Summary of recent advances
The Drosophila respiratory organ (tracheal system) consists of epithelial tubes, the morphogenesis of which is controlled by distinct sets of signaling pathways and transcription factors. The downstream events controlling tube formation and shape are only now beginning to be identified. Here we review recent insight into the communication between neighboring tracheal cells, their interactions with the surrounding matrix, and the impact of these processes on tube morphogenesis. We focus on cell-cell interactions that drive rearrangement of cells within the epithelium and that are essential for maintenance of epithelial integrity, and also on cell-matrix interactions that play key roles in determining and maintaining the size and shape of tube lumens.
Introduction
In Drosophila, tracheal cells initially specified in thickenings of the embryonic epidermal epithelium invaginate via an Epidermal growth factor receptor (Egfr)-dependent mechanism to generate 10 pairs of tracheal sacs, each composed of ~ 80 cells organized in a single epithelial layer [1–4]. Genetic screens have identified many of the key regulators of tracheal cell fate and branching morphogenesis, including components of a Fibroblast Growth Factor (Branchless FGF/Breathless FGFR) signaling pathway that play a central role in multiple steps of tracheal network formation. Branchless FGF signals from stereotyped positions outside of the tracheal system [5] activate tracheal Breathless FGFR [6–8] to initiate the developmental programs that shape the tracheal sacs into a network of interconnected tubes of three distinct cellular architectures (Figure 1, reviewed in [9,10]). The cell biological and genetic mechanisms of Drosophila tracheal development have been extended to describe many aspects of vertebrate epithelial and endothelial tube morphogenesis [11–16], and current advances are also likely to inform our understanding of vertebrate organogenesis.
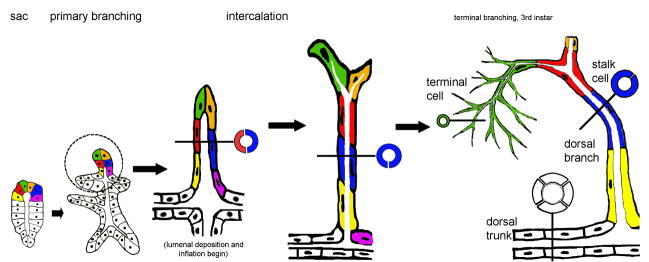
Figure 1. Three distinct tube types generated by tracheal branching morphogenesis.
From left to right: During early stages of embryogenesis, tracheal cells invaginate and form tracheal sacs composed of roughly 80 cells arranged in a polarized epithelial monolayer (“sac” schematic). Six cells are colored coded (yellow, red, green, orange, blue and magenta) to allow them to be followed over time. In response to a Branchless FGF chemoattractant cue, tip cells initiate the primary branching program, and six primary branches bud from the tracheal sac (“primary branching” schematic). Cells within the hashed circle (dorsal trunk anterior branch, to left, dorsal branch at top) are schematized at later developmental time points shown to the right. The cells of the dorsal branch are initially arranged side by side such that a cross-section view (black line) reveals the profile of two cells (red and blue) surrounding the tube lumen (“intercalation” schematic). The cells remodel their cell-cell contacts, changing neighbors (note: blue cell no longer shares a cell-cell junction with the orange or magenta cells) and intercalating to form a longer thinner tube. In a cross-sectional view, the mature dorsal branch tube is a single cell (blue) in circumference. The dorsal branch tip cells (green and orange) become specified as terminal and fusion cells, the former undergoes extensive branching during larval life, while the latter anastamoses with a fusion cell from the contra-lateral side to produce a continuous tube spanning the dorsal midline. By the end of embryogenesis, tubes of three distinct cellular architectures are present in the tracheal system. These distinct tube types are easily recognized in the third instar larvae, where terminal cells have ramified extensively, producing dozens of branched terminal tubes (“terminal branching, 3rd instar” schematic). The tubes from a single terminal cell (green) spread over areas of 100 microns or more, and are a micron or less in diameter. In cross section the tubes are revealed to be “seamless.” In contrast, the dorsal branch stalk cells (red, blue, yellow) wrap around a lumenal space and seal into a tube by forming autocellular adherins and septate junctions–represented by the single seam visible in cross section (blue). Dorsal trunk tubes are several cells (white) in circumference and the cells that compose them organize into a tube by making intercellular adherins and septate junctions –in cross section, a junctional seam is visible between all cells.
CELL-CELL INTERACTIONS IN TRACHEAL MORPHOGENESIS
Here, we review cell-cell interactions required for branching of new tubes from the tracheal sac, maintenance of epithelial integrity, and intercalation of cells in a tube to alter tube size and shape.
Competition–knowing one’s place
Budding of new branches from the tracheal epithelium (primary branching) towards a Branchless FGF chemoattractant cue [5] is led by specialized tip cells that actively migrate and pull trailing stalk cells along behind them. Cells must communicate with each other to sort into leading tip cells and trailing stalk cells, and this communication takes the form of competition [17]. Tip cells are postulated to generate a lateral inhibitory cue proportional to their FGFR activity, and thus enforce a follower stalk cell fate upon their neighbors (Figure 2). Consistent with this model, Notch is required cell autonomously to restrict tip cell number: in Notch mutant embryos almost all cells of the new branch become tip cells [17,18], and tracheal expression of the Notch intracellular domain (constitutively active Notch) either inhibits or completely blocks branch outgrowth [17]. Moreover, in mosaic larvae, cells lacking Notch activity became tip cells at a frequency approaching 90% (Ghabrial and Krasnow, unpublished). Laser ablation confirms that tip cells are the source of a lateral inhibitory signal, as their elimination results in the transformation of neighboring stalk cells into tip cells [19]. Because stalk cells appear poised to become tip cells, doing so within 20 minutes of the cell ablations, it will be important to determine whether competition involves a non-transcriptional/non-canonical Notch pathway.
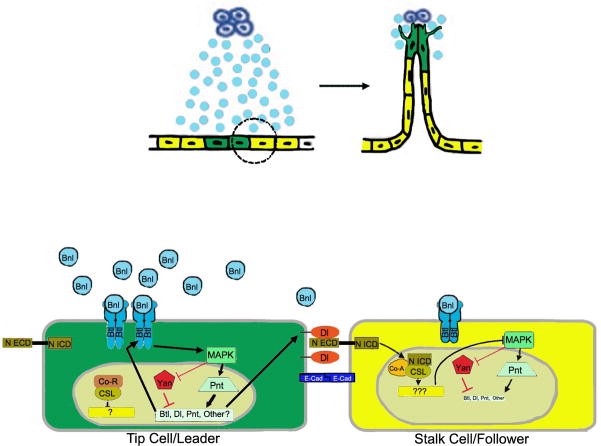
Figure 2. Model for tip cell selection.
In the top panel, dorsal branch cells in the tracheal epithelium are schematized receiving a signal (light blue) from FGF-secreting cells (dark blue). Two cells are selected as tip cells (green) while the other cells will become followers (yellow). Interactions between a leader and follower cell (circled) are shown in the enlarged bottom panel. The two tracheal epithelial cells are held together by adherens junctions, featuring homophilic interactions between Drosophila E-cadherin (E-Cad) on the surface of the two cells. Initial slight differences in FGF signaling are amplified by positive and negative feedback loops. Breathless FGFR signaling (FGF: Branchless/Bnl, FGFR: Breathless/Btl) through the canonical mitogen associated protein kinase (MAPK) pathway–here represented only the terminal kinase in the pathway, MAPK–results in phosphorylation of the ETS box transcription factors Pointed (Pnt) and Yan (encoded by anterior open). Phosphorylation of Pointed activates transcription of breathless, Delta (Dl), and pointed itself. Transcription of breathless and pointed is expected to increase FGFR pathway activity, while Delta (Dl) activates Notch (extracellular (N ECD) and intracellular (N ICD) domains indicated). Proteolytic processing of the ligated Notch receptor releases the N ICD, which associates with the transcription factor CSL (CBF1/suppressor of hairless/Lag1, light gold oval), and co-activator (Co-A, in Drosophila, mastermind). Activation of Notch antagonizes MAPK, downregulating the FGFR pathway and Delta expression in the follower cell.
The mechanisms governing branching morphogenesis in the airsac primordia (larval tracheal cells that give rise to the pupal and adult tracheal system during metamorphosis) [20,21] show substantial overlap with those for primary branching of the embryonic tracheal sacs, including a prominent role of FGF signaling; however at least some key players are different. For example, the Egfr pathway was found to be critical for cell survival and proliferation [20], but no role for Notch was reported. Instead, secretion of Matrix Metalloprotease 2 (MMP2) from tip cells serves as a lateral inhibitory cue by reducing the amount of active FGF ligand available to neighboring cells [22]. Strikingly, this feedback appears to be at least somewhat specific to the FGFR pathway, as MMP2 is unable to similarly inhibit Egfr signaling [22].
Epithelial integrity – holding it all together
Like all epithelial cells, the cells in tracheal sacs are connected to their neighbors by cell-cell junctions. Tracheal cells adhere to each other through homophilic interactions between Cadherin proteins (Drosophila E-cadherin) expressed on opposing cell surfaces. Basal to the adherens junctions are septate junctions; these are composed of claudins and other proteins that form a paracellular barrier, and thus are functionally and molecularly equivalent to vertebrae tight junctions. Maintenance and remodeling of these junctions are essential to tracheal tube morphogenesis.
Egfr signaling, required for tracheal invagination, plays an unexpected but critical role in maintenance of the tracheal epithelium as it reorganizes from 20 individual sacs into an interconnected tubular network. Overexpression of Mapk phosphatase 3 (Mkp3), a negative regulator of Egfr-dependent ERK/Mapk activity, depletes E-cadherin based cell-cell adhesion resulting in fragmentation of the tracheal epithelium under the stress of primary branching [1] – the cells of the branches separate, and the tubes become discontinuous. Loss of Mkp3 activity had the opposite effect, stiffening the epithelium and delaying cell movement. Even after primary branching, the level of Egfr activity within the tracheal system appears to be critical for normal morphogenesis, as over-activation of Egfr due to loss of receptor tyrosine phosphatase function causes all single-celled tubes to become grossly dilated [23].
Intercalation – making tubes longer and narrower
Multiple cells initially circumscribe the lumen of primary branch tubes, but in many instances these cells rearrange, exchanging neighbors by remodeling adherens junctions, thus forming longer tubes of fewer cells in circumference. This process of intercalation occurs, for example, in the dorsal branches of the tracheal system, in which tubes that are two cells in circumference become longer tubes that are a single cell in circumference [3,24] (see Figure 1). Wingless/Wnt (Wg)-dependent expression of the transcription factor Spalt in the dorsal trunk (the primary branch of greatest diameter) acts to repress intercalation [24], while Decapentaplegic/transforming growth factor-β (Dpp)-dependent expression of the transcription factors, Knirps and Knirps-related in dorsal branches represses Spalt and promotes intercalation [25]. Another transcription factor, Tramtrack, [26] has also been found to be required for intercalation of the dorsal branch cells. The factors regulated downstream of these transcription factors remain mostly unknown, although studies indicate that the adherens junction protein, E-cadherin (encoded by shotgun), is at least an indirect target. Cell surface levels of E-cadherin are modulated by lysosomal targeting or Rab11-mediated recycling of endocytosed E-cadherin [27]. The balance tips in favor of Rab11-mediated recycling to the plasma membrane, keeping cell surface E-cadherin levels high, in the dorsal trunk tubes that remain multiple cells in circumference, and in favor of lysosomal targeting, reducing cell surface E-cadherin levels, in the dorsal branch tubes that remodel adherens junctions and intercalate. Thus, down-regulation of surface E-cadherin is a precondition for cell intercalation. Drosophila Src kinases promote remodeling by targeting E-cadherin for degradation; a simultaneous stimulation of E-cadherin mRNA transcription is important for maintaining epithelial integrity [28]. Another junctional protein, Polychaetoid (Pyd) [29], the Drosophila Zonula Occludins-1 (ZO-1) orthologue, has been implicated in dorsal branch intercalation and proposed to be a transcriptional target of Tramtrack [26]. Mutations in pyd result in partially penetrant intercalation defects, in which pairs of dorsal branch cells fail to remodel their junctions to form autocellular tubes (see Figure 1, intercalation), and instead retain their initial arrangement in tubes of two cells in circumference. While Pyd isoforms localize to adherens and septate junctions, the molecular role of Pyd in dorsal branch intercalation is not known. Mutations in three other loci have been identified in a forward genetic-mosaic screen of the third chromosome that also confer partially penetrant intercalation defects (Ghabrial, Levi, and Krasnow; submitted). In addition to these genetic requirements for tracheal cell rearrangement, mechanical force generated by tip cell pulling on stalk cells is also critical, since releasing tension on the dorsal branch by laser ablation of the connection to the dorsal trunk blocks intercalation and lengthening of the tube, but permits the remaining dorsal branch cells to migrate and connect properly to the contra-lateral dorsal branch [19]. These data suggest several distinct molecular pathways intersect to regulate junctional remodeling and intercalation of tracheal cells during tube morphogenesis.
ESSENTIAL ROLES OF MATRIX CONTACTS IN TUBE MORPHOGENESIS
Tracheal cells are polarized, with their apical membranes lining the tube lumen and in contact with a lumenal matrix, and their basolateral membranes apposed to the extracellular matrix surrounding the tube. Here we examine the cell-matrix interactions that are critical for making, shaping and maintaining epithelial tubes. Interactions between a tracheal cell’s lumenal matrix and apical membrane are central to making tubes of the appropriate length and diameter, while interactions between the basolateral membrane and extracellular matrix are critical to tube maintenance.
Lumenal matrix – shaping and coordinating
A lumenal matrix composed of Zona Pelucida (ZP) domain proteins, chitin modifying enzymes, chitin and other less well-defined components (such as the antigen recognized by mAb2A12) has proven to be critical to embryonic tracheal morphogenesis. Nascent liquid-filled tubes complete three steps en route to becoming mature airways: first, a secretory burst deposits proteins into the lumenal space; next, tubes expand diametrically; finally, lumenal proteins and liquid are cleared and replaced with gas [30–32].
Tracheal branches grow to characteristic diameters under precise genetic control [32]. During embryonic development, apical secretion (visualized with fluorescent reporters, ANF-GFP and Gasp-GFP) immediately precedes expansion in tube diameter [31], and is presumed to directly contribute to it (Figure 3). COPI and COPII transport vesicles mediate trafficking between endoplasmic reticulum (ER) and Golgi compartments and are required for the burst of apical secretion [31,33–36]. Although the mechanism by which apical secretion induces tube expansion is not clear, it may do so directly by increasing apical membrane, or indirectly, by triggering a mechanical (eg altering the apical extracellular matrix) or chemical change in the lumen that in turn impinges on the epithelial cells.
Figure 3. Regulation of tube diameter and length.
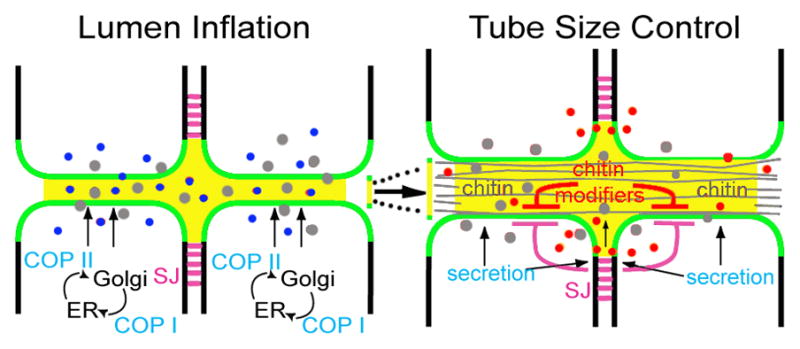
The COP I/II secretion apparatus (light blue) is required for a burst of apical secretion (apically secreted proteins–dark blue; chitin–grey) that correlates with inflation of the tube lumen (lumenal matrix –yellow). Later apical secretion of the chitin modifiers, Serp and Verm (red), is important in limiting the growth of the apical membrane (green) along the long axis of the dorsal trunk tube (eg. to regulate tube length). During tube expansion, chitin cable (grey lines) formation is essential for uniform diametric growth. Proteins localized to the septate junctions (SJ–magenta lines) provide an additional level of regulation on tube size by limiting the expansion of apical polarity proteins, and thus restricting expansion of the apical membrane domain.
Secretion of chitin fibers into the tracheal lumen is detected prior to tube expansion and continues throughout the period of rapid diametric growth [37]. Mutants defective in cable formation fail to expand the tube lumen at dorsal trunk fusion points (where primary dorsal trunk branches from neighboring hemisegments anastamose), while excessively dilating the tube lumen throughout the rest of the dorsal trunk [38–41]. Thus, the chitin cable coordinates the behavior of the tracheal cells that surround it, and stabilizes the epithelium during diametric expansion. Additionally, the chitin cable also restricts tube length, since mutations affecting the chitin-modifying proteins, Serpentine (Serp) and Vermiform (Verm), lead to increased dorsal trunk length [42,43]. How does the chitin cable exert this influence over tube diameter and length? Rigid chitin polymers are likely to impose physical constraints on cell and tube shape.
Tube length is also dependent upon septate junction proteins: mutations in a number of septate junction components result in lengthened tubes [44–51]. Septate junctions, like vertebrate tight junctions, act as a paracellular diffusion barrier (constituted, in part, by claudin family proteins [48,52,53]) that is critical to the ability of all epithelial tubes to transport and modify gasses and liquids. Septate junctions additionally act as a landmark for targeting of basolateral polarity proteins such as Discs Large (Dlg), Lethal Giant Larvae (Lgl), and Scribble (Scrib). This latter function of septate junctions is required in regulating tube length, rather than the trans-epithelial diffusion barrier per se, since embryos mutant for dlg and scrib are defective in tube length without affecting barrier function [44]. However, the contribution of septate junctions to tube morphogenesis is more complex, as they also direct secretion of chitin modifiers Serp and Verm into the lumen [42,43,54] (Figure 3). Indeed, requirement for septate junction proteins in tube length regulation can be partially explained by the role of Serp and Verm in deactylation of chitin, which is presumed to generate shorter and more rigid chitin fibrils. Septate junction-independent apical secretion is likewise required for tube length regulation: disruption of Rho-Diaphanous-Myosin V transport perturbs lumenal deposition of 2a12 antigen and ZP protein Piopio (but not Verm or Serp) and results in excessive tube length [55].
Mutations in convoluted also cause excessive tube length, and reveal a length-regulatory pathway that is independent of septate junctions, chitin cable formation, and chitin deacetylase secretion. The convoluted gene encodes acid labile subunit (ALS), a protein that complexes with and regulates Insulin-like growth factors (IGFs); unexpectedly, Convoluted regulates tube size via an IGF-independent pathway that EM analysis reveals to be important in lumenal matrix organization [47].
Interestingly, cell-cell interactions also appear to play a role in the regulation of tube length: the novel protein encoded by the putative planar cell polarity regulator, serrano, causes a deficit of apical membrane addition when overexpressed and a mild increase when knocked out [56]. The role of planar cell polarity proteins in tracheal morphogenesis is not yet well understood, but Serrano is proposed to regulate aspects of planar cell polarity via a direct physical interaction with Disheveled.
Extracellular Matrix – staying tubular via cytoskeletal attachments
In addition to the multicellular and autocellular tubes generated by the cells of the dorsal trunk and branches, subcellular, “seamless” tubes (see Figure 1) are formed within the terminal cells and branch extensively to bring oxygen to target tissues. Each terminal cell may extend dozens of branched cellular processes that ramify on internal tissues; each cellular process contains a seamless tube. Unlike tubes dependent upon intercellular or autocellular junctions for their formation, seamless tubes are thought to form by “cell hollowing [57]” in which vesicles are trafficked to the middle of the cell and fuse to form apical membrane de novo, thus hollowing out a lumen within the middle of the cell (although see [58], for an alternative model). The genetic and molecular mechanisms by which seamless tubes are made and shaped are not well understood, but adhesion between terminal branch tubes and the surrounding extracellular matrix (ECM) has been found to be critical for seamless tube maintenance in larvae [59]. In mutant terminal cells disconnected from the ECM (eg cells mutant for talin/rhea/tendrils, the α-integrins multiple edematous wings and inflated, or the β-integrin, myospheroid) cellular extensions, and the seamless tubes within them, retract into the soma. These mutant cells will appear to have fewer cellular extensions that are each perforated by multiple seamless tubes. Mutations in vine, recently identified as the gamma subunit of the triC chaperonin complex, cause a very similar phenotype (Ghabrial, Levi and Krasnow; unpublished). While the triC complex is important for folding of many proteins, its role in folding of actin and tubulin have long been appreciated [60], and may account for the observed tube maintenance defects, since disruption of the microtubule network by the expression of microtubule-severing proteins can partially phenocopy mutation of vine (Boaz P. Levi, PhD thesis, Stanford University, 2006; Ghabrial, Levi, and Krasnow, unpublished). We speculate that a cytoskeletal connection between the ECM/basolateral membrane and the apical membrane is required to stabilize the tube lumen, which appears to be under tension. Cytoskeleton attachment sites on the apical membrane are likely to include transmembrane proteins of the ZP domain family, such as Dumpy [61].
Conclusions
Over the last 5 years a shift in emphasis in the studies of tube morphogenesis has become apparent, as investigators have begun to go beyond the deciphering of signaling pathways, to an increasingly sophisticated exploration of cell shape, dynamic remodeling of cell junctions, and epithelial cell behavior. These new approaches, combined with live imaging studies and the additional tubulogenesis genes identified in recent sets of genetic mosaic screens ([62,63], Ghabrial, Levi, and Krasnow; submitted) promises that the next five years will bring a similar wealth of new insights into the cellular and subcellular bases of making and shaping branched tubular networks.
Acknowledgments
The authors apologize to those whose work we were unable to include within the limited confines of this review. We thank Dena Alpert for her insightful comments on the manuscript. JS has received support from NIH Developmental Biology training grant (T32-HD007516-12) and is currently supported by an NIH NRSA postdoctoral fellowship (F32-GM090438). ASG gratefully acknowledges research support from the NIH (1R01GM089782-01A1), the March of Dimes (Basil O’Connor award), a McCabe Scholar award, an ACS pilot grant, and the University of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cela C, Llimargas M. Egfr is essential for maintaining epithelial integrity during tracheal remodelling in Drosophila. Development. 2006;133:3115–3125. doi: 10.1242/dev.02482. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura M, Inoue Y, Hayashi S. A wave of EGFR signaling determines cell alignment and intercalation in the Drosophila tracheal placode. Development. 2007;134:4273–4282. doi: 10.1242/dev.010397. [DOI] [PubMed] [Google Scholar]
- 3.Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA. Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development. 1996;122:1395–1407. doi: 10.1242/dev.122.5.1395. [DOI] [PubMed] [Google Scholar]
- 4.Brodu V, Casanova J. The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination. Genes Dev. 2006;20:1817–1828. doi: 10.1101/gad.375706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- 6.Klambt C, Glazer L, Shilo BZ. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 1992;6:1668–1678. doi: 10.1101/gad.6.9.1668. [DOI] [PubMed] [Google Scholar]
- 7.Lee T, Hacohen N, Krasnow M, Montell DJ. Regulated Breathless receptor tyrosine kinase activity required to pattern cell migration and branching in the Drosophila tracheal system. Genes Dev. 1996;10:2912–2921. doi: 10.1101/gad.10.22.2912. [DOI] [PubMed] [Google Scholar]
- 8.Shishido E, Higashijima S, Emori Y, Saigo K. Two FGF-receptor homologues of Drosophila: one is expressed in mesodermal primordium in early embryos. Development. 1993;117:751–761. doi: 10.1242/dev.117.2.751. [DOI] [PubMed] [Google Scholar]
- 9.Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA. Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol. 2003;19:623–647. doi: 10.1146/annurev.cellbio.19.031403.160043. [DOI] [PubMed] [Google Scholar]
- 10.Cabernard C, Neumann M, Affolter M. Cellular and molecular mechanisms involved in branching morphogenesis of the Drosophila tracheal system. J Appl Physiol. 2004;97:2347–2353. doi: 10.1152/japplphysiol.00435.2004. [DOI] [PubMed] [Google Scholar]
- 11.Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- 12.Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol. 2010;341:34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz A, Simons M. Branching morphogenesis. Circ Res. 2008;103:784–795. doi: 10.1161/CIRCRESAHA.108.181818. [DOI] [PubMed] [Google Scholar]
- 14.Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. Bioessays. 2008;30:303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- 15.Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4:11–18. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- 16.Lu P, Werb Z. Patterning mechanisms of branched organs. Science. 2008;322:1506–1509. doi: 10.1126/science.1162783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Ghabrial AS, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–749. doi: 10.1038/nature04829. In this paper the cell autonomous requirement for the Breathless FGFR is tested in a series of genetic mosaic experiments, revealing that primary branch morphogenesis can occur normally with only a single leading cell able to perceive the Branchless FGF cue. The concept of competition among cells for leading positions is established, and the role for the Notch pathway in restricting tip cell number is demonstrated. [DOI] [PubMed] [Google Scholar]
- 18.Ikeya T, Hayashi S. Interplay of Notch and FGF signaling restricts cell fate and MAPK activation in the Drosophila trachea. Development. 1999;126:4455–4463. doi: 10.1242/dev.126.20.4455. [DOI] [PubMed] [Google Scholar]
- 19**.Caussinus E, Colombelli J, Affolter M. Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr Biol. 2008;18:1727–1734. doi: 10.1016/j.cub.2008.10.062. In this paper the cell autonomous requirement for the Breathless FGFR is tested in a series of genetic mosaic experiments, revealing that primary branch morphogenesis can occur normally with only a single leading cell able to perceive the Branchless FGF cue. The concept of competition among cells for leading positions is established, and the role for the Notch pathway in restricting tip cell number is demonstrated. [DOI] [PubMed] [Google Scholar]
- 20*.Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev Cell. 2005;9:831–842. doi: 10.1016/j.devcel.2005.10.008. The requirements for Egfr and Breathless FGFR pathways during development of the airsac are established. The Egfr pathway is found to be required for cell division and cell survival while the FGFR pathway is found to be required for cells to occupy positions at the migrating tip of the airsac. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 22**.Wang Q, Uhlirova M, Bohmann D. Spatial restriction of FGF signaling by a matrix metalloprotease controls branching morphogenesis. Dev Cell. 18:157–164. doi: 10.1016/j.devcel.2009.11.004. MMP2 is identified as a transcriptional target of FGF signaling in the airsac, and is shown to act in a lateral inhibition/negative feed back loop in which MMP2 produced by tip cells acts as an extracellular inhibitor of FGF activity. [DOI] [PubMed] [Google Scholar]
- 23*.Jeon M, Zinn K. Receptor tyrosine phosphatases control tracheal tube geometries through negative regulation of Egfr signaling. Development. 2009;136:3121–3129. doi: 10.1242/dev.033597. A role for receptor tyrosine phosphatases in Egfr-dependent regulation of tubulogenesis is identified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro C, Neumann M, Affolter M. Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr Biol. 2004;14:2197–2207. doi: 10.1016/j.cub.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 25.Chen CK, Kuhnlein RP, Eulenberg KG, Vincent S, Affolter M, Schuh R. The transcription factors KNIRPS and KNIRPS RELATED control cell migration and branch morphogenesis during Drosophila tracheal development. Development. 1998;125:4959–4968. doi: 10.1242/dev.125.24.4959. [DOI] [PubMed] [Google Scholar]
- 26.Araujo SJ, Cela C, Llimargas M. Tramtrack regulates different morphogenetic events during Drosophila tracheal development. Development. 2007;134:3665–3676. doi: 10.1242/dev.007328. [DOI] [PubMed] [Google Scholar]
- 27*.Shaye DD, Casanova J, Llimargas M. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol. 2008;10:964–970. doi: 10.1038/ncb1756. The role of vesicular trafficking in the regulation of E-cadherin levels during intercalation is explored. Spalt expression inhibits intercalation of the dorsal trunk cells and correlates with subapical accumulation of Rab11 (and endosomal recycling). The trafficking of Rab11 cargo, E-cadherin, appears to be regulated by Spalt at the level of the Rab11-effector, dRip11. [DOI] [PubMed] [Google Scholar]
- 28.Shindo M, Wada H, Kaido M, Tateno M, Aigaki T, Tsuda L, Hayashi S. Dual function of Src in the maintenance of adherens junctions during tracheal epithelial morphogenesis. Development. 2008;135:1355–1364. doi: 10.1242/dev.015982. [DOI] [PubMed] [Google Scholar]
- 29.Jung AC, Ribeiro C, Michaut L, Certa U, Affolter M. Polychaetoid/ZO-1 is required for cell specification and rearrangement during Drosophila tracheal morphogenesis. Curr Biol. 2006;16:1224–1231. doi: 10.1016/j.cub.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 30.Behr M, Wingen C, Wolf C, Schuh R, Hoch M. Wurst is essential for airway clearance and respiratory-tube size control. Nat Cell Biol. 2007;9:847–853. doi: 10.1038/ncb1611. [DOI] [PubMed] [Google Scholar]
- 31**.Tsarouhas V, Senti KA, Jayaram SA, Tiklova K, Hemphala J, Adler J, Samakovlis C. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell. 2007;13:214–225. doi: 10.1016/j.devcel.2007.06.008. Seminal examination of secretory and endocytic mechanisms driving tubulogenesis. [DOI] [PubMed] [Google Scholar]
- 32.Beitel GJ, Krasnow MA. Genetic control of epithelial tube size in the Drosophila tracheal system. Development. 2000;127:3271–3282. doi: 10.1242/dev.127.15.3271. [DOI] [PubMed] [Google Scholar]
- 33.Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 34.Grieder NC, Caussinus E, Parker DS, Cadigan K, Affolter M, Luschnig S. gammaCOP is required for apical protein secretion and epithelial morphogenesis in Drosophila melanogaster. PLoS One. 2008;3:e3241. doi: 10.1371/journal.pone.0003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Forster D, Armbruster K, Luschnig S. Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr Biol. 2009;20:62–68. doi: 10.1016/j.cub.2009.11.062. Identifies a cell autonomous role for secretion in regulating tube expansion. [DOI] [PubMed] [Google Scholar]
- 36.Jayaram SA, Senti KA, Tiklova K, Tsarouhas V, Hemphala J, Samakovlis C. COPI vesicle transport is a common requirement for tube expansion in Drosophila. PLoS One. 2008;3:e1964. doi: 10.1371/journal.pone.0001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Devine WP, Lubarsky B, Shaw K, Luschnig S, Messina L, Krasnow MA. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc Natl Acad Sci U S A. 2005;102:17014–17019. doi: 10.1073/pnas.0506676102. Define role for lumenal chitinous filament in tube expansion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jurgens G, Kluding G, Nusslein-Volhard C, Wieschaus E. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. II. Zygotic loci on the third chromosome. Roux’s Archives of Developmental Biology. 1984;193:283–295. doi: 10.1007/BF00848157. [DOI] [PubMed] [Google Scholar]
- 39.Moussian B, Soding J, Schwarz H, Nusslein-Volhard C. Retroactive, a membrane-anchored extracellular protein related to vertebrate snake neurotoxin-like proteins, is required for cuticle organization in the larva of Drosophila melanogaster. Dev Dyn. 2005;233:1056–1063. doi: 10.1002/dvdy.20389. [DOI] [PubMed] [Google Scholar]
- 40*.Moussian B, Tang E, Tonning A, Helms S, Schwarz H, Nusslein-Volhard C, Uv AE. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development. 2006;133:163–171. doi: 10.1242/dev.02177. Define role for lumenal chitinous filament in tube expansion. [DOI] [PubMed] [Google Scholar]
- 41.Ostrowski S, Dierick HA, Bejsovec A. Genetic control of cuticle formation during embryonic development of Drosophila melanogaster. Genetics. 2002;161:171–182. doi: 10.1093/genetics/161.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Luschnig S, Batz T, Armbruster K, Krasnow MA. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr Biol. 2006;16:186–194. doi: 10.1016/j.cub.2005.11.072. Back to back publications establishing the role of chitin modifiers Serp and Verm in restricting tube length, and demonstrating a role for septate junction proteins in their apical secretion. [DOI] [PubMed] [Google Scholar]
- 43**.Wang S, Jayaram SA, Hemphala J, Senti KA, Tsarouhas V, Jin H, Samakovlis C. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr Biol. 2006;16:180–185. doi: 10.1016/j.cub.2005.11.074. Back to back publications establishing the role of chitin modifiers Serp and Verm in restricting tube length, and demonstrating a role for septate junction proteins in their apical secretion. [DOI] [PubMed] [Google Scholar]
- 44.Laprise P, Paul SM, Boulanger J, Robbins RM, Beitel GJ, Tepass U. Epithelial polarity proteins regulate Drosophila tracheal tube size in parallel to the luminal matrix pathway. Curr Biol. 2010;20:55–61. doi: 10.1016/j.cub.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Llimargas M, Strigini M, Katidou M, Karagogeos D, Casanova J. Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system. Development. 2004;131:181–190. doi: 10.1242/dev.00917. [DOI] [PubMed] [Google Scholar]
- 46.Paul SM, Ternet M, Salvaterra PM, Beitel GJ. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130:4963–4974. doi: 10.1242/dev.00691. [DOI] [PubMed] [Google Scholar]
- 47.Swanson LE, Yu M, Nelson KS, Laprise P, Tepass U, Beitel GJ. Drosophila convoluted/dALS is an essential gene required for tracheal tube morphogenesis and apical matrix organization. Genetics. 2009;181:1281–1290. doi: 10.1534/genetics.108.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164:313–323. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu VM, Yu MH, Paik R, Banerjee S, Liang Z, Paul SM, Bhat MA, Beitel GJ. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development. 2007;134:999–1009. doi: 10.1242/dev.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul SM, Palladino MJ, Beitel GJ. A pump-independent function of the Na, K-ATPase is required for epithelial junction function and tracheal tube-size control. Development. 2007;134:147–155. doi: 10.1242/dev.02710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hijazi A, Masson W, Auge B, Waltzer L, Haenlin M, Roch F. boudin is required for septate junction organisation in Drosophila and codes for a diffusible protein of the Ly6 superfamily. Development. 2009;136:2199–2209. doi: 10.1242/dev.033845. [DOI] [PubMed] [Google Scholar]
- 52.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 53.Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5:611–620. doi: 10.1016/s1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 54*.Tonning A, Hemphala J, Tang E, Nannmark U, Samakovlis C, Uv A. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev Cell. 2005;9:423–430. doi: 10.1016/j.devcel.2005.07.012. Define role for lumenal chitinous filament in tube expansion. [DOI] [PubMed] [Google Scholar]
- 55*.Massarwa R, Schejter ED, Shilo BZ. Apical secretion in epithelial tubes of the Drosophila embryo is directed by the Formin-family protein Diaphanous. Dev Cell. 2009;16:877–888. doi: 10.1016/j.devcel.2009.04.010. Identify an important Diaphanous-dependent secretory pathway. [DOI] [PubMed] [Google Scholar]
- 56*.Chung S, Vining MS, Bradley PL, Chan CC, Wharton KA, Jr, Andrew DJ. Serrano (sano) functions with the planar cell polarity genes to control tracheal tube length. PLoS Genet. 2009;5:e1000746. doi: 10.1371/journal.pgen.1000746. Intriguing study implicating planar cell polarity proteins in tubulogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 58*.Gervais L, Casanova J. In vivo coupling of cell elongation and lumen formation in a single cell. Curr Biol. 2010;20:359–366. doi: 10.1016/j.cub.2009.12.043. Study of apical polarity protein localization within terminal cells that challenges the established “cell hollowing” model of seamless tube formation. [DOI] [PubMed] [Google Scholar]
- 59.Levi BP, Ghabrial AS, Krasnow MA. Drosophila talin and integrin genes are required for maintenance of tracheal terminal branches and luminal organization. Development. 2006;133:2383–2393. doi: 10.1242/dev.02404. [DOI] [PubMed] [Google Scholar]
- 60.Spiess C, Meyer AS, Reissmann S, Frydman J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol. 2004;14:598–604. doi: 10.1016/j.tcb.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jazwinska A, Ribeiro C, Affolter M. Epithelial tube morphogenesis during Drosophila tracheal development requires Piopio, a luminal ZP protein. Nat Cell Biol. 2003;5:89–5901. doi: 10.1038/ncb1049. [DOI] [PubMed] [Google Scholar]
- 62.Baer MM, Bilstein A, Leptin M. A clonal genetic screen for mutants causing defects in larval tracheal morphogenesis in Drosophila. Genetics. 2007;176:2279–2291. doi: 10.1534/genetics.107.074088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Metzstein MM, Krasnow MA. Functions of the nonsense-mediated mRNA decay pathway in Drosophila development. PLoS Genet. 2006;2:e180. doi: 10.1371/journal.pgen.0020180. [DOI] [PMC free article] [PubMed] [Google Scholar]