Abstract
Resveratrol is a phytochemical with chemopreventive activity in preclinical rodent models of colorectal carcinogenesis. Antiproliferation is one of many chemopreventive modes of action it has been shown to engage. Concentrations of resveratrol which can be achieved in human tissues after oral administration have not yet been defined. The purpose of this study was to measure concentrations of resveratrol and its metabolites in colorectal tissue of humans who ingested resveratrol. Twenty patients with histologically confirmed colorectal cancer consumed 8 daily doses of resveratrol at 0.5 or 1.0g prior to surgical resection. Resveratrol was found to be well tolerated. Normal and malignant biopsy tissue samples were obtained before dosing. Parent compound plus its metabolites resveratrol-3-O-glucuronide, resveratrol-4′-O-glucuronide, resveratrol-3-O-sulfate, resveratrol-4′-O-sulfate, resveratrol sulfate glucuronide and resveratrol disulfate were identified by high pressure liquid chromatography (HPLC) with UV or mass spectrometric detection in colorectal resection tissue. Quantitation was achieved by HPLC/UV. Cell proliferation, as reflected by Ki-67 staining, was compared in pre- and post-intervention tissue samples. Resveratrol and resveratrol-3-O-glucuronide were recovered from tissues at maximal mean concentrations of 674 and 86.0nmol/g, respectively. Levels of resveratrol and its metabolites were consistently higher in tissues originating in the right side of the colon compared to the left. Consumption of resveratrol reduced tumor cell proliferation by 5% (P=0.05). The results suggest that daily oral doses of resveratrol at 0.5 or 1.0g produce levels in the human gastrointestinal tract of an order of magnitude sufficient to elicit anti-carcinogenic effects. Resveratrol merits further clinical evaluation as a potential colorectal cancer chemopreventive agent.
Keywords: Resveratrol, tissue levels, metabolism
Introduction
Resveratrol, (trans-3,5,4′-trihydroxystilbene) is a polyphenolic phytochemical contained in grapes, peanuts and mulberries, which has been shown to prevent malignancies in a variety of target tissues in preclinical models, prominently the colorectum (1). Resveratrol ameliorated formation of aberrant crypt foci (ACF) and/or adenocarcinoma incidence in the colon of rats which had been exposed to the carcinogens azoxymethane or N,N-dimethylhydrazine (2-4). It interfered with adenoma development in the ApcMin mouse, a model of colorectal malignancies associated with an Apc mutation (5, 6). Resveratrol also inhibited colorectal inflammation and carcinogenesis in a murine model of ulcerative colitis (7). These promising preclinical results, together with safety concerns associated with the use of nonsteroidal anti-inflammatory drugs such as aspirin and selective cyclooxygenase (COX) inhibitors in colorectal cancer chemoprevention (8, 9), support consideration of resveratrol for clinical development as a colorectal cancer chemopreventive agent. A recent pilot study of resveratrol at repeated daily doses of up to 5g for 29 days in healthy volunteers showed that it is safe, although at the 2.5 and 5g dose levels it caused reversible diarrhea in some individuals (manuscript submitted). In an earlier pharmacokinetic pilot study, consumption of a single dose of resveratrol at 5.0g, the highest dose employed, generated average peak plasma concentrations of 2.4nmol/mL (10), not dramatically below levels at which resveratrol elicits biochemical effects relevant to cancer chemoprevention in cells in vitro (∼10nmol/mL) (1). Levels of metabolic resveratrol conjugates exceeded those of parent agent by up to almost six-fold, consistent with the poor systemic availability of resveratrol (10). The bioavailability of resveratrol administered either as single agent (10-14) or as integral constituent of a dietary mixture (11,15-17) has been the focus of several recent clinical studies. However, it is not known whether consumption of resveratrol by humans can achieve target organ concentrations commensurate with pharmacological activity observed in preclinical models. Such knowledge is essential to optimize the design of future intervention studies aimed at preventing malignancies.
Prominent among the many modes of action by which resveratrol is considered to exert its chemopreventive efficacy is inhibition of proliferation of preneoplastic or malignant cells (18). In the light of the current interest in resveratrol as a potential colorectal cancer preventive agent, we wished to measure levels of resveratrol and its metabolites in the human colorectum in order to help define doses which may be employed in future colorectal cancer chemoprevention intervention studies of this agent. In addition, we wanted to determine plasma levels of resveratrol and/or its metabolites which accompany those measured in the colorectum. To achieve these aims, patients with confirmed colorectal cancer, who were to undergo surgical resection of their malignancy, received resveratrol at 0.5 or 1g daily for 8 days prior to surgery. Concentrations of parent agent and metabolites were determined in surgically removed tissue and in plasma. Finally, the hypothesis was tested that consumption of resveratrol at these doses may be associated with an anti-proliferative effect in the target tissue. To that end, colorectal cell proliferation reflected by immunohistochemical staining for Ki-67 was compared in tumor tissue obtained by endoscopy prior to intervention and during surgical resection.
Materials and Methods
Patients
The study was approved by the Nottingham UK Research Ethics Committee. Twenty patients with resectable colorectal cancer were recruited into the study at the University Hospitals of Leicester, UK. Patients met the following eligibility criteria: histological diagnosis of colorectal adenocarcinoma, disease amenable to surgical resection; age>18 years; WHO performance status of 0-2; hemoglobin >10g/dL; ALT and serum bilirubin <2.5× and <1.5× the upper limit of normal, respectively; creatinine <140μmol/L. Exclusion criteria included: unfitness for general anesthesia, active peptic ulcer disease; pregnancy or lactation; excessive alcohol intake (more than 21 and 14 units weekly for men and women, respectively); radiotherapy or chemotherapy treatment within 28 days before enrolment, medication within 14 days of enrolment that could interfere with cell proliferation (anticoagulants including warfarin, nonsteroidal anti-inflammatory drugs, steroids). Patients were asked to abstain from consumption of foods and drinks containing resveratrol during the study period and gave written informed consent.
Intervention
Resveratrol was administered as uncoated, immediate release caplets containing 0.5g of resveratrol, supplied by Pharmascience Inc., Montreal, Quebec, Canada. Patients (10 per dose level) received resveratrol, prior to surgical resection, at either 0.5 or 1.0g. The choice of dose was based on the results of a recent phase I repeat dose pharmacokinetic study of resveratrol daily doses of 0.5-5.0g in healthy volunteers, in whom the 0.5 and 1.0g doses ingested daily for 29 days was very well tolerated (manuscript submitted). Resveratrol was taken in the evening, between the hours of 17:00-22:00 each day for 8 days. The last dose was administered on the evening prior to surgical resection. Study participants were assessed for adverse events, graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0, 2006).
Sample preparation
Blood and colorectal tissues were collected pre-dosing at diagnostic endoscopy and post-dosing at resection surgery. At endoscopy, up to twelve biopsies were collected, six each of malignant tissue and macroscopically normal tissue ∼5-10 cm away from the malignancy, in addition to those required for standard diagnostic purposes. The mean time periods between last dose and surgical resection or between last dose and blood collection were 18h (range 15-23h) and 17.8h (range 11-21h), respectively. Right sided tumors were resected by right hemicolectomy, left-sided by anterior resection or sigmoid colectomy. Resected bowel tissue was placed on ice and protected from light. One malignant tissue sample (including mucosa) and, where possible, between 2 and 6 histologically normal tissue samples were taken from each patient. Sample weight was ∼0.5-1.0g. Normal tissue was taken at a distance of ∼5 and/or ∼10cm from the proximal and distal resection margins with a similar distance to the tumor; some samples were also taken from the margins. For each patient half the number of samples were snap-frozen and kept at -80°C until analysis, the other half were fixed in formalin for 24h and embedded in paraffin wax.
Venous blood was collected in lithium heparin tubes pre-dose and at the time of resection. Tubes were chilled on ice and protected from light. Blood samples were kept on ice and centrifuged (3000×g, 4°C, 15min); the supernatant was removed and frozen. Plasma and tissues were kept at -80°C until analysis, which was performed within 6 months.
Analysis of resveratrol and resveratrol metabolites
Frozen tissue samples were mixed with liquid nitrogen and ground, then weighed and homogenized with 3 parts (w/v) of HEPES buffer. Samples of tissue homogenate or plasma were extracted with methanol and analyzed by HPLC/UV as described previously (10, 19). Separation was achieved on a Waters Atlantis C18 column (4.6mm × 150mm 3μm, Waters, Elstree, UK) in combination with a Waters Atlantic C18 guard column (4.6mm × 20mm, 3μm). Quantitation of resveratrol using a gradient HPLC/UV system (Waters Alliance 2695, Manchester, UK) was performed using an internal standard (naringenin) and a method which had previously been validated, in terms of inter- and intra-day variability, recovery, accuracy and precision. Tissue and plasma samples were extracted and analysed in duplicate and the mean value used. The extraction efficiency for resveratrol from tissue homogenate was 95.2±2.8% (mean±SD, n=7). The limit of quantitation was 175pmol resveratrol/g tissue and 22pmol/mL plasma, whilst the limit of detection was half of these values. Resveratrol and its metabolites in biomatrices were stable under the storage and assay conditions. As authentic resveratrol metabolites were not available in sufficient quantities as reference materials, metabolite amounts were calculated as “resveratrol equivalents”, on the assumption that recovery characteristics and relationship between peak area ratio and concentration were the same as for parent resveratrol. Authentic resveratrol-3-O-sulfate (provided by Pharmascience, Montreal), resveratrol-4′-O-sulfate, resveratrol-3-O-glucuronide and resveratrol-4′-O-glucuronide became available during the course of the study by in-house synthesis, permitting HPLC peak identification, so that resveratrol metabolites could be identified by co-chromatography. Metabolite identity was confirmed by liquid chromatography-tandem mass spectrometry (LC/MS/MS) with selected reaction monitoring (SRM), operated in the negative ion mode using a Waters Alliance 2695 series HPLC with a Waters Quattro Ultima Pt. triple quadrupole mass spectrometer (Waters, Manchester, UK) under chromatographic conditions described previously (10, 19). Definitive isomer identification was not possible for resveratrol disulfate and resveratrol sulfate glucuronide.
Analysis of cell proliferation
Colorectal tumor sections, obtained pre-intervention and at the time of surgery, were stained for Ki-67 (mouse anti-human monoclonal antibody, Dako, Ely, UK). Briefly, paraffin-embedded sections (4μm) mounted on polysine-coated slides were dewaxed (65°C, 20min) and hydrated through a graded series of alcohol rinses. The antigen was unmasked by microwaving sections (20min) in Tris-EDTA buffer (pH 9). Endogenous peroxidase activity was inactivated by incubation of slides with hydrogen peroxide (3%, 10min); nonspecific binding was blocked with protein block solution. Sections were incubated with primary antibody (dilution 1:2000) overnight at 4°C. After washing (PBS) sections, the tissue-antibody reaction was visualized using a commercial kit (Dako). Representative fields were selected in biopsies and superficial regions of the resected tumor of all patients and in normal surgical specimens from 5 patients on the 0.5g dose. The total number of epithelial cells, and the number of positively (brown) staining epithelial cells were counted in six adjacent high power fields (magnification ×400; Leitz Orthoplan microscope, Leica DC 300 camera) for each sample, by two independent observers. Analyses were performed blinded. Differences in counts between observers were less than 10%, and both recorded the same differences between cohorts. Acqusition software was Adobe Photoshop version 7. Numbers of epithelial cells counted in samples stained for Ki-67 were as follows: 1670±625 in pre- and 1592±472 (mean±SD, n=21 sections) in post-intervention malignant tissue, 1343±135 in pre- and 1666±335 (n=5 sections) in post-intervention normal tissue. Values quoted under Results denote percentage of cells which stained positively.
Statistical analysis
Statistical comparison of results for Ki-67 immunostaining of pre- and post-intervention tissues was made either by paired Students t or in the case of the normal tissue by nonparametric Wilcoxon-Mann Whitney test because of the small sample size, using Statistical Package for the Social Sciences (SPSS) version 13 (Windows XP). P values of 0.05 were considered to indicate significance.
Results
Patient demographics and safety of resveratrol
The demographics of the twenty patients with confirmed colorectal cancer who were recruited into the study and the nature, stage and location of their tumors are described in Table 1. One patient presented with 2 synchronous colorectal tumors, in the sigmoid colon and cecum, which were resected during the same surgical operation. Patients ingested resveratrol at 0.5 or 1.0g daily for 8 days prior to surgery. There were no resveratrol-related adverse events, and it was well tolerated at both doses.
Table 1.
Characteristics of patients who were recruited into the study (A), and of their tumors (B)
| A | |
|---|---|
| Patient characteristics | |
| Age (and range) (years) | 66.8±17.2 (46-83) |
| Males:females | 9:11 |
| Caucasian:Asian | 18:2 |
| BMI (and range) (kg/m2) | 25.3±2.68 (17.4-29.2) |
| B | ||
|---|---|---|
| Tumor characteristics | ||
| Location | Cecum | 6 |
| Hepatic flexure/transverse colon | 2 | |
| Sigmoid colon | 10 | |
| Recto-sigmoid | 3 | |
| Operation | Laparoscopy | 8 |
| Laparotomy | 13 | |
| Resection | Right hemicolectomy | 8 |
| Left colectomy/sigmoid colectomy | 2 | |
| Anterior resection | 11 | |
| Histology | Differentiation - moderate:poor | 19:2 |
| Lymphocytic invasion:extravascular invasion | 4:5 | |
| Complete excision (R0):incomplete excision (R1) | 20:1 | |
| Dukes staging | A:B:C1 | 5:7:9 |
NOTE: One patient presented with two colorectal tumors
Identification of resveratrol and its metabolites in colorectal tissue
Resveratrol and its metabolites were recovered from human colorectal tissue and identified by HPLC/UV co-chromatography with authentic reference material. Identity was confirmed by suitable SRM mass transitions of mass-to-charge ratios (m/z) determined by HPLC/MS/MS analysis. Fig.1A shows a representative HPLC/UV chromatogram of colorectal tissue extracts from a patient who had ingested 8 doses of resveratrol, juxtaposed for comparison with a chromatogram from another patient, who had discontinued intervention 7 days prior to bowel resection and was replaced in the study (patient choice), and no longer had detectable resveratrol species in his/her tissue. Resveratrol afforded a substantial peak in the majority of samples. The following species could be identified by co-chromatography with authentic reference material and by mass spectrometry (Fig. 1B): resveratrol (m/z 227>184), resveratrol-3-O-glucuronide and resveratrol-4′-O-glucuronide (m/z 403>227), resveratrol-3-O-sulfate and resveratrol-4′-O-sulfate (m/z 307>227), resveratrol disulfate (m/z 387>227) and resveratrol sulfate glucuronide (m/z 483>227) (Fig.1B). The HPLC eluent containing the latter peak was collected and the analyte identified by transitions of m/z 483>227 (loss of sulfate and glucuronide), 483>403 (loss of sulfate), 483>307 (loss of glucuronide) and 227>184 (characteristic transition for resveratrol). Resveratrol sulfate glucuronide was a prominent metabolite in colorectal tissue of five out of the ten patients on the 0.5g dose and in nine of the ten patients on 1.0g. Overall, these findings contrast with results obtained previously in the plasma of healthy volunteers, in whom the amount of resveratrol recovered was relatively small compared to that of its metabolites, with resveratrol sulfate glucuronide being only a minor metabolite (10).
Figure 1.
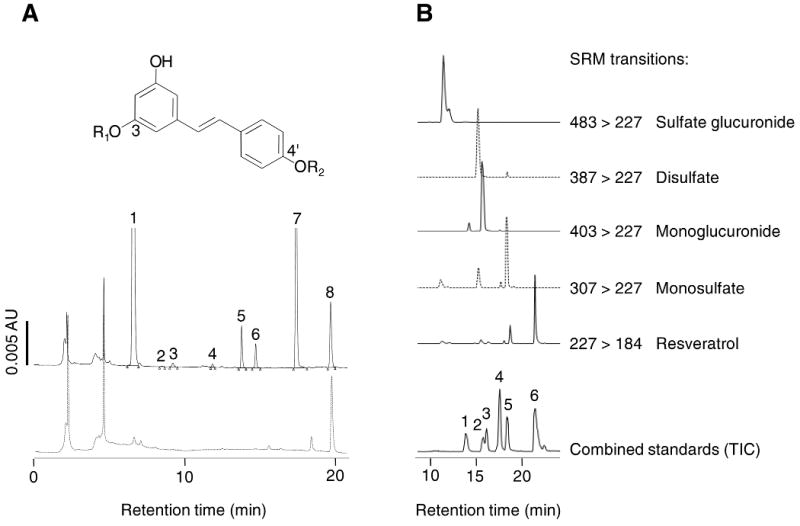
Figure 1 A. HPLC/UV chromatograms of extracts of normal colon tissue resected from a patient who had received resveratrol 1.0g daily for 8 days (solid line) and a patient who had refrained from resveratrol ingestion during the 7 days prior to resection (broken line). Identity of resveratrol-derived species as established by co-chromatography with authentic reference material and LC/MS/MS analysis is indicated above the peaks. Insert shows structures of resveratrol and its metabolites: R1, R2=H: resveratrol “7”, R1=sulfate R2=H: resveratrol-3-O-sulfate “6”, R1=H, R2=sulfate: resveratrol-4′-O-sulfate “5”, R1=glucuronide, R2=H: resveratrol-3-O-glucuronide “4”, R1=H, R2=glucuronide: resveratrol-4′-O-glucuronide “2”. Positions of the sulfonic and glucuronic acid moieties in resveratrol disulfate “3” and resveratrol sulfate glucuronide “1” are probably 3 and 4′, but this needs confirmation by 1H-NMR. Naringenin “8” was the internal standard. For details of tissue procurement, extraction and chemical analysis see Materials and Methods.
Figure 1 B. LC/MS selected reaction monitoring (SRM) of transitions for the identification of resveratrol metabolites in extracts of colon tissue taken from a patient who had received 0.5g of resveratrol for 8 days. Metabolites identified were resveratrol sulfate glucuronide (m/z 483>227), resveratrol disulfate (m/z 387>227) resveratrol-3-O-glucuronide and resveratrol-4′-O-glucuronide (m/z 403>227), resveratrol-3-O-sulfate and resveratrol-4′-O-sulfate (307>227). Resveratrol was also identified (m/z 227>184). The total ion current from the analysis of a mixture of authentic standards is also shown for comparison. The mixture contained resveratrol-4′-O-glucuronide (1), resveratrol-3-O-glucuronide (2), dehydrated resveratrol glucuronide (exhibits the transition 385>227 and is not present in the patient samples) (3), resveratrol-4′-O-sulfate (4), resveratrol-3-O-sulfate (5) and resveratrol (6).
Concentrations of resveratrol and its metabolites in colorectal tissue
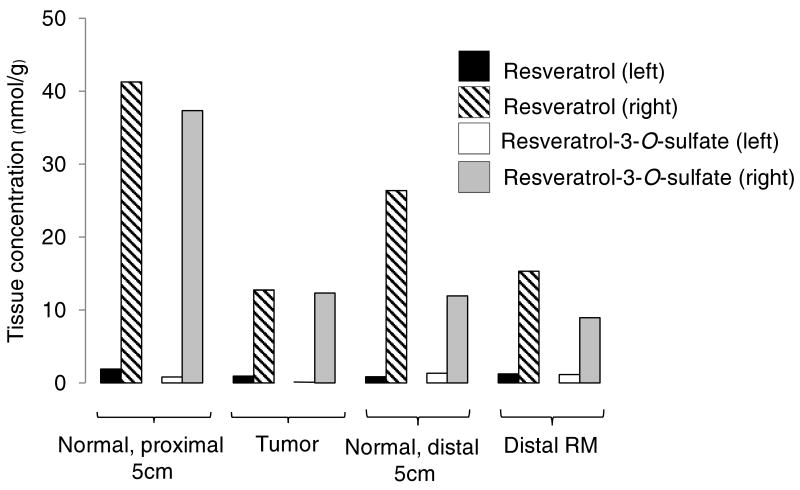
Parent agent and five metabolites were quantitated in tumor and normal tissues from patients on resveratrol (Table 2 and Table 3). Individual values varied substantially from each other, reflected by coefficients of variation in some cases exceeding 200%, both between specific tissue sites for each individual and for each site between patients. In most tissues, concentrations of resveratrol and its metabolic conjugates were higher in samples of right-sided origin (cecum, ascending colon, hepatic flexure/transverse colon) than in those from the left side (splenic flexure, descending colon, sigmoid colon, rectum) (Table 2 and Table 3). Highest mean concentrations of parent resveratrol after the 0.5 and 1.0g doses were 18.6 and 674nmol/g (or nmol/mL, assuming 1mL weighs 1g), respectively, in normal tissue localized proximal to the tumor on the right side, and 8.33 and 94.1nmol/g, respectively, in right-sided tumors. Maximal mean tissue concentrations determined for resveratrol metabolites (in nmol resveratrol equivalents/g) were 86.0 for resveratrol-3-O-glucuronide at the 0.5g dose level and 67.2 for resveratrol-3-O-sulfate in patients on 1.0g resveratrol, both observed in normal right-sided colorectal tissue proximal to the tumor (Table 2 and Table 3 respectively). The difference in levels between left- and right-sided tumors is best illustrated in a patient bearing two colorectal tumors, in whom the concentrations of resveratrol-derived species were considerably higher in the cecal (right-sided) than the sigmoid colonic (left-sided) tumor (Figure 2).
Table 2.
Concentrations of resveratrol and its major metabolites in normal tissue (proximal or distal to the tumor) and tumor tissue, obtained from the right (cecum, ascending colon, hepatic flexure) or left side (splenic flexure, descending colon, sigmoid colon, rectum) of the colorectum, in patients who received resveratrol daily for 8 days at 0.5g.
| Species | Tissue Levels (nmol/g)* | |||||
|---|---|---|---|---|---|---|
| Proximal to Tumor | Tumor | Distal to Tumor | ||||
| Left | Right | Left | Right | Left | Right | |
| Resveratrol | 0.67±0.72 (0–3.0) |
18.6±17.4 (0–45.9) |
0.63±0.69 (0–1.80) |
8.33±6.06 (3.1–15.0) |
0.48±0.47 (0–1.04) |
4.94±4.82 (1.95–13.5) |
| Resveratrol-3-O-glucuronide | loq** | 86.0±125 (0–317) |
lod | 0.73±1.26 (0–2.18) |
loq | 0.64±0.46 (0–0.94) |
| Resveratrol-4′-O-glucuronide | 0.17±0.34 (0–0.93) |
7.91±11.2 (0–28.2) |
loq | 0.29±0.50 (0–0.87) |
0.28±0.43 (0–0.99) |
lod |
| Resveratrol sulfate glucuronide | 17.1±20.8 (0–61.1) |
44.5±47.9 (0–149) |
12.8±15.9 (0–34.6) |
5.09±7.86 (0–14.2) |
20.0±39.7 (0–121) |
lod |
| Resveratrol-3-O-sulfate | 0.82±0.77 (0–2.84) |
34.0±48.6 (0.67–128) |
0.44± 0.70 (0–1.90) |
3.09±2.13 (0.68–4.75) |
0.94±1.17 (0–3.40) |
2.37±0.43 (1.86–2.93) |
| Resveratrol-4′-O-sulfate | loq | 1.21±1.78 (0–4.52) |
0.25± 0.43 (0–1.02) |
0.26±0.46 (0–0.79) |
lod | 0.17±0.38 (0–0.85) |
Values are the mean±SD of 7 samples from tissues in the left side and 3 from the right side, with one tumor sample and between 1 and 3 normal tissue samples (proximal or distal to the tumor) per patient. Range in brackets.
loq=close to or below the limit of quantitation; lod=below the limit of detection.
Table 3.
Concentrations of resveratrol and its major metabolites in normal tissue (proximal or distal to the tumor) and tumor tissue, obtained from the right (cecum, ascending colon, hepatic flexure) or left side (splenic flexure, descending colon, sigmoid colon, rectum) of the colorectum, in patients who received resveratrol daily for 8 days at 1.0g.
| Species | Tissue Levels (nmol/g)* | |||||
|---|---|---|---|---|---|---|
| Proximal to Tumor | Tumor | Distal to Tumor | ||||
| Left | Right | Left | Right | Left | Right | |
| Resveratrol | 1.21±1.33 (0–5.23) |
674±1303 (10.1–3774) |
2.07±3.27 (0.30–8.68) |
94.1±89.2 (12.7–195) |
2.07±3.25 (0.28–9.40) |
62.5±76.2 (10.7–272) |
| Resveratrol-3-O-glucuronide | 0.19±0.22 (0–0.61) |
10.8±17.2 (0–50.8) |
lod** | 0.75±0.70 (0–1.85) |
0.28±0.29 (0–0.71) |
0.83±0.81 (0–3.24) |
| Resveratrol-4′-O-glucuronide | 0.43±0.41 (0–1.22) |
1.71±1.87 (0–5.31) |
0.38±0.47 (0–1.05) |
0.53±0.79 (0–1.78) |
0.53±0.48 (0–1.35) |
0.83±0.77 (0–2.37) |
| Resveratrol sulfate glucuronide | 19.5±4.82 (10.7–27.3) |
27.1±21.6 (10.4–94.6) |
20.7±5.04 (11.7–25.4) |
29.1±12.8 (16.4–50.6) |
25.2±17.2 (4.55–51.8) |
18.4±5.76 (9.6–29.4) |
| Resveratrol-3-O-sulfate | 0.67±0.56 (0–1.73) |
67.2±119 (3.87–366) |
0.16±0.39 (0–0.95) |
10.4±13.6 (1.80–33.4) |
1.10±1.21 (0–3.57) |
5.82±3.02 (1.59–11.9) |
| Resveratrol-4′-O-sulfate | lod | 3.43±3.36 (0.32–12.6) |
lod | 3.09±4.72 (0–11.33) |
loq | 0.90±0.70 (0–2.18) |
Values are the mean±SD of 6 samples from tissues on the left side and 5 from the right side, with one tumor sample and between 1 and 3 normal tissue samples (proximal or distal to the tumor) per patient. Range in brackets.
lod=below the limit of detection; loq=close to or below the limit of quantitation.
Figure 2.
Concentrations of resveratrol and resveratrol-3-O-sulfate in normal colon and tumor tissue of a patient with a cecal (right-sided) and a sigmoid colonic tumor (left-sided), who had received resveratrol at a dose of 1.0g daily for 8 days. Normal tissue samples were taken at a distance of 5cm proximal or distal from tumor, and from the distal resection margin (RM). Differences in levels between the left and right side, similar to those shown here, were observed for resveratrol disulfate and the two resveratrol monoglucuronides, but not for resveratrol sulfate glucuronide.
Concentrations of resveratrol and its metabolites in plasma
Plasma was obtained from these patients at the point of surgery and analyzed for resveratrol-derived species. Resveratrol was present at levels close to, or below, the limit of detection. Five resveratrol conjugates circulated at quantifiable concentrations and these were identified by mass spectrometry as the two monoglucuronides, resveratrol-3-O-sulfate, resveratrol sulfate glucuronide and resveratrol disulfate (Table 4). The highest mean level, 22.3nmol/mL, was observed for resveratrol sulfate glucuronide in patients on 1.0g resveratrol.
Table 4.
Concentrations of resveratrol and its major metabolites in the plasma of patients who received resveratrol daily for 8 days at 0.5 or 1.0g
| Plasma Levels (nmol/mL)* | ||
|---|---|---|
| Species | 0.5g | 1.0g |
| Resveratrol | loq** | loq |
| Resveratrol-3-O-glucuronide | loq | 0.24±0.13 (0.07-0.50) |
| Resveratrol-4′-O-glucuronide | 0.04±0.06 (0-0.16) |
0.24±0.17 (0.05-0.57) |
| Resveratrol sulfate glucuronide | 13.4±16.5 (0-34.9) |
22.3±10.1 (6.67-36.3) |
| Resveratrol disulfate | 0.31±0.20 (0-0.59) |
0.60±0.81 (0.17-2.86) |
| Resveratrol-3-O-sulfate | 0.13±0.15 (0-0.52) |
0.59±0.41 (0.17-1.33) |
| Resveratrol- 4′-O-sulfate | loq | loq |
Values are the mean±SD of 10 patients per dose (and range).
loq=close to or below the limit of quantitation.
Effect of resveratrol on colorectal cell proliferation
Ki-67 is a granular component of the nucleolus expressed exclusively in proliferating cells and commonly used as a surrogate marker of cell growth. Levels of Ki-67 were measured immunohistochemically in epithelial cells of biopsy and resection tissues. In all patients collectively, tumor cell Ki-67 staining was reduced from 88.0±6.64% in pre-dose biopsy samples to 83.2±10.0% in post-intervention surgical tissue (n=20, P=0.05 paired Students T test). When the 0.5 and 1.0g dose groups were analyzed separately, resveratrol consumption still decreased tumor cell Ki-67 staining, by 5.6 and 1.9%, respectively, albeit this reduction was not significant. Ki-67 staining was also assessed in normal tissue of 5 patients on the 0.5g dose, and staining was reduced from 74.6±20.6% in biopsies to 67.6±15.4% in surgical tissues (P=0.05, Wilcoxon-Mann Whitney test).
Cursory analysis of tumor tissue before (biopsy) and after intervention (resection tissue) did not show any histopathological differences.
Discussion
The results outlined above define for the first time concentrations of resveratrol and its metabolites in the colorectum of individuals who ingested resveratrol repeatedly. The highest mean resveratrol tissue concentrations observed, 18.6 and 674nmol/g for the 0.5 and 1.0g dose levels, respectively, are close to, or exceed, 36nmol/g, the mean concentration of resveratrol measured in the gastrointestinal tract of ApcMin mice on resveratrol at 0.2% in the diet (6). This gastrointestinal tissue level of resveratrol accompanied reduction of ApcMin adenoma number by 27%, compared to mice on control diet. Parent resveratrol accounted for a much larger proportion of total resveratrol species in colorectal tissue than in the plasma at an equivalent time point post-dosing, which supports the notion that the colorectum may be a suitable target for chemoprevention by oral resveratrol. The colorectal tissue concentrations described here for resveratrol are above those achieved after repeated consumption of curcumin at 3.6g, another putatively chemopreventive polyphenol, which were 7.7 and 12.7nmol/g in tumor and normal tissue, respectively (20). In human-derived colon cells in vitro, resveratrol has been shown to elicit biochemical effects commensurate with anti-carcinogenesis, such as growth inhibition and apoptosis induction, at concentrations exceeding ∼10nmol/mL (21-23). For example, the mean IC50 values for the resveratrol-mediated inhibition of growth of human-derived HT-29 or HCA-7 colon cancer cells were 19.9 and 26.2nmol/mL, respectively (23). Although the concentrations measured here in the colorectum of patients were highly variable, they suggest that the doses administered, 0.5 and 1.0g, can give colorectal tissue levels associated with chemopreventive activity. It is important to note that these doses are similar to, or considerably above, those which have been shown to prevent colorectal malignancies in preclinical models. Daily doses of resveratrol in rodents which, when administered for an extended period of time, exerted chemopreventive activity, and their corresponding counterparts in a 70kg human (obtained by body surface area extrapolation (24)), were as follows: 15 or 240mg/kg in the ApcMin mouse (5, 6) equating to 81mg and 1.3g in humans; 0.2 mg/kg in rats exposed to azoxymethane (2) equating to 1.9mg in humans; 8mg/kg in rats which received N,N-dimethylhydrazine (3) equating to 75mg in humans; the human equivalent doses which reduced azoxymethane-induced colon cancer in a mouse model of colitis were 116 and 232mg (7).
We recovered resveratrol conjugates at quantifiable concentrations from the colorectum, with resveratrol-3-O-glucuronide furnishing the highest value at 86nmol resveratrol equivalents/g. This finding is important as it has been speculated that resveratrol conjugates may contribute to the pharmacological efficacy of its parent (1), a theory which still requires experimental verification. Concentrations of resveratrol and its metabolites varied depending on the anatomical site from where the tissue originated, with generally higher values in the right-sided than left-sided colorectum. Feces are transported from the cecum on the right side across the transverse to the sigmoid colon and rectum on the left, and resveratrol tissue concentrations are likely to be related to those in the feces. So it is conceivable that concentrations are higher in right-sided tissues as they come into contact with fecal resveratrol earlier than those on the left side. Furthermore, the feces are liquid in the right-sided colorectum becoming more solid as they pass onto the left, and the fluid environment of the right colon is probably better suited to the permeation and absorption of small molecular weight species such as resveratrol than the semi-solid environment on the left (25). Whilst plasma levels of parent resveratrol which accompanied tissue levels were at, or below, the limit of quantitation, resveratrol monoglucuronides, resveratrol-3-O-sulfate, resveratrol disulfate and resveratrol sulfate glucuronide circulated at quantifiable levels, consistent with the low systemic availability of resveratrol and its avid conjugative metabolism (1). The implication of this finding is that the presence of resveratrol conjugates in the circulation may be exploited as markers of adherence in future intervention trials of resveratrol.
There was an indication that resveratrol exerted a small reduction in cell proliferation in colorectal tissue after ingestion. Whilst the biological importance of such a slight decrease is debatable, its observation suggests that, in principle, resveratrol can exert a pharmacological effect in the human colorectum. It needs to be stressed that pharmacodynamic data based on the comparison of phenomena in biopsies with those in resection tissue have to be interpreted with utmost caution, because of differences in localization, method of surgical procurement and size of samples. Nevertheless, compromising cell proliferation is one of the modes of action by which resveratrol is thought to exert its chemopreventive efficacy, and the slight reduction in cell proliferation observed here is consistent with the effect of resveratrol on colorectal cells in vitro (21-23) and colorectal tissue in rats in vivo (3).
In conclusion, the results described here suggest that daily doses of resveratrol at 0.5 and 1.0g can furnish levels in the human gastrointestinal tract that are of an order of magnitude sufficient to elicit pharmacological effects. Therefore, resveratrol merits further clinical evaluation as a potential alternative to non-steroidal anti-inflammatory agents and selective COX inhibitors in colorectal cancer chemoprevention.
Acknowledgments
We thank Sarah Porter, Tracey Cook and Simone Daly for help with patient identification and Mike Thomas and John Jameson (all at University Hospitals of Leicester) for provision of resection material.
Grant Support
The study was supported by US National Cancer Institute contract NCI-N01-CN-25025, programme grant C325/A6691 from Cancer Research UK and an Experimental Cancer Medicine Centre grant (Cancer Research UK and UK Department of Health).
Footnotes
Disclosure of Potential Conflicts of Interest
T Booth, employment, Pharmascience, Montreal Canada. The other authors declared no potential conflict of interest.
References
- 1.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 2.Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21(CIP) expression. Carcinogenesis. 2000;21:1619–22. [PubMed] [Google Scholar]
- 3.Sengottuvelan M, Viswanathan P, Nalini N. Chemopreventive effect of trans-resveratrol - a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis. 2006;27:1038–46. doi: 10.1093/carcin/bgi286. [DOI] [PubMed] [Google Scholar]
- 4.Sengottuvelan M, Nalini N. Dietary supplementation of resveratrol suppresses colonic tumour incidence in 1,2-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Brit J Nutr. 2006;96:145–53. doi: 10.1079/bjn20061789. [DOI] [PubMed] [Google Scholar]
- 5.Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer. 2001;39:102–7. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- 6.Sale S, Tunstall RG, Ruparelia KC, Potter GA, Steward WP, Gescher AJ. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene (DMU 212). on adenoma development in the ApcMin+ mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int J Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- 7.Cui X, Jin Y, Hofseth AB, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res. 2010;3:549–59. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dube C, Rostom A, Lewin G, et al. The use of aspirin for primary prevention of colorectal cancer: A systematic review prepared for the US Preventive Services Task Force. Ann Intern Med. 2007;146:365–75. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- 9.Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. N Engl J Med. 2006;355:950–2. doi: 10.1056/NEJMe068158. [DOI] [PubMed] [Google Scholar]
- 10.Boocock DJ, Faust GES, Patel KR, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers & Prev. 2007;16:1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 11.Meng XF, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J Agric Food Chem. 2004;52:935–42. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 12.Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Disp. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 13.Almeida L, Vaz-da-Silva M, Falcao A, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Molec Nutr Food Res. 2009;53:S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 14.Nunes T, Almeida L, Rocha JF, et al. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J Clin Pharmacol. 2009;49:1477–82. doi: 10.1177/0091270009339191. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg DA, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 16.Vitaglione P, Sforza S, Galaverna G, et al. Bioavailability of trans-resveratrol from red wine in humans. Molec Nutr Food Res. 2005;49:495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 17.Zamora-Ros R, Urpi-Sarda M, Lamuela-Raventos RM, et al. Diagnostic performance of urinary resveratrol metabolites as a biomarker of moderate wine consumption. Clin Chem. 2006;52:1373–80. doi: 10.1373/clinchem.2005.065870. [DOI] [PubMed] [Google Scholar]
- 18.Athar M, Back JH, Tang X, et al. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–83. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boocock DJ, Patel K, Faust GES, et al. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J Chromatog B. 2007;848:182–7. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcea G, Jones DJL, Berry DP, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers & Prev. 2005;14:120–5. [PubMed] [Google Scholar]
- 21.Schneider Y, Vincent F, Duranton B, et al. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. 2000;158:85–91. doi: 10.1016/s0304-3835(00)00511-5. [DOI] [PubMed] [Google Scholar]
- 22.Fuggetta MP, Lanzilli G, Tricarico M, et al. Effect of resveratrol on proliferation and telomerase activity of human colon cancer cells in vitro. J Exp & Clin Cancer Res. 2006;25:189–93. [PubMed] [Google Scholar]
- 23.Sale S, Verschoyle RD, Boocock D, et al. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Brit J Cancer. 2004;90:736–44. doi: 10.1038/sj.bjc.6601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–44. [PubMed] [Google Scholar]
- 25.Hebden JM, Gilchrist PJ, Perkins AC, Wilson CG, Spiller RC. Stool water content and colonic drug absorption: Contrasting effects of lactulose and codeine. Pharm Res. 1999;16:1254–9. doi: 10.1023/a:1014805815499. [DOI] [PubMed] [Google Scholar]