Abstract
The extracellular matrix (ECM) is a key regulator of cell and tissue function. Traditionally, the ECM has been thought of primarily as a physical scaffold that binds cells and tissues together. However, the ECM also elicits biochemical and biophysical signaling. Controlled proteolysis and remodeling of the ECM network regulate tissue tension, generate pathways for migration, and release ECM protein fragments to direct normal developmental processes such as branching morphogenesis. Collagens are major components of the ECM of which basement membrane type IV and interstitial matrix type I are the most prevalent. Here we discuss how abnormal expression, proteolysis and structure of these collagens influence cellular functions to elicit multiple effects on tumors, including proliferation, initiation, invasion, metastasis, and therapy response.
Introduction
Many of the processes that regulate tissue and organ development are hijacked in cancer [1]. For example, the epithelial migration and invasion occurring in mammary carcinomas are morphologically and molecularly similar to epithelial branching morphogenesis in mammary gland development [2,3]. However, while epithelial invasion is stringently regulated in development, solid tumors display deregulated and persistent invasion. In both instances, the extracellular matrix (ECM) provides a physical scaffold for cell adhesion and migration, it influences tissue tension and it signals to cells through ECM receptors. Proteolysis of the ECM regulates cellular migration by modifying the structure of the ECM scaffold and by releasing ECM fragments with biological functions. ECM proteolysis is therefore tightly controlled in normal tissues but typically deregulated in tumors.
Collagens are major constituents of the ECM, representing as much as 30% of total mammalian protein mass ([4], see Box 1). Type I collagen is the main structural protein in the interstitial ECM [5]. Type IV collagen is a key component of the basement membrane (BM), which is found at the basal surface of epithelial and endothelial cells and is essential for tissue polarity [6]. Epithelial invasion in both branching morphogenesis and cancer requires that the cells must interact with these collagens. The BM is breached as both normal and transformed epithelial cells invade into the interstitial tissue. It is also compromised at the site of the vasculature by metastasizing cancer cells [7].
Box 1. Collagen structure.
At least 46 distinct collagen polypeptide α-chains have been identified in vertebrates and they can be assembled into 28 different collagens [103].
Collagens are categorized according to their structural properties in the ECM. These include the classic fibrillar and network forming types, the FACITs (fibril-associated collagens with interrupted triple helices), the MACITs (membrane-associated collagens with interrupted triple helices), and the MULTIPLEXINs (multiple triple-helix domains and interruptions) [103].
Collagens are composed of three polypeptide α-chains, which can be either homo- or hetero-trimers. In the endoplasmic reticulum, the α-chains are packed into a tight triple-helical structure forming the collagenous domain [5].
The tight packing of the collagen triple-helix is facilitated by repeated Glycine-X-Y motifs in the collagenous domain of the collagen molecules (4-hydroxyproline is often found in the Y position) [5].
The α-chains also contain non-collagenous domains, which are proteolytically removed in the fibrillar collagens (e.g., types I, II, III). For other collagens, non-collagenous domains are important for supramolecular network formation, which for example is mediated by the C-terminal non-collagenous (NC1) domain of type IV collagen.
Collagens are maturated by posttranslational modifications including proteolytic processing of the N- and C-terminus for the fibrillar types (e.g., type I collagen), hydroxylation of peptidyl prolyl and lysyl residues, sulfilimine linking (type IV collagen), glycosylation of hydroxylysine residues by galactose and glucose, and enzymatic (lysyl-oxidase (LOX)-mediated) and non-enzymatic (glycation-mediated) covalent crosslinking [4,33,104].
The non-collagenous domains can upon proteolytic removal exert new functions. Such collagen-derived proteolytic fragments include endostatin (from type XVIII collagen), restin (from type XV collagen) and tumstatin (from type IV collagen) that have anti-angiogenic and tumor growth inhibitory functions [4,105].
The desmoplastic response in cancer
Fibrosis is an accumulation of ECM proteins, including type I collagen [8]. Organ fibrosis and cancer are associated, although the association may simply reflect collagen accumulation due to increased activity of inflammatory and tumorigenic factors such as TGF-β [9]. Nevertheless, many malignancies are associated with a strong fibrotic reaction, termed “desmoplasia”, which is characterized by an accumulation of fibrillar collagen types I and III and increased degradation of type IV collagen [10-12]. Such fibrotic foci correlate with adverse prognosis in mammary carcinomas [13]. Desmoplasia has also been observed at metastatic sites where it may facilitate the successful establishment of metastases [14,15]. Indeed, increased expression of type I collagen and many of its modifying enzymes is frequently observed in the gene expression signatures associated with increased risk of metastasis [16,17].
Architectural changes of fibrillar collagen in cancer
The architecture of the collagen scaffolds in tumors is severely altered. Tumor-associated collagens are often linearized and crosslinked reflecting elevated deposition and significant posttranslational modification ([18,19] and Figure 1). This physical restructuring of interstitial collagen progressively stiffens the ECM which thereafter elicits diverse effects on cellular differentiation, gene expression, proliferation, survival and migration [20-23]. These cellular effects can in turn significantly modify tumor progression and influence treatment response.
Figure 1. Changes in the type I collagen scaffold with tumor progression.
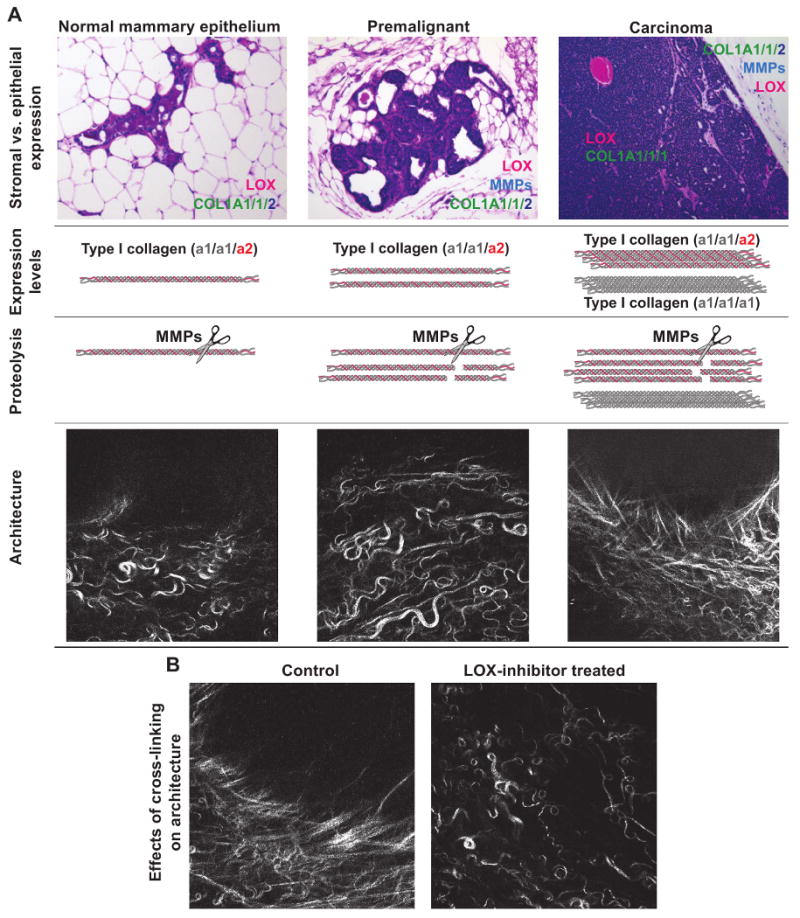
(A) Relative levels of stromal vs. epithelial expression of type I collagen, the collagen crosslinking enzyme lysyl oxidase (LOX) and the collagenolytic matrix metalloproteinases (MMPs) during tumor progression. In early stage tumors, LOX is high in the stromal cells, and in late stage tumors, its expression also increases in carcinoma cells. In late stage tumors, the carcinoma cells begin to express an increased ratio of the α1-chain to α2-chain of type I collagen. The net result is an increase in both the normal type I collagen α1,α1,α2,-heterotrimer and in an MMP-resistant type I collagen α1,α1,α1,-homotrimer. The carcinoma-associated changes in collagen and collagen remodeling enzymes modify the architecture of the collagen scaffold such that early, thin and relaxed collagens (curly fibrils) progressively thicken and linearize coinciding with tumor progression and invasion.
(B) Inhibition of collagen crosslinking through LOX-inhibition prevents collagen remodeling and maintains a normal collagen architecture.
Photomicrographs are from [19] and reprinted with permission.
The linearization of interstitial collagen in invasive tumors is, at least in part, due to an increased number of covalent crosslinks between collagen molecules [19]. Collagen crosslinking is predominantly catalyzed by enzymes such as lysyl oxidase (LOX). During the early stages of breast carcinogenesis, LOX is synthesized by stromal cells, likely in response to TGF-β. In late stage tumors, LOX is induced also in the carcinoma cells in response to hypoxia [24,25]. In a mouse model of ErbB2-induced breast carcinoma, treatment with LOX inhibitors, before tumors form, decreases ECM crosslinking and prevents tissue stiffening ([19], and Figure 1B). This in turn inhibits focal adhesion maturation and decreases growth factor receptor signaling and concomitantly reduces tumor incidence and size and delays tumor progression. The cancer cell-secreted LOX enzymes in late stage cancer may also promote metastasis by regulating the behavior of the cancer cells and modifying the ECM of the metastatic niche [26,27]. Consistently, increased expression of LOX and its related family members correlate clinically with tumor progression and elevated metastatic risk [27,28].
Fibronectin binds collagen and regulates collagen fibril organization [29]. Stretching of fibronectin stimulates its fibrillogenesis by revealing cryptic binding sites within the unfolded molecules, leading in turn to increased fibronectin rigidity [30]. Increased rigidity greatly increases binding forces between fibronectin and its receptor α5β1 integrin [31]. The size, density and rigidity of fibronectin fibrils in vivo therefore influence the function of the collagen fibrils, and vice versa. This dynamic and reciprocal relationship between collagens and fibronectin likely plays a role in tumor progression. Indeed, fibronectin deposition has been implicated as an early step in metastasis [15].
SPARC (secreted protein acidic and rich in cysteine) is a glycoprotein that participates in ECM organization and binds to types I and IV collagen [32]. In a murine model of pancreatic cancer, SPARC deficiency reduces expression levels of types I, III and IV collagen and decreases collagen fibrillogenesis [32]. Nevertheless, these animals show elevated metastasis, possibly due to an abnormal vascular BM that facilitates intra- and extravasation of the cancer cells.
Sugars such as glucose and ribose induce covalent bonds with lysine residues in collagen fibrils to introduce non-enzymatic and random crosslinking, and these glycation adducts in turn form intermolecular covalent links [33]. Consistently, Diabetes Mellitus patients with uncontrolled glucose metabolism have increased numbers of glucose adducts on long-lived proteins like collagen [34]. These patients also have an elevated risk of developing tumors [35], suggesting that collagen glycation and the resulting ECM stiffening could be possible risk factors.
Collagen fibers as highways for migration
The collagen fibers surrounding the normal epithelial structures in soft tissues such as the mammary gland and lung are typically curly and anisotropic. However, following tumor initiation many of the fibers progressively thicken and linearize ([18,19], and Figure 1A). This linearization is most notable adjacent to the tumor vasculature and in areas with cancer cell invasion [18,19,36]. Linearized fibers are stiffer than curly ones and the resulting increased ECM stiffness can substantially potentiate growth factor-dependent cell migration [19,37]. These abnormal collagen fibers could promote metastasis by fostering cell migration into the interstitial matrix and towards the vasculature. Indeed, intravital imaging shows that cancer cells and leukocytes migrate rapidly in collagen-rich regions on the collagen fibers [36,38,39]. Cancer cells might exploit these remodeled “linear” collagen fibers as invasion “highways” analogous to the preferential migration of glioma cancer cells along the matrix associated with blood vessels and rigid myelin sheath bundles [40] (Figure 2). The mechanisms whereby matrix rigidity could enhance cancer cell migration likely involve activation of collagen receptors, including integrins [41] and discoidin domain receptor (DDR) 1 [42] (see Box 2 for more on collagen receptors), and modulation of growth factor receptor signaling. Interestingly, an unusual form of type I collagen, a homotrimer of the α1 chains (in contrast to the normal α1/α1/α2 heterotrimer, see Figure 1A), enhances carcinoma cell migration in vitro [43]. Moreover, the homotrimers are secreted solely by carcinoma cells and are resistant to cleavage by matrix metalloproteinases (MMPs) [43].
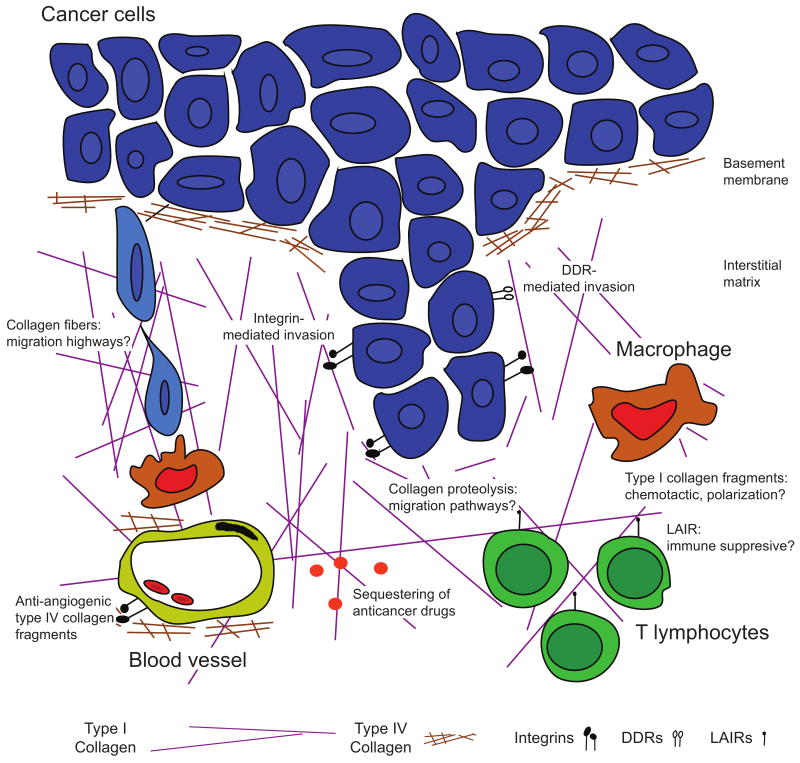
Figure 2. Cellular effects of the collagen scaffold in carcinomas.
Types I and IV collagen influence multiple steps in tumor evolution. Type IV collagen is degraded as carcinomas break through the basement membrane to invade. It is breached again as cells intravasate en route to form metastases. Proteolysis of type IV collagen in vascular basement membranes results in generation of fragments with anti-angiogenic activity acting through integrins. Type I collagen fibers mediate invasion at several levels. Uncleaved fibers may act as “highways” for cell migration, possibly facilitated by macrophages. Both integrin and discoidin domain receptor (DDR) mediated signaling can facilitate cell invasion. This type of invasion may also require the generation of pathways through the collagen scaffold by proteolysis. The immune reaction against tumors may be regulated by collagen: most immune cells express leukocyte-associated Ig-like receptors (LAIRs), which upon collagen binding inhibits immune cell activation. Macrophages may also be regulated by type I collagen fragments, which are chemotactic for macrophage precursors and possibly involved in regulation of their polarization.
Box 2. Collagen receptors – not just anchoring poles.
Collagen signals are mediated to cells via a variety of receptors, including integrins, discoidin domain receptors (DDRs), leukocyte-associated Ig-like receptors (LAIRs), mannose receptor family members and glycoprotein VI (reviewed in [91]).
Integrins are composed of α and β units. Native collagens are recognized by four integrins: α1β1, α2β1, α10β1 and α11β1. Integrin α1β1 binds both type I and IV collagen whereas α2β1 only binds type I collagen [91].
DDRs are tyrosine kinase receptors activated by collagen [106]. Both DDR1 and DDR2 are activated by intact fibrillar collagens, including type I. DDR1, but not DDR2, also is activated by type IV collagen [106].
LAIRs bind collagens at Glycine-Proline-Hydroxyproline repeats [107]. They are expressed on most immune cells and the interaction between LAIR-1 and collagen inhibits immune cell activation (reviewed in [83]).
Several members of the mannose receptor family bind collagen, including the mannose receptor and uPARAP/endo180 [91]. The main function of these receptors appears to be to internalize collagen for intracellular degradation.
Proteolysis of collagen – effects on cancer beyond path generation
Although cells migrate along collagen fibers, collagen in tissues also represents a physical barrier against invasion [44]. Thus, collagen degradation by proteases, including cathepsins and MMPs, and uptake of the degraded collagen is important for cancer cell invasion [10,45,46]. For many cells, proteolysis of types I and IV collagen is essential for migration through the ECM [7,45,47-49]. Proteolysis of the ECM generates pathways for cells to migrate through [50-53]. In addition, proteolysis of types I and IV collagen can also reveal RGD sequences in the molecules that activate αv integrins [54,55].
Cleavage of type I collagen by MMP1, -8, -13 and -14 (MT1-MMP) results in generation of characteristic fragments that are 3/4 and 1/4 of the length of the native molecule ([56,57], Figure 1A). These fragments may act as antagonists of full length collagen because they bind but fail to activate α2β1 integrin [58]. However, the fragments might also promote cellular migration and survival by activating αvβ3 integrin [59-62].
DDR signaling is also affected by collagen proteolysis. Intact type I collagen can inhibit cancer cell proliferation via DDR2 activation, but this growth restriction is released by MMP-mediated proteolysis [63]. This fits well with the overlap between a DDR2 binding site and the MMP14 cleavage site in type I collagen [64,65].
Collagen proteolysis is also a critical step in angiogenesis [66,67]. However, non-collageneous (NC) domains of collagens (see Box 1) released by proteolysis can also inhibit tumor angiogenesis. For example, endostatin, a c-terminal fragment of type XVIII collagen, inhibits endothelial cell migration and thus tumor angiogenesis [4]. Several other inhibitors of angiogenesis are generated by proteolysis of type IV collagen [6], including tumstatin, a fragment of the type IV α3 chain generated by MMP9 [68]. The type IV collagen-derived anti-angiogenic fragments affect endothelial cellular functions by modulating αvβ3 and αvβ5 integrin signaling [68,69]. The ability of these fragments to inhibit angiogenesis suggests that they act as antagonists of αvβ3 and αvβ5 integrins, because these integrins are normally activated on endothelial cells by matrix components surrounding actively remodeling blood vessels (e.g., vitronectin, fibrinogen, and fibronectin).
Collagen as a regulator of response to therapy
Resistance to cancer therapy can be caused by cancer cell intrinsic mechanisms, such as overexpression of anti-apoptotic genes, but factors in the tumor microenvironment can also regulate therapy response [1].
Types I and IV collagen can induce chemoresistance by directly interacting with integrins on cancer cells [70,71]. The level and structural organization of collagen can also indirectly influence therapeutic efficacy by regulating drug delivery. In many tumors, drug delivery is impaired by an increased interstitial fluid pressure. The increased interstitial fluid pressure is due in part to a leaky vasculature and sparse or nonfunctional lymphatics [72]. However, increased ECM stiffness and a dense collagen interstitial fiber network can also influence interstitial fluid pressure. For example, deficiency in fibromodulin, which binds to collagen to stabilize the fibrils [73], decreases collagen fibril size in tumors and reduces interstitial fluid pressure [74]. Consistently, collagenase treatment of tumors decreases interstitial pressure and enhances drug delivery [75,76]. A dense collagen network can also directly impede the diffusion of large molecular weight drugs to compromise treatment efficiency [77,78]. Finally, drug delivery can also be inhibited by binding and sequestering of drugs to components in the ECM, including collagens [79].
Improved drug responses have been achieved when the collagen content in tumors has been reduced. This can for example be accomplished by vaccinating mice against fibroblast-activating protein, a proteinase expressed by carcinoma-associated fibroblasts. As a result, the carcinoma-associated fibroblasts are killed, leading to a reduction in the amount of type I collagen in the tumors and improved drug delivery and efficacy of chemotherapy [80]. An increased delivery and efficacy of chemotherapy is also achieved by depletion of tumor-associated fibrotic stroma through inhibition of Hedgehog signaling in a mouse model of pancreatic ductal adenocarcinoma [81].
Interactions between collagen and the tumor immune infiltrate
A variety of immune cells are present in tumors and many of these accumulate and migrate within regions of dense fibrillar collagen [36,38,82]. How might the dense fibrillar collagen influence the function of immune cells? ECM stiffness promotes integrin-mediated adhesion assembly [21], which could influence e.g., T cell activation. Another possibility is via collagen-mediated activation of leukocyte-associated Ig-like receptors (LAIRs). LAIRs are highly expressed on most immune cells and can through their ITIMs (immunoreceptor tyrosine-based inhibition motifs) inhibit immune cell activation (reviewed in [83]). Although it is not clear whether LAIRs and integrins cooperate, activation of LAIRs is a plausible mechanism whereby high levels of deposited tumor collagen could lead to inhibition of an anti-tumor immune response.
Collagen can regulate leukocyte infiltration into tumors. Activation of the collagen receptor DDR1 is necessary for macrophage infiltration into atherosclerotic plaques [84]. Consistently, type I collagen and collagen fragments are chemotactic for monocytes (macrophage precursors) and neutrophils ([85,86] and references therein).
Collagen may regulate the balance between tumor-inhibiting and tumor-promoting effects of immune cells. For example, culturing macrophages on type I collagen reduces their cytotoxicity against cancer cells [87], suggesting that this inhibits the polarization of the macrophages to the tumoricidal M1-like type. The possibility that the collagen scaffold can regulate macrophage polarization is further supported by the increase in pro-tumorigenic, M2-like macrophages observed in tumors of Sparc-/- mice with an abnormal collagen scaffold [32].
Collagen influences the immune cell infiltrate, but immune cells also influence collagen architecture. Macrophages regulate mammary epithelial invasion during normal development [88]. This may in part be through their ability to initiate the remodeling and reorganization of the collagen fibers surrounding the developing epithelium [89], probably achieved through secretion of a repertoire of soluble factors such as MMPs. Macrophages can also take up collagen for intracellular degradation via binding to the glycoprotein Mfge8 [90], the mannose receptor, and uPARAP/endo180 [91].
Collagen and regulation of differentiation
Matrix stiffness can determine stem cell lineage specification and direct mesenchymal stem cell differentiation into bone, neurons or muscle cells [92]. During bone development, inhibition of MMP-mediated cleavage of type I collagen leads to osteopenia, a loose bone structure, rather than increased bone formation [93], suggesting that an abnormal collagen scaffold modifies the balance between bone-forming osteoblasts and bone-resorbing osteoclasts. Indeed, the collagenolytic activity of MMP14 regulates the differentiation of mesenchymal stem cells into bone-producing osteoblasts in 3-dimensional (3D) collagen matrices [47]. By analogy, the modified levels, fibril organization and proteolysis of collagens in tumors could influence the differentiation state of cancer cells. Interestingly, type I collagen and Matrigel (which contains BM constituents such as laminin-111 and type IV collagen) increase engraftment of cancer cells in mice [94-96]. So how does collagen influence tumor engraftment? One potential clue is that the percentage of cancer cells that express stem cell markers increases when the cells are exposed to type I collagen [97]. Furthermore, breast cancer cells with stem cell-like characteristics express increased levels of types I, IV and XVIII collagen, suggesting that an ECM autocrine circuit might promote tumor evolution [98].
Integrins are strong contenders for linking collagen and cancer cell differentiation [41]. Integrin αvβ3 is a marker of luminal progenitor cells in the mammary epithelium and β3 integrin (also known as CD61) is also a marker of a cancer stem cell-like population [99,100]. Integrin αvβ3 is not activated by intact type I collagen, but by MMP-generated collagen fragments [59-62] and by stretched/denatured fibronectin, suggesting that collagen remodeling and stiffening could regulate stem cell differentiation by modulating the activity of this integrin. Indeed, collagen remodeling by MMP14 regulates the differentiation of adipocytes from preadipocytes [23]. Whether these effects are due to a reduction in tissue rigidity or through generation of bioactive collagen fragments remains to be determined.
The challenges ahead
The overall architecture of the ECM is affected by collagen concentration, posttranslational modification (e.g., crosslinking) and proteolysis. In cancer, all of these levels of collagen metabolism are deregulated, resulting in an abnormal ECM architecture. However, to determine how this influences tumor evolution is challenging.
The study of the effects of collagen architecture on tumor evolution using in vitro assays has been informative, but a major concern is the ability to accurately replicate the complicity of the ECM architecture found in vivo. There is therefore a strong need to study collagen structure/function in vivo and to develop tractable methods to manipulate biochemical composition, architectural features and mechanical properties of collagen while simultaneously monitoring cancer cell behavior. To address these concerns, second harmonic generation using two-photon microscopy has been used in live animals to monitor how epithelial and stromal cells interact with and initiate collagen remodeling to regulate its architecture [36,39,49,78,89,101]. In addition, recent work with Atomic Force Microscopy has yielded high resolution force “heat” maps that demonstrate the existence of stiffness tracts that register with regions of collagen fiber enlargement and linearization (Lopez et al., Submitted).
Collagens are often used in vitro as barriers that cells must cross in invasion assays. Yet, it is clear that collagen has much more complex cellular effects than merely acting as an inert scaffolding protein and migration barrier. Indeed, such a simplistic view betrays the elegant reciprocal relationship between the ECM and cell behavior [102]. Our limited understanding of the effects of collagen in cancer is well illustrated by the findings described above: both increased [19] and decreased [32] deposition of collagen can be associated with increased malignancy. These findings suggest that many of the effects of collagen are mediated by its architecture or by the dynamics of its remodeling rather than solely by protein level.
Analysis of human tumors has revealed an association between collagen expression or collagen modifying enzymes and poor prognosis [16,17,27,28,57], supporting the notion that collagen remodeling is highly relevant to human cancer progression. The challenge for ECM biologists is to deconstruct the collagen “code”, or in other words, to determine just how the structure of the collagen triple helix translates into cellular effects to promote malignancy.
Acknowledgments
We thank Dr. Mark Sternlicht for his contribution to Figure 1. This work was supported by NIH grants U01CA141451 to M.E. and U54CA143836 and CA138818-01A1 to V.M.W. and DOD grant W81XWH-05-1-0330 to V.M.W., as well as funding from the Breast Cancer Alliance, the Susan G. Komen for the Cure, and Long Island 2 Day Walk to Fight Breast Cancer to M.E. M.G.R. was supported by Rigshospitalet, Augustinus fonden, Dagmar Marshalls fond, and the European Association for Cancer Research (EACR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Egeblad M, Nakasone E, Werb Z. The tumor as an organ: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Boot-Handford RP, Tuckwell DS. Fibrillar collagen: the key to vertebrate evolution? A tale of molecular incest. Bioessays. 2003;25:142–151. doi: 10.1002/bies.10230. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 7.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]; * A comprehensive review on how different cell types cross the basement membrane during development and in cancer.
- 8.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11:97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huijbers IJ, Iravani M, Popov S, Robertson D, Al-Sarraj S, Jones C, Isacke CM. A role for fibrillar collagen deposition and the collagen internalization receptor endo180 in glioma invasion. PLoS One. 2010;5:e9808. doi: 10.1371/journal.pone.0009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu GG, Risteli L, Makinen M, Risteli J, Kauppila A, Stenback F. Immunohistochemical study of type I collagen and type I pN-collagen in benign and malignant ovarian neoplasms. Cancer. 1995;75:1010–1017. doi: 10.1002/1097-0142(19950215)75:4<1010::aid-cncr2820750417>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Kauppila S, Stenback F, Risteli J, Jukkola A, Risteli L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J Pathol. 1998;186:262–268. doi: 10.1002/(SICI)1096-9896(1998110)186:3<262::AID-PATH191>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Hasebe T, Sasaki S, Imoto S, Mukai K, Yokose T, Ochiai A. Prognostic significance of fibrotic focus in invasive ductal carcinoma of the breast: a prospective observational study. Mod Pathol. 2002;15:502–516. doi: 10.1038/modpathol.3880555. [DOI] [PubMed] [Google Scholar]
- 14.Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper documents an association between risk of metastasis and a gene expression signature that includes COL1A1.
- 17.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]; * This paper documents an association between risk of metastasis and a gene expression signature that includes COL1A1 and COL1A2.
- 18.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper demonstrates that collagen crosslinking during breast tumorigenesis stiffens the ECM to promote focal adhesions, enhancing PI3 kinase (PI3K) activity, and fostering invasion of an oncogene-initiated epithelium.
- 20.Paszek MJ, Weaver VM. The tension mounts: mechanics meets morphogenesis and malignancy. J Mammary Gland Biol Neoplasia. 2004;9:325–342. doi: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 21.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]; ** This paper demonstrated for the first time that tissue stiffness promotes malignant behavior by inducing Rho-dependent cell tension. Furthermore, by reducing cancer cell tension, the tumor behavior was normalized towards a non-invasive, non-proliferating tissue. The clustering of integrins induced by tissue stiffness or by myosin-induced contractility perturbs epithelial morphogenesis and induces a tumor-like behavior by disrupting adherens junctions, destabilizing tissue polarity, and enhancing growth.
- 22.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]; ** This paper shows that the differentiation of adipocytes requires MMP14-mediated proteolysis that modulates pericellular collagen rigidity.
- 24.Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud JA, Sommer P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am J Pathol. 1997;150:497–507. [PMC free article] [PubMed] [Google Scholar]
- 25.Santhanam AN, Baker AR, Hegamyer G, Kirschmann DA, Colburn NH. Pdcd4 repression of lysyl oxidase inhibits hypoxia-induced breast cancer cell invasion. Oncogene. 2010 doi: 10.1038/onc.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper shows that lysyl oxidase is critical for defining metastastic sites through a mechanism that involves type IV collagen modification and recruitment of myeloid cells.
- 27.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 28.Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, et al. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer Res. 2003;63:1657–1666. [PubMed] [Google Scholar]
- 29.Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- 30.Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, Gourdon D, Nelson BJ, Vogel V. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci U S A. 2009;106:18267–18272. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 32.Arnold SA, Rivera LB, Miller AF, Carbon JG, Dineen SP, Xie Y, Castrillon DH, Sage EH, Puolakkainen P, Bradshaw AD, et al. Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. Dis Model Mech. 2010;3:57–72. doi: 10.1242/dmm.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper shows that organization of the collagen network by SPARC regulates metastasis and polarization of pro-tumorigenic, alternatively activated macrophages in mice.
- 33.Furber JD. Extracellular glycation crosslinks: prospects for removal. Rejuvenation Res. 2006;9:274–278. doi: 10.1089/rej.2006.9.274. [DOI] [PubMed] [Google Scholar]
- 34.Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10:339–352. doi: 10.1016/0891-5849(91)90040-a. [DOI] [PubMed] [Google Scholar]
- 35.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–1167. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 36.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 37.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J, Cermak L, Bottinger EP, Singer RH, White JG, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res. 2002;62:6278–6288. [PubMed] [Google Scholar]
- 40.Giese A, Kluwe L, Laube B, Meissner H, Berens ME, Westphal M. Migration of human glioma cells on myelin. Neurosurgery. 1996;38:755–764. [PubMed] [Google Scholar]
- 41.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makareeva E, Han S, Vera JC, Sackett DL, Holmbeck K, Phillips CL, Visse R, Nagase H, Leikin S. Carcinomas Contain a Matrix Metalloproteinase-Resistant Isoform of Type I Collagen Exerting Selective Support to Invasion. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-09-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper demonstrates that the type I collagen α1 homotrimer, found in carcinomas, is resistant to matrix metalloproteinases and enhances proliferation and migration of invasive cancer cells. This suggests that invasive cancer cells use MMP-resistant type I collagen homotrimers for directed migration.
- 44.Liotta LA. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 45.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper discusses the importance of proteolysis of the collagen network for cancer cell migration.
- 46.Curino AC, Engelholm LH, Yamada SS, Holmbeck K, Lund LR, Molinolo AA, Behrendt N, Nielsen BS, Bugge TH. Intracellular collagen degradation mediated by uPARAP/Endo180 is a major pathway of extracellular matrix turnover during malignancy. J Cell Biol. 2005;169:977–985. doi: 10.1083/jcb.200411153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu C, Li XY, Hu Y, Rowe RG, Weiss SJ. MT1-MMP controls human mesenchymal stem cell trafficking and differentiation. Blood. 2010;115:221–229. doi: 10.1182/blood-2009-06-228494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A comprehensive review on how ECM architecture regulates different types of cell migration.
- 51.Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008;68:7247–7249. doi: 10.1158/0008-5472.CAN-08-0784. [DOI] [PubMed] [Google Scholar]
- 52.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]; ** This paper demonstrates the importance of pericellular fibrillar collagen remodeling as well as force generation for cellular migration.
- 53.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–1031. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 55.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 57.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 58.Messent AJ, Tuckwell DS, Knauper V, Humphries MJ, Murphy G, Gavrilovic J. Effects of collagenase-cleavage of type I collagen on alpha2beta1 integrin-mediated cell adhesion. J Cell Sci. 1998;111(Pt 8):1127–1135. doi: 10.1242/jcs.111.8.1127. [DOI] [PubMed] [Google Scholar]
- 59.Stringa E, Knauper V, Murphy G, Gavrilovic J. Collagen degradation and platelet-derived growth factor stimulate the migration of vascular smooth muscle cells. J Cell Sci. 2000;113(Pt 11):2055–2064. doi: 10.1242/jcs.113.11.2055. [DOI] [PubMed] [Google Scholar]
- 60.Montgomery AM, Reisfeld RA, Cheresh DA. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci U S A. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fera E, O'Neil C, Lee W, Li S, Pickering JG. Fibroblast growth factor-2 and remodeled type I collagen control membrane protrusion in human vascular smooth muscle cells: biphasic activation of Rac1. J Biol Chem. 2004;279:35573–35582. doi: 10.1074/jbc.M400711200. [DOI] [PubMed] [Google Scholar]
- 62.Petitclerc E, Stromblad S, von Schalscha TL, Mitjans F, Piulats J, Montgomery AM, Cheresh DA, Brooks PC. Integrin alpha(v)beta3 promotes M21 melanoma growth in human skin by regulating tumor cell survival. Cancer Res. 1999;59:2724–2730. [PubMed] [Google Scholar]
- 63.Wall SJ, Werner E, Werb Z, DeClerck YA. Discoidin domain receptor 2 mediates tumor cell cycle arrest induced by fibrillar collagen. J Biol Chem. 2005;280:40187–40194. doi: 10.1074/jbc.M508226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J Biol Chem. 2008;283:6861–6868. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- 65.Wu H, Byrne MH, Stacey A, Goldring MB, Birkhead JR, Jaenisch R, Krane SM. Generation of collagenase-resistant collagen by site-directed mutagenesis of murine pro alpha 1(I) collagen gene. Proc Natl Acad Sci U S A. 1990;87:5888–5892. doi: 10.1073/pnas.87.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seandel M, Noack-Kunnmann K, Zhu D, Aimes RT, Quigley JP. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–2332. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- 67.Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–767. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cooke VG, Kalluri R. Chapter 1. Molecular mechanism of type IV collagen-derived endogenous inhibitors of angiogenesis. Methods Enzymol. 2008;444:1–19. doi: 10.1016/S0076-6879(08)02801-2. [DOI] [PubMed] [Google Scholar]
- 70.Armstrong T, Packham G, Murphy LB, Bateman AC, Conti JA, Fine DR, Johnson CD, Benyon RC, Iredale JP. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–7437. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 71.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, Chilvers ER, Dransfield I, Donnelly SC, Strieter R, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]; ** This paper demonstrates that adhesion to ECM components, including type IV collagen, can suppress chemotherapy-induced apoptosis through activation of β1 integrin.
- 72.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 73.Svensson L, Aszodi A, Reinholt FP, Fassler R, Heinegard D, Oldberg A. Fibromodulin-null mice have abnormal collagen fibrils, tissue organization, and altered lumican deposition in tendon. J Biol Chem. 1999;274:9636–9647. doi: 10.1074/jbc.274.14.9636. [DOI] [PubMed] [Google Scholar]
- 74.Oldberg A, Kalamajski S, Salnikov AV, Stuhr L, Morgelin M, Reed RK, Heldin NE, Rubin K. Collagen-binding proteoglycan fibromodulin can determine stroma matrix structure and fluid balance in experimental carcinoma. Proc Natl Acad Sci U S A. 2007;104:13966–13971. doi: 10.1073/pnas.0702014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gade TP, Buchanan IM, Motley MW, Mazaheri Y, Spees WM, Koutcher JA. Imaging intratumoral convection: pressure-dependent enhancement in chemotherapeutic delivery to solid tumors. Clin Cancer Res. 2009;15:247–255. doi: 10.1158/1078-0432.CCR-08-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 77.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 78.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 79.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 80.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper demonstrates that the killing of tumor-associated fibroblasts, by using a DNA vaccine targeting fibroblast activation protein, resulted in decreased type I collagen expression and significantly better uptake of and response to chemotherapeutic drugs.
- 81.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper demonstrates that the delivery and efficacy of the chemotherapeutic drug gemcitabine is improved by coadministration of a drug that depletes tumor-associated stromal tissue by inhibition of the Hedgehog signaling pathway in mice.
- 82.Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1:155–167. doi: 10.1242/dmm.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyaard L. The inhibitory collagen receptor LAIR-1 (CD305) J Leukoc Biol. 2008;83:799–803. doi: 10.1189/jlb.0907609. [DOI] [PubMed] [Google Scholar]
- 84.Franco C, Britto K, Wong E, Hou G, Zhu SN, Chen M, Cybulsky MI, Bendeck MP. Discoidin domain receptor 1 on bone marrow-derived cells promotes macrophage accumulation during atherogenesis. Circ Res. 2009;105:1141–1148. doi: 10.1161/CIRCRESAHA.109.207357. [DOI] [PubMed] [Google Scholar]
- 85.Postlethwaite AE, Kang AH. Collagen- and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976;143:1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]; * This paper demonstrates that a tripeptide (N-acetyl Pro-Gly-Pro) derived from the breakdown of collagen causes chemotaxis of neutrophils through CXC receptors.
- 87.Kaplan G. In vitro differentiation of human monocytes. Monocytes cultured on glass are cytotoxic to tumor cells but monocytes cultured on collagen are not. J Exp Med. 1983;157:2061–2072. doi: 10.1084/jem.157.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 89.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–3229. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 90.Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, et al. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713–3722. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007;26:146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]; * A comprehensive review on the structure and function of the different collagen receptors.
- 92.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 93.Egeblad M, Shen HC, Behonick DJ, Wilmes L, Eichten A, Korets LV, Kheradmand F, Werb Z, Coussens LM. Type I collagen is a genetic modifier of matrix metalloproteinase 2 in murine skeletal development. Dev Dyn. 2007;236:1683–1693. doi: 10.1002/dvdy.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Del Buono R, Pignatelli M, Hall PA. Control of differentiation in a rectal adenocarcinoma cell line: the role of diffusable and cell-associated factors. J Pathol. 1991;164:59–66. doi: 10.1002/path.1711640111. [DOI] [PubMed] [Google Scholar]
- 95.Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer Sci. 2009;100:1166–1172. doi: 10.1111/j.1349-7006.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kirkland SC. Type I collagen inhibits differentiation and promotes a stem cell-like phenotype in human colorectal carcinoma cells. Br J Cancer. 2009;101:320–326. doi: 10.1038/sj.bjc.6605143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 100.Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 101.Perentes JY, McKee TD, Ley CD, Mathiew H, Dawson M, Padera TP, Munn LL, Jain RK, Boucher Y. In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nat Methods. 2009;6:143–145. doi: 10.1038/nmeth.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper describes the use of multiphoton laser scanning microscopy to determine the rate of remodeling of the collagen network by tumor-associated stromal cells in live mice.
- 102.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 103.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A comprehensive review on the biochemistry of collagen.
- 104.Vanacore R, Ham AJ, Voehler M, Sanders CR, Conrads TP, Veenstra TD, Sharpless KB, Dawson PE, Hudson BG. A sulfilimine bond identified in collagen IV. Science. 2009;325:1230–1234. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper demonstrates that a sulfilimine bond is responsible for the covalent crosslinking of adjoining protomers of type IV collagen in the basement membrane.
- 105.Mundel TM, Kalluri R. Type IV collagen-derived angiogenesis inhibitors. Microvasc Res. 2007;74:85–89. doi: 10.1016/j.mvr.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18:1108–1116. doi: 10.1016/j.cellsig.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 107.Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJ, et al. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J Exp Med. 2006;203:1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]