Abstract
Purpose
Histone deacetylase inhibitors (HDACi) are targeted anti-cancer agents with a well-documented ability to act synergistically with cytotoxic agents. We recently demonstrated that the HDACi valproic acid (VPA) sensitizes osteosarcoma cells to doxorubicin (DOX) in vitro and in vivo. As there are no published reports on the clinical utility of HDACi in dogs with spontaneous cancers, we sought to determine a safe and biologically effective dose of VPA administered prior to a standard dose of DOX.
Methods
21 dogs were enrolled into eight cohorts in an accelerated dose-escalation trial consisting of pre-treatment with oral VPA followed by DOX on a three-week cycle. Blood and tumor tissue were collected for determination of serum VPA concentration and evaluation of pharmcodynamic effects by immunofluorescence cytochemistry and immunohistochemistry. Serum and complete blood counts were obtained for determination of changes in DOX pharmacokinetics or hematologic effects.
Results
All doses of VPA were well tolerated. Serum VPA concentrations increased linearly with dose. DOX pharmacokinetics were comparable to those in dogs receiving DOX alone. A positive correlation was detected between VPA dose and histone hyperacetylation in PBMC. No potentiation of DOX-induced myelosuppression was observed. Histone hyperacetylation was documented in tumor and PBMC. Responses included 2/21 complete, 3/21 partial, 5/21 stable disease, and 11/21 progressive disease.
Conclusions
VPA can be administered to dogs at doses up to 240 mg/kg/day prior to a standard dose of DOX. In addition, we have developed the PK/PD tools necessary for future studies of novel HDACi in the clinical setting of canine cancer.
Keywords: valproate, HDAC, canine, Adriamycin
Introduction
Histone deacetylase (HDAC) enzymes, responsible for the removal of acetyl groups from N-terminal tails of histone proteins, play a crucial role in chromatin plasticity and have recently been identified as one of the most promising therapeutic targets in cancer therapy (1). The rationale behind the use of HDAC inhibitors stems from the discovery of epigenetically silenced tumor suppressor genes in a number of model systems resulting in tumor cell addiction to altered signaling pathways (2, 3). In addition, an imbalance in the activity of histone acetyltransferases, the enzymes responsible for lysine acetylation, and HDAC plays a crucial role in the development and progression of some tumors (4). A number of HDAC inhibitors have demonstrated anti-tumor activity in vitro and in vivo, either as single agents or in combination with other chemotherapeutics, and have shown efficacy in hematologic and solid tumors through inhibition of proliferation by cell cycle arrest, effects on terminal differentiation, and apoptosis (5-13). A few of these agents have entered into clinical trials, and of the HDAC inhibitors, SAHA (Vorinostat®) and romidepsin (Istodax®) have received FDA approval for use in humans as single agents in the treatment of cutaneous T-cell lymphoma (CTCL). For the most part, these agents are well tolerated, demonstrate target modulation in tumor tissues, and show some single-agent efficacy in solid and hematologic malignancies (8, 14, 15). However, these agents are likely to provide the most benefit when combined with other treatment modalities such as chemotherapy or radiotherapy.
While early-phase studies in humans provide promising results and describe some biological parameters for determining potential susceptibility to HDACi containing treatment protocols, it has also been clearly demonstrated that the current drug development pipeline for novel anti-cancer therapies in many cases fails to properly identify agents that provide long-term improvements in patient survival. The use of spontaneously occurring tumors in pet dogs has the potential to improve current drug development and modeling strategies (16-18). This realization has led to the completion and publication of the first canine clinical trial conducted by the National Cancer Institute's Comparative Oncology Trials Consortium (COTC) evaluating safety, toxicity, tumor specificity, and efficacy of a novel tumor endothelium targeting agent (RGD-A-TNF) being developed as a therapy for human cancers (18). The translational utility of the canine cancer model stems from the greater similarities to human cancers when compared to murine models. Unlike murine models, dogs are relatively outbred, immunocompetent animals with spontaneously occurring tumors experiencing spontaneous metastasis and therapy resistance, representing a spectrum of tumor histotypes that have biology similar to that found in humans. When compared with murine tumors, the relatively large size of canine tumors more closely approximates human solid tumors with respect to important biological factors such as hypoxia and clonal variation, and allows for multiple samplings of tumor tissue over time. The relatively rapid time course of disease progression, when compared with human cancer, allows for more rapid assessment of therapeutic endpoints than is possible in many human clinical trials (19, 20). In addition, the lack of a meaningful standard of care for many types of canine cancer alleviates the obligation to start with single-agent Phase I trials and allows immediate evaluation of novel agents in combination protocols, where they are most likely to show a benefit.
While there have been some reports on the in vitro sensitivity of canine cancer cells to HDAC inhibitors (21, 22), there have been no reports on the clinical use of HDAC inhibitors in canine cancer patients.
The anti-epileptic drug valproic acid (VPA), belonging to the short-chain fatty acid class of HDACi, has shown efficacy in a variety of tumor models, especially when combined with other forms of therapy (5, 6, 15, 23-26). We previously showed that pre-treatment of canine and human osteosarcoma cells with VPA results in sensitization to doxorubicin (DOX), partially via increasing nuclear DOX accumulation, resulting in decreased proliferation and increased apoptosis (22). This was also demonstrated in an in vivo xenograft model of canine osteosarcoma (22). Here we report the pharmacokinetic and pharmacodynamic results of a Phase I trial of oral VPA in tumor-bearing dogs given prior to a standard dose of DOX. We hypothesized that VPA could be safely administered to dogs for 48 hours prior to a standard dose of DOX, would demonstrate target modulation in PBMC and tumor tissues, and would have no effects on DOX PK.
Methods
Chemicals and antibodies
Divalproex sodium extended-release tablets (Depakote® ER) were purchased from Cardinal Health (Dublin, OH). Doxorubicin (DOX) was purchased from Bedford Laboratories (Bedford, OH). Controls for the valproic acid assay were purchased from Cliniqa (San Marcos, CA). Anti acetyl-histone H3 and H4 and total histone H3 and H4 antibodies were purchased from Upstate Biotechnology (Waltham, MA). FITC-conjugated goat anti-rabbit antibody was purchased from Bethyl Laboratories (Montgomery, TX). Horseradish peroxidase-conjugated goat anti-rabbit IgG antibody was purchased from Pierce (Rockford, IL). Daunorubicin hydrocholoride was purchased from Sigma (St. Louis, MO), and doxorubicinol hydrochloride from Toronto Research Chemicals, Inc. (North York, Ontario, Canada). All other reagents were of analytical grade.
Patient recruitment
All dogs in this study were pet dogs presenting as patients to the Colorado State University Animal Cancer Center. Study participation was offered in cases where standard therapy had failed or had been declined by the dog's owner, or in cases of advanced disease where no meaningful standard therapy exists. Dogs were treated in accordance with the “NIH Guidelines for Care and Use of Laboratory Animals”. Protocol approval was obtained from the Institutional Animal Care and Use Committee and the Colorado State University Veterinary Teaching Hospital Clinical Review Board. Signed informed consent and consent to necropsy were obtained from all owners.
This study was open to dogs with histologically or cytologically confirmed neoplasia. Dogs with regional or distant metastasis or advanced local disease were included if a survival time of greater than 6 weeks was expected. Dogs were required to be free of other severe complicating concurrent disease conditions, and required to have adequate laboratory and clinical indices to safely undergo therapy (specifically: total bilirubin not exceeding 1.5× normal; creatinine not exceeding 2× normal; at least 2,500 neutrophils/μL, 75,000 platelets/μL, and a hematocrit of at least 28%). Treatment related adverse events were graded based on guidelines set forth in the Veterinary Comparative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE) (27). A VCOG performance status of 0-1 was required for study inclusion; (0, normal activity; 1, restricted activity [decreased activity from pre-disease status]; 2, compromised [ambulatory only for vital activities, consistently defecates and urinates in acceptable areas]; 3, disabled [dog needs to be force-fed, is unable to confine urination and defecation to acceptable areas], and; 4, dead). Prior chemotherapy and radiation therapy were allowed with a 3-week or 6-week washout period, respectively. In addition, a 72-hour washout from prednisone was required if being used as an antineoplastic drug. No concurrent anti-neoplastic therapy was allowed, and prior DOX exposure could not exceed 90 mg/m2.
Pretreatment procedures and evaluations
A complete blood count (CBC), serum biochemistry profile, and urinalysis were performed prior to enrollment in the study and staging was performed as appropriate for specific tumor type. 10 mL of heparinized whole blood was collected for PBMC separation, and 14-Ga needle core biopsies were obtained from accessible tumors using local anesthesia or brief sedation.
Treatments
All dogs were given oral divalproex (VPA) for 48 hours prior to DOX administration with an initial loading dose of 2X the intended maintenance dose, and two additional doses were administered on the day of DOX administration (total of 6 doses, or 72 hours of therapy). The initial maintenance dose of 30 mg/kg was chosen as the starting point as this is one-half of the putative anti-convulsant dose for VPA in dogs (28). Doses were escalated according to an accelerated dose-escalation protocol whereby one dog was enrolled in each cohort and the cohort was expanded to six only if toxicity (grade 3 or higher) was encountered in the first dog. All dogs received a standard dose of DOX (30 mg/m2, or 1 mg/kg if <15 kg) as initial treatment on day 3, between the morning and evening VPA doses. DOX dose was reduced by 20% for subsequent treatments if grade 3 or 4 toxicities were observed after the first dose.
Monitoring procedures and evaluations
A CBC and blood chemistry were obtained 48 hours after initiation of VPA therapy. Serum, plasma, and heparinized whole blood were collected immediately prior to the morning VPA dose on day 3 for determination of VPA trough concentrations and VPA pharmacodynamic evaluation. Serum was collected after DOX administration at 0, 10, 20, 30, 60, 120, 240, 360 minutes, and 24 hours for evaluation of DOX pharmacokinetics. Tumor biopsies were obtained again 48 hours after initiation of VPA therapy. A CBC and blood chemistry were obtained 7 and 21 days following DOX administration. Owners were asked to fill out Quality of Life/Pain questionnaires prior to study, after 48 hours of VPA therapy, and at 7 and 21 days following DOX administration. The VPA/DOX combination was continued on an every 3 week basis until disease progression or maximal cumulative DOX dose (5 cycles or 150 mg/m2) was reached. Removal from the study resulted from either disease progression, decreased quality of life (VCOG score >2), cumulative DOX dose > 150 mg/m2, or owner request. Tumor response in dogs with osteosarcoma that had amputation performed was assessed by radiographic evaluation of the size and number of lung metastases.
Valproic acid analysis in serum
Serum trough VPA concentrations were determined using the Cedia® Valproic Acid II Assay (Microgenics; Fremont, CA) on a Hitachi 917 System Analyzer (Roche; Indianapolis, IN). Results were graphed as serum trough VPA concentration vs. maintenance dose.
Doxorubicin and doxorubicinol analysis in plasma by LC/MS/MS
Doxorubicin and doxorubicinol were measure in dog plasma using an LC/MS/MS assay. Positive ion electrospray ionization (ESI) mass spectra were obtained with a MDS Sciex 3200 Q-TRAP triple quadrupole mass spectrometer (Applied Biosystems, Inc., Foster City, CA) with a turbo ionspray source interfaced to an Agilent 1200 Series Binary Pump SL HPLC system (Santa Clara, CA). Samples were chromatographed with a Phenomenex Prodigy, 5μm, C18 100 Å, 150 × 2.00 mm column (Phenomenex, Torrance, CA). An LC gradient was employed with mobile phase A consisting of 10 mM ammonium acetate plus 0.1% acetic acid (pH 4.4) and mobile phase B consisting of acetonitrile. Chromatographic separation was achieved holding mobile phase B steady at 10% from 0-2 min, increasing mobile phase B linearly from 10% to 90% from 2-9 min, holding steady from 9-11 minutes, and decreasing linearly from 90% to 10% from 11-13 minutes, followed by re-equilibration from 13-15 minutes. The sample injection volume was 50 μL and the analysis run time was 15 minutes. The mass spectrometer settings were optimized as follows: turbo ionspray temperature, 350°C; ion spray voltage, 5500 V; declustering potential (DP), 20 V; entrance potential (EP), 5.0 V; collision energy (CE), 40 V; collision cell exit potential (CXP), 5.0 V; curtain gas, N2, (CUR), 50 units; collision gas, N2, (CAD), medium. Samples were quantified by internal standard reference method in the MRM mode monitoring ion transitions m/z 544→361 amu for DOX, m/z 546→363 amu for doxorubicinol, and m/z 528→321 for the internal standard, daunorubicin. Scan times were 200 ms and Q1 and Q3 were both operated in unit resolution mode.
Analytical standards (2.5-500 ng/ml), quality control (QC) and unknowns were all prepared by adding 500 μl of unknown or spiked blank plasma samples to 1.5 ml microcentrifuge tubes containing 5 μl of 10 μM daunorubicin solution followed by brief vortexing. Plasma proteins were then precipitated by the addition of 500 μl of acetonitrile followed by 10 min vortex mixing. Samples were then centrifuged at 18,000 RCF for 10 min and the supernatant collected and transferred to autosampler vials for analysis. The lower limit of quantitation for the assay was 2.5 ng/ml for both doxorubicin and doxorubicinol with accuracy and precision (CV%) of 91.7% ± 5.7% and 95.3% ± 3.8%, respectively.
Immunohistochemistry
Deparaffinized sections of pre- and post-VPA tumor biopsies were stained for histone H3 acetylation after antigen retrieval using DakoCytomation Target Retrieval Solution pH 9 (Dako, Carpinteria, CA). Prepared sections were incubated with anti AcH3 at 1:50 overnight at 4°C followed by goat anti-rabbit HRP at 1:250 for 1.5 hrs at room temperature followed by diaminobenzidine (DAB) (Vector Labs, Burlingame, CA) staining and hematoxylin counterstain. Images were obtained using a Zeiss Axioplan 2 microscope coupled with a Zeiss AxioCam HRc camera. For blinded comparison of acetylated histones between pre- and post-VPA samples, an overall H-score was given to each image, obtained by multiplying the percent of cells within each field with positive nuclear staining (0-100; determined subjectively) by an overall stain intensity score (0-no evident staining to 3-intense staining). Seven images were obtained for each pre- and post-treatment sample and H-score results were averaged.
Immunofluorescence
Isolated peripheral blood mononuclear cells (PBMC) from pre- and post-VPA blood samples were stained for acetylated histones H3 using total histone H3 as a control. PBMC were isolated using Lymphocyte Separation Media [(LSM); Mediatech, Inc., Herndon, VA]. Briefly, two volumes of PBS with 5mM EDTA were added to heparinized blood samples, which were then underlaid with eight mL of LSM. Samples were spun at 400 × g for 20 minutes and the lymphocyte layer was aspirated and washed three times with one volume of PBS/EDTA. Isolated PBMC were stored in liquid nitrogen until evaluation was performed. PBMC were cytospun onto slides and fixed in 95% ethanol/5% glacial acetic acid at -20°C for 5 minutes prior to permeabilization using 0.2% Tween 20 in PBS. Nonspecific binding was blocked with 1% BSA in PBST for one hour at room temp, followed by overnight incubation with polyclonal rabbit anti-acetyl histone H3 (1:200) or anti-total H3 (1:50) then 1.5 hours with goat anti-rabbit-FITC (1:250). Slides were then rinsed three times and mounted using VectaShield plus DAPI® (Vector Labs). Images were obtained using a Zeiss Axioplan 2 microscope coupled with a Zeix AxioCam HRc camera. For blinded comparison of acetylated histones between samples, a semi-automated computerized image analysis program was developed using AxioVision Rel. 4.5 software (Zeiss) that measured the fluorescence of each cell within a field and reported the average fluorescence for each field. Seven images were obtained from each sample and the results were averaged.
Western analysis
For evaluation of tumor tissue histone acetylation, snap-frozen pre- and post- VPA biopsy samples were lysed in buffer containing T-PER Protein extraction reagent (Pierce), 1 mM NaVO4, 1 mM PMSF, Complete Mini protease inhibitor (Roche), and 1% SDS, transferred to 1.5 mL microfuge tubes and passed through a 25 gauge needle 7-10 times before centrifugation at 10,000 × g for 10 minutes. Protein concentration of lysates was determined via BCA assay (Pierce). Lysates were loaded into a denaturing 4-12% Bis-Tris gel (Invitrogen, Carslbad, CA) and electrotransferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 5% non-fat dry milk in TBST (40 mM Tris pH 7.6, 300 mM NaCl, and 0.5% Tween-20) for one hour at room temperature and incubated in a 1:2500 dilution of rabbit polyclonal anti-acetyl H3, or total H3, in blocking solution overnight at 4°C. After three washes in TBST, membranes were incubated in a 1:20,000 dilution of HRP-conjugated goat anti-rabbit IgG for 1.5 hours at room temperature. Immunoreactive proteins were detected using SuperSignal® West Pico Chemiluminescent Substrate (Pierce) and analyzed by autoradiography. Densitometry was performed using Image J software available online from the NIH (http://rsb.info.nih.gov/ij/index.html).
Statistical analyses
Determination of correlations between serum trough VPA levels and dose, WBC parameters and VPA dose, and fold induction of PBMC histone hyperacetylation versus dose were performed using linear regression. DOX pharmacokinetic parameters were calculated by non-compartmental analysis as previously described (29) and comparisons between our data and historical controls were made by unpaired two-tailed T-test. Comparison of H-scores between pre and post treatment samples was done by two-tailed T-test. Statistical analysis was performed using GraphPad Prism® (GraphPad Software, La Jolla, CA). For all comparisons, a P-value less than 0.05 was considered significant.
Results
Dose-escalation trial
An accelerated dose-escalation design was used to govern dose escalation toward a MTD for oral administration of VPA. In all, 21 dogs met the inclusion criteria and were enrolled in the study. All dogs underwent pre-treatment evaluation of blood chemistry and CBC, and pre-treatment biopsies were obtained from accessible tumors. Age, weight, sex, and breed, and tumor type was recorded for each patient (Table 1). At enrollment, 10 of 21 patients (47%) had documented metastatic disease, and 8 of 21 (38%) had received prior chemotherapy and/or radiation or had been enrolled in a previous clinical trial.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients (N=21) |
% |
|---|---|---|
| Sex | ||
| Male | 11 | 52.4 |
| Female | 10 | 47.6 |
| Age, years | ||
| Median | 9 | |
| Range | 6.2-13.4 | |
| Weight, kg | ||
| Median | 30.1 | |
| Range | 4.7-42.4 | |
| Breed | ||
| Purebred | 16 | 76.2 |
| Mixed | 5 | 23.8 |
| Tumor Histology | ||
| Lymphoma | 5 | 23.8 |
| Melanaoma | 4 | 19 |
| Osteosarcoma | 3 | 14.3 |
| Carcinoma | 3 | 14.3 |
| Soft Tissue Sarcoma | 3 | 14.3 |
| Hemangiosarcoma | 1 | 4.8 |
| Mast Cell | 1 | 4.8 |
| Cutaneous T-cell Lymphoma | 1 | 4.8 |
| Dose Cohort, mg/kg (loading/maintenance) | ||
| 30/15 | 2 | 9.5 |
| 60/30 | 1 | 4.7 |
| 90/45 | 1 | 4.7 |
| 120/60 | 1 | 4.7 |
| 150/75 | 1 | 4.7 |
| 180/90 | 6 | 28.6 |
| 210/105 | 6 | 28.6 |
| 240/120 | 3 | 14.3 |
Oral VPA was well tolerated in all dose-escalation cohorts with only a single Grade 3 metabolic event reported (ALKP > 5× ULN) at the 180/90 mg/kg cohort in a patient with Stage 5 lymphoma. In addition, a single Grade 2 anorexia was reported in a patient at the 210/105 mg/kg cohort. Other owner-documented side effects were reported as mild and included lethargy, decreased appetite, and diarrhea. A total of 69 treatment cycles were administered with an average of 3.3 cycles per patient (range 1 to 5). No maximum tolerated dose (MTD) was reached, as the highest dose failed to produce any dose-limiting toxicity. Escalation was halted due to compliance issues with the number of tablets required; owners were required to administer as many as 19 tablets for loading doses followed by up to 9 tablets twice per day thereafter.
Two dogs required dose reductions in DOX after the first treatment cycle because of neutropenia; one patient at the 60 mg/kg maintenance dose exhibited febrile grade 3 neutropenia (neutrophil count 500-999/μL) and another at the 105 mg/kg maintenance dose with non-febrile grade 3 neutropenia.
Responses were evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) (30) and included 2/21 (10%) complete responses (both lymphoma), 3/21 (14%) partial responses (lymphoma, melanoma, lung carcinoma), 5/21 (24%) dogs with stable disease through 5 treatment cycles (osteosarcoma, renal cell carcinoma, apocrine gland adenocarcinoma, melanoma, soft-tissue sarcoma), and 11/21 (58%) progressive disease.
Pharmacokinetics
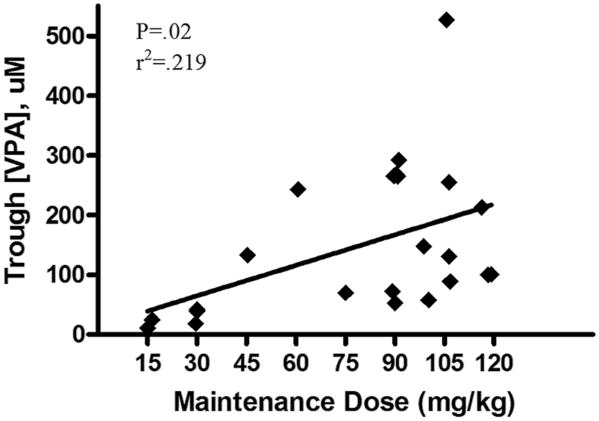
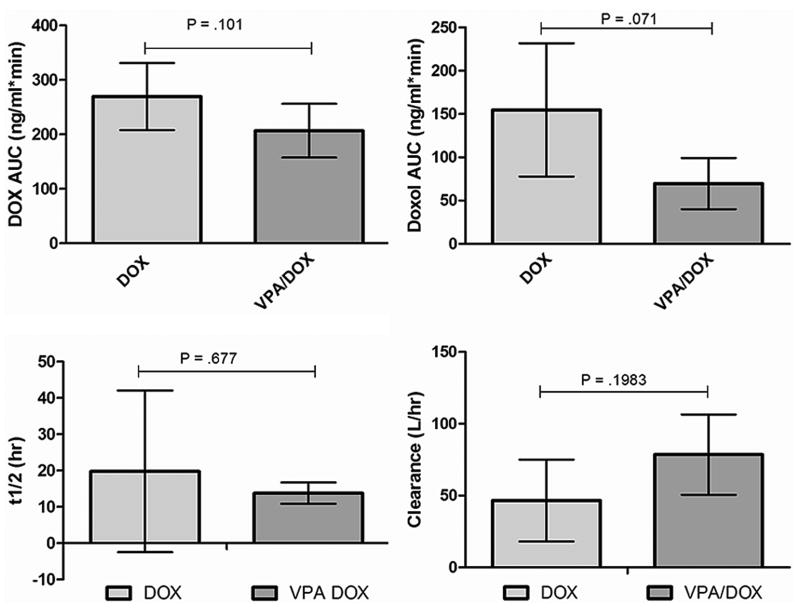
For determination of serum trough VPA concentrations, blood was obtained 48 hours after initiation of therapy, immediately prior to administration of the fifth dose. A linear correlation between the administered maintenance dose and serum trough VPA concentration existed in the patient population (Fig. 1). In addition, we evaluated DOX pharmacokinetic parameters including AUC, clearance, and half-life in three dogs in the highest dose cohort. To determine if administration of VPA altered DOX pharmacokinetics, our PK results were compared to those of two previously reported studies evaluating DOX PK in dogs receiving single agent therapy (31, 32). As shown in Figure 2, there were no significant differences in any of the evaluated PK parameters between our study dogs and historical controls.
Fig. 1.
Serum was collected after 48 hours of divalproex (VPA) therapy for determination of trough VPA concentration by serum chemistry analyzer. Results were plotted against the actual maintenance dose received and demonstrate that trough VPA concentration increases linearly with dose.
Fig. 2.
Serial serum samples were collected after IV doxorubicin (DOX) administration in three dogs in the highest VPA dose cohort (240 mg/kg/day) for determination of AUC, half-life, and clearance as well as AUC of the major metabolite, doxorubicinol by HPLC tandem M/S. Results were compared to those previously published for dogs receiving DOX alone and show no significant alterations in DOX PK parameters for VPA pre-treated dogs.
Pharmacodynamics
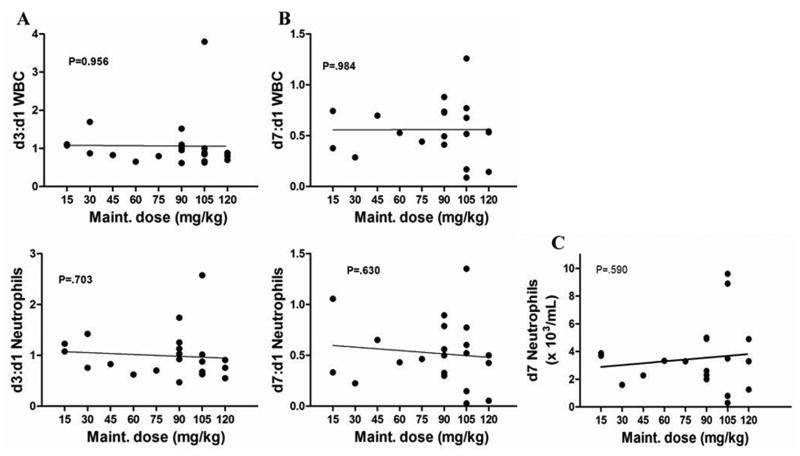
In order to determine if VPA therapy was associated with any myelosuppression, or potentiated DOX-induced myelosuppression, we evaluated patient complete blood counts (CBC) prior to and 48 hours after initiation of VPA therapy as well as 7 days after DOX administration. By examining the ratio of total WBC as well as neutrophil counts from day three to day one, we observed no correlation between dose administered and myelosuppression (Fig. 3a). The WBC nadir following DOX is typically around day 7 post administration, and a comparison of nadir WBC and neutrophil count with VPA dose revealed no potentiation of DOX induced myelosuppression (Fig. 3b and 3c). Correlations between absolute neutrophil count and administered dose were also evaluated and results were similar to those observed for total WBC (Fig. 3). Taken together, these data indicate that VPA administered at doses up to 240 mg/kg/day does not induce any significant myelosuppression or potentiate DOX induced myelosuppression.
Fig. 3.
Complete blood counts were obtained prior to and 48 hours after initiation of VPA as well as on day 7 post DOX. A) Ratio of WBC (top) and neutrophils (bottom) on day 3 to pre-treatment shows no correlation between VPA dose and myelosuppression. B) Ratio of WBC (top) and neutrophils (bottom) on day 7 (DOX nadir) to pre-treatment and C) absolute neutrophil counts on day 7 demonstrate that VPA pre-treatment does not potentiate DOX-induced myelosuppression.
Immunofluorescence
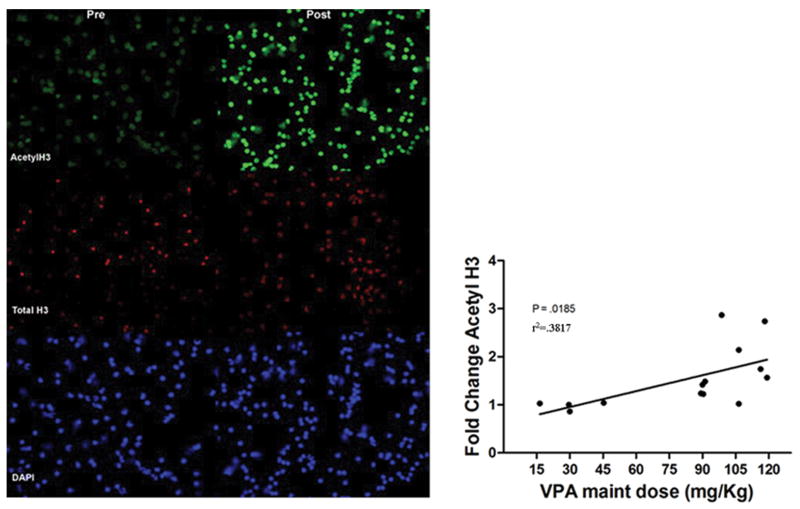
As described in the Methods section, blood was collected prior to and 48 hours after administration of VPA for separation of PBMC and evaluation of histone hyperacetylation. Fourteen matched samples were available for comparison of pre- and post-VPA histone acetylation by fluorescence immunocytochemistry. Tumor types represented in dogs providing PMBC for analysis included adenocarcinoma, lymphoma, osteosarcoma, soft tissue sarcoma, renal cell carcinoma, melanoma, hemangiosarcoma, cutaneous T-cell lymphoma, and pulmonary carcinoma. The results were recorded as fold change induction of acetylated histone H3, with total histone or total histone H3 as a control. As shown in Fig. 4, there was a significant correlation between the fold induction of acetyl H3 and the administered VPA maintenance dose, suggesting that histone hyperacetylation is useful as a PD marker to evaluate 48 hour VPA exposure in future clinical trials.
Fig. 4.
Left: Photomicrograph of PBMC in a dog before and 48 hours after initiation of VPA (240/120 mg/kg cohort) demonstrating an increase in acetylation of histone H3 (top) with no change in staining intensity of total histone (middle); nuclei are stained with DAPI (bottom). Right: Comparison of fold change in histone H3 acetylation vs maintenance dose received shows a positive correlation between dose and target modulation in PBMC.
Immunohistochemistry and Western blot
Nine dogs had tumors that were amenable to biopsy pre- and post-VPA treatment. For eight of these dogs, formalin fixed paraffin embedded samples were evaluated for induction of histone hyperacetylation by immunohistochemistry, while snap-frozen biopsy samples were used for detection of target modulation by western blot. As shown in Fig. 5, histone hyperacetylation in tumor tissues could be detected by IHC, and Western blot confirmed similar induction in these same dogs (data not shown). There was no direct correlation between administered VPA dose and magnitude of tumor histone acetylation by IHC, nor was there a positive correlation between tumor and PBMC histone hyperacetylation (data not shown).
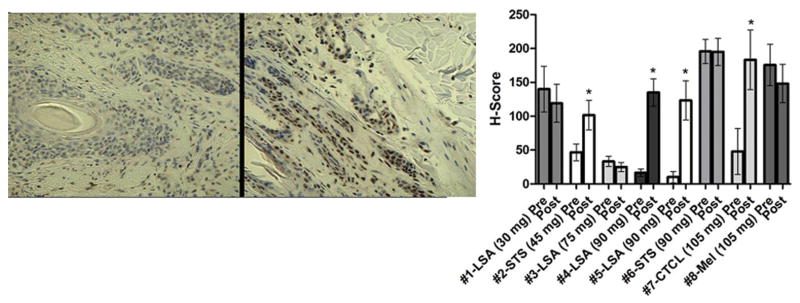
Fig. 5.
Accessible tumors were biopsied before and 48 hours after initiation of VPA. Left: Photomicrograph of IHC for acetylated histone H3 in a dog with cutaneous T-cell lymphoma pre and post VPA (210/105 mg/kg cohort) demonstrating significant induction of hyperacetylation. Right: Graphical representation of IHC comparisons for all evaluable biopsy samples. Objective responses were measured in dogs 3, 4, and 5. * indicates significant increase in histone H3 acetylation (P<.05).
Discussion
Here we report the results of a Phase I clinical trial evaluating the use of combined VPA/DOX therapy in dogs with spontaneously occurring tumors, aimed at defining a safe and biologically effective dose of VPA to use in future efficacy trials, as well as validating a toolbox of pharmacodynamic assays that can be applied to future studies of novel HDACi. The dosing scheme of a 48 hour pre-treatment period with an HDAC inhibitor prior to DOX was based upon previous findings by our own group as well as others of superior in vitro chemosensitization when compared to co-administration in a number of cell types, as well as in vivo activity in a canine osteosarcoma xenograft model (6, 22, 33). Additional benefits of pulse-dosing of VPA compared to continuous administration include the ability to give higher doses, because of the washout period between treatment cycles, as well as ease of compliance and reduced patient cost.
Eight dose cohorts, ranging from 30 mg/kg/day up to 240 mg/kg/day, were evaluated. This study failed to determine a MTD as the highest dose was well tolerated by patients. Consistent with Phase I reports of VPA as well as other HDACi in humans, the toxicities encountered were generally mild. (8, 14, 34-36) The most commonly reported adverse events were Grade 1 anorexia and lethargy and Grade 1 diarrhea. A single Grade 3 metabolic toxicity (increase in serum ALP > 5× ULN) was reported in a patient with Stage V lymphoma in the 180 mg/kg/day cohort and was most likely reflective of disease progression during the 48 hour VPA pre-treatment time period. This was only observed in the first treatment cycle for this patient, with ALP levels returning to near normal after the first cycle and remaining stable for the remainder of the study. In addition, a single Grade 2 anorexia was reported in a dog in the 210 mg/kg/day cohort but no further toxicity was reported after expansion of that cohort. The number of dogs requiring DOX dose reduction in our study does not exceed that expected for dogs receiving DOX alone (37). Although no MTD was reached as there was no reported dose limiting toxicity in the highest dose cohort, the number of tablets required and owner compliance became a limiting factor in further dose escalation. However, it has been suggested that the traditional MTD dosing of anti-cancer agents may not be optimal when using targeted agents, as the determination of biologically effective dose is a more relevant endpoint and these agents may have the most efficacy with doses below MTD (38-41). It is interesting to note that human Phase I studies have identified a much lower dose of 140 mg/kg/day as the MTD for VPA (14), while we observed no dose limiting toxicities in dogs at 240 mg/kg/day. This is likely due to PK differences (i.e. volume of distribution or bioavailability) between species as 48 hour trough serum concentrations in human patients appear to be much higher than those seen in dogs given comparable doses on a mg/kg basis. This may also be explained by interspecies allometric scaling parameters that would suggest dogs require higher administered doses to achieve the same drug exposure. However, our data may also suggest that dogs require lower overall VPA exposure for biologic efficacy as, in spite of lower serum VPA concentrations compared to human patients, objective responses were observed in our Phase I study with a few responses observed in traditionally anthracycline-resistant tumors. It is possible that higher doses could be administered to dogs that would result in plasma concentrations approximating those seen in human patients, and novel oral formulations of VPA that allow higher doses to be administered with fewer tablet numbers could help to further elucidate the relationships between dose, exposure, and response in dogs. However, trough serum levels of up to 0.5 mM were reached in this study; a level that was shown to be capable of chemosensitization in vitro in our previous work (22). We did not observe any obvious cardiotoxicity in any dogs, although this was not directly evaluated in this study and the total cumulative DOX dose was low (< 150mg/m2).
Serum trough VPA concentrations increased linearly with the administered maintenance dose. The relative weakness of this correlation could be explained by discrepancies in the times that VPA was actually administered to dogs and those reported by owners, or by reduced drug exposure due to post-administration emesis and/or passage of partially digested pills as was reported by a few owners. Pharmacodynamic evaluation of fold change in histone H3 acetylation in fourteen evaluable PBMC samples also correlated with administered dose, but did not correlate with trough VPA levels (data not shown). This would suggest that the use of a pharmacodynamic endpoint, in this case direct target modulation, may give a better estimate of overall drug exposure and activity during the 48 hour treatment period than determining VPA concentrations at a single time point. In this study, PBMC acetylation was not a predictor of objective response. In addition, tumor histotype did not appear to correlate with degree of target modulation in PBMC. However, these assessments may be limited by the small sample size in this study.
We measured DOX pharmacokinetics in three dogs to ensure that VPA pre-treatment had no effects on DOX elimination. Since VPA PK was linear in our dog population, it would be safe to assume that any changes to DOX PK would be manifest in the highest dose cohort, and these three dogs were used for DOX PK comparisons. We did not anticipate any alterations in DOX PK as the mechanisms of metabolism are non-overlapping; VPA is primarily metabolized by complete beta-oxidation, while DOX metabolism occurs primarily via reduction by aldo-keto reductase to form doxorubicinol (31, 42). There were no significant changes in PK of DOX or the major metabolite, doxorubicinol, when compared to two separate historical control populations receiving DOX alone, suggesting that no alterations in DOX dosing are required in dogs receiving combination therapy. Our results of a lack of potentiation of anthracycline-induced side effects are consistent with a report in humans evaluating a combination of VPA and epirubicin, although this report did not specifically evaluate AUC, clearance, or half-life of epirubicin in VPA pre-treated patients (14)
Treatment responses were evaluated by RECIST criteria. Two complete responses were observed, both in dogs with lymphoma. Responses in lymphoma with DOX alone are not unexpected as reported remission rates for treatment-naïve lymphoma in dogs range from 60-85% (43). For this reason, the number of lymphoma dogs in the study was limited to five. Three dogs experienced a partial remission (PR) and included one lymphoma, one melanoma, and one carcinoma. In five dogs, stable disease persisted until treatment was stopped after reaching the maximum DOX dose as opposed to disease progression. Eleven dogs were removed from the study because of progressive disease. No dogs were removed from study due to decreased quality of life (VCOG > 2) or owner request. Although a portion of the objective responses measured may be attributable to DOX alone, it is interesting to note that two of the responses (one PR and one SD) were seen in melanoma, a tumor that is generally resistant to anthracycline therapy in dogs (44).
In addition to demonstrating target modulation in PBMC following VPA treatment, we also found histone H3 hyperacetylation of tumor samples in 4/8 (50%) evaluable samples by IHC, and western blot of these samples confirmed histone hyperacetylation. Tumor tissue target modulation did not appear to predict those dogs with a measurable objective response; however, intrinsic DOX sensitivity of each tumor type may overshadow this type of prediction in this combination study. There was no apparent correlation between administered dose and target modulation in tumor tissue, supporting the idea that dosing to toxicity does not increase likelihood of enhanced therapeutic effect. The lack of correlation between dose and tumor target modulation could also be explained by sampling error; obtaining small biopsies from large heterogeneous tumors may result in post-treatment sampling of areas with different basal acetylation levels or varying blood flow resulting in differences in drug exposure.
In conclusion, this is the first study to evaluate the safety and clinical utility of HDACi in dogs with spontaneous cancer, an extremely useful model for bridging the gap between mouse and human clinical studies. We used a sustained-release formulation of VPA given for 48 hours prior to a standard dose of DOX and demonstrated that serum trough VPA level increased linearly with the dose administered. In addition, we found no evidence that the administration of VPA altered AUC, half-life, or clearance of DOX. VPA administration did not result in significant myelosuppression nor did it potentiate DOX-induced myelosuppression at VPA doses up to 240 mg/kg/day. We were able to demonstrate target modulation, specifically histone H3 hyperacetylation, in both normal and tumor tissues after administration of VPA, and the magnitude of PBMC hyperacetylation correlated positively with the administered dose of VPA. Interestingly, serum trough VPA concentrations did not correlate with magnitude of histone hyperacetylation suggesting that this particular PD marker may be a better overall determinant of 48 hour VPA exposure than trough VPA concentrations. Objective responses were observed in this Phase I study and it is of interest to note that a few responses were seen in traditionally anthracycline-resistant tumors. In this study, we have demonstrated the safety of HDACi in dogs with cancer and have developed the tools necessary for rigorous PK/PD evaluation in future trials of VPA or other HDACi.
Statement of Translational Relevance.
Histone deacetylase (HDAC) enzymes have emerged as an important target in cancer therapy and a growing list of compounds that inhibit HDAC are being evaluated for their ability to enhance the anti-tumor activity of chemotherapy. Traditional pre-clinical murine models often fail to accurately predict the antitumor activity or toxicity observed in human clinical trials. We describe a Phase I pharmacokinetic and pharmacodynamic study of a combination of valproic acid and doxorubicin in spontaneously occurring cancers in canines, a model that, for some tumor types, more closely recapitulates the setting encountered in human clinical trials. Our study shows that valproic acid can be safely administered at biologically active doses with only mild side effects and does not alter the pharmacokinetics of doxorubicin. We have developed the PK/PD tools necessary for future efficacy studies of novel HDACi-containing combinations in canine cancer patients providing the potential for better informed human clinical trial decisions.
Acknowledgments
We would like to thank Dr. Pamela Munster for insightful discussions on clinical trail design, Brad Charles for technical assistance with immunohistochemistry on patient derived samples, and Dr. Christie Anderson for her help with scheduling, sample acquisition, and client communication throughout the clinical trial. We would also like to thank the owners of all the patients enrolled in the trial for their willingness to participate.
Financial Support: Morris Animal Foundation, NIH NCRR T32-RR-007072-06, American Cancer Society RSG-04-219-01
Literature Cited
- 1.Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Molecular oncology. 2007;1:19–25. doi: 10.1016/j.molonc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinberg AP, Tycko B. The history of cancer epigenetics. Nature Reviews Cancer. 2004;4:143–53. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 3.Zhu P, Martin E, Mengwasser J, Schlag P, Janssen KP, Gottlicher M. Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer cell. 2004;5:455–63. doi: 10.1016/s1535-6108(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 4.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nature reviews. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre J, Angels-Moral M, Bozzo J. Combination therapy with valproic acid in cancer: initial clinical approach. Drugs of the Future. 2006;31 [Google Scholar]
- 6.Marchion DC, Bicaku E, Daud AI, Sullivan DM, Munster PN. In vivo synergy between topoisomerase II and histone deacetylase inhibitors: predictive correlates. Mol Cancer Ther. 2005;4:1993–2000. doi: 10.1158/1535-7163.MCT-05-0194. [DOI] [PubMed] [Google Scholar]
- 7.Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer research. 2003;63:7291–300. [PubMed] [Google Scholar]
- 8.Kelly WK, Richon VM, O'Connor O, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–88. [PubMed] [Google Scholar]
- 9.Gilbert J, Gore SD, Herman JG, Carducci MA. The Clinical Application of Targeting Cancer through Histone Acetylation and Hypomethylation. Clinical Cancer Research. 2004;10:4589–96. doi: 10.1158/1078-0432.CCR-03-0297. [DOI] [PubMed] [Google Scholar]
- 10.Karagiannis TC, El-Osta A. Clinical potential of histone deacetylase inhibitors as stand alone therapeutics and in combination with other chemotherapeutics or radiotherapy for cancer. Epigenetics. 2006;1:121–6. doi: 10.4161/epi.1.3.3328. [DOI] [PubMed] [Google Scholar]
- 11.Jaboin J, Wild J, Hamidi H, et al. MS-27-275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer research. 2002;62:6108–15. [PubMed] [Google Scholar]
- 12.Woo HJ, Lee SJ, Choi BT, Park YM, Choi YH. Induction of apoptosis and inhibition of telomerase activity by trichostatin A, a histone deacetylase inhibitor, in human leukemic U937 cells. Exp Mol Pathol. 2007;82:77–84. doi: 10.1016/j.yexmp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Mai A, Massa S, Rotili D, et al. Histone Deacetylation in Epigenetics: An Attractive Target for Anticancer Therapy. Medicinal Research Reviews. 2005;25:261–309. doi: 10.1002/med.20024. [DOI] [PubMed] [Google Scholar]
- 14.Munster P, Marchion D, Bicaku E, et al. Phase I trial of histone deacetylase inhibition by valproic acid followed by the topoisomerase II inhibitor epirubicin in advanced solid tumors: a clinical and translational study. J Clin Oncol. 2007;25:1979–85. doi: 10.1200/JCO.2006.08.6165. [DOI] [PubMed] [Google Scholar]
- 15.Munster P, Marchion D, Bicaku E, et al. Clinical and biological effects of valproic acid as a histone deacetylase inhibitor on tumor and surrogate tissues: phase I/II trial of valproic acid and epirubicin/FEC. Clin Cancer Res. 2009;15:2488–96. doi: 10.1158/1078-0432.CCR-08-1930. [DOI] [PubMed] [Google Scholar]
- 16.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS medicine. 2009;6:e1000161. doi: 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna C, London C, Vail D, Mazcko C, Hirschfeld S. Guiding the optimal translation of new cancer treatments from canine to human cancer patients. Clin Cancer Res. 2009;15:5671–7. doi: 10.1158/1078-0432.CCR-09-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paoloni MC, Tandle A, Mazcko C, et al. Launching a novel preclinical infrastructure: comparative oncology trials consortium directed therapeutic targeting of TNFalpha to cancer vasculature. PloS one. 2009;4:e4972. doi: 10.1371/journal.pone.0004972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimmelman J, Nalbantoglu J. Faithful companions: a proposal for neurooncology trials in pet dogs. Cancer research. 2007;67:4541–4. doi: 10.1158/0008-5472.CAN-06-3792. [DOI] [PubMed] [Google Scholar]
- 20.Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–80. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 21.Kisseberth WC, Murahari S, London CA, Kulp SK, Chen CS. Evaluation of the effects of histone deacetylase inhibitors on cells from canine cancer cell lines. American journal of veterinary research. 2008;69:938–45. doi: 10.2460/ajvr.69.7.938. [DOI] [PubMed] [Google Scholar]
- 22.Wittenburg L, Bisson L, Rose B, Thamm DH. The histone deacetylase inhibitor valproic acid sensitizes human and canine osteosarcoma to doxorubicin. Cancer Chemotherapy Pharmacology. 2010 doi: 10.1007/s00280-010-1287-z. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuchmann M, Schulze-Bergkamen H, Fleischer B, et al. Histone deacetylase inhibition by valproic acid down-regulates c-FLIP/CASH and sensitizes hepatoma cells towards CD95- and TRAIL receptor-mediated apoptosis and chemotherapy. Oncol Rep. 2006;15:227–30. doi: 10.3892/or.15.1.227. [DOI] [PubMed] [Google Scholar]
- 24.Yamanegi K, Yamane J, Hata M, et al. Sodium valproate, a histone deacetylase inhibitor, decreases the secretion of soluble Fas by human osteosarcoma cells and increases their sensitivity to Fas-mediated cell death. Journal of cancer research and clinical oncology. 2009;135:879–89. doi: 10.1007/s00432-008-0522-z. [DOI] [PubMed] [Google Scholar]
- 25.Das CM, Aguilera D, Vasquez H, et al. Valproic acid induces p21 and topoisomerase-II (alpha/beta) expression and synergistically enhances etoposide cytotoxicity in human glioblastoma cell lines. J Neurooncol. 2007 doi: 10.1007/s11060-007-9402-7. [DOI] [PubMed] [Google Scholar]
- 26.Catalano MG, Fortunati N, Pugliese M, et al. Valproic acid, a histone deacetylase inhibitor, enhances sensitivity to doxorubicin in anaplastic thyroid cancer cells. J Endocrinol. 2006;191:465–72. doi: 10.1677/joe.1.06970. [DOI] [PubMed] [Google Scholar]
- 27.Veterinary Co-operative Oncology Group - Common Terminology Criteria for Adverse Events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Veterinary and comparative oncology. 2004;2:195–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 28.Plumb DC. In: Plumb's Veterinary Drug Handbook. Sixth. Plumb DC, editor. Ames, Iowa: Blackwell Publishing; 2008. pp. 916–8. [Google Scholar]
- 29.Wagner J. Noncompartmental and System Analysis Pharmacokinetics for the Pharmaceutical Scientist. Lancaster, PA: Technomic Publishing Company, Inc.; 1993. pp. 83–99. [Google Scholar]
- 30.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 31.Gustafson DL, Rastatter JC, Colombo T, Long ME. Doxorubicin pharmacokinetics: Macromolecule binding, metabolism, and excretion in the context of a physiologic model. Journal of pharmaceutical sciences. 2002;91:1488–501. doi: 10.1002/jps.10161. [DOI] [PubMed] [Google Scholar]
- 32.Selting KA, Ogilvie GK, Gustafson DL, et al. Evaluation of the effects of dietary n-3 fatty acid supplementation on the pharmacokinetics of doxorubicin in dogs with lymphoma. American journal of veterinary research. 2006;67:145–51. doi: 10.2460/ajvr.67.1.145. [DOI] [PubMed] [Google Scholar]
- 33.Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM, Munster PN. Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. Journal of cellular biochemistry. 2004;92:223–37. doi: 10.1002/jcb.20045. [DOI] [PubMed] [Google Scholar]
- 34.de Bono JS, Kristeleit R, Tolcher A, et al. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin Cancer Res. 2008;14:6663–73. doi: 10.1158/1078-0432.CCR-08-0376. [DOI] [PubMed] [Google Scholar]
- 35.Siu LL, Pili R, Duran I, et al. Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. J Clin Oncol. 2008;26:1940–7. doi: 10.1200/JCO.2007.14.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogilvie GK, Reynolds HA, Richardson RC, et al. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. Journal of the American Veterinary Medical Association. 1989;195:1580–3. [PubMed] [Google Scholar]
- 37.Daud AI, Dawson J, DeConti RC, et al. Potentiation of a topoisomerase I inhibitor, karenitecin, by the histone deacetylase inhibitor valproic acid in melanoma: translational and phase I/II clinical trial. Clin Cancer Res. 2009;15:2479–87. doi: 10.1158/1078-0432.CCR-08-1931. [DOI] [PubMed] [Google Scholar]
- 38.Rowinsky EK. The pursuit of optimal outcomes in cancer therapy in a new age of rationally designed target-based anticancer agents. Drugs. 2000;60 1:1–14. doi: 10.2165/00003495-200060001-00001. discussion 41-2. [DOI] [PubMed] [Google Scholar]
- 39.Fox E, Curt GA, Balis FM. Clinical trial design for target-based therapy. The oncologist. 2002;7:401–9. doi: 10.1634/theoncologist.7-5-401. [DOI] [PubMed] [Google Scholar]
- 40.Jain RK, Lee JJ, Hong D, et al. Phase I oncology studies: evidence that in the era of targeted therapies patients on lower doses do not fare worse. Clin Cancer Res. 16:1289–97. doi: 10.1158/1078-0432.CCR-09-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoekstra R, Verweij J, Eskens FA. Clinical trial design for target specific anticancer agents. Investigational new drugs. 2003;21:243–50. doi: 10.1023/a:1023581731443. [DOI] [PubMed] [Google Scholar]
- 42.Silva MF, Ruiter JP, Overmars H, et al. Complete beta-oxidation of valproate: cleavage of 3-oxovalproyl-CoA by a mitochondrial 3-oxoacyl-CoA thiolase. The Biochemical journal. 2002;362:755–60. doi: 10.1042/0264-6021:3620755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vail D, MacEwen EG, Young KM. Canine Lymphoma and Lymphoid Leukemias. In: Withrow SJ, MacEwen EG, editors. Small Animal Clinical Oncology. Third. Philadelphia: W.B. Saunders; 2001. pp. 558–90. [Google Scholar]
- 44.Vail D, Withrow SJ. Tumors of the Skin and Subcutaneous Tissues. In: Withrow SJ, MacEwen EG, editors. Small Animal Clinical Oncology. Third. Philadelphia: W.B. Saunders; 2001. pp. 233–60. [Google Scholar]