Abstract
Amplification or overexpression of MDM2 promotes a variety of human tumors by degrading tumor suppressor proteins such as p53. Phosphorylation of MDM2 on serines 166 and 186 by the survival kinase Akt inhibits p53-mediated apoptosis. However, it is unclear whether this pathway contributes to normal or malignant pathophysiology in vivo. To address these questions, we generated transgenic mice expressing the Akt-phosphorylated form of MDM2 (MDM2DDS166D/S186D) in the mammary epithelium. Activation of MDM2 delayed mammary gland involution and accelerated tumor progression in MMTV/neu transgenic mice by inhibiting apoptosis in a manner associated with decreased p53 expression. Our findings offer in vivo evidence that activation of MDM2 by Akt contributes to mammary development and tumorigenesis.
Introduction
Amplification/overexpression of murine double minute 2 (MDM2) is associated with a variety of human tumors. MDM2 was first indentified as a negative regulator of p53, a well-known tumor suppressor which prevents tumorigenesis in response to stressors and oncogenic factors(1, 2). MDM2 is known to bind to the transactivation domain of p53 and inhibit its transactivity. As a Ring finger-containing E3 ubiquitin ligase, MDM2 can also promote p53 degradation through an ubiquitin-mediated proteolytic process(3).
The serine/threonine protein kinase Akt, also known as protein kinase B (PKB), plays a critical role in tumorigenesis by regulating a variety of cellular processes, including preventing cells from undergoing apoptosis(4, 5). The importance of Akt in promoting cell survival was demonstrated by the study that targeted expression of constitutively activated Akt to the mammary epithelium of transgenic mice significantly delayed the mammary involution with impaired apoptosis(6). A number of Akt/PKB downstream targets have been indentified which are associated with apoptotic functions, such as NF-κB(7), Bad(8), Forkhead transcription factors (9, 10) and p21Cip1/WAF1(11). In addition, activated Akt can inhibit p53-meditated apoptosis through phosphorylation of its downstream target MDM2 on serine166 and serine186(12–14). The phosphorylation of MDM2 stimulates the translocation of MDM2 to the nucleus to bind to p53, and then targets p53 for degradation by the proteosome(13, 14). Activated Akt was also reported to phosphorylate MDM2 at serine188, although its function is unclear(15).
However, the Akt/MDM2/p53 pathway was largely studied in the in vitro culture system. To directly study the role of Akt activated MDM2 in mammary development and tumorigenesis, we generated transgenic mice expressing the Akt-phosphorylated form of MDM2 (MDM2DDS166D/S186D) in the mammary epithelium. Activation of MDM2 delayed mammary gland involution and accelerated tumor progression in MMTV/neu transgenic mice by inhibiting apoptosis in a manner associated with decreased p53 expression. Our findings offer in vivo evidence that activation of MDM2 by Akt contributes to mammary development and tumorigenesis.
Materials and methods
Generation of transgenics
The cDNA encoding MDM2 and MDM2DD (S166D/S186D) were subcloned respectively from PCDNA3 vector into the p206 vector(6, 14). Generation of transgenic mice was described previously(6). The genotypes of transgenic mice were identified by transgnene specific PCR and further confirmed by Southern blot analysis described previously(6).
Primary antibodies
Primary antibodies used include: rabbit polyclonal anti-MDM2 (N-20; Santa Cruz Biotechnology Inc); rabbit polyclonalanti-p53 (FL-393; Santa Cruz Biotechnology Inc); anti-p53 antibody (FL-393; Santa Cruz Biotechnology Inc); anti-p21 antibody (ab7960; abcam); anti-FOXO3a antibody (H-144; Santa Cruz Biotechnology Inc).
Isolation of primary mammary epithelial cells (MECs)
Primary mammary epithelial cells were isolated from FBV/n and MMTV/MDM2DD mammary glands by digesting with 2 mg/ml collagenase and 100 units/ml hyaluronidase (sigma) for 3 hours. The cells were incubated with 2% FBS DMEM medium in Matrigel-coated plate.
Results and Discussion
Generation of transgenic mice
To study the role of Akt-activated MDM2 in mammary development and tumorigenesis, we generated transgenic mice expressing MDM2 or Akt-phosphorylated form of MDM2(MDM2DD) in the mammary epithelium. MDM2DD mimics the active phosphorylated state of MDM2 by Akt, which was generated by substituting both the serine residues at 166 and 186 with aspartic acid. Full length cDNA of MDM2 or MDM2DD were placed under the mouse mammary tumor virus (MMTV) promoter (Fig. 1A). Genotypes of transgenic mice were identified by transgnene specific PCR (data not shown). Transgene expressions were examined at RNA level by quantitative RT-PCR (Fig. 1B, 1C) and at protein level by immunohistochemical staining (Fig. 1D–1I). The transgenes were uniformly expressed in the mammary epithelium, with the expression of MDM2DD predominantly in the nucleus and the expression of MDM2 in both cytoplasm and nucleus, while very little MDM2 was expressed in FVB/n control mice. The results were consistent with previous study which indicated that AKT mediated MDM2 Ser166 and Ser168 phosphorylations are critical for the translocation of MDM2 to nucleus (14).
Figure 1.
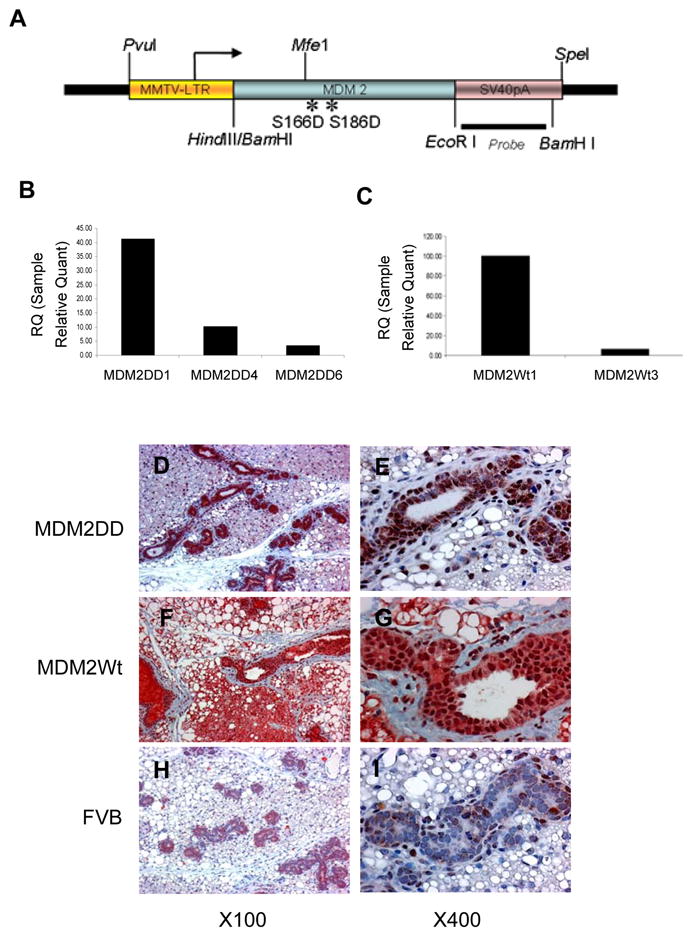
Generation of transgenic mice. (A) Structure of the transgene. (B and C) Quantitative RT-PCR analysis of MDM2DD transcript expressions in MMTV/MDM2DD (B) and MMTV/MDM2 (C) strains. (D–I) Immunohistochemical analysis of MDM2 expressions on pregnant 7 days mammary glands from MMTV/MDM2DD (D, E), MMTV/MDM2 (F, G) and FVB/n (H, I) mice.
MMTV/MDM2DD mice exhibited delayed mammary involution
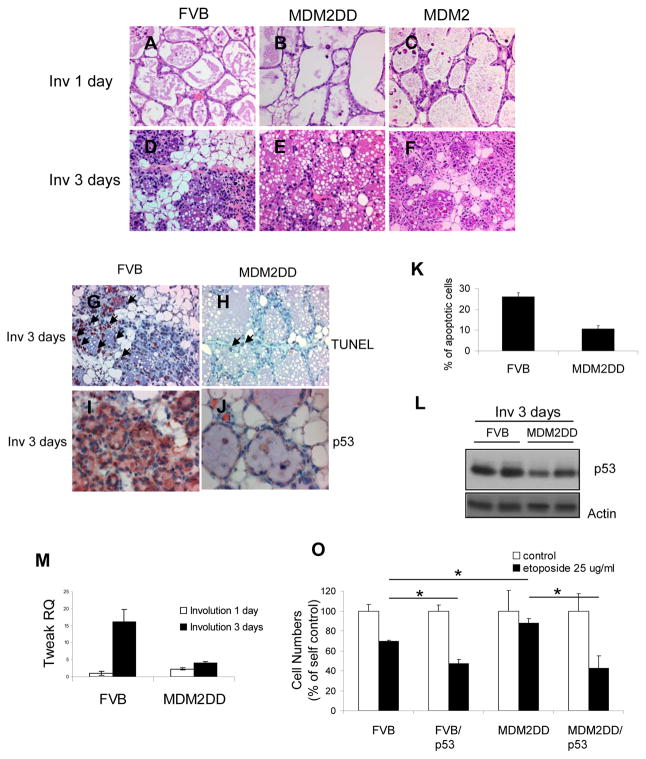
To determine the contribution of MDM2 and MDM2DD to the mammary gland development, we observed the morphological changes by whole mount and histological analysis, and checked the functional differentiations by β-casein RNA expressions, on virgin, pregnancy and lactation mammary glands. The results suggested that overexpression of MDM2 or MDM2DD had no effect on puberty, pregnancy and lactation of mammary gland (Supplement Fig. 1 and 2). Interestingly, the involution process was affected. 1 day postparturition, mammary glands from both MMTV/MDM2 and MMTV/MDM2DD mice showed no difference compared to those from FVB/n mice (Fig. 2A, 2B and 2C). The alveoli were expanded and surrounded with single-layered epithelial cells. 3 days postweaning, the FVB/n mice showed that the majority of lobuloalveolar structures were collapsed. In addition, ducts began to appear and fat cells were obvious (Fig. 2D). Mammary glands from MMTV/MDM2 mice showed similar morphology as the control mice (Fig. 2F), whereas MMTV/MDM2DD mice showed delayed involution progression with alveoli still expanding and surrounded with single-layered epithelial cells (Fig. 2E). These observations indicated that expression of MDM2DD delayed mammary gland involution in transgenic mice.
Figure 2.
MMTV/MDM2DD mice showed delayed mammary involution. (A-F) Histological patterns on involution 1 day and 3 days mammary glands from FVB/n (A, D), MMTV/MDM2DD (B, E) and MMTV/MDM2 (C, F) mice. (G, H) TUNEL analysis on involution 3 days mammary glands from FVB/n (E) and MMTV/MDM2DD (G) mice. Arrows indicated the apoptotic cells. (I, J) Immunohistochemical analysis of p53 expression on mammary glands from FVB/n (I) and MMTV/MDM2DD (J) mice. (K) Apoptotic indices of FVB/n and MDM2DD mice at involution 3 days. (L) Immunoblot analysis of p53 expression on mammary glands from FVB/n and MMTV/MDM2DD mice. (M) Quantitative RT-PCR analysis of Tweak transcript expression in mammary glands from MMTV/MDM2DD and FVB/n mice. (O) Cell number counting on MECs from MMTV/MDM2DD and FVB/n mammary glands with etoposide (25ug/ml) treatment for 3 days. Re-expressing of p53 was achieved by p53-containing lentivirus infection for 2 days.
MMTV/MDM2DD mice showed decreased epithelial apoptosis and p53 expression
Since apoptosis is the major characteristic during mammary involution, TUNEL assay was performed to assess whether the delayed mammary involution in MMTV/MDM2DD mice was associated with defected apoptosis. Significantly less apoptosis was found in MMTV/MDM2DD glands (10.7%) as compared with FVB/n glands (26%) (Fig. 2G, 2H and 2K). Previous studies showed that Tweak, LIF and TGFb3 expression was increased during mammary gland involution, which can induce apoptosis(16), we therefore compared their RNA expression in MDM2DD mice with FVB/n mice and found that the increased RNA expression of these genes in FVB/n mice during involution were inhibited in the MDM2DD mice (Fig 2M, Supplement Fig. 3A and 3B). These observations suggested MDM2DD slows mammary gland involution by inhibiting apoptosis, which corresponded with the decreased expressions of genes known to be involved in involution.
Earlier study showed that Akt activated MDM2 has an increased ability to degrade p53, we then examined whether the defective apoptosis in mammary glands from MMTV/MDM2DD mice was dependent on the change of p53 expression. We first examined the expression of p53 on mammary glands by immunohistochemical staining and immunoblotting and found that there was less p53 expression in MMTV/MDM2DD mice at involution 3 days compared to that in the FVB/n mice (Fig. 2I, 2J and 2L). Next, we treated primary mammary epithelial cells (MECs) isolated from MMTV/MDM2DD and FVB/n mice with etoposide and found that there was significantly less cell death in MDM2DD-epxressing MECs compared to those from FVB/n mice (p<0.01). Re-expressing p53 in MDM2DD-expressing MECs increased the cell death to the similar level of those from p53-expressing wild-type MECs (Fig. 2O). These results suggested that the impaired apoptosis during mammary gland involution in MDM2DD mice was dependent on the decreased expression of p53.
Although the functionality of MDM2 is not only dependent on its ability to degrade p53, MDM2 becomes more closely related to p53 function after it is activated by Akt. (14). This close regulation was proved in the current study that expression of MDM2DD in the transgenic mice delayed the mammary gland involution, which was related to decreased expression of p53 (Fig. 2J, 2L and 2O). It was also reported that transgenic mice expressing myr-Akt1 in the salivary glands showed a significant increase in MDM2 phosphorylation and reduction in apoptosis and p53 expression after gamma irradiation(17). These results indicate that Akt-activated MDM2 is critical in suppressing p53-dependent apoptosis in vivo.
Previous study showed that MDM2 transgenic mice driven by β-lactoglobulin promoter (BLG/MDM2) displayed lactation defects during mammary gland development which was independent of p53 expression (18). There are 2 reasons accounting for the differences in regarding to the affect of MDM2 to the mammary gland development shown in two studies. First, the hormone regulations on these two promoters are different, the MDM2 expression level and expression duration are different in these two transgenic mice. Second, these two transgenic mice were generated in two different genomic background (BLG/MDM2 in C57B1/6J and MMTV/MDM2 in FVB/n), which has been shown to have an impact on the phenotype of mice.
Coexpression of MDM2DD and neu results in accelerated tumor formation
To explore the roles of MDM2 and MDM2DD in mammary tumorigenesis, cohorts of virgin female MMTV/MDM2 and MMTV/MDM2DD mice were monitored for tumor formation. None of these two groups developed mammary tumors after a year of observation.
We next examined whether MDM2DD expression could collaborate with other oncogenes in promoting mammary tumorigenesis. We generated bitransgenic mice coexpressing either MDM2 or MDM2DD, with activated neu, in the mammary epithelium, by interbreeding MMTV/neu mice with MMTV/MDM2 and MMTV/MDM2DD mice respectively. Cohorts of virgin female bitransgenics and MMTV/neu mice were monitored for tumor formation. The results revealed that expression of MDM2DD accelerated the tumor formation in MMTV/neu mice (Fig. 3A). 50% of the bitransgenic mice showed tumor formation at 165 days (n=27) as compared with 179 days (n=32) from MMTV/neu mice (p<0.01). However, expression of MDM2 did not accelerate the tumor formation in MMTV/neu mice (Fig. 3B).
Figure 3.
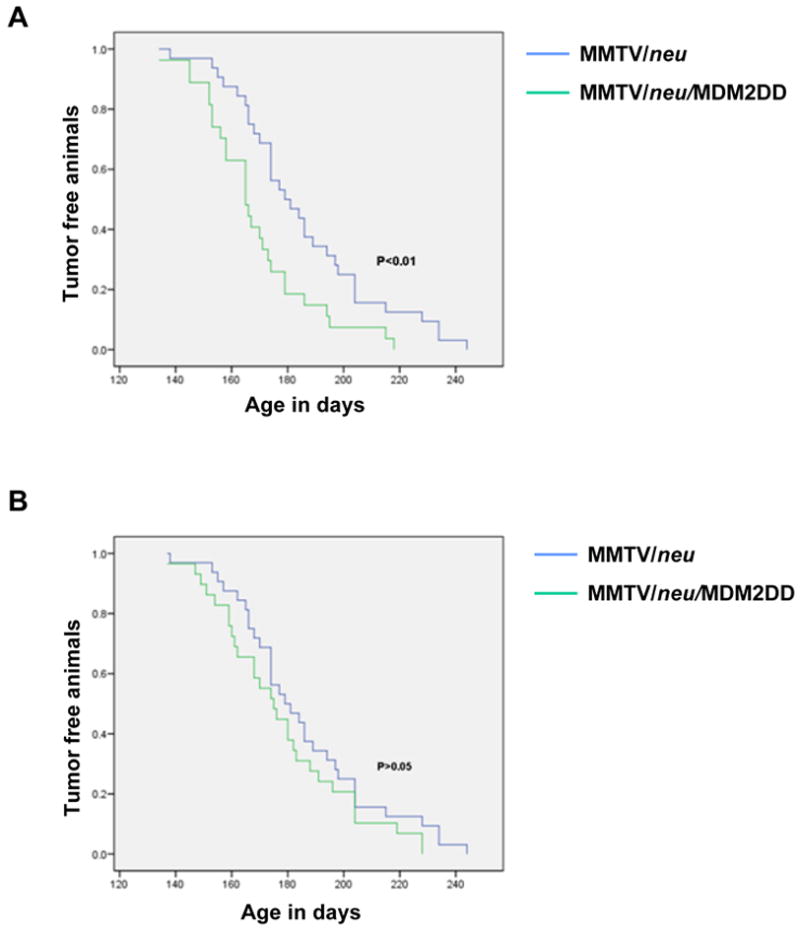
MDM2DD in affecting tumor onset. (A) MDM2DD accelerated tumor onset in MMTV/neu mice (P<0.01 by Mantel-Cox test). (B) MDM2 did not accelerate tumor onset in MMTV/neu mice (P>0.01 by Mantel-Cox test).
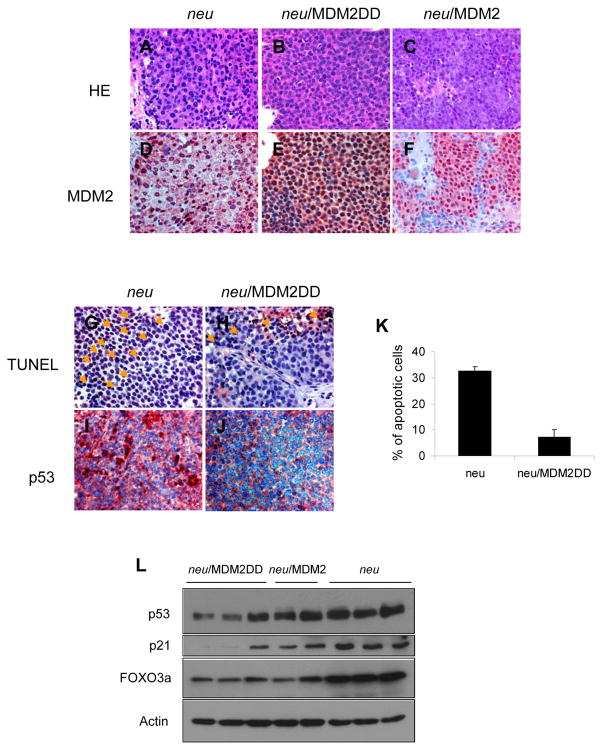
To confirm that the accelerated tumor formation in MMTV/neu/MDM2DD bitransgenic mice was due to the transgene expression, immunohistochemical staining was performed on mammary tumors. The result revealed that expressions of MDM2 were detected in bitransgenic mammary tumors (Figure 4D, 4E and 4F). Nuclear localization of MDM2 is important for its function in accelerating tumorigenesis because many MDM2 targeted tumor suppressor genes are predominantly localized in nucleus. Our data demonstrated that although MMTV/neu can promote nuclear localization of MDM2 to some extent, the amount of nuclear localized MDM2 was still less in MDM2 expressing tumors than in MDM2DD expressing tumors (Fig 4E, 4F), which might explain why MDM2 did not have a significant effect on tumorigenesis in MMTV/neu mice comparing to MDM2DD. This raises an interesting question that nuclear localized MDM2 (MDM2DD) might be more potent in inducing tumor formation than wild type MDM2 which is distributed in both cytoplasm and nucleus.
Figure 4.
MDM2DD inhibited apoptosis and decreased p53 expression in MMTV/neu tumor. (A–C) Histological patterns of MMTV/neu (A), MMTV/neu/MDM2DD (B) and MMTV/neu/MDM2 (C) tumors. (D–F) Immunohistochemical analysis of MDM2 expressions in MMTV/neu (D), MMTV/neu/MDM2DD (E) and MMTV/neu/MDM2 (F) tumors. (G, H) TUNEL analysis in MMTV/neu (G) and MMTV/neu/MDM2DD (H) tumors. Arrows indicated the apoptotic cells. (I, J) Immunohistochemical analysis of p53 expressions in MMTV/neu (I) and MMTV/neu/MDM2DD (J) tumors. (K) Apoptotic indices of MMTV/neu and MMTV/neu/MDM2DD mammary tumors. (L) Immunoblot analysis of p53, p21 and FOXO3a expressions in mammary tumors from MMTV/neu, MMTV/neu/MDM2DD and MMTV/neu/MDM2 mice.
As overexpression of MDM2DD in mammary gland was associated with an inhibition of apoptosis during involution, we therefore examined whether the accelerated tumor formation in bitransgenics was related to the survival advantage provided by MDM2DD. The degrees of apoptosis in mammary tumors from MMTV/neu/MDM2DD and MMTV/neu mice were measured. The results revealed that MDM2DD mammary tumors had a decrease in apoptosis (7.25%) compared to neu tumors (32.5%) (Fig. 4G, 4H and 4K). Since MDM2DD has increased ability to degrade p53, we next examined whether expression changes of p53 was related to MDM2DD in mammary tumors and showed that expression of p53 and its target p21 was decreased in bitransgenic mammary tumors (Fig. 4I, 4J and 4L). Interestingly, when another MDM2 target FOXO3a was examined, FOXO3a expression levels were indeed lower in the MDM2/MDM2DD mammary tumors than that of the neu expressing tumors (Fig. 4L). However, we did not observe a significant difference between MDM2 and MDM2DD expressing mammary tumors suggesting that there may be a threshold level for the reduction of FOXO3a that was already reached with wildtype MDM2 and MDM2DD is not able to reduce it further. Thus, the MDM2-mediated downregulation of FOXO3a may not significantly contribute to the differential response between MDM2 and MDM2DD mice. These observations suggested that MDM2DD accelerates mammary tumor formation in MMTV/neu mice with an impaired apoptosis, which correlates with decreased p53 expression.
Taken together, these observations suggest that expression of Akt activated MDM2 in mammary epithelium inhibits the apoptosis during mammary gland involution and accelerates the tumor formation in MMTV/neu mice. The expression of activated MDM2 corresponded with the decreased expression of p53 in vivo.
Supplementary Material
Acknowledgments
NIH PO1 CA099031, DOD COE W81WXH-06-2-0033, National Breast Cancer Foundation, Inc., Patel Memorial Breast Cancer Endowment Fund and MD Anderson-China Medical University Hospital Sister Institution Fund (to M-C. H.). In memory of Mrs. Serena Lin-Guo for her courageous fight against breast cancer.
References
- 1.Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–3. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 2.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–45. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 3.Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res. 2003;1:993–1000. [PubMed] [Google Scholar]
- 4.Huang WC, Hung MC. Induction of Akt activity by chemotherapy confers acquired resistance. J Formos Med Assoc. 2009;108:180–94. doi: 10.1016/S0929-6646(09)60051-6. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Hung MC. Signaling intricacies take center stage in cancer cells. Cancer Res. 2005;65:2511–5. doi: 10.1158/0008-5472.CAN-05-0189. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson J, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol Cell Biol. 2001;21:2203–12. doi: 10.1128/MCB.21.6.2203-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–4. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 8.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 9.Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–60. doi: 10.1016/s0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 10.Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–7. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–52. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 12.Ashcroft M, Ludwig RL, Woods DB, et al. Phosphorylation of HDM2 by Akt. Oncogene. 2002;21:1955–62. doi: 10.1038/sj.onc.1205276. [DOI] [PubMed] [Google Scholar]
- 13.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 15.Milne D, Kampanis P, Nicol S, et al. A novel site of AKT-mediated phosphorylation in the human MDM2 onco-protein. FEBS Lett. 2004;577:270–6. doi: 10.1016/j.febslet.2004.09.081. [DOI] [PubMed] [Google Scholar]
- 16.Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. 2004;6:R92–109. doi: 10.1186/bcr754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limesand KH, Schwertfeger KL, Anderson SM. MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells. Mol Cell Biol. 2006;26:8840–56. doi: 10.1128/MCB.01846-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundgren K, Montes de Oca Luna R, McNeill YB, et al. Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev. 1997;11:714–25. doi: 10.1101/gad.11.6.714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.