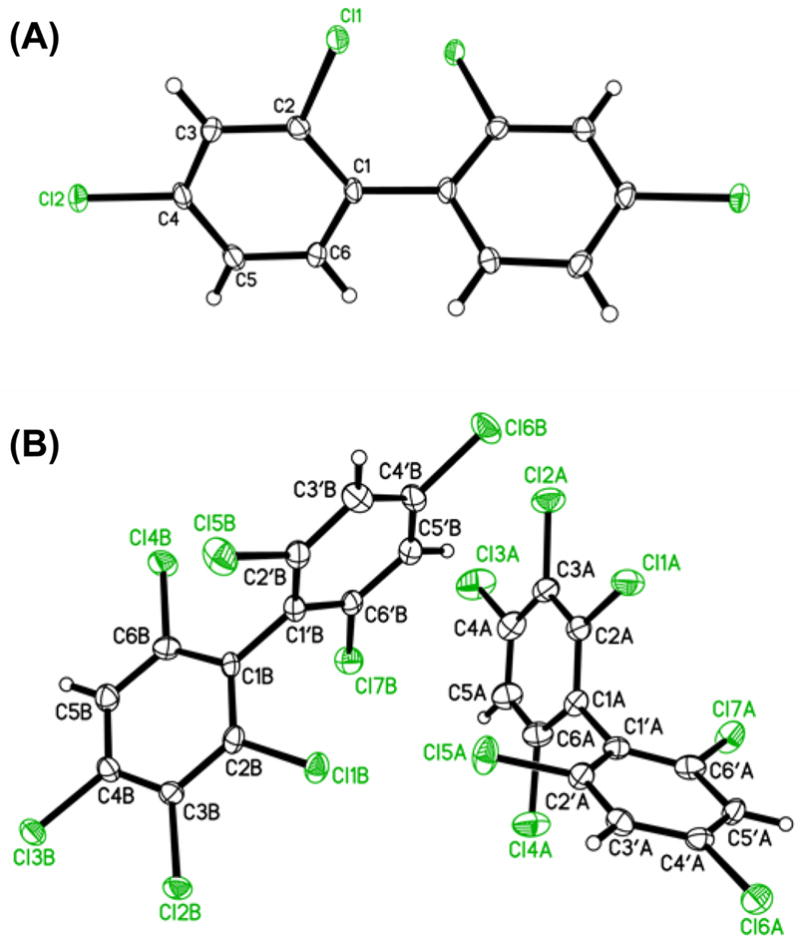
Molecular structure of (A) PCB 47 (3e) and (B) PCB 184 (3p) showing the atom labeling scheme. Displacement ellipsoids are drawn at the 50% probability level.
In this and in earlier studies (Lehmler et al., 2002; Shaikh et al., 2008; Vyas et al., 2006), the calculated dihedral angles of PCB derivatives (73.0° and 89.9° for PCB 47 and PCB 184, respectively) are larger compared to the solid state dihedral angles. This difference between solid-state and calculated dihedral angles is the result of crystal packing effects, which allow the biphenyl system to adopt an energetically less favourable conformation to maximize intermolecular interactions, and thus the lattice energy in the crystal. Similarly, PCB congeners (especially PCB congeners with 2 or less ortho chlorine substituents) are somewhat more flexible and can adopt an energetically unfavourable conformation because of ligand-receptor interactions.
3. Conclusions
The synthesis of large quantities of pure and well-characterized non-dioxin-like PCB congeners with multiple ortho chlorine substituents represents a significant synthetic challenge because “classic” synthetic approaches, such as the Suzuki and the Ullmann coupling reactions, have limitations, such as poor yields and the formation of undesired and toxic byproducts. The present study investigated several modifications that expand the scope of these reactions, and thus allow the straightforward synthesis of large (gram) quantities of PCB congeners with multiple ortho substituents. Performing the Suzuki coupling reaction at elevated temperatures (110°C) yielded PCB congeners with a 2,2′ chlorine substitution pattern in good yields; however, no PCB congeners with three or four ortho chlorine substituents were obtained under these reaction conditions. The modified Ullmann coupling reaction performed with copper-bronze/CuCl in NMP at 110°C gave good yields of symmetrical PCB congeners. In addition, unsymmetrical Ullmann coupling reactions performed in solution were employed to synthesize nitro-PCB derivatives that are potentially useful synthetic intermediates. The PCB congeners synthesized with the modified Ullmann coupling reaction were used to synthesize several PCB derivatives, including sulfur containing PCB metabolites. Overall, the modified coupling reactions can by used to synthesize PCB derivatives with multiple ortho substituents, and thus expand the strategies that are available for the synthesis of PCB derivatives for environmental and toxicological studies.
4. Experimental
All PCB derivatives were characterized by 1H and 13C NMR spectroscopy and GC-MS in electron impact (EI) mode. Their purity was assessed by gas chromatography and combustion analysis as described previously (Kania-Korwel et al., 2004; Lehmler and Robertson, 2001a, b). A detailed description of the instruments used and the analytical data of the PCB derivatives are provided in the Supplementary Material. A detailed description of the synthesis of the PCB 136 (3j) and PCB 155 (3l) derivatives, including PCB 184 (3p), are also provided in the Supplementary Material. In short, the nitration of PCB 136 (3j) and PCB 155 (3l) was performed as described by Waller and co-workers (Waller et al., 1999). The resulting nitro-PCBs 4a and 4b were reduced to 5a and 5b using sodium hydrosulfite (Waller et al., 1999). PCB methyl sulfides 6a and 6b and PCB 184 were synthesized from the corresponding NH2-PCBs (Mizutani et al., 1978). PCB methyl sulfides 6a and 6b were oxidized using H2O2 as described by Bergman and Wachtmeister (Bergman and Wachtmeister, 1978).
4.1. Typical experimental procedure for the Suzuki coupling reaction
A 2M Na2CO3 solution (2 eq.), boronic acid 2a–d (1.5 eq.) dissolved in 270 mL toluene:ethanol (9:1, v/v) and Pd(PPh3)4 (3 mol%) were added under argon to a solution of the respective chlorinated iodo- or bromobenzene 1a–e (15–20 mmol) in toluene (30 mL). The pale yellow reaction mixture was heated to 110 °C under argon until all the starting iodo- or bromobenzenes were completely consumed (16–24 h). The reaction mixture was allowed to cool to room temperature and washed with 2M NaOH (2 × 100 mL). The aqueous phase was extracted with diethylether (1 × 50 mL). The combined organic phase was washed with water (2 × 100 mL); dried over MgSO4; filtered and concentrated in vacuo to give a dark yellow oil. Column chromatography on silica gel using hexane as eluent followed by recrystallization from methanol gave the desired PCB congeners in good-to-excellent yields (Table 1).
4.1.1. 2,2′-Dichlorobiphenyl (PCB 4, 3a)
Off-white solid; 1H NMR (400 MHz, CDCl3): δ/ppm 7.29–7.39 (m, 6 H), 7.50–7.53 (m, 2 H); 13C NMR (100 MHz, CDCl3): δ/ppm 126.7, 129.4, 129.6, 131.4, 133.7, 138.6; Anal. Calcd for C12H8Cl2: C, 64.60; H, 3.61; Found: C, 64.62; H, 3.60; GC-MS (m/z, relative abundance %): 222 (M·+, 86), 187 (M-Cl, 50), 152 (M-2Cl, 100); IR (KBr pellet, ν, cm−1): 1002, 1052, 1079, 1453, 3054 and 3061 (aromatic C-H stretch).
4.2. Typical experimental procedure for the modified Ullmann Coupling reaction
Freshly activated copper-bronze powder (200 mg/mmol) (Kleiderer and Adams, 1933) was added in small portions to a solution of the respective chlorinated iodobenzene 1a–b,f–i (10 mmol) and CuCl (200 mg, 20 mol%) in N-methyl-pyrrolidinone (30 mL) under nitrogen and the mixture was heated to 110 °C for 17–48 h. The reaction mixture was allowed to cool to room temperature and diluted with ethyl acetate (50 mL). The mixture was filtered over a short column of Celite to remove copper-bronze, and the column was washed with ethyl acetate (50 mL). The organic phase was washed with sat. NH4OH (2 × 100 mL) and water (1 × 100 mL); dried over MgSO4; filtered and concentrated in vacuo to give a dark brown oil. Column chromatography on silica gel using hexane as eluent followed by recrystallization from methanol gave the symmetrically substituted polychlorinated biphenyls 3a,e,i–l in moderate to good yields (Table 2).
4.2.1. 2,2′,6,6′-Tetrachlorobiphenyl (PCB 54, 3i)
Colorless crystalline solid; 1H NMR (400 MHz, CDCl3): δ/ppm 7.32–7.36 (m, 2 H), 7.46 (“d”, J = 8.0 Hz, 4 H); 13C NMR (100 MHz, CDCl3): δ/ppm 128.1, 130.4, 135.3, 135.4; Anal. Calcd for C12H6Cl4: C, 49.36; H, 2.07; Found: C, 49.18; H, 2.03; GC-MS (m/z, relative abundance %): 290 (M·+, 79), 220 (M-2Cl, 61), 150 (M-4Cl, 16); IR (KBr pellet, ν, cm−1): 1179, 1116, 1187, 1202, 1408, 1425, 1432, 3058 and 3084 (aromatic C-H stretch).
4.3. Unsymmetrical Ullmann Coupling reaction without copper(I)chloride
To a mixture of freshly activated copper-bronze powder (500 mg) (Kleiderer and Adams, 1933) and 2,4-dichloro-iodobenzene 1b (1.1 g, 4.0 mmol), a solution of 1-iodo-2-nitrobenzene 1j (0.25 g, 1.0 mmol) in N-methyl-pyrrolidinone (5 mL) was added drop wise over 4 h period under nitrogen while the reaction mixture was heated at 150 °C. The mixture was stirred for an additional 30 min. at that temperature, allowed to cool to room temperature and diluted with ethyl acetate (15 mL). The mixture was filtered over a short column of Celite to remove copper-bronze and the column was washed with ethyl acetate (15 mL). The organic phase was washed with sat. NH4OH (2 × 30 mL) and water (1 × 50 mL); dried over MgSO4; filtered and concentrated in vacuo to give a dark brown oil. Column chromatography on silica gel using hexane as eluent gave PCB 47 as byproduct (30 mg, 5%, calculated based on 1b). Subsequently, 2,4-dichloro-2′-nitrobiphenyl (3n) was eluted from the column with EtOAc:hexane (5:95, v/v). Recrystallization from methanol yielded 3n as a pale yellow crystalline solid in 67% yield.
4.3.1. 2,4-Dichloro-2′-nitrobiphenyl (3n)
Pale yellow crystalline solid; yield, 67%; mp 70–71 °C (95% aq. methanol); 1H NMR (400 MHz, CDCl3): δ/ppm 7.21 (d, J = 8.2 Hz, 1 H), 7.34 (m, 2 H), 7.48 (d, J = 2.0 Hz, 1 H), 7.59 (d“t”, J = 7.7 Hz, J = 1.2 Hz, 1 H), 7.70 (d“t”, J = 7.7 Hz, J = 1.2 Hz, 1H), 8.12 (dd, J = 7.7 Hz, J = 1.2 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ/ppm 124.8, 127.5, 129.5, 129.6, 130.8, 132.5, 133.4, 133.5, 133.8, 134.9, 136.0, 148.7; Anal. Calcd for C12H7Cl2NO2: C, 53.76; H, 2.63; N, 5.22; Found: C, 53.72; H, 2.56; N, 5.23; GC-MS (m/z, relative abundance %): 267 (M·+, 1), 232 (M-Cl, 100), 202 (27), 186 (M-Cl-NO2, 25), 150 (M-2Cl-H-NO2, 26), 139 (12); IR (KBr pellet, ν, cm−1): 811, 854, 1104, 1350 (N-O symmetrical stretch), 1524 (N-O asymmetrical stretch), 3057 and 3091 (aromatic C-H stretch).
4.4. Unsymmetrical Ullmann Coupling reaction with copper(I)chloride
To a mixture of freshly activated copper-bronze powder (200 mg/mmol) (Kleiderer and Adams, 1933), CuCl (40 mg, 20 mol%) and 2,4-dichloro-iodobenzene 1b (0.55 g, 2.0 mmol), a solution of 1-iodo-2-nitrobenzene 1j (0.25 g, 1.0 mmol) in N-methyl-pyrrolidinone (10 mL) was added drop wise over a 8 h period under nitrogen while the reaction mixture was heated at 110 °C. The mixture was allowed to stir for an additional 16 h at that temperature, after which it was cooled to room temperature and diluted with ethyl acetate (15 mL). The mixture was filtered over a short column of Celite to remove copper-bronze and the column was washed with ethyl acetate (15 mL). The organic phase was washed with sat. NH4OH (2 × 30 mL) and water (1 × 50 mL); dried over MgSO4; filtered and concentrated in vacuo to give a dark brown oil. Column chromatography on silica gel using hexane as eluent gave unreacted 2,4-dichloro-iodobenzene. Subsequently, elution with 25–30% EtOAc in hexane yielded 2,2′-dinitrobiphenyl (3o) as pale yellow solid (92%).
5.5. X-ray crystal structure analysis
X-ray diffraction data were collected at 90.0(2) K on either a Nonius KappaCCD or a Bruker-Nonius X8 Proteum diffractometer with graded-multilayer focusing optics as described previously (Lehmler et al., 2002; Shaikh et al., 2008). Crystal data and relevant details of the structure determinations are presented in the Supplementary Material. CCDC-684977 (for 3e), 684975 (for 4b), -684976 (for 5b) and -684978 (for 3p) contain the supplementary crystallographic data for this paper. Copies of the data can be obtained free of charge on application to the CCDC, 12 Union Road, Cambridge CB2 1EZ, U.K. (fax, (+44)1223-336- 033; e-mail, deposit@ccdc.cam.ac.uk).
Supplementary Material
Acknowledgments
This research was supported by grants ES05605, ES012475 and ES013661 from the National Institute of Environmental Health Sciences, NIH, (HJL and LWR) and MRI grant #0319176 (SP) from the National Science Foundation. Contents of this manuscript are solely the reponsibility of the authors and do not necessarily represent the official views of the NIEHS/NIH or NSF.
References
- Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone, 3-methylcholanthrene or mixed-type inducers to cytosolic Ah receptor. Chem Biol Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Bauer U, Amaro AR, Robertson LW. A new strategy for the synthesis of polychlorinated biphenyl metabolites. Chem Res Toxicol. 1995;8:92–95. doi: 10.1021/tx00043a012. [DOI] [PubMed] [Google Scholar]
- Bergman A, Wachtmeister CA. Synthesis of methylthio- and methylsulfonyl-polychlorobiphenyls via nucleophilic aromatic substitution of certain types of polychlorobiphenyls. Chemosphere. 1978;7:949–956. [Google Scholar]
- Bolgar M, Cunningham J, Cooper R, Kozloski R, Hubball J, Miller DP, Crone T, Kimball H, Janooby A, Miller B, Fairless B. Physical, spectral and chromatographic properties of all 209 individual PCB congeners. Chemosphere. 1995;31:2687–2705. [Google Scholar]
- Denomme MA, Bandiera S, Lambert I, Copp L, Safe L, Safe S. Polychlorinated biphenyls as phenobarbitone-type inducers of microsomal enzymes. Structure-activity relationships for a series of 2,4-dichloro-substituted congeners. Biochem Pharmacol. 1983;32:2955–2963. doi: 10.1016/0006-2952(83)90402-1. [DOI] [PubMed] [Google Scholar]
- Fanta PE. The Ullmann Synthesis of Biaryls. Synthesis. 1974:9–21. doi: 10.1021/cr60119a004. [DOI] [PubMed] [Google Scholar]
- Goldstein JA, Hass JR, Linko P, Harvan DJ. 2,3,7,8-Tetrachlorodibenzofuran in a commercially available 99% pure polychlorinated biphenyl isomer identified as the inducer of hepatic cytochrome P-448 and aryl hydrocarbon hydroxylase in the rat. Drug Metab Dispos. 1978;6:258–264. [PubMed] [Google Scholar]
- Hansen LG. The ortho side of PCBs: Occurrence and disposition. Boston: Kluwer Academic Publishers; 1999. [Google Scholar]
- Kania-Korwel I, Parkin S, Robertson LW, Lehmler HJ. Synthesis of polychlorinated biphenyls and their metabolites with a modified Suzuki coupling. Chemosphere. 2004;56:735–744. doi: 10.1016/j.chemosphere.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Kleiderer EC, Adams R. Stereochemistry of biphenyls. XXXI. Preparation and properties of 2,2′,6,6′-tetrafluoro-3,3′-dicarboxy-5,5′-dichlorobiphenyl. J Am Chem Soc. 1933;55:4219–4225. [Google Scholar]
- Kodavanti PRS. Intracellular signaling and developmental neurotoxicity. In: Zawia NH, editor. Molecular Neurotoxicology: environmental agents and transcription-transduction coupling. Boca Roton, FL: CRC press; 2004. pp. 151–182. [Google Scholar]
- Lehmler H-J, Parkin S, Robertson LW. The three-dimensional structure of 3,3′,5′-trichloro-4-methoxybiphenyl, a “coplanar” polychlorinated biphenyl (PCB) derivative. Chemosphere. 2002;46:485–488. doi: 10.1016/s0045-6535(01)00177-1. [DOI] [PubMed] [Google Scholar]
- Lehmler H-J, Robertson LW. Synthesis of hydroxylated PCB metabolites with the Suzuki-coupling. Chemosphere. 2001a;45:1119–1127. doi: 10.1016/s0045-6535(01)00052-2. [DOI] [PubMed] [Google Scholar]
- Lehmler H-J, Robertson LW. Synthesis of polychlorinated biphenyls using the Suzuki-coupling. Chemosphere. 2001b;45:137–143. doi: 10.1016/s0045-6535(00)00546-4. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Yamamoto K, Tajima K. Sulfur-containing metabolites of chlorobiphenyl isomers, a comparative study. J Agric Food Chem. 1978;26:862–866. doi: 10.1021/jf60218a054. [DOI] [PubMed] [Google Scholar]
- Moron M, Sundström G, Wachtmeister CA. Polychlorinated biphenyls. VI. 2,3,7,8-Tetrachlorodibenzofuran, a critical byproduct in the synthesis of 2,2′,4,4′,5,5′-hexachloro-biphenyl by the Ullmann reaction. Acta Chem Scand. 1973;27:3121–3122. doi: 10.3891/acta.chem.scand.27-3121. [DOI] [PubMed] [Google Scholar]
- Newman MS, Logue MW. Synthesis of 6,6′-diethynyldiphenic anhydride. J Org Chem. 1971;36:1398–1401. [Google Scholar]
- Robertson LW, Hansen LG. Recent advances in the environmental toxicology and health effects of PCBs. Lexington: University Press of Kentucky; 2001. [Google Scholar]
- Schuetz EG, Brimer C, Schuetz JD. Environmental xenobiotics and the antihormones cyproterone acetate and spironolactone use the nuclear hormone pregnenolone X receptor to activate the CYP3A23 hormone response element. Mol Pharmacol. 1998;54:1113–1117. doi: 10.1124/mol.54.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz EG, Wrighton SA, Safe SH, Guzelian PS. Regulation of cytochrome P-450p by phenobarbital and phenobarbital-like inducers in adult rat hepatocytes in primary monolayer culture and in vivo. Biochemistry. 1986;25:1124–1133. doi: 10.1021/bi00353a027. [DOI] [PubMed] [Google Scholar]
- Shaikh NS, Parkin S, Lehmler H-J. The Ullmann coupling reaction: A new approach to tetraarylstannanes. Organometallics. 2006;25:4207–4214. [Google Scholar]
- Shaikh NS, Parkin S, Luthe G, Lehmler H-J. The three-dimensional structure of 3,3′,4,4′-tetrachlorobiphenyl, a dioxin-like polychlorinated biphenyl (PCB) Chemosphere. 2008;70:1694–1698. doi: 10.1016/j.chemosphere.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, McKinney JD. 2,2′,4,4′,6,6′-hexachlorobiphenyl. Acta Cryst. 1979;B35:259–262. [Google Scholar]
- Suzuki A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles. J Organomet Chem. 1999;576:147–168. [Google Scholar]
- Suzuki H, Enya T, Hisamatsu Y. Synthesis and characterization of some nitrobenzanthrones. Suspected new mutagens in atmospheric environment. Synthesis. 1997:1273–1276. [Google Scholar]
- Vyas SM, Parkin S, Lehmler HJ. 2,2′,3,4,4′,5,5′-Heptachlorobiphenyl (PCB 180) Acta Cryst E. 2006;E62:o2905–o2906. [Google Scholar]
- Waller SC, He YA, Harlow GR, He YQ, Mash EA, Halpert JR. 2,2′,3,3′,6,6′-hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem Res Toxicol. 1999;12:690–699. doi: 10.1021/tx990030j. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhang D, Liebeskind LS. Ambient temperature, Ullmann-like reductive coupling of aryl, heteroaryl, and alkenyl halides. J Org Chem. 1997;62:2312–2313. doi: 10.1021/jo9700078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.